New Glass-Ceramics in the System Ca2SiO4-Ca3(PO4)2—Phase Composition, Microstructure, and Effect on the Cell Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of the Glass-Ceramics
2.2.2. Characterization of the Glass-Ceramics
2.2.3. Materials and Methods for Assessing Cell Viability
Cell Line and Culturing
Wright Cell Staining
MTT Assay for Cell Growth
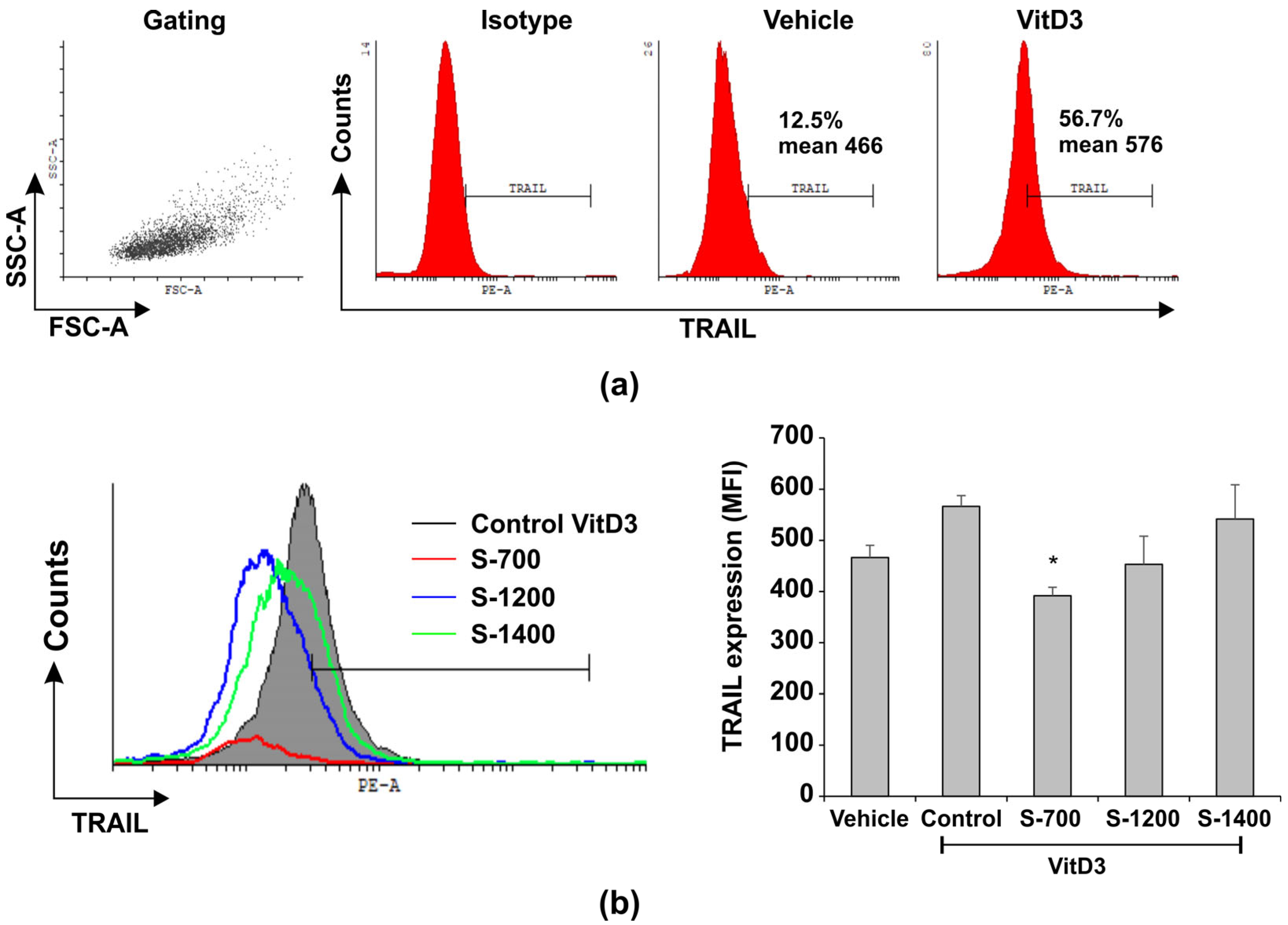
Flow Cytometry for Evaluation of Surface TRAIL
2.3. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Glass-Ceramics
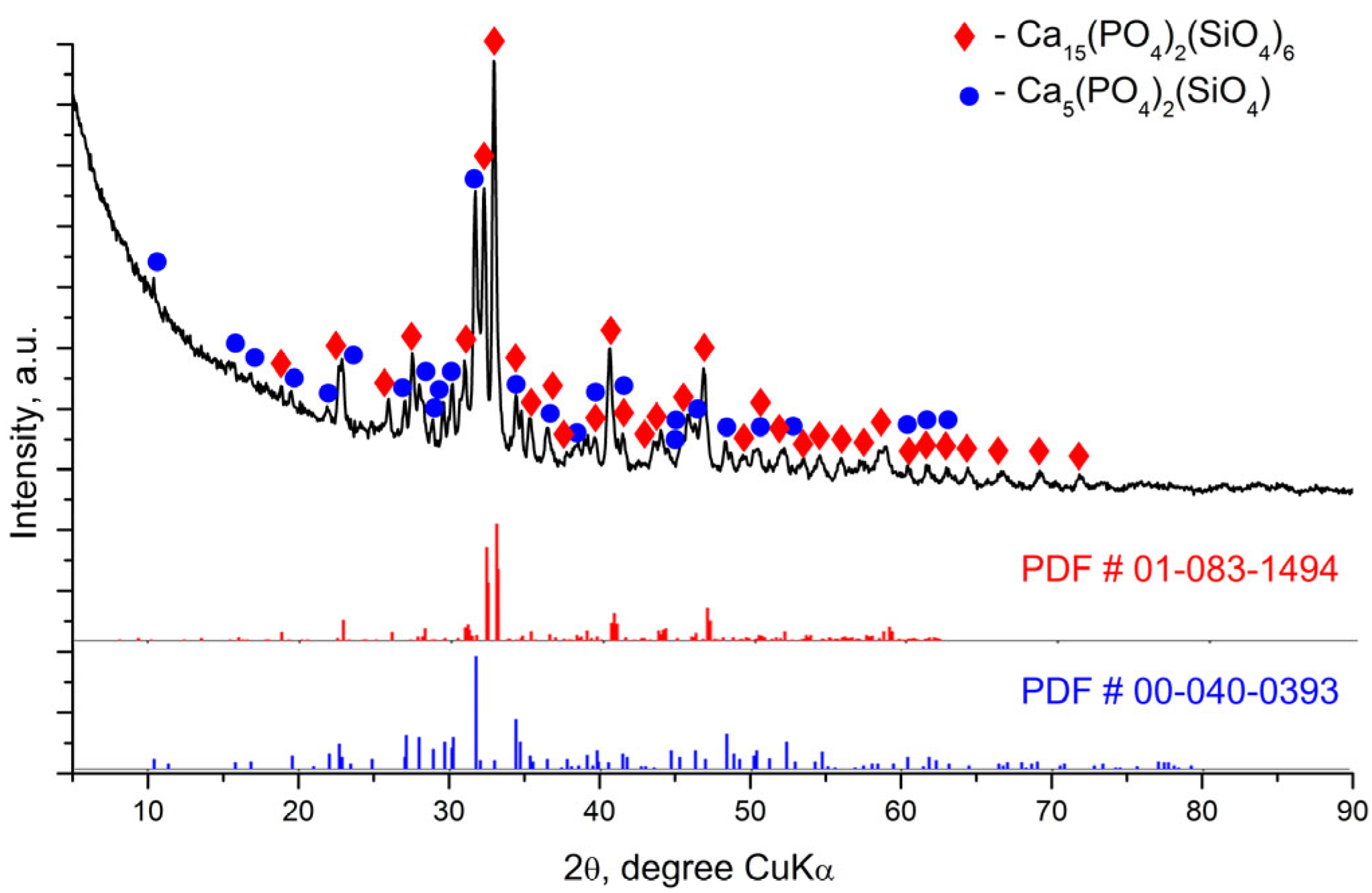
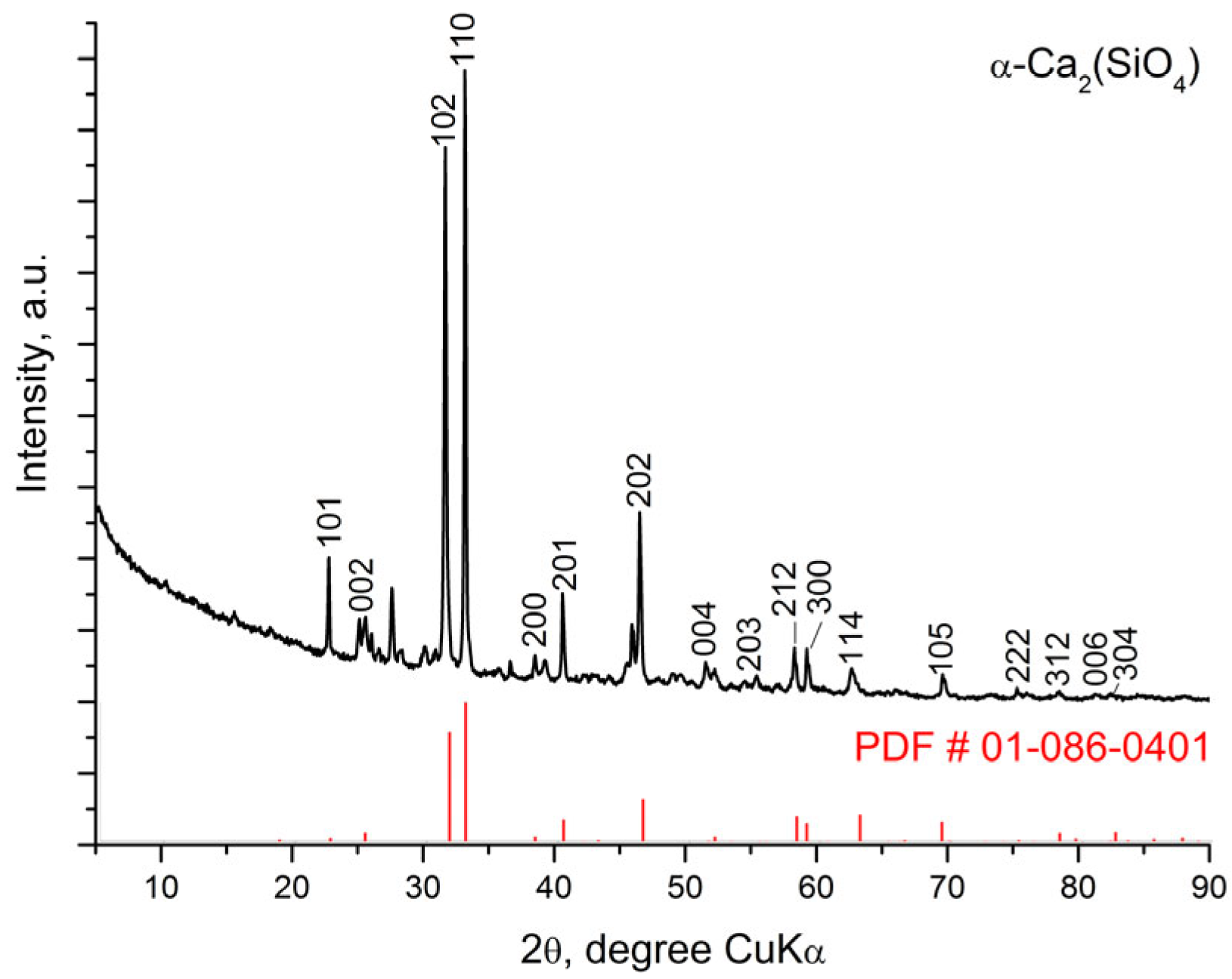
3.2. X-Ray Diffraction Analysis (XRD) of the Glass-Ceramics Synthesized After Heating at Different Tempratures
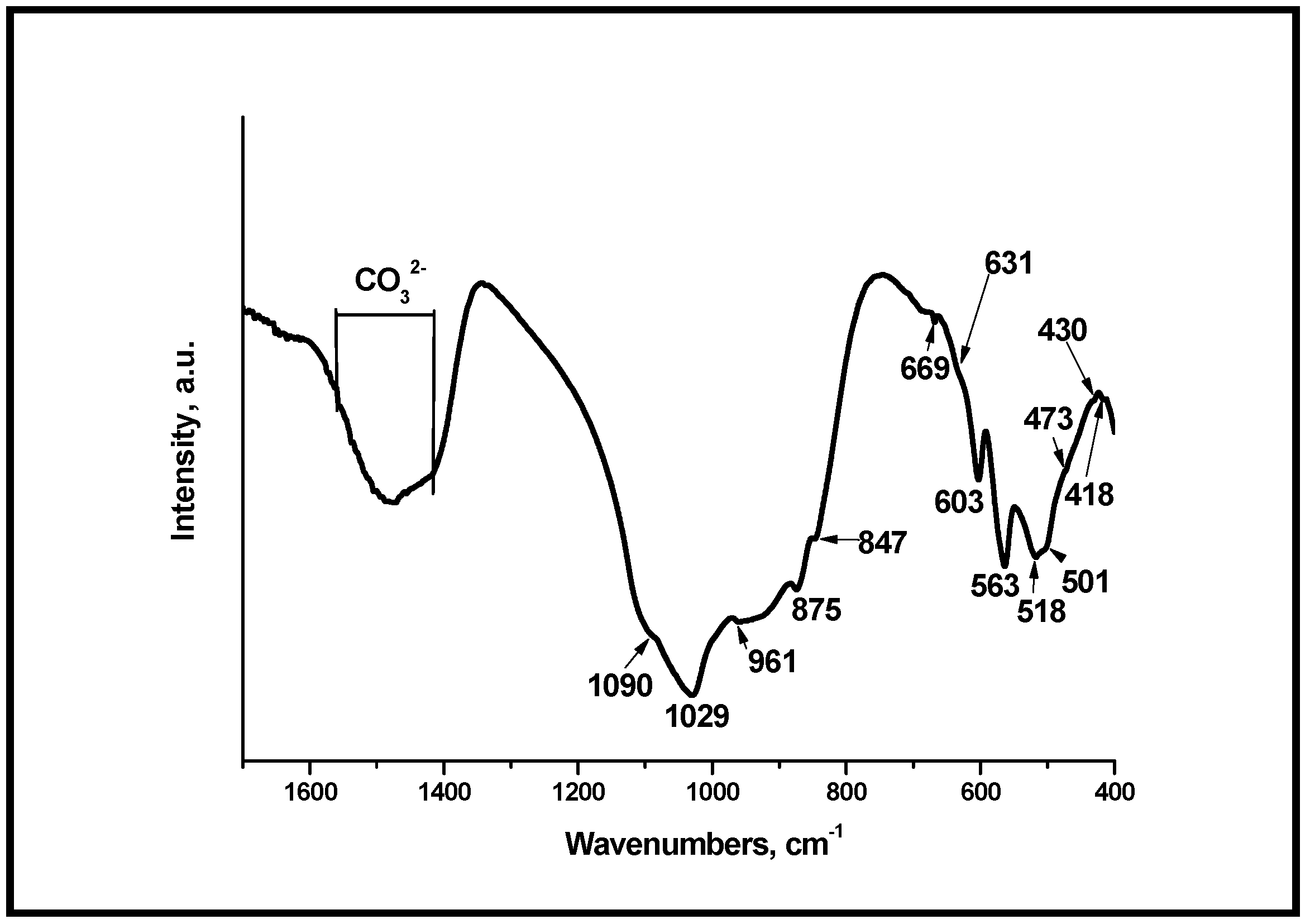
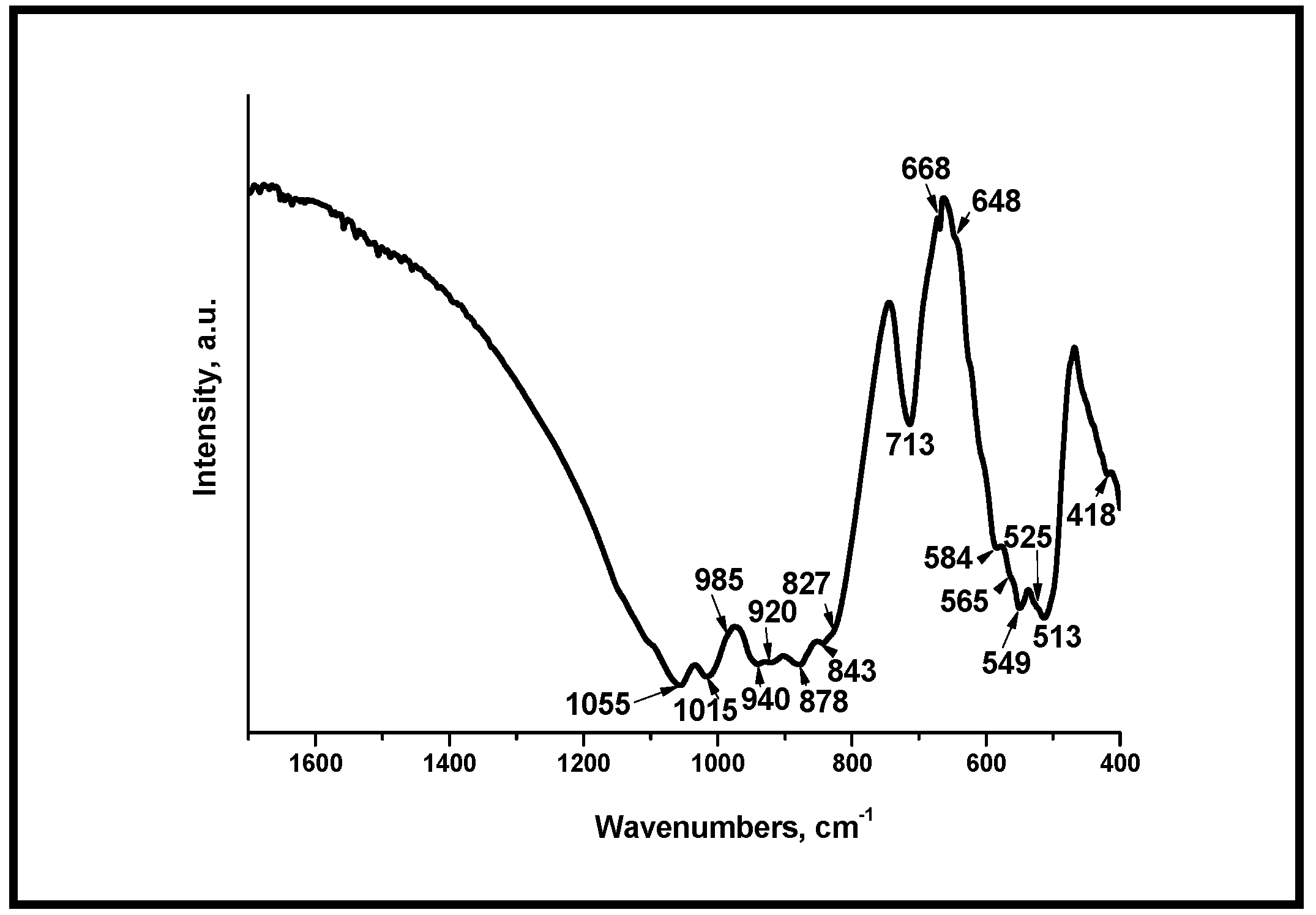
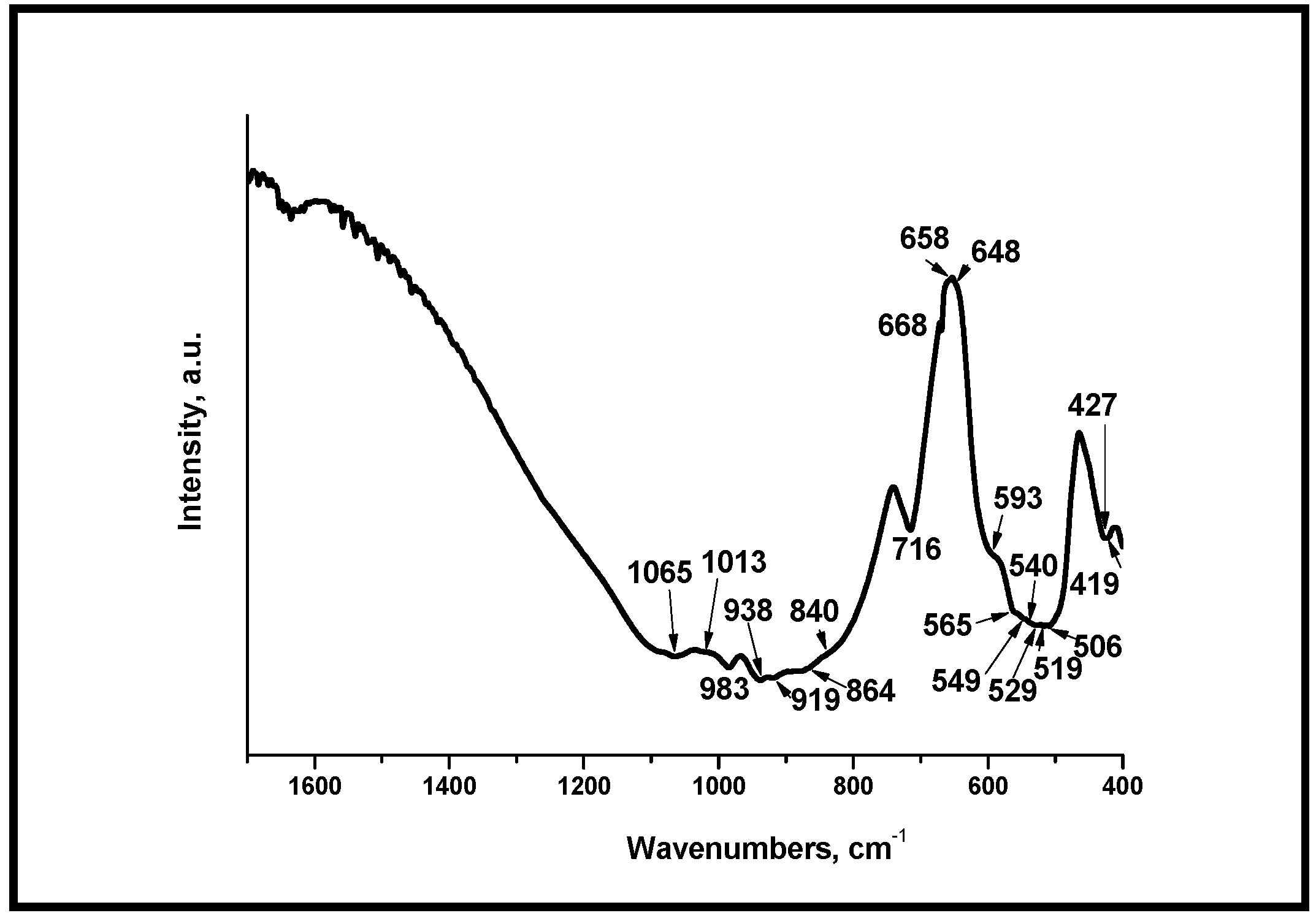
3.3. Fourier Transform Infrared Spectroscopy (FTIR) of the Glass-Ceramics Synthesized After Heating at Different Temperatures
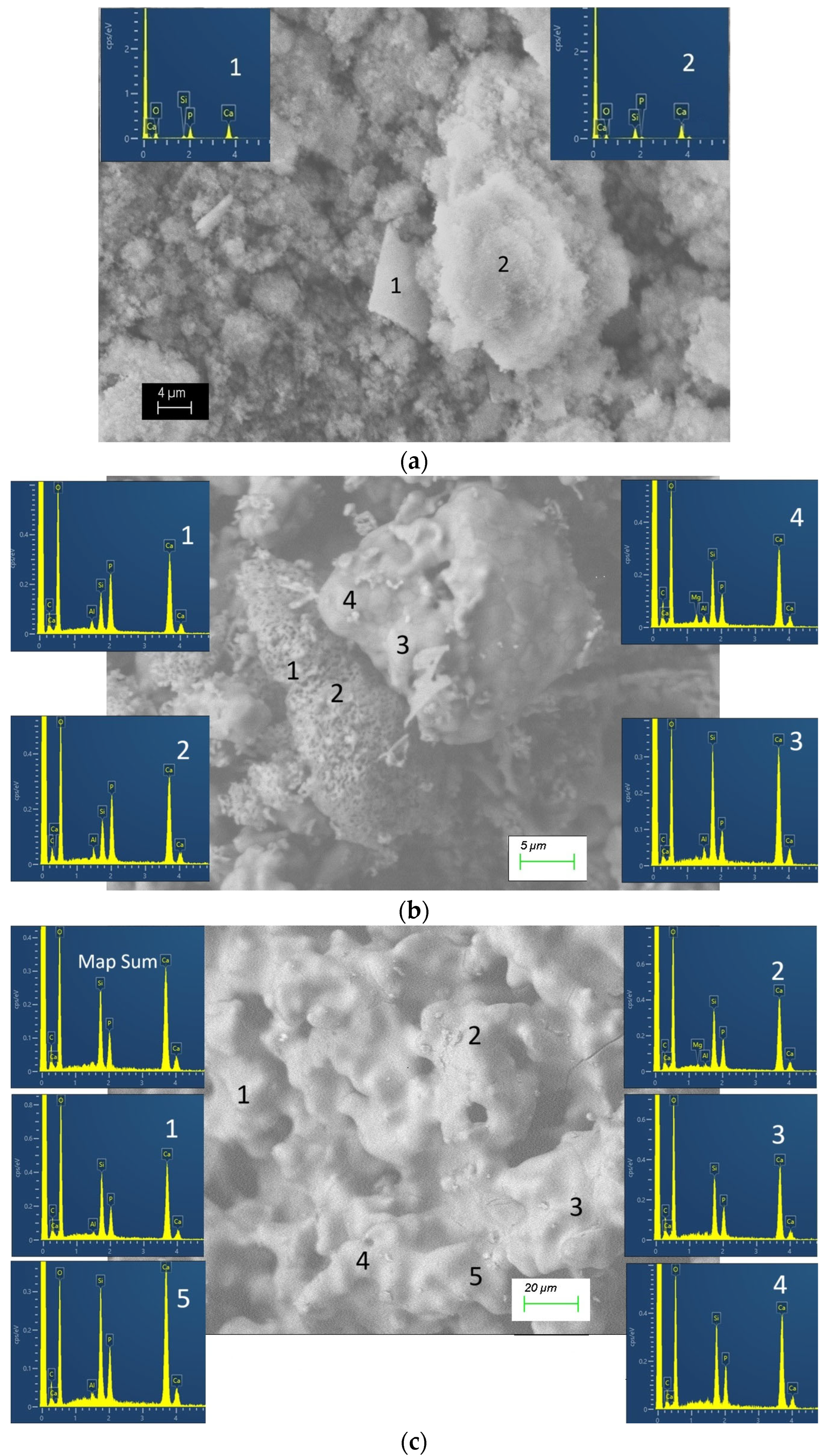
3.4. Analyses of the Glass-Ceramic Materials by Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
3.5. Adsorption-Texture Parameters
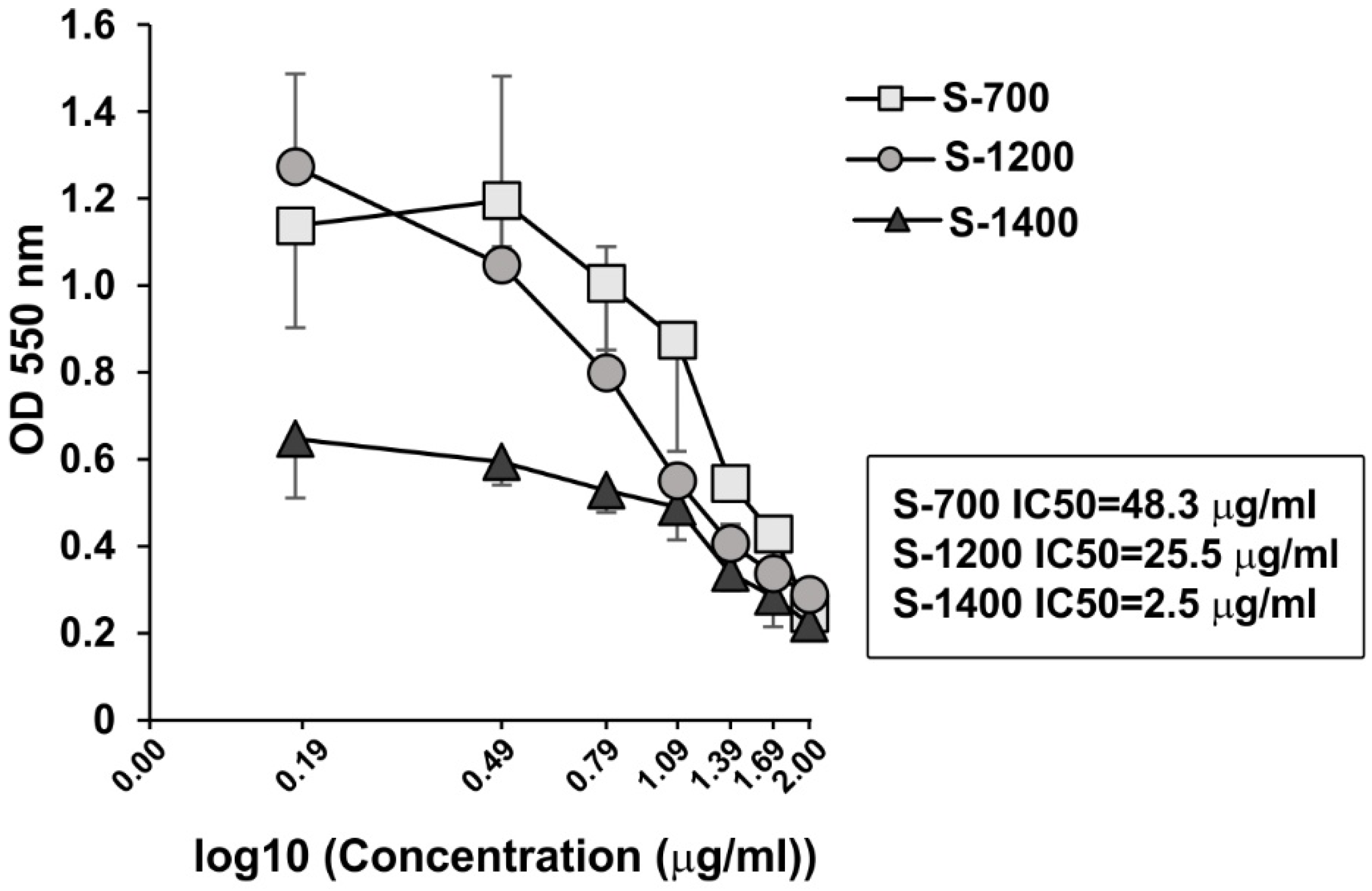
3.6. Effect of the Glass-Ceramic Materials on Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, I.M.; Velasquez, P.A.; De Aza, P.N. Synthesis and stability of α-tricalcium phosphate doped with dicalcium silicate in the system Ca3(PO4)2–Ca2SiO4. Mater. Charact. 2010, 61, 761–767. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Roy, S.; Basu, B. Mechanical and tribological characterization of human tooth. Mater. Charact. 2008, 59, 747–756. [Google Scholar] [CrossRef]
- Pritchard, A.; Nielsen, B.D. Silicon Supplementation for Bone Health: An Umbrella Review Attempting to Translate from Animals to Humans. Nutrients 2024, 16, 339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–662. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. A silicon requirement for normal skull formation in chicks. J. Nutr. 1980, 110, 352–359. [Google Scholar] [CrossRef]
- Knabe, C.; Houshmand, A.; Berger, G.; Ducheyne, P.; Gildenhaar, R.; Kranz, I.; Stiller, M. Effect of rapidly resorbable bone substitute materials on the temporal expression of the osteoblastic phenotype in vitro. J. Biomed. Mater. Res. Part A 2008, 84, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Langstaff, S.; Sayer, M.; Smith, T.J.N.; Pugh, S.M. Resorbable bioceramics based on stabilized calcium phosphates. Part II: Evaluation of biological response. Biomaterials 2001, 22, 135–150. [Google Scholar] [CrossRef]
- García Carrodeguas, R.; de Aza, A.; Jimenez, J.; De Aza, P.N.; Pena, P.; López-Bravo, A.; De Aza, S. Preparation and in vitro characterization of wollastonite doped tricalcium phosphate bioceramics. Key Eng. Mater. 2008, 361, 237–240. [Google Scholar]
- Minarelli Gaspar, A.M.; Saska, S.; García Carrodeguas, R.; de Aza, A.; Pena, P.; De Aza, P.N.; de Aza, S. Biological response to wollastonite doped α-tricalcium phosphate implants in hard and soft tissues in rats. Key Eng. Mater. 2009, 396, 7–10. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- De Val, J.E.M.S.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Martinez, I.M.; Granero-Marin, J.M.; Negri, B.; Chiva-Garcia, F.; Gonzalez Martinez, J.M.; de Aza, P.N. New block graft of α-TCP with silicon in critical size defects in rabbits: Chemical characterization, histological, histomorphometric and micro-CT study. Ceram. Int. 2012, 38, 1563–1570. [Google Scholar] [CrossRef]
- Wesselsky, A.; Jensen, O.M. Synthesis of pure Portland cement phases. Cem. Concr. Res. 2009, 39, 973–980. [Google Scholar] [CrossRef]
- Nicoleau, L.; Nonat, A.; Perrey, D. The di-and tricalcium silicate dissolutions. Cem. Concr. Res. 2013, 47, 14–30. [Google Scholar] [CrossRef]
- Baraček, J.; Palou, M.; Másilko, J.; Wasserbauer, J.; Šoukal, F.; Opravil, T.; Boháč, M. Application of sol-gel method to investigate the influence of P2O5 on the course of reactions in CaO-SiO2 system. Mater. Sci. Forum 2015, 851, 92–97. [Google Scholar] [CrossRef]
- De Val, J.E.M.S.; Calvo-Guirado, J.L.; Marín, J.M.G.; Moreno, G.G.; Mazón, P.; de Aza, P.N. Material characterization and in vivo behavior of dicalcium silicate cement modified with phosphorus. Ceram. Int. 2016, 42, 952–960. [Google Scholar] [CrossRef]
- Zuleta, F.; Murciano, A.; Gehrke, S.A.; Maté-Sánchez de Val, J.E.; Calvo-Guirado, J.L.; De Aza, P.N. A New Biphasic Dicalcium Silicate Bone Cement Implant. Materials 2017, 10, 758. [Google Scholar] [CrossRef]
- Kaou, M.H.; Balázsi, C.; Balázsi, K. Structural and Morphological Investigation of Calcium-Silicate-Based Bioceramics Prepared from Eggshell via Conventional Approach. Inorganics 2025, 13, 43. [Google Scholar] [CrossRef]
- Radulica, N.; Sanz, J.L.; Lozano, A. Dentin bond strength of calcium silicate-based materials: A systematic review of in vitro studies. Appl. Sci. 2024, 14, 104. [Google Scholar] [CrossRef]
- Ismail, H.; Mohamad, H. Bioactivity and Biocompatibility Properties of Sustainable Wollastonite Bioceramics from Rice Husk Ash/Rice Straw Ash: A Review. Materials 2021, 14, 5193. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, H.J. Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass. J. Funct. Biomater. 2025, 16, 188. [Google Scholar] [CrossRef]
- Radwanski, M.; Piwonski, I.; Szmechtyk, T.; Sauro, S.; Lukomska-Szymanska, M. Microstructural and Elemental Characterization of Calcium Silicate-Based Sealers. Nanomaterials 2025, 15, 756. [Google Scholar] [CrossRef]
- Cucuruz, A.; Ghițulică, C.D.; Voicu, G.; Bogdan, C.A.; Dochiu, V. Calcium silicate (CaSiO3) scaffolds with applications in tissue engineering. Chem. Proc. 2023, 13, 18. [Google Scholar]
- Li, H.W.; Sun, J.Y. Effects of dicalcium silicate coating ionic dissolution products on human mesenchymal stem-cell proliferation and osteogenic differentiation. J. Int. Med. Res. 2011, 39, 112–128. [Google Scholar] [CrossRef]
- Cheng, W.; Chang, J. Fabrication and characterization of polysulfone-dicalcium silicate composite films. J. Biomater. Appl. 2006, 20, 361–376. [Google Scholar] [CrossRef]
- Primus, C.M.; Tay, F.R.; Niu, L.N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef]
- Stead, J.E.; Ridsdale, C.H. Crystals in basic converter slag. J. Chem. Soc. Trans. 1887, 51, 601–610. [Google Scholar] [CrossRef]
- De Aza, P.N.; Zuleta, F.; Velasquez, P.; Vicente-Salar, N.; Reig, J.A. α′H-Dicalcium silicate bone cement doped with tricalcium phosphate: Characterization, bioactivity and biocompatibility. J. Mater. Sci. Mater. Med. 2014, 25, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.M.; Velásquez, P.; De Aza, P.N. The Sub-System α-TCPss-Silicocarnotite Within the Binary System Ca3(PO4)2–Ca2SiO4. J. Am. Ceram. Soc. 2012, 95, 1112–1117. [Google Scholar] [CrossRef]
- De Aza, P.N.; García-Bernal, D.; Cragnolini, F.; Velasquez, P.; Meseguer-Olmo, L. The effects of Ca2SiO4–Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. Mater. Sci. Eng. C 2013, 33, 4009–4020. [Google Scholar] [CrossRef]
- Mazón, P.; De Aza, P.N. Porous scaffold prepared from α′L-Dicalcium silicate doped with phosphorus for bone grafts. Ceram. Int. 2018, 44, 537–545. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; Baudin, C.; De Aza, P.N. Nurse′s A-Phase: Synthesis and Characterization in the Binary System Ca2SiO4–Ca3(PO4)2. J. Am. Ceram. Soc. 2015, 98, 3042–3046. [Google Scholar] [CrossRef]
- Engin, N.O.; Tas, A.C. Manufacture of macroporous calcium hydroxyapatite bioceramics. J. Eur. Ceram. Soc. 1999, 19, 2569–2572. [Google Scholar] [CrossRef]
- Ros-Tárraga, P.; Mazón, P.; Meseguer-Olmo, L.; De Aza, P.N. Revising the subsystem Nurse’s a-phase-silicocarnotite within the system Ca3(PO4)2–Ca2SiO4. Materials 2016, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; De la Casa-Lillo, M.A.; De Aza, S.; De Aza, P.N. The System Ca3(PO4)2–Ca2SiO4: The Sub-System Ca2SiO4–7CaO.P2O5.2SiO2. J. Am. Ceram. Soc. 2011, 94, 4459–4462. [Google Scholar] [CrossRef]
- Radev, L.; Hristov, V.; Michailova, I.; Samuneva, B. Sol-gel bioactive glass-ceramics Part I: Calcium phosphate silicate/wollastonite glass-ceramics. Open Chem. 2009, 7, 317–321. [Google Scholar] [CrossRef]
- Radev, L.; Hristov, V.; Michailova, I.; Fernandes, H.M.V.; Salvado, M.I.M. In vitro bioactivity of biphasic calcium phosphate silicate glass-ceramic in CaO-SiO2-P2O5 system. Process. Appl. Ceram. 2010, 4, 15–24. [Google Scholar] [CrossRef]
- Radev, L. Influence of thermal treatment on the structure and in vitro bioactivity of sol-gel prepared CaO-SiO2-P2O5 glass-ceramics. Process. Appl. Ceram. 2014, 8, 155–166. [Google Scholar] [CrossRef]
- Mihailova, I.; Dimitrova, P.; Radev, L. α/β-TCP silicate glass-ceramic obtained by sol–gel: Structure and in vitro bioactivity. Bol. Soc. Esp. Cerám. V. 2024, 63, 330–345. [Google Scholar] [CrossRef]
- Saalfeld, H.; Klaska, Κ.H. The crystal structure of 6Ca2SiO4·1Ca3(PO4)2. Z. Für Krist. Cryst. Mater. 1981, 155, 65–74. [Google Scholar]
- Widmer, R.; Gfeller, F.; Armbruster, T. Structural and Crystal Chemical Investigation of Intermediate Phases in the System Ca2SiO4–Ca3(PO4)2–CaNaPO4. J. Am. Ceram. Soc. 2015, 98, 3956–3965. [Google Scholar] [CrossRef]
- Papynov, E.; Shichalin, O.; Buravlev, I.; Belov, A.; Portnyagin, A.; Mayorov, V.; Merkulov, E.; Kaidalova, T.; Skurikhina, Y.; Turkutyukov, V.; et al. CaSiO3-HAp Structural Bioceramic by Sol-Gel and SPS-RS Techniques: Bacteria Test Assessment. J. Funct. Biomater. 2020, 11, 41. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Buravlev, I.Y.; Mayorov, V.Y.; Fedorets, A.N.; Buravleva, A.A.; Lembikov, A.O.; Gritsuk, D.V.; Kapustina, O.V.; et al. CaSiO3 -HAp Metal-Reinforced Biocomposite Ceramics for Bone Tissue Engineering. J. Funct. Biomater. 2023, 14, 259. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Yarusova, S.B.; Ivanov, N.P.; Papynov, E.K.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Myagchilov, A.V.; Fedorets, A.N.; Rastorguev, V.L.; et al. Calcium silicate solid-state matrices from boric acid production waste for 60Co removal and immobilization by spark plasma sintering. J. Water Process Eng. 2024, 59, 105042. [Google Scholar] [CrossRef]
- Choudhury, S. A review of the sol-gel process and its application. Int. Educ. Res. J. 2024, 10, 122–125. [Google Scholar] [CrossRef]
- Yoneda, T.; Alsina, M.M.; Garcia, J.L.; Mundy, G.R. Differentiation of HL-60 cells into cells with the osteoclast phenotype. Endocrinology 1991, 129, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.; Doncheva, T.; Kostova, N.; Uzunova, I.; Latinova, N.; Gerasimova, V.; Dat, N.T.; Giang, D.H.; Luyen, N.T. Biological effect of alkaloid enriched fractions and reticuline from the Stephania dielsiana on promyelocyte HL-60 cell line. Rev. Bras. Farmacogn. 2025, 35, 734–753. [Google Scholar] [CrossRef]
- Srinivasan, B.; Lloyd, M.D. Dose-Response Curves and the Determination of IC50 and EC50 Values. J. Med. Chem. 2024, 67, 17931–17934. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, S. FTIR spectrum analysis to predict the crystalline and amorphous phases of hydroxyapatite: A comparison of vibrational motion to reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef]
- Giera, A.; Manecki, M.; Bajda, T.; Rakovan, J.; Kwaśniak-Kominek, M.; Marchlewski, T. Arsenate substitution in lead hydroxyl apatites: A Raman spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P. Formate incorporation in the structure of Ca-deficient apatite: Rietveld structure refinement. J. Solid State Chem. 2003, 174, 132–140. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using Fourier transform infrared spectroscopy. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012, 12, 251–263. [Google Scholar]
- Wang, M.; Qian, R.; Bao, M.; Gu, C.; Zhu, P. Raman, FT-IR and XRD study of bovine bone mineral and carbonated apatites with different carbonate levels. Mater. Lett. 2018, 210, 203–206. [Google Scholar] [CrossRef]
- Yasar, O.F.; Liao, W.C.; Mathew, R.; Yu, Y.; Stevensson, B.; Liu, Y.; Edén, M. The carbonate and sodium environments in precipitated and biomimetic calcium hydroxy-carbonate apatite contrasted with bone mineral: Structural insights from solid-state nmr. J. Phys. Chem. C 2021, 125, 10572–10592. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2002, 59, 697–708. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Gerasimov, K.B.; Ishchenko, A.V.; Dudina, D.V. A novel approach to the synthesis of silicocarnotite. Mater. Lett. 2016, 164, 255–259. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Yilgor, E.; Yagci, M.B.; Unal, U.; Yilgor, I. Effect of reaction solvent on hydroxyapatite synthesis in sol–gel process. R. Soc. Open Sci. 2017, 4, 171098. [Google Scholar] [CrossRef] [PubMed]
- Mohan Babu, M.; Syam Prasad, P.; Venkateswara Rao, P.; Hima Bindu, S.; Prasad, A.; Veeraiah, N.; Özcan, M. Influence of ZrO2 addition on structural and biological activity of phosphate glasses for bone regeneration. Materials 2020, 13, 4058. [Google Scholar] [CrossRef]
- Saidani, S.; Smith, A.; El Hafiane, Y.; Tahar, L.B. Role of dopants (B, P and S) on the stabilization of β-Ca2SiO4. J. Eur. Ceram. Soc. 2021, 41, 880–891. [Google Scholar] [CrossRef]
- Mabrouk, A.; Bachar, A.; Atbir, A.; Follet, C.; Mercier, C.; Tricoteaux, A.; Leriche, A.; Hampshire, S. Mechanical properties, structure, bioactivity and cytotoxicity of bioactive Na-Ca-Si-P-O-(N) glasses. J. Mech. Behav. Biomed. Mater. 2018, 86, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Nandakumar, A.; Anilbhai, N.; Boccaccini, A.R.; Jones, J.R.; Jell, G. The effect of Si species released from bioactive glasses on cell behaviour: A quantitative review. Acta Biomater. 2023, 170, 39–52. [Google Scholar] [CrossRef]
- Piatti, E.; Miola, M.; Verné, E. Tailoring of bioactive glass and glass-ceramics properties for in vitro and in vivo response optimization: A review. Biomater. Sci. 2024, 12, 4546–4589. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.J.; Tsai, H.F.; Wu, C.S.; Chyuan, I.-T.; Hsu, P.-N. TRAIL inhibits RANK signaling and suppresses osteoclast activation via inhibiting lipid raft assembly and TRAF6 recruitment. Cell Death Dis. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Hu, Z.; Guo, X.; Xiao, Y.; Liu, X.; de Bruijn, J.D.; Bao, C.; Yuan, H. Genesis of osteoclasts on calcium phosphate ceramics and their role in material-induced bone formation. Acta Biomater. 2023, 157, 625–638. [Google Scholar] [CrossRef]



| Oxides | Nominal Composition Ca15(PO4)2(SiO4)6 (Mass %) | Experimental Composition (Mass %) |
|---|---|---|
| CaO | 62.60 | 59.24 ± 3.15 |
| SiO2 | 26.83 | 26.32 ± 1.38 |
| P2O5 | 10.56 | 12.78 ± 0.67 |
| MgO | - | 0.50 ± 0.06 |
| Al2O3 | - | 0.10 ± 0.02 |
| Fe2O3 | - | 0.06 ± 0.01 |
| MnO | - | 0.04 ± 0.01 |
| Crystalline Phases | β-Ca2(SiO4) | Ca5(PO4)3(OH) Hydroxyapatite |
|---|---|---|
| Weight fraction, % | 54 (2) | 46 (2) |
| Symmetry | ||
| Crystal System | Monoclinic | Hexagonal |
| Space group | SG P121/c1 (14) | SG P63/m (176) |
| Unit Cell Parameters | ||
| a, Å | 5.40 (3) | 9.42 (4) |
| b, Å | 6.77 (3) | 9.42 (4) |
| c, Å | 10.83 (5) | 6.91 (3) |
| β, ° | 118.33 (8) |
| Crystalline Phases | Ca15(PO4)2(SiO4)6 | Ca5(PO4)2(SiO4) Silicocarnotite |
|---|---|---|
| Weight fraction, % | 63 (1) | 21 (1) |
| Symmetry Crystal System Space group Unit Cell Parameters a, Å b, Å c, Å | Orthorhombic SG Pnm21 (31) 21.67 (1) 9.367 (5) 6.835 (2) | Orthorhombic SG Pnma (62) 6.723 (3) 15.433 (6) 10.092 (4) |
| Crystalline Phases | CaSiO3 Pseudowollastonite | Ca5(PO4)3(OH) Hydroxyapatite |
| Weight fraction, % | 11 (1) | 5 (1) |
| Symmetry Crystal System Space group Unit Cell Parameters a, Å b, Å c, Å β, ° | Monoclinic SG C12/c1 (15) 11.796 (9) 6.851 (5) 10.524 (8) 111.61 (6) | Hexagonal SG P63/m (176) 9.38 (1) 9.38 (1) 6.934 (9) |
| Crystalline Phases | α-Ca2SiO4 | CaSiO3 Pseudowollastonite | Ca15(PO4)2(SiO4)6 |
|---|---|---|---|
| Weight fraction, % | 68 (3) | 21 (1) | 11 (1) |
| Symmetry Crystal System Space group Unit Cell Parameters a, Å b, Å c, Å β, ° | Hexagonal SG P63/mmc (194) 5.3963 (6) 5.3963 (6) 7.090 (1) | Monoclinic SG C12/c1 (15) 6.848 (4) 11.815 (7) 19.97 (2) 90.57 (7) | Orthorhombic SG Pnm21 (31) 21.593 (1) 9.335 (1) 7.017 (1) |
| Samples | |||
|---|---|---|---|
| Parameters | S-700 | S-1200 | S-1400 |
| SBET (m2 g−1) | 55 | 3 | 1.8 |
| Vtotal (cm3 g−1) | 0.320 | 0.006 | 0.003 |
| Daverage (nm) | 23 | 7.9 | 6.1 |
| Vmi (cm3 g−1) | 0.003 | - | - |
| Smi (m2 g−1) | 7 | - | - |
| Sext (m2 g−1) | 48 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihailova, I.; Dimitrova, P.; Avdeev, G.; Ivanova, R.; Georgiev, H.; Nedkova-Shtipska, M.; Teodosieva, R.; Radev, L. New Glass-Ceramics in the System Ca2SiO4-Ca3(PO4)2—Phase Composition, Microstructure, and Effect on the Cell Viability. Materials 2025, 18, 3887. https://doi.org/10.3390/ma18163887
Mihailova I, Dimitrova P, Avdeev G, Ivanova R, Georgiev H, Nedkova-Shtipska M, Teodosieva R, Radev L. New Glass-Ceramics in the System Ca2SiO4-Ca3(PO4)2—Phase Composition, Microstructure, and Effect on the Cell Viability. Materials. 2025; 18(16):3887. https://doi.org/10.3390/ma18163887
Chicago/Turabian StyleMihailova, Irena, Petya Dimitrova, Georgi Avdeev, Radostina Ivanova, Hristo Georgiev, Milena Nedkova-Shtipska, Ralitsa Teodosieva, and Lachezar Radev. 2025. "New Glass-Ceramics in the System Ca2SiO4-Ca3(PO4)2—Phase Composition, Microstructure, and Effect on the Cell Viability" Materials 18, no. 16: 3887. https://doi.org/10.3390/ma18163887
APA StyleMihailova, I., Dimitrova, P., Avdeev, G., Ivanova, R., Georgiev, H., Nedkova-Shtipska, M., Teodosieva, R., & Radev, L. (2025). New Glass-Ceramics in the System Ca2SiO4-Ca3(PO4)2—Phase Composition, Microstructure, and Effect on the Cell Viability. Materials, 18(16), 3887. https://doi.org/10.3390/ma18163887






