Correlative Raman Spectroscopy–SEM Investigations of Sintered Magnesium–Calcium Alloys for Biomedical Applications
Abstract
1. Introduction
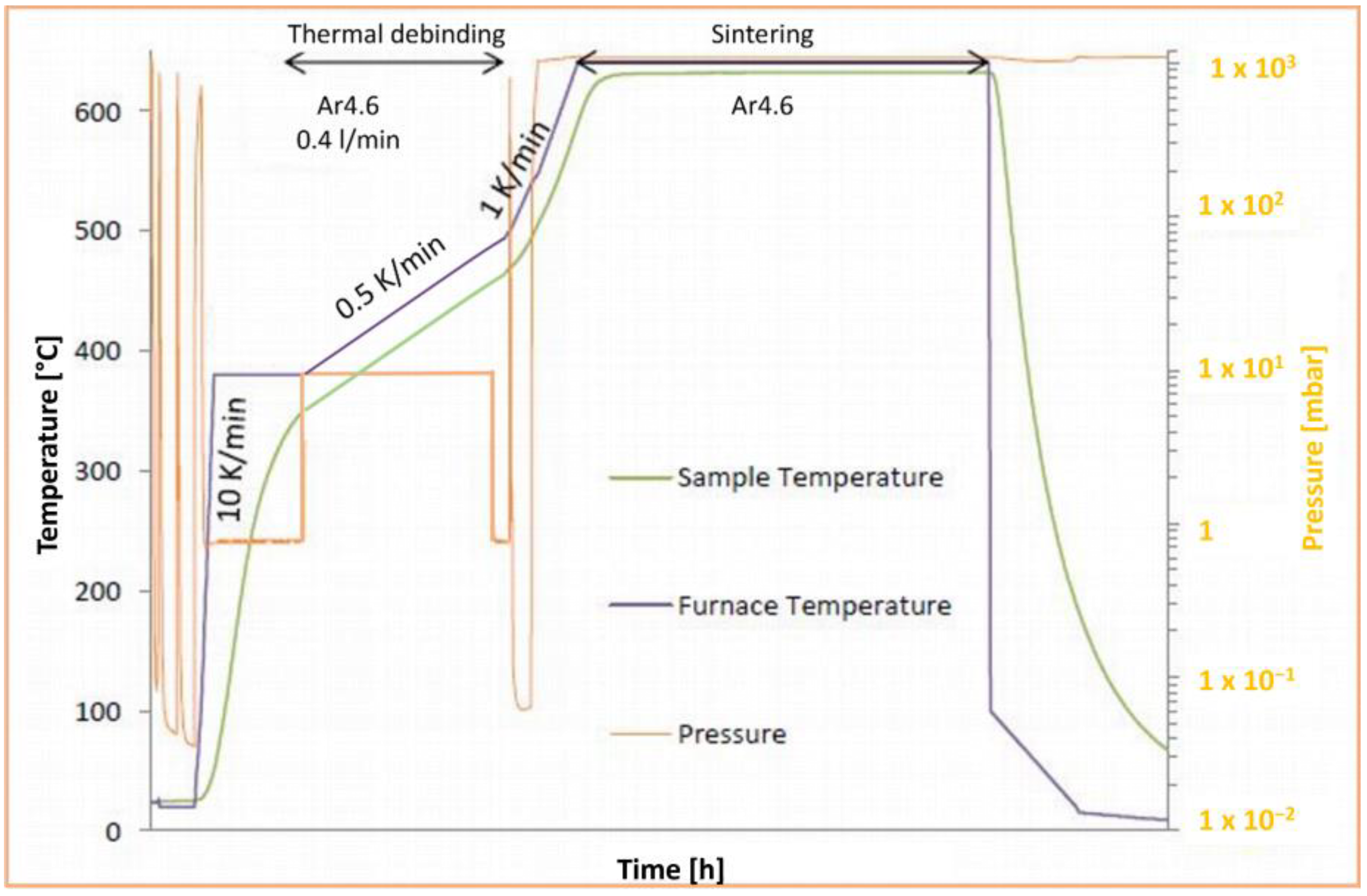
2. Materials and Methods
3. Results
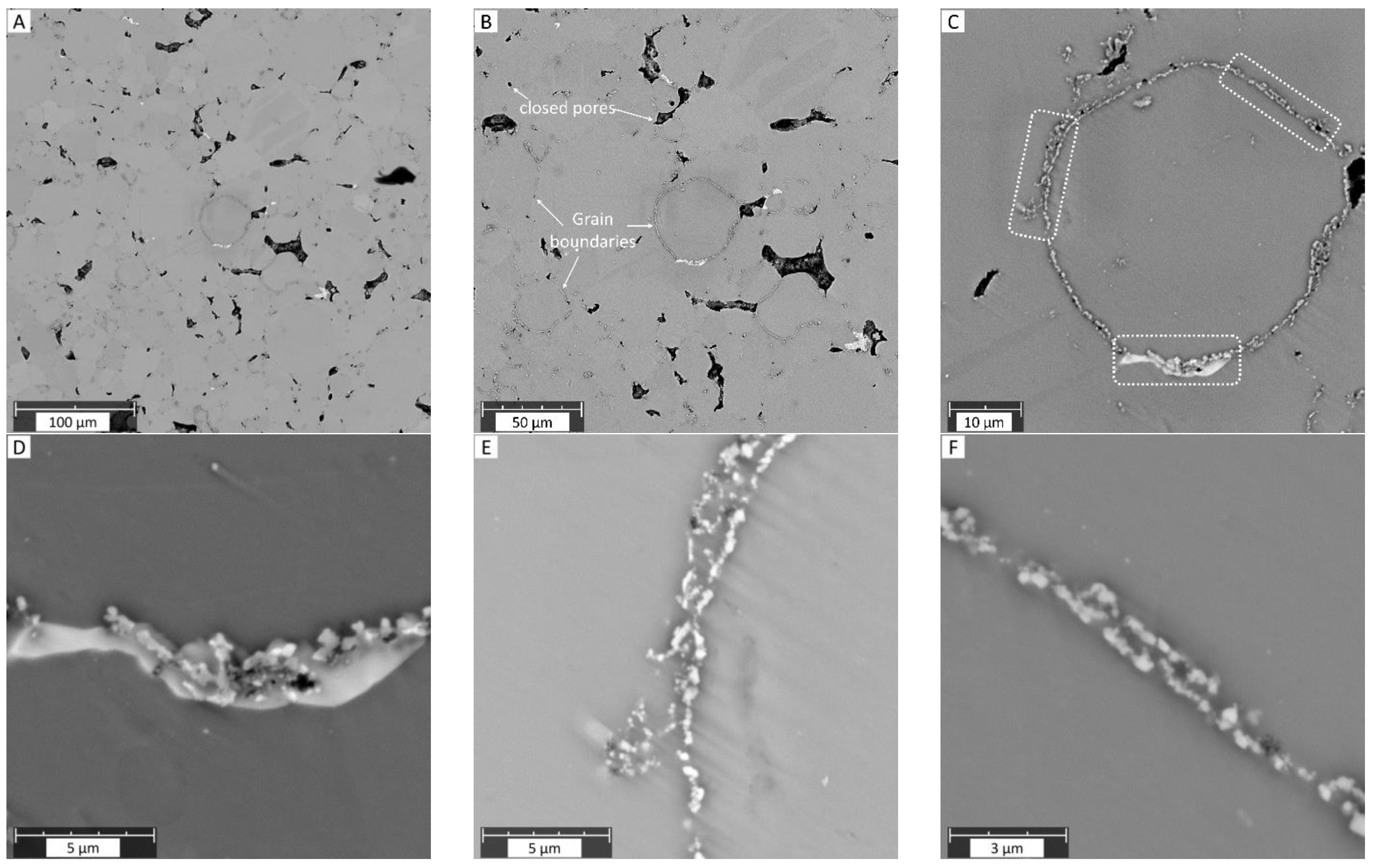
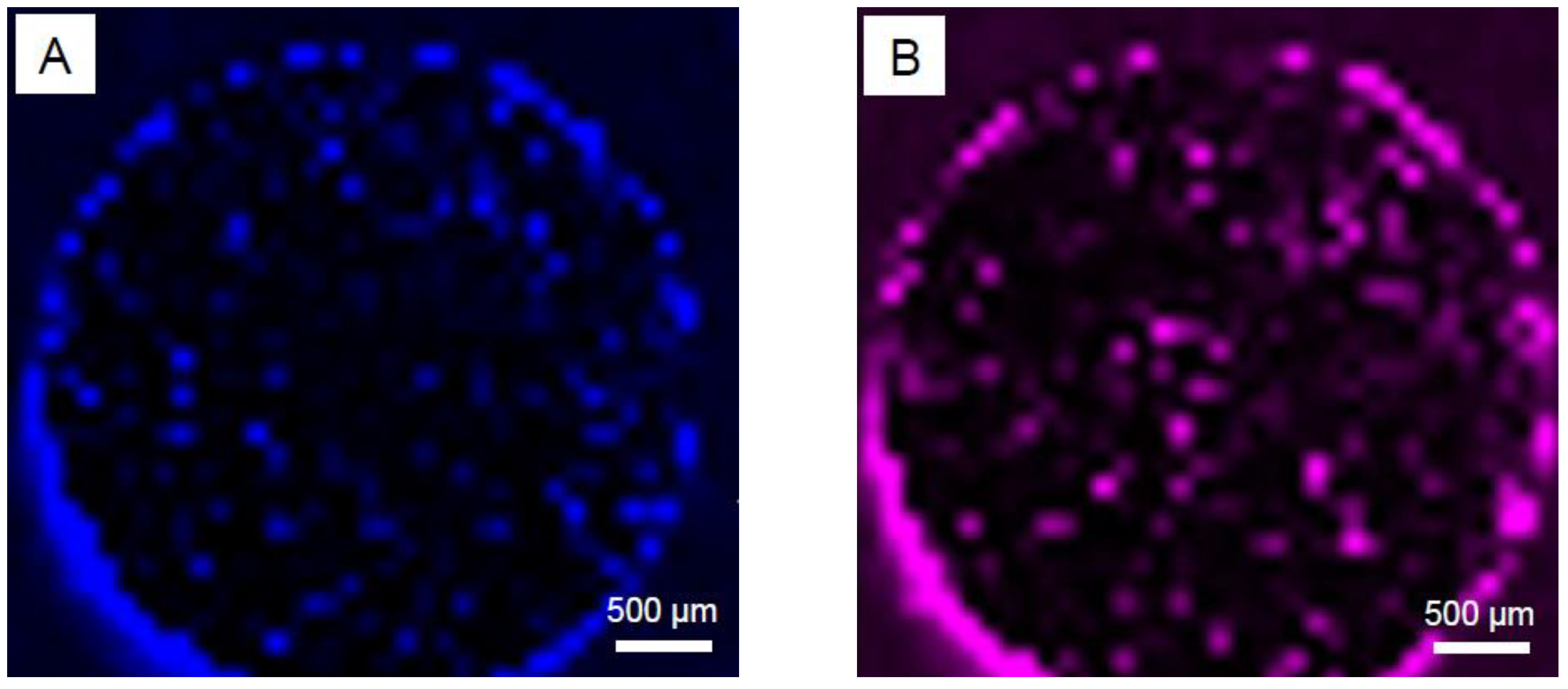
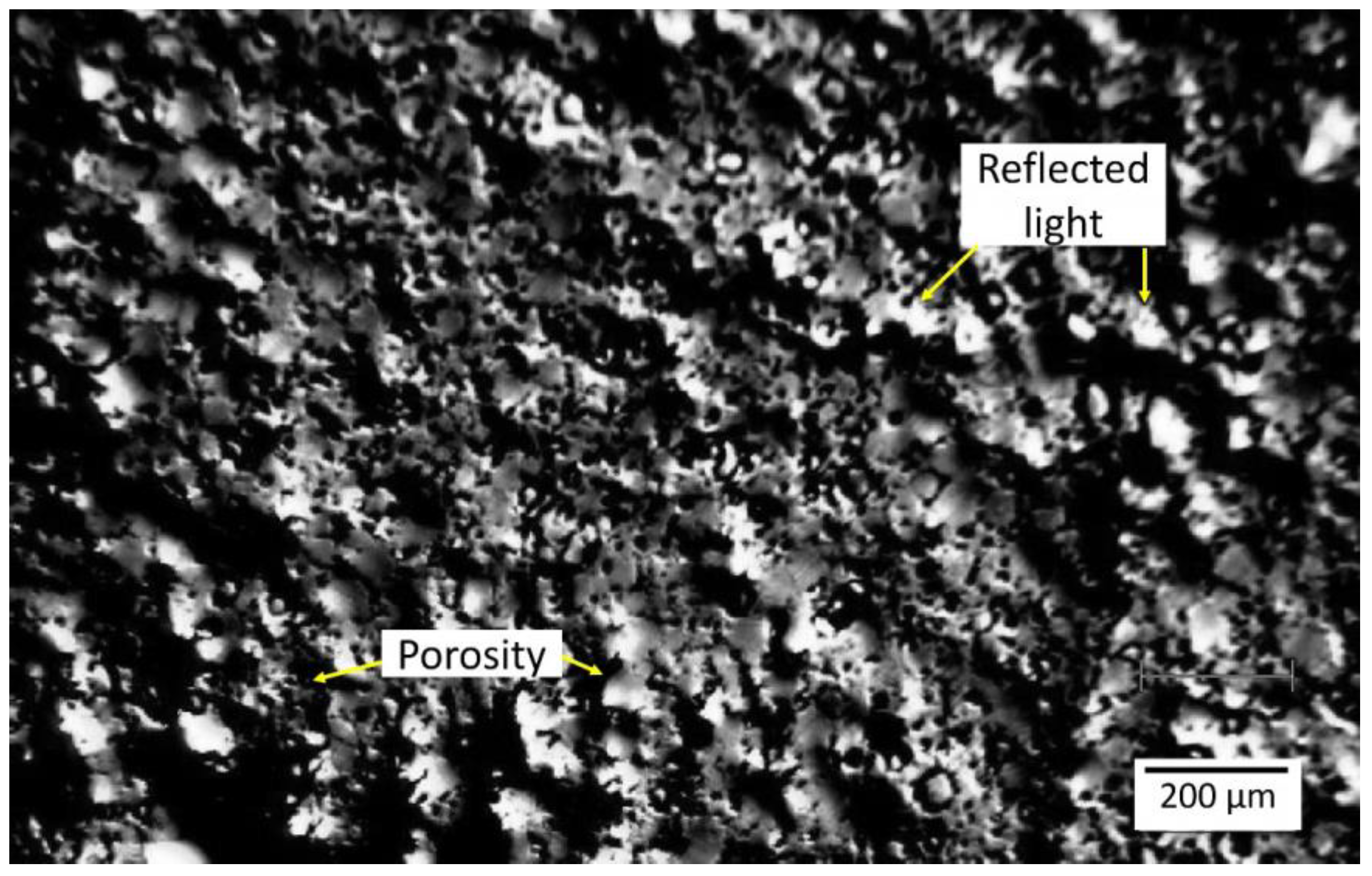
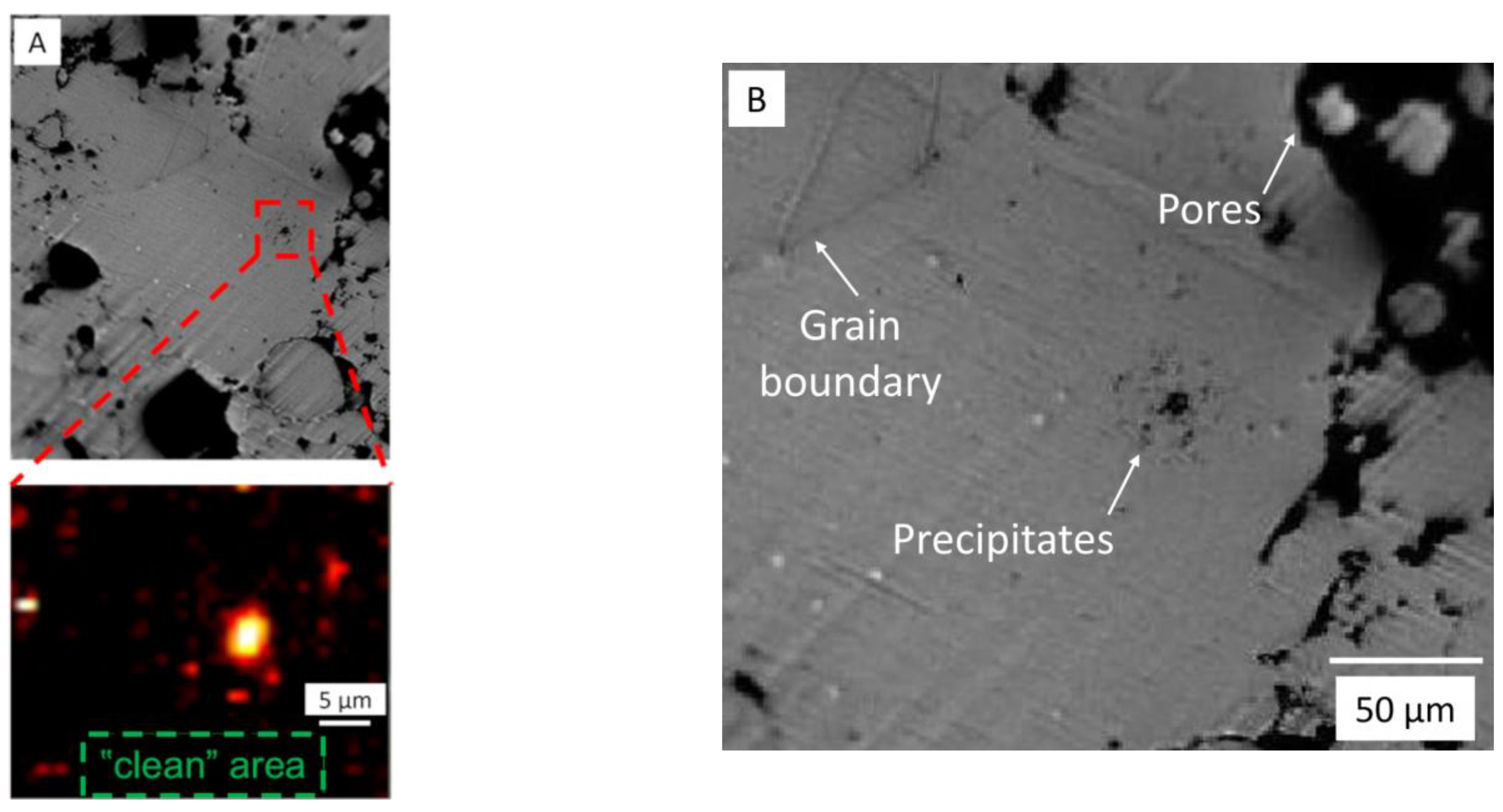
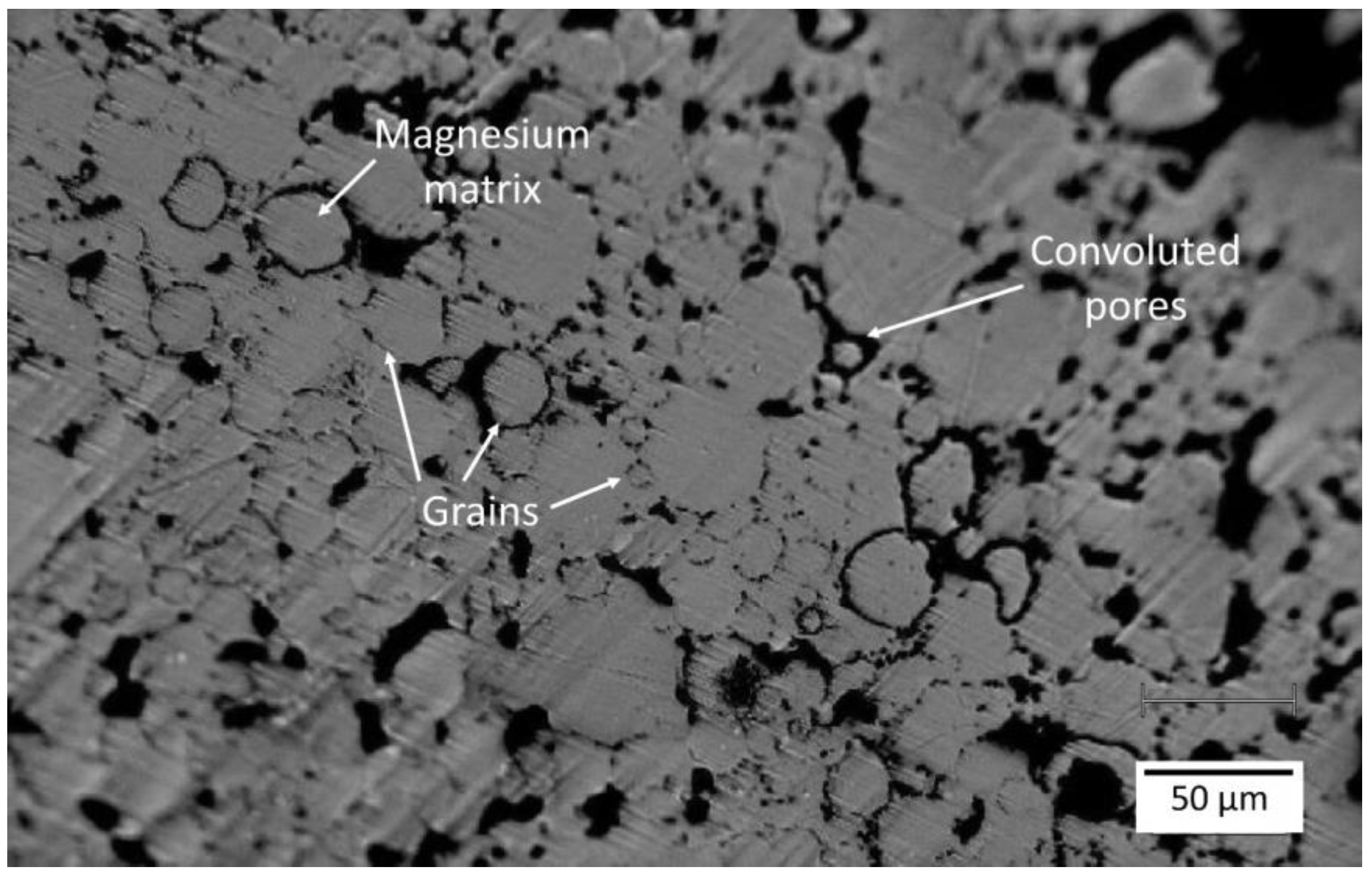
3.1. SEM Studies
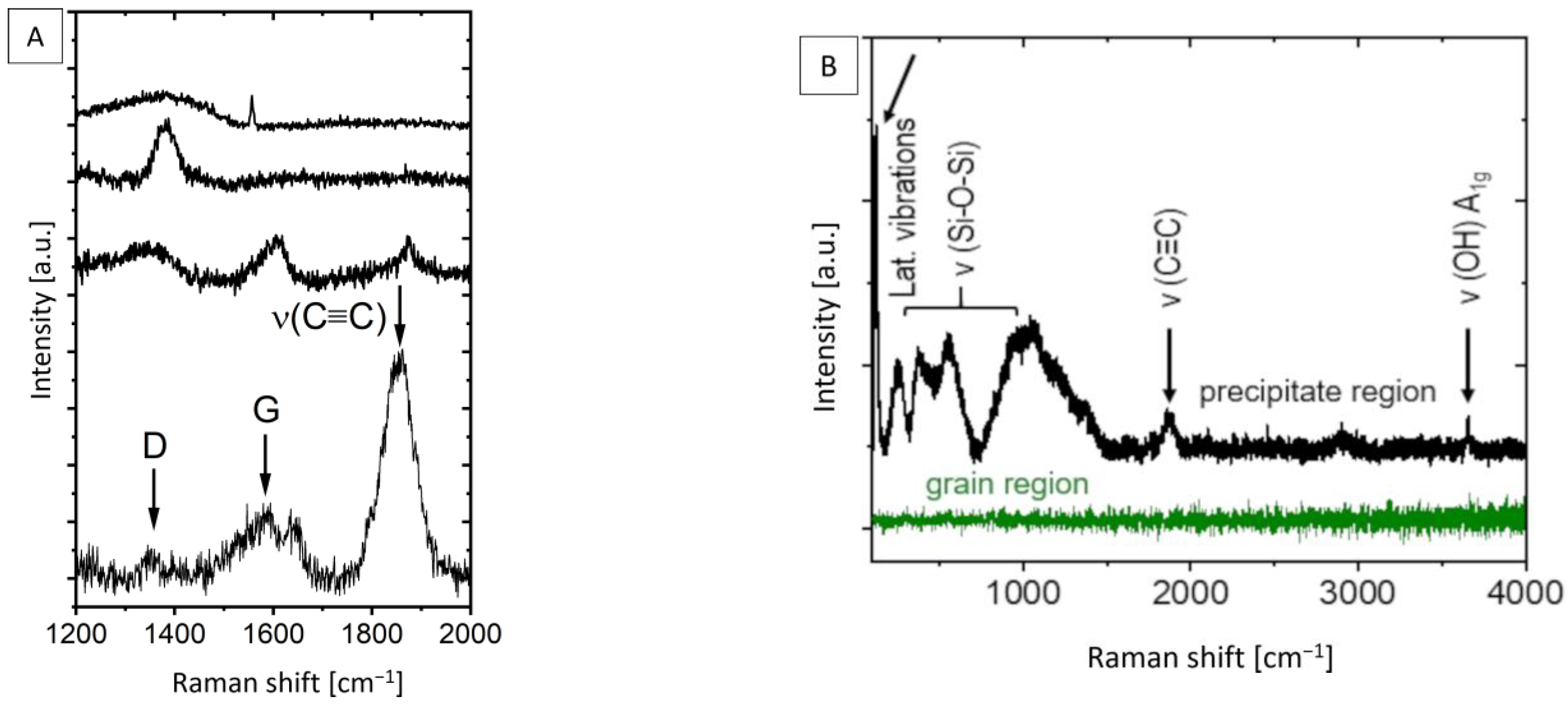
3.2. Micro-Raman Spectroscopy Studies
4. Discussion
5. Conclusions
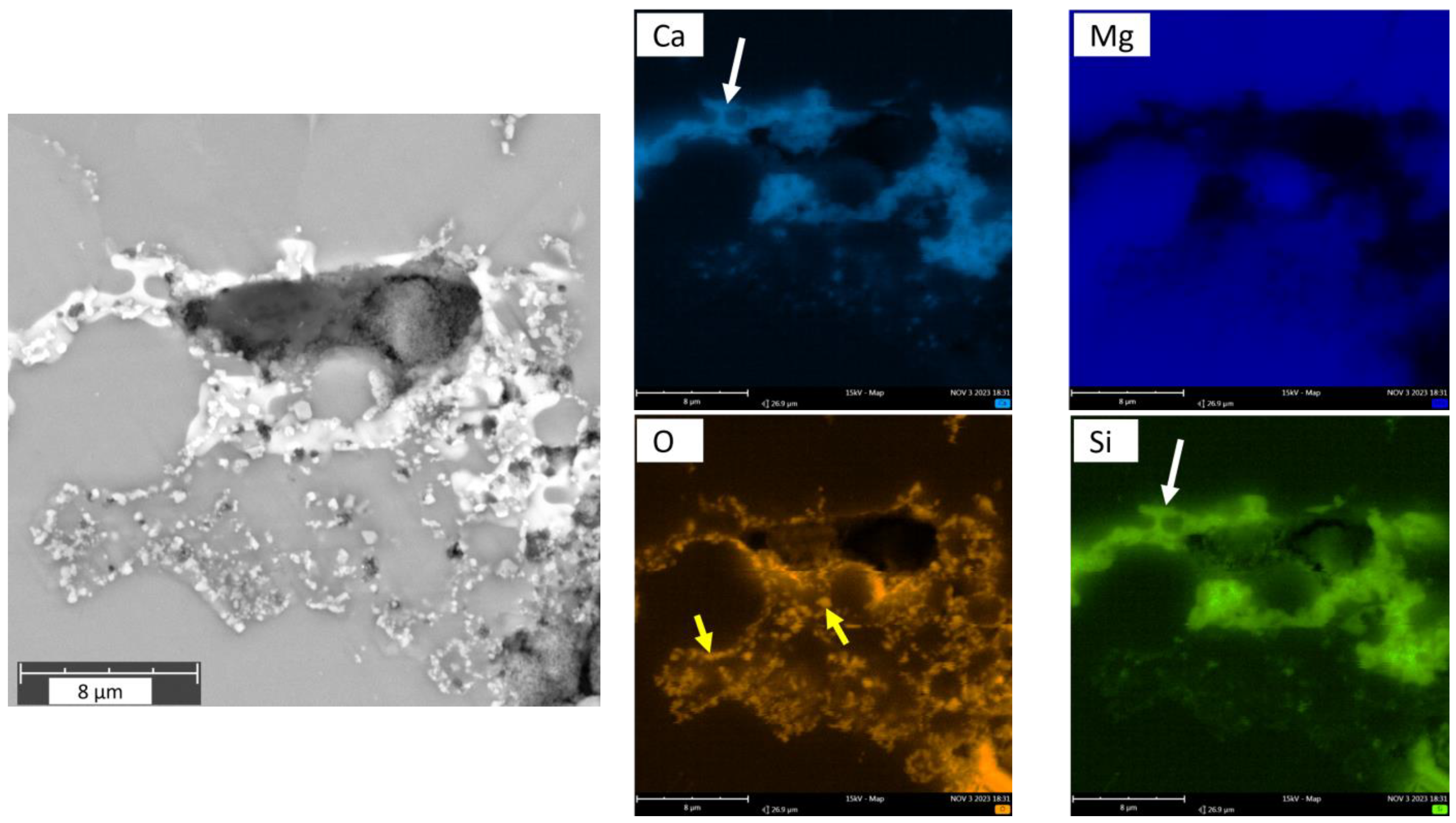
- Contrary to the previous reports that PM-processed Mg materials generally exhibit a homogenous microstructure, the current findings show a certain microstructural inhomogeneity in the form of Ca/Si-rich phase and oxide phase segregations, as well as the carbon compound distribution along certain grain boundaries in MIM Mg-0.6Ca material.
- The sintered MIM Mg-0.6Ca material contains residual carbon products in the form of free carbon and carbides, as confirmed by the stretching modes at ~1370 cm−1/~1560 cm−1 and ~1865 cm−1 from Raman spectroscopy, respectively.
- These carbon compounds are a result of the reactions between the backbone polymer PPcoPE in the used binder system and the bulk material constituents during the thermal debinding and sintering stages of MIM Mg-0.6Ca material. The thermal decomposition of magnesium carbides at sintering temperatures leads to free carbon in the final material. The detected C≡C stretching mode (~1865 cm−1) indicates the formation of CaC2.
- Additionally, the presence of impurities such as SiO2 and Ca/Si-rich phases is confirmed by EDX analysis, attributing these phases to the common Si impurity in the starting powders.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Boroni, M. A review of degradation properties of Mg based biodegradable implants. Res. Rev. Mater. Sci. Chem. 2012, 1, 15–58. [Google Scholar]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Ding, Y.-F.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; El-Aziz, A.M.; Breitinger, H.-G. Study of the degradation behavior and the biocompatibility of Mg–0. 8Ca alloy for orthopedic implant applications. J. Magnes. Alloys 2019, 7, 249–257. [Google Scholar]
- Negahban, A.; Shamsi, M.; Sedighi, M. Advances in the modification of magnesium-based biomaterials to address corrosion and corrosion-fatigue: A review of developments and prospects. J. Mater. Res. Technol. 2024, 30, 4064–4108. [Google Scholar] [CrossRef]
- Feser, K.; Kietzmann, M.; Bäumer, W.; Krause, C.; Bach, F.W. Effects of Degradable Mg-Ca Alloys on Dendritic Cell Function. J. Biomater. Appl. 2010, 25, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, J.; Yu, L.; Wang, K.; Zhang, W.; Fan, H.; Deng, Z.; Wu, J.; Wang, K. Corrosion behavior investigation of gallium coating on magnesium alloy in simulated body fluid. J. Mater. Res. Technol. 2023, 27, 225–236. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Manonukul, A. A Review on Material Extrusion Additive Manufacturing of Metal and How It Compares with Metal Injection Moulding. Metals 2022, 12, 429. [Google Scholar] [CrossRef]
- Wolff, M.; Marvi-Mashhadi, M.; Nidadavolu, E.; Lüneburg, H.; Ebel, T.; Willumeit-Römer, R. Comparison between compression tested and simulated Mg-6.3Gd bone scaffolds produced by binder based additive manufacturing technique. J. Magnes. Alloys 2023, 11, 2750–2762. [Google Scholar] [CrossRef]
- Azadi, A.; Ebel, T.; Wolff, M.; O’Cearbhaill, E.; Celikin, M. Additive manufacturing of magnesium alloys for biomedical applications: Critical review on sinterability and alloy development. J. Mater. Res. Technol. 2025, 35, 6986–7007. [Google Scholar] [CrossRef]
- Wolff, M.; Ebel, T.; Dahms, M. Sintering of Magnesium. Adv. Eng. Mater. 2010, 12, 829–836. [Google Scholar] [CrossRef]
- Burke, P.; Petit, C.; Yakoubi, S.; Kipouros, G. Thermal Effects of Calcium and Yttrium Additions on the Sintering of Magnesium Powder. In Magnesium Technology; Springer International Publishing: Cham, Switzerland, 2011; pp. 481–484. [Google Scholar]
- Seong, J.W.; Kim, W.J. Development of biodegradable Mg–Ca alloy sheets with enhanced strength and corrosion properties through the refinement and uniform dispersion of the Mg2Ca phase by high-ratio differential speed rolling. Acta Biomater. 2015, 11, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.R.; Kumar, T.S.S.; Chakkingal, U. A review on recent advancements in biodegradable Mg-Ca alloys. J. Magnes. Alloys 2022, 10, 2094–2117. [Google Scholar] [CrossRef]
- Nidadavolu, E.P.S.; Krüger, D.; Zeller-Plumhoff, B.; Tolnai, D.; Wiese, B.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R. Pore characterization of PM Mg–0.6Ca alloy and its degradation behavior under physiological conditions. J. Magnes. Alloys 2021, 9, 686–703. [Google Scholar]
- Makkar, P.; Sarkar, S.K.; Padalhin, A.R.; Moon, B.-G.; Lee, Y.S.; Lee, B.T. In vitro and in vivo assessment of biomedical Mg–Ca alloys for bone implant applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Schaper, J.; Wolff, M.; Ebel, T.; Willumeit, R. Sintering of Mg and its alloys under Hydrogen Atmospheres. In Proceedings of the Euro PM2018 Congress & Exhibition, Bilbao, Spain, 14–18 October 2018. [Google Scholar]
- Herranz, G. Control of carbon content in metal injection molding (MIM). In Handbook of Metal Injection Molding; Heaney, D.F., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 265–304. [Google Scholar]
- Hofstetter, J.; Martinelli, E.; Pogatscher, S.; Schmutz, P.; Povoden-Karadeniz, E.; Weinberg, A.M.; Uggowitzer, P.J.; Löffler, J.F. Influence of trace impurities on the in vitro and in vivo degradation of biodegradable Mg–5Zn–0.3Ca alloys. Acta Biomater. 2015, 23, 347–353. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Wang, C.; Snihirova, D.; Jin, Y.; Zhang, Y.; Zheludkevich, M.L. Approaching “stainless magnesium” by Ca micro-alloying. Mater. Horiz. 2021, 8, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Garamus, V.M.; Wieland, D.C.F.; Moosmann, J.P.; Beckmann, F.; Lottermoser, L.; Serdechnova, M.; Blawert, C.; Fazel, M.; Nidadavolu, E.P.S.; Limberg, W.; et al. Three-Dimensional Distribution of Titanium Hydrides After Degradation of Magnesium/Titanium Hybrid Implant Material—A Study by X-Ray Diffraction Contrast Tomography. J. Compos. Sci. 2025, 9, 396. [Google Scholar] [CrossRef]
- Bazhenov, V.; Li, A.; Iliasov, A.; Bautin, V.; Plegunova, S.; Koltygin, A.; Komissarov, A.; Abakumov, M.; Redko, N.; Shin, K.S. Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. J. Funct. Biomater. 2022, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Kiani, F.; Lin, J.; Vahid, A.; Munir, K.; Wen, C.; Li, Y. Microstructures, mechanical properties, corrosion, and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications. J. Magnes. Alloys 2023, 11, 110–136. [Google Scholar] [CrossRef]
- Farr, N.T.H.; Hughes, G.M.; Rodenburg, C. Monitoring Carbon in Electron and Ion Beam Deposition Within FIB-SEM. Materials 2021, 14, 3034. [Google Scholar] [CrossRef]
- Ahrens, L.; Mikulics, M.; Schröder, S.; Mayer, J.; Hardtdegen, H.H. Laser-Micro-Annealing of Microcrystalline Ni-Rich NCM Oxide: Towards Micro-Cathodes Integrated on Polyethylene Terephthalate Flexible Substrates. Materials 2025, 18, 680. [Google Scholar] [CrossRef]
- Reimers, J.; Mikulics, M.; Lipinska-Chwalek, M.; Zeller-Plumhoff, B.; Kibkalo, L.; Kruth, M.; Willumeit-Römer, R.; Mayer, J.; Hardtdegen, H.H. Towards Correlative Raman Spectroscopy–STEM Investigations Performed on a Magnesium–Silver Alloy FIB Lamella. Nanomaterials 2025, 15, 430. [Google Scholar] [CrossRef]
- Maltseva, A.; Shkirskiy, V.; Lefèvre, G.; Volovitch, P. Effect of pH on Mg(OH)2 film evolution on corroding Mg by in situ kinetic Raman mapping (KRM). Corros. Sci. 2019, 153, 272–282. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Egorkin, V.S.; Ustinov, A.Y.; Sergienko, V.I.; Gnedenkov, S.V. The detailed corrosion performance of bioresorbable Mg-0.8Ca alloy in physiological solutions. J. Magnes. Alloys 2022, 10, 1326–1350. [Google Scholar] [CrossRef]
- Knigge, S.; Mueller, M.; Fricke, L.; Schilling, T.; Glasmacher, B. In Vitro Investigation of Corrosion Control of Magnesium with Degradable Polycaprolactone Coatings for Cardiovascular Grafts. Coatings 2023, 13, 94. [Google Scholar] [CrossRef]
- ISO 2740:2023; Sintered Metal Materials, Excluding Hardmetals—Tensile Test Pieces. ISO: Geneva, Switzerland, 2023.
- Schaper, J.; Wolff, M.; Wiese, B.; Ebel, T.; Willumeit, R. Powder Metal Injection Moulding and Heat Treatment of AZ81 Mg Alloy. J. Mater. Process. Technol. 2019, 267, 241–246. [Google Scholar] [CrossRef]
- Wolff, M.; Helmholz, H.; Luczak, M.; Strerath, D.; Ebel, T.; Willumeit-Römer, R. In Situ X-ray Synchrotron Radiation Analysis, Tensile- and Biodegradation Testing of Redox-Alloyed and Sintered MgCa-Alloy Parts Produced by Metal Injection Moulding. Metals 2022, 12, 353. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.G.; Suckert, M.R.; Dahms, M.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Magnesium Powder Injection Molding (MIM) of Orthopedic Implants for Biomedical Applications. JOM 2016, 68, 1191–1197. [Google Scholar] [CrossRef]
- Nidadavolu, P.E.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Dahms, M. On the Determination of Magnesium Degradation Rates under Physiological Conditions. Materials 2016, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Mikulics, M.; Adam, R.; Chen, G.; Chakraborty, D.; Cheng, J.; Pericolo, A.; Komissarov, I.; Bürgler, D.E.; Heidtfeld, S.F.; Serafini, J.; et al. Determination of Thermal Damage Threshold in THz Photomixers Using Raman Spectroscopy. Crystals 2023, 13, 1267. [Google Scholar] [CrossRef]
- Konar, S.; Nylén, J.; Svensson, G.; Bernin, D.; Edén, M.; Ruschewitz, U.; Häussermann, U. The many phases of CaC2. J. Solid State Chem. 2016, 239, 204–213. [Google Scholar] [CrossRef]
- Ruschewitz, U. Binary and ternary carbides of alkali and alkaline-earth metals. Coord. Chem. Rev. 2003, 244, 115–136. [Google Scholar] [CrossRef]
- Dawson, P.; Hadfield, C.D.; Wilkinson, G.R. The polarized infra-red and Raman spectra of Mg(OH)2 and Ca(OH)2. J. Phys. Chem. Solids 1973, 34, 1217–1225. [Google Scholar] [CrossRef]
- Duffy, T.S.; Meade, C.; Fei, Y.; Mao, H.-K.; Hemley, R.J. High-pressure phase transition in brucite, Mg(OH)2. Am. Mineral. 1995, 80, 222–230. [Google Scholar] [CrossRef]
- Krbata, M.; Kohutiar, M.; Escherova, J.; Klučiar, P.; Studeny, Z.; Trembach, B.; Beronská, N.; Breznická, A.; Timárová, L. Continuous Cooling Transformation of Tool Steels X153CrMoV12 and 100MnCrW4: Analysis of Microstructure and Hardness Changes. Appl. Mech. 2025, 6, 16. [Google Scholar] [CrossRef]
- Novák, J. Zur Kenntnis der Magnesiumcarbide: I. Mitteilung. Z. Für Phys. Chem. 1910, 73, 513–546. [Google Scholar] [CrossRef]
- Schaper, J.G. Magnesium Polyolefin Interactions During Thermal Debinding in the MIM Process of Magnesium. Ph.D. Thesis, Christian-Albrechts Universität Kiel, Kiel, Germany, 2019. [Google Scholar]
- Schaper, J. Sintering of Mg and its Alloys under Hydrogen Atmospheres. Preprints 2021. Preprints:202102.0295.v1. [Google Scholar]
- de Oro Calderon, R.; Gierl-Mayer, C.; Danninger, H. Interstitial chemistry in sintering of metallic materials—Often overlooked but decisive. Eur. J. Mater. 2022, 2, 381–406. [Google Scholar] [CrossRef]
- Nylén, J.; Konar, S.; Lazor, P.; Benson, D.; Häussermann, U. Structural behavior of the acetylide carbides Li2C2 and CaC2 at high pressure. J. Chem. Phys. 2012, 137, 224507. [Google Scholar] [CrossRef]
- Reckeweg, O.; Baumann, A.; Mayer, H.A.; Glaser, J.; Meyer, H.-J. Über die Koexistenz von tetragonalem und monoklinem CaC2: Strukturelle und spektroskopische Untersuchungen an Erdalkalimetallacetyliden, MC2 (M = Ca, Sr, Ba). Z. Für Anorg. Allg. Chem. 1999, 625, 1686–1692. [Google Scholar] [CrossRef]
- Kang, S.-J.L. What We Should Consider for Full Densification when Sintering. Materials 2020, 13, 3578. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, J.; Li, J.; Li, H.; Zhao, Z. Mechanical and corrosion performance of Mg alloy via 3DP by full liquid phase sintering. Powder Technol. 2022, 399, 117138. [Google Scholar] [CrossRef]
- Gouadec, G.; Colomban, P. Raman Spectroscopy of nanomaterials: How spectra relate to disorder, particle size and mechanical properties. Prog. Cryst. Growth Charact. Mater. 2007, 53, 1–56. [Google Scholar] [CrossRef]
- Angel, R.J.; Murri, M.; Mihailova, B.; Alvaro, M. Stress, strain and Raman shifts. Z. Für Krist. Cryst. Mater. 2019, 234, 129–140. [Google Scholar] [CrossRef]
- Becker, M.; Scheel, H.; Christiansen, S.; Strunk, H.P. Grain orientation, texture, and internal stress optically evaluated by micro-Raman spectroscopy. J. Appl. Phys. 2007, 101, 063531. [Google Scholar] [CrossRef]
- Nidadavolu, E.; Wolff, M.; Ebel, T.; Willumeit-Römer, R. Effect of Powder Surface Oxides in Ensuring a Reproducible Homogenous Microstructure in Powder Processed Mg-0.6Ca Alloy. In Proceedings of the EUROPM2024 Congress and Exhibition, Malmo, Sweden, 29 September–2 October 2024. [Google Scholar]
- Li, J.; Kipouros, G.J.; Burke, P.; Bibby, C. Investigation of Surface Film Formed on Fine Mg Particles. Microsc. Microanal. 2009, 15, 362–363. [Google Scholar] [CrossRef]
| Metallic Powders | Elemental Composition [wt.%] | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Cu | Ca | Si | Mn | Zn | Mg | |
| Pure Mg (d50 = 22 µm) | 0.0018 | 0.0006 | 0.0002 | 0.0041 | 0.0221 | 0.0110 | 0.0027 | Bal. |
| Master alloy Mg-10Ca | 0.0021 | 0.0003 | 0.0012 | 10.214 | 0.0200 | 0.0150 | 0.0034 | Bal. |
| Binder Component | Abbreviation | Manufacturer |
|---|---|---|
| Paraffin wax [50 wt.%] | PW58 | Merck |
| Paraffin wax [10 wt.%] | PW57 | Merck |
| Stearic acid [5 wt.%] | StA | Merck |
| Polypropylene-copolymer-polyethylene [35 wt.%] | PPcoPE | -- 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nidadavolu, E.; Mikulics, M.; Wolff, M.; Ebel, T.; Willumeit-Römer, R.; Zeller-Plumhoff, B.; Mayer, J.; Hardtdegen, H.H. Correlative Raman Spectroscopy–SEM Investigations of Sintered Magnesium–Calcium Alloys for Biomedical Applications. Materials 2025, 18, 3873. https://doi.org/10.3390/ma18163873
Nidadavolu E, Mikulics M, Wolff M, Ebel T, Willumeit-Römer R, Zeller-Plumhoff B, Mayer J, Hardtdegen HH. Correlative Raman Spectroscopy–SEM Investigations of Sintered Magnesium–Calcium Alloys for Biomedical Applications. Materials. 2025; 18(16):3873. https://doi.org/10.3390/ma18163873
Chicago/Turabian StyleNidadavolu, Eshwara, Martin Mikulics, Martin Wolff, Thomas Ebel, Regine Willumeit-Römer, Berit Zeller-Plumhoff, Joachim Mayer, and Hilde Helen Hardtdegen. 2025. "Correlative Raman Spectroscopy–SEM Investigations of Sintered Magnesium–Calcium Alloys for Biomedical Applications" Materials 18, no. 16: 3873. https://doi.org/10.3390/ma18163873
APA StyleNidadavolu, E., Mikulics, M., Wolff, M., Ebel, T., Willumeit-Römer, R., Zeller-Plumhoff, B., Mayer, J., & Hardtdegen, H. H. (2025). Correlative Raman Spectroscopy–SEM Investigations of Sintered Magnesium–Calcium Alloys for Biomedical Applications. Materials, 18(16), 3873. https://doi.org/10.3390/ma18163873






