Double-Layer Sol–Gel Modifications on Titanium Alloy Substrates—Physicochemical Properties Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Metal Substrate Preparation
2.2. Preparation of SiO2/BN and SiO2/TiN Samples
2.2.1. Synthesis of Hybrid Sols
2.2.2. Coatings Deposition
2.3. Coatings Characterization
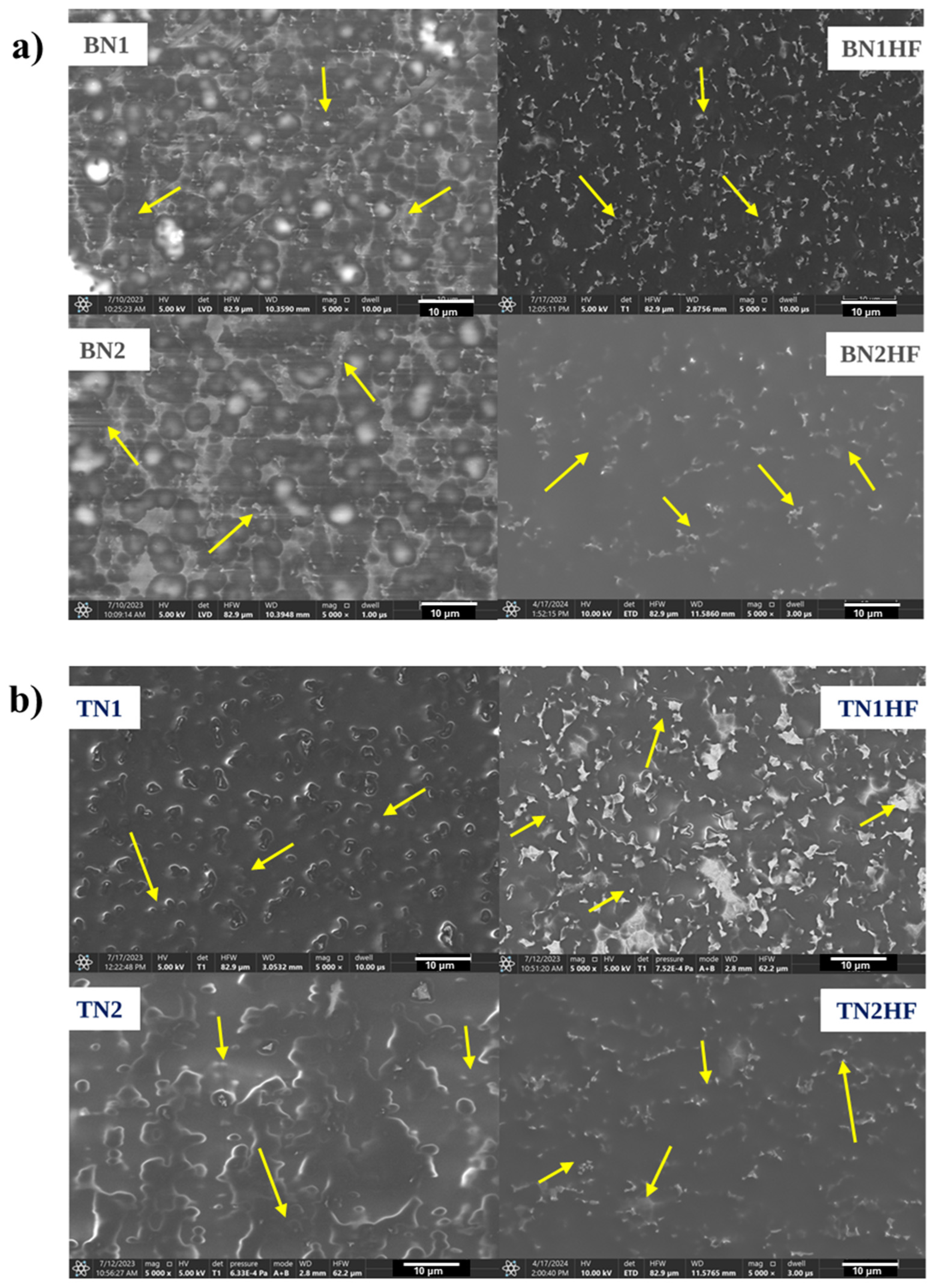
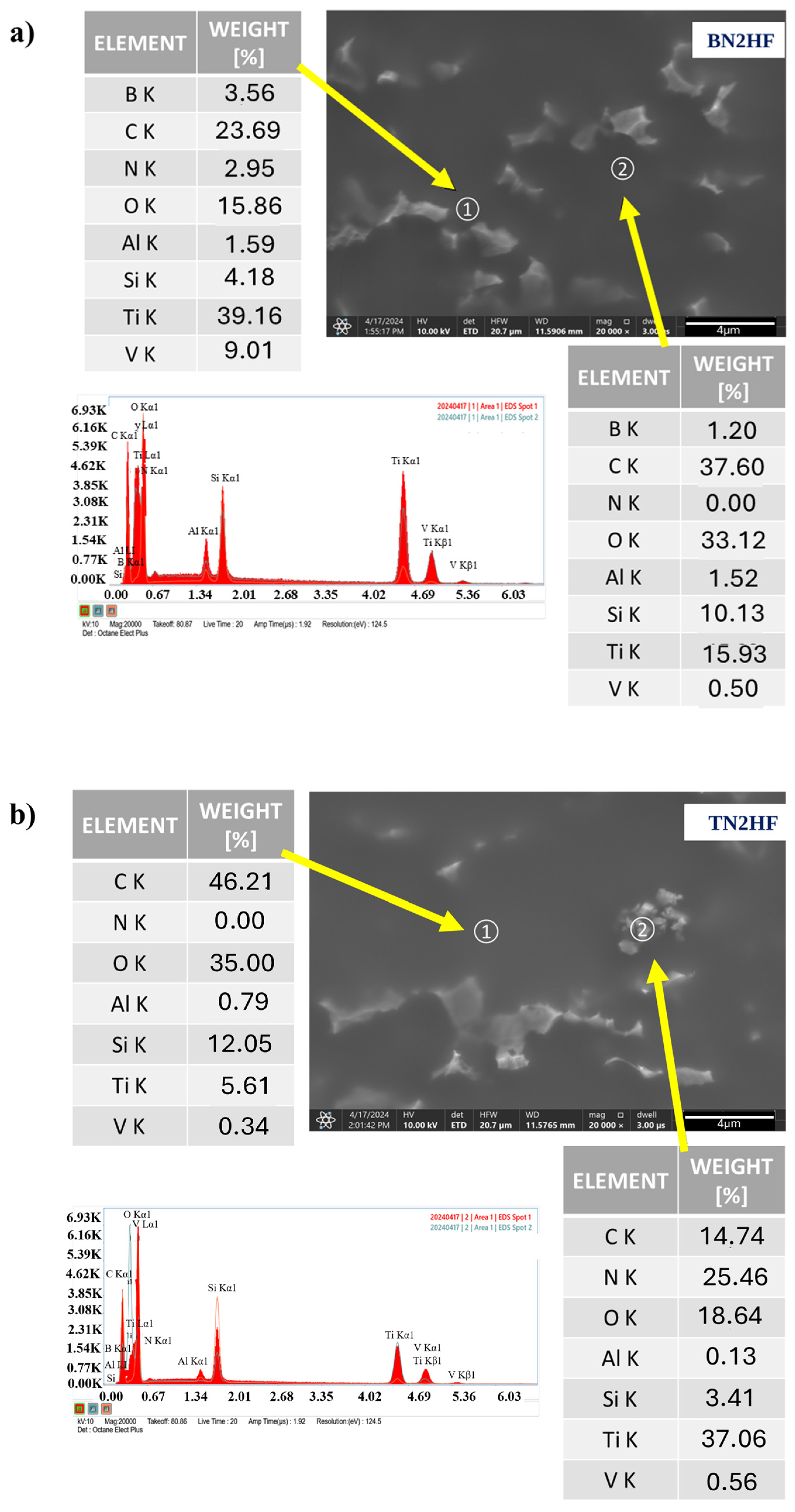
2.3.1. Scanning Electron Microscopy
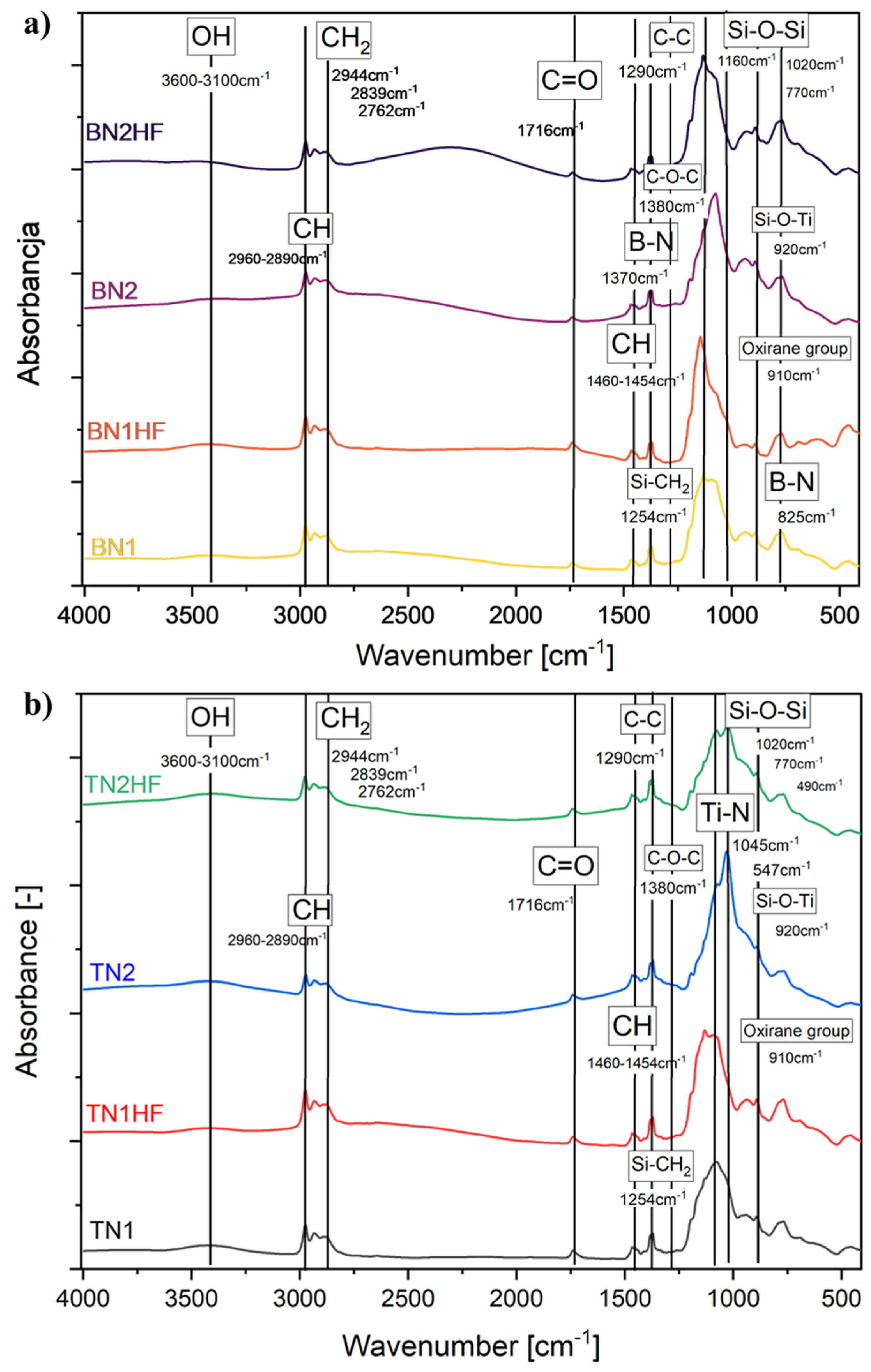
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. XRD X-Ray Diffractometry
2.3.4. Roughness
2.3.5. Surface Wettability
2.3.6. Surface Free Energy
2.3.7. Mechanical Properties
2.3.8. Corrosion Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2024, 17, 114. [Google Scholar] [CrossRef]
- Haydar, H.J.; Al-Deen, J.; AbidAli, A.K.; Mahmoud, A.A. Improved Performance of Ti6Al4V Alloy in Biomedical Applications—Review. J. Phys. Conf. Ser. 2021, 1973, 121–146. [Google Scholar] [CrossRef]
- Guijiang, W.; Meiying, T.; Shokouh, A.; Jie, L.; Vasilievich, V.U.; Binghao, W.; Jia Liu, L.; Lu, L.; Liqiang, W. An overview of surface modification, A way toward fabrication of nascent biomedical Ti–6Al–4V alloys. J. Mater. Res. Technol. 2023, 24, 5896–5921. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Bowen, J.; Zelzer, M.; Stamboulis, A. Selective modification of Ti6Al4V surfaces for biomedical applications. RSC Adv. 2020, 10, 17642–17652. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Corrosion in Titanium Dental Implants/Prostheses—A Review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Gogolewski, D.; Kozior, T.; Zmarzły, P.; Mathia, T.G. Morphology of models manufactured by slm technology and the ti6al4v titanium alloy designed for medical applications. Materials 2021, 14, 6249. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, N.; Kumar, D.; Hegab, H. Design, development and tribological characterization of Ti–6Al–4V/hydroxyapatite composite for bio-implant applications. Mater. Chem. Phys. 2020, 243, 122662. [Google Scholar] [CrossRef]
- Fojt, J. Ti-6Al-4V alloy surface modification for medical applications. Appl. Surf. Sci. 2012, 262, 163–167. [Google Scholar] [CrossRef]
- Abraham, M.A.; Joseph, S.; Mathew, A.T.; Thomas, A.S.; Abraham, M.E.; Sali, G.; Rajesh, P. Different Surface Modifications of Titanium Implant: A Review. IOSR-JDMS 2021, 20, 49–53. [Google Scholar]
- Zhang, Y.; Attarilar, S.; Wang, L.; Lu, W.; Yang, J.; Fu, Y. A Review on Design and Mechanical Properties of Additively Manufactured NiTi Implants for Orthopedic Applications. Int. J. Bioprint. 2021, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Attarilar, S.; Salehi, M.T.; Al-Fadhalah, K.J.; Djavanroodi, F.; Mozafari, M. Functionally graded titanium implants: Characteristic enhancement induced by combined severe plastic deformation. PLoS ONE 2019, 14, e0221491. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8, 576969. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Liang, L.; Hong, H.; Zhang, Z.; Shen, B. Electrochemical behaviour of EPD synthesized graphene coating on titanium alloys for orthopedic implant application. Procedia CIRP 2018, 71, 322–328. [Google Scholar] [CrossRef]
- Shah, A.; Fasehah, S.N.; Hassan, M.A.; Daud, R.; Che Kob, C.G. Improvement of Corrosion Resistance of Tin Coated on Titanium Alloy for Biomedical Application. J. Phys. Conf. Ser. 2021, 1823, 012111. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Solcan, C.; Hritcu, L.D.; Spataru, M.C. Research Progress of Titanium-Based Alloys for Medical Devices. Biomedicines 2021, 11, 2997. [Google Scholar] [CrossRef]
- Ayata, S.; Ensinger, W. Ion beam sputter coating in combination with sol-gel dip coating of Al alloy tube inner walls for corrosion and biological protection. Surf. Coat. Technol. 2018, 340, 121–125. [Google Scholar] [CrossRef]
- Zanurin, A.; Johari, N.A.; Alias, J.; Ayu, H.M.; Redzuan, N.; Izman, S. Research progress of sol-gel ceramic coating: A review. Mater. Today Proc. 2021, 48, 1849–1854. [Google Scholar] [CrossRef]
- Babiarczuk, B.; Szczurek, A.; Donesz-Sikorska, A.; Rutkowska, I.; Krzak, J. The influence of an acid catalyst on the morphology, wettabillity, adhesion and chemical structure properties of TiO2 and ZrO2 sol-gel thin films. Surf. Coat. Technol. 2016, 285, 134–145. [Google Scholar] [CrossRef]
- Tarzanagh, Y.J.; Seifzadeh, D.; Rajabalizadeh, Z.; Habibi-Yangjeh, A.; Khodayari, A.; Sohrabnezhad, S. Sol-gel/MOF nanocomposite for effective protection of 2024 aluminum alloy against corrosion. Surf. Coat. Technol. 2019, 380, 125038. [Google Scholar] [CrossRef]
- Juan-Díaz, M.J.; Martínez-Ibáñez, M.; Lara-Sáez, I.; da Silva, S.; Izquierdo, R.; Gurruchaga, M.; Goñi, I.; Suay, J. Development of hybrid sol–gel coatings for the improvement of metallic biomaterials performance. Prog. Org. Coat. 2016, 96, 42–51. [Google Scholar] [CrossRef]
- Romero Pareja, R.; López Ibáñez, R.; Martín, F.; Ramos-Barrado, J.R.; Leinen, D. Corrosion behaviour of zirconia barrier coatings on galvanized steel. Surf. Coat. Technol. 2006, 200, 6606–6610. [Google Scholar] [CrossRef]
- Ugas-Carrión, R.; Sittner, F.; Yekehtaz, M.; Flege, S.; Brötz, J.; Ensinger, W. Influence of stabilizing agents on structure and protection performance of zirconium oxide films. Surf. Coat. Technol. 2010, 204, 2064–2067. [Google Scholar] [CrossRef]
- Tiringer, U.; Durán, A.; Castro, Y.; Milošev, I. Self-Healing Effect of Hybrid Sol-Gel Coatings Based on GPTMS, TEOS, SiO2 Nanoparticles and Ce(NO3)3 Applied on Aluminum Alloy 7075-T6. J. Electrochem. Soc. 2018, 165, C213–C225. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Bashir, Z.; Mohsin, M.; Riaz, S.; Naseem, S. Sol-gel synthesized boron nitride (BN) thin films for antibacterial and magnetic applications. Optik 2021, 243, 167502. [Google Scholar] [CrossRef]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Qing, Y.Q.; Yang, C.N.; Shang, Y.; Sun, Y.Z.; Liu, C.S. Facile approach in fabricating hybrid superhydrophobic fluorinated polymethylhydrosiloxane/TiO2 nanocomposite coatings. Colloid Polym. Sci. 2015, 293, 1809–1816. [Google Scholar] [CrossRef]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L.; Owen, J.R. A sol-gel route to titanium nitride conductive coatings on battery materials and performance of TiN-coated LiFePO4. J. Mater. Chem. A 2017, 5, 2251–2260. [Google Scholar] [CrossRef]
- Blees, M.H.; Winkelman, G.B.; Balkenende, A.R.; Den Toonder, J.M.J. The effect of friction on scratch adhesion testing: Application to a sol-gel coating on polypropylene. Thin Solid Film. 2000, 359, 1–13. [Google Scholar] [CrossRef]
- Basso, M.; Colusso, E.; Tancon, M.; Bortolin, S.; Mirafiori, M.; Guglielmi, M.; Del Col, D.; Martucci, A.; Martucci, A. Hydrophobic hybrid silica sol-gel coating on aluminium: Stability evaluation during saturated vapour condensation. J. Non-Cryst. Solids X 2022, 17, 100–143. [Google Scholar] [CrossRef]
- Das, M.; Basu, A.K.; Ghatak, S.; Joshi, A.G. Carbothermal synthesis of boron nitride coating on PAN carbon fiber. J. Eur. Ceram. Soc. 2009, 29, 2129–2134. [Google Scholar] [CrossRef]
- Göncü, Y.; Geçgin, M.; Bakan, F.; Ay, N. Electrophoretic deposition of hydroxyapatite-hexagonal boron nitride composite coatings on Ti substrate. Mater. Sci. Eng. C 2017, 79, 343–353. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Yue, X.; Huo, J.; Ye, F.; Liu, J.; Shi, F.; Ma, J. New BN/SiOC aerogel composites fabricated by the sol-gel method with excellent thermal insulation performance at high temperature. Mater. Des. 2020, 185, 108–217. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, J.; Wang, F.; Liu, L.; Liu, H.; Yan, C.; Guo, Z. Sol-gel synthesized hexagonal boron nitride/titania nanocomposites with enhanced photocatalytic activity. Appl. Surf. Sci. 2019, 465, 154–163. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, S.; Ter-Ovanessian, B.; Hermange, K.; Huo, Y.; Normand, B. Enhancing the barrier effect of sol-gel derived inorganic coating by doping h-BN nanosheet. Appl. Surf. Sci. 2021, 544, 148849. [Google Scholar] [CrossRef]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coat. Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Pandit, S.; Gaska, K.; Mokkapati, V.R.S.S.; Forsberg, S.; Svensson, M.; Kádár, R.; Mijakovic, I. Antibacterial effect of boron nitride flakes with controlled orientation in polymer composites. RSC Adv. 2019, 9, 33454–33459. [Google Scholar] [CrossRef] [PubMed]
- Çamurlu, H.E.; Akarsu, E.; Arslan, O.; Mathur, S. Nanocomposite glass coatings containing hexagonal boron nitride nanoparticles. Ceram. Int. 2016, 42, 8856–8862. [Google Scholar] [CrossRef]
- ISO 4288:1996; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Rules and Procedures for the Assessment of Surface Texture. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 4624:2002; Paints and Varnishes—Pull-off Test for Adhesion. International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 10993-15; Biological Evaluation of Medical Devices—Part 15: Identification and Quantification of Degradation Products from Metals and Alloys. International Organization for Standardization: Geneva, Switzerland, 2000.
- King, F.L.; Yepuri, V.; Singh, G.R. Sol-gel Spin Coated Hydrophilic Composite Titania-Silica Coatings on the Glass Substrates for Automotive Applications. E3S Web Conf. 2024, 509, 01001. [Google Scholar] [CrossRef]
- Cho, A.R.; Park, S.-Y. Synthesis of titania- and silica-polymer hybrid materials and their application as refractive index-matched layers in touch screens. Opt. Mater. Express 2015, 5, 690–703. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Nguyen, T.; Vu, D.; Dinh, D.; Nguyen, A.H.; Ly, T.; Dao, P.H.; Nguyen, T.L.; Bach, L.G.; Thai, H. Modification of Titanium Dioxide Nanoparticles with 3-(Trimethoxysilyl)propyl Methacrylate Silane Coupling Agent. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Cutz, L.; Tiringer, U.; de Jong, W.; Mol, A. Hybrid sol-gel coatings for reducing wettability and storage degradation of biomass pellets. Mater. Chem. Phys. 2023, 304, 127861. [Google Scholar] [CrossRef]
- Shams-Ghahfarokhi, F.; Khoddami, A.; Mazrouei-Sebdani, Z.; Rahmatinejad, J.; Mohammadi, H. A new technique to prepare a hydrophobic and thermal insulating polyester woven fabric using electro-spraying of nano-porous silica powder. Surf. Coat. Technol. 2019, 366, 97–105. [Google Scholar] [CrossRef]
- Cambon, J.B.; Ansart, F.; Bonino, J.P.; Turq, V. Effect of cerium concentration on corrosion resistance and polymerization of hybrid sol-gel coating on martensitic stainless steel. Prog. Org. Coat. 2012, 75, 486–493. [Google Scholar] [CrossRef]
- Wanag, A.; Sienkiewicz, A.; Rokicka-Konieczna, P.; Kusiak-Nejman, E.; Morawski, A.W. Influence of modification of titanium dioxide by silane coupling agents on the photocatalytic activity and stability. J. Environ. Chem. Eng. 2020, 8, 103917. [Google Scholar] [CrossRef]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Venkateswara Rao, A. Porous superhydrophobic silica films by sol-gel process. Microporous Mesoporous Mater. 2010, 130, 115–121. [Google Scholar] [CrossRef]
- Kostoglou, N.; Polychronopoulou, K.; Rebholz, C. Thermal and chemical stability of hexagonal boron nitride (h-BN) nanoplatelets. Vacuum 2015, 112, 42–45. [Google Scholar] [CrossRef]
- Harrison, H.B.; Lamb, J.T.; Nowlin, K.S.; Guenthner, A.J.; Ghiassi, K.B.; Kelkar, A.D.; Alston, J.R. Quantification of hexagonal boron nitride impurities in boron nitride nanotubes: Via FTIR spectroscopy. Nanoscale Adv. 2019, 1, 1693–1701. [Google Scholar] [CrossRef]
- Barth, K.L.; Fukarek, W.; Maucher, H.P.; Plass, M.F.; Lunk, A. In situ characterization of cubic boron nitride film growth in the IR spectral region. Thin Solid Film. 1998, 313–314, 697–703. [Google Scholar] [CrossRef]
- Čolović, B.; Kisić, D.; Jokanović, B.; Rakočević, Z.; Nasov, I.; TrajkovskaPetkoska, A.; Jokanović, V. Wetting properties of titanium oxides, oxynitrides and nitrides obtained by DC and pulsed magnetron sputtering and cathodic arc evaporation. Mater. Sci.-Pol. 2019, 37, 173–181. [Google Scholar] [CrossRef]
- Choi, D.; Kumta, P.N. Nanocrystalline TiN Derived by a Two-Step Halide Approach for Electrochemical Capacitors. J. Electrochem. Soc. 2006, 153, A2298–A2303. [Google Scholar] [CrossRef]
- Lewandowska, M.; Garbacz, H.; Fabianowski, W.; Polak, B.; Lewandowska-Szumieł, M. Modyfikacje Powierzchni Siateczek Tytanowych Przeznaczonych Na Implanty. Inżynieria Biomateriałów 2004, 7, 60–62. [Google Scholar]
- Melentiev, R.; Fang, F.; Narala, S.K.R. Influence of different pretreatments on Ti-6Al-4V surface integrity and scratch-resistance of epoxy coating: Analysis of topography, microstructure, chemistry and wettability. Surf. Coat. Technol. 2020, 404, 126436. [Google Scholar] [CrossRef]
- Vlcak, P.; Fojt, J.; Drahokoupil, J.; Brezina, V.; Sepitka, J.; Horazdovsky, T.; Miksovsky, J.; Cerny, F.; Lebeda, M.; Haubner, M. Influence of surface pre-treatment with mechanical polishing, chemical, electrochemical and ion sputter etching on the surface properties, corrosion resistance and MG-63 cell colonization of commercially pure titanium. Mater. Sci. Eng. C 2020, 115, 111065. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; la Monaca, A.; Murray, J.; Speidel, A.; Ushmaev, D.; Clare, A.; Axinte, D.; M’Saoubi, R. Surface integrity in metal machining—Part I: Fundamentals of surface characteristics and formation mechanisms. Int. J. Mach. Tools Manuf. 2021, 162, 103687. [Google Scholar] [CrossRef]
- la Monaca, A.; Liao, Z.; Axinte, D. A digital approach to automatically assess the machining-induced microstructural surface integrity. J. Mater. Process. Technol. 2020, 282, 116703. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Kaps, S.; Adelung, R.; Scharnberg, M.; Faupel, F.; Milenkovic, S.; Hassel, A.W. Determining superhydrophobic surfaces from an expanded Cassie Baxter equation describing simple wettability experiments. arXiv 2014. [Google Scholar] [CrossRef]
- Belan, J.; Uhricik, M.; Hanusova, P.; Vasko, A. The Ti6Al4V Alloy Microstructure Modification Via Various Cooling Rates, its Influence on Hardness and Microhardness. Manuf. Technol. 2020, 20, 560–565. [Google Scholar] [CrossRef]
- Tiringer, U.; van Dam, J.P.B.; Abrahami, S.T.; Terryn, H.; Kovač, J.; Milošev, I.; Mol, J.M.C. Scrutinizing the importance of surface chemistry versus surface roughness for aluminium/sol-gel film adhesion. Surf. Interfaces 2021, 26, 101417. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008. [Google Scholar] [CrossRef]
- Atanacio, A.J.; Latella, B.A.; Barbé, C.J.; Swain, M.V. Mechanical properties and adhesion characteristics of hybrid sol-gel thin films. Surf. Coat. Technol. 2005, 192, 354–364. [Google Scholar] [CrossRef]
- Xie, Y.; Hawthorne, H.M. Measuring the adhesion of sol-gel derived coatings to a ductile substrate by an indentation-based method. Surf. Coat. Technol. 2003, 172, 42–50. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, M.; Zhang, Z.; Yi, X.; Yan, J.; Zhou, Z.; Fang, D.; He, Y.; Li, Y. Corrosion Behavior of Nitrided Layer of Ti6Al4V Titanium Alloy by Hollow Cathodic Plasma Source Nitriding. Materials 2023, 16, 2961. [Google Scholar] [CrossRef] [PubMed]

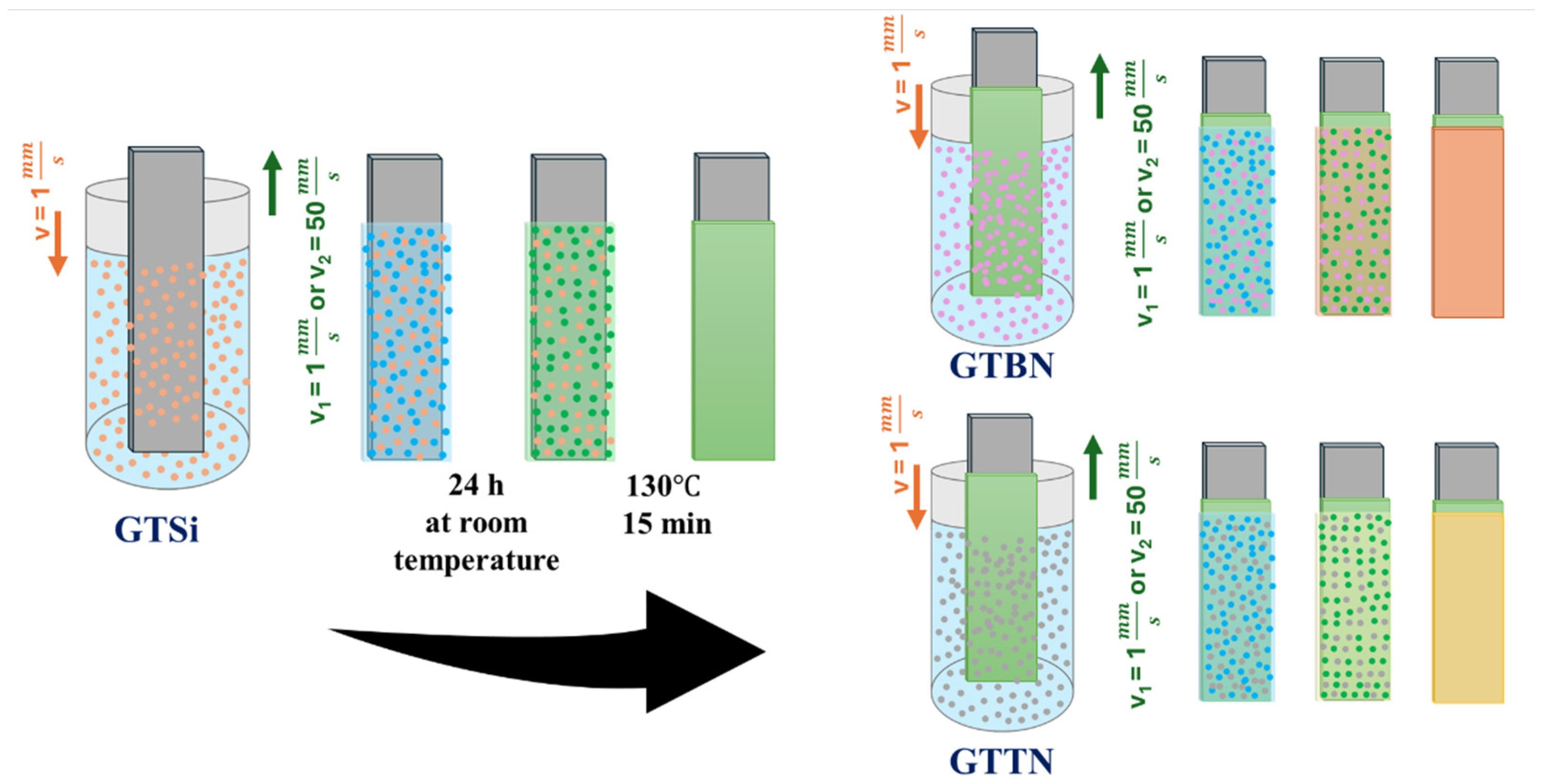
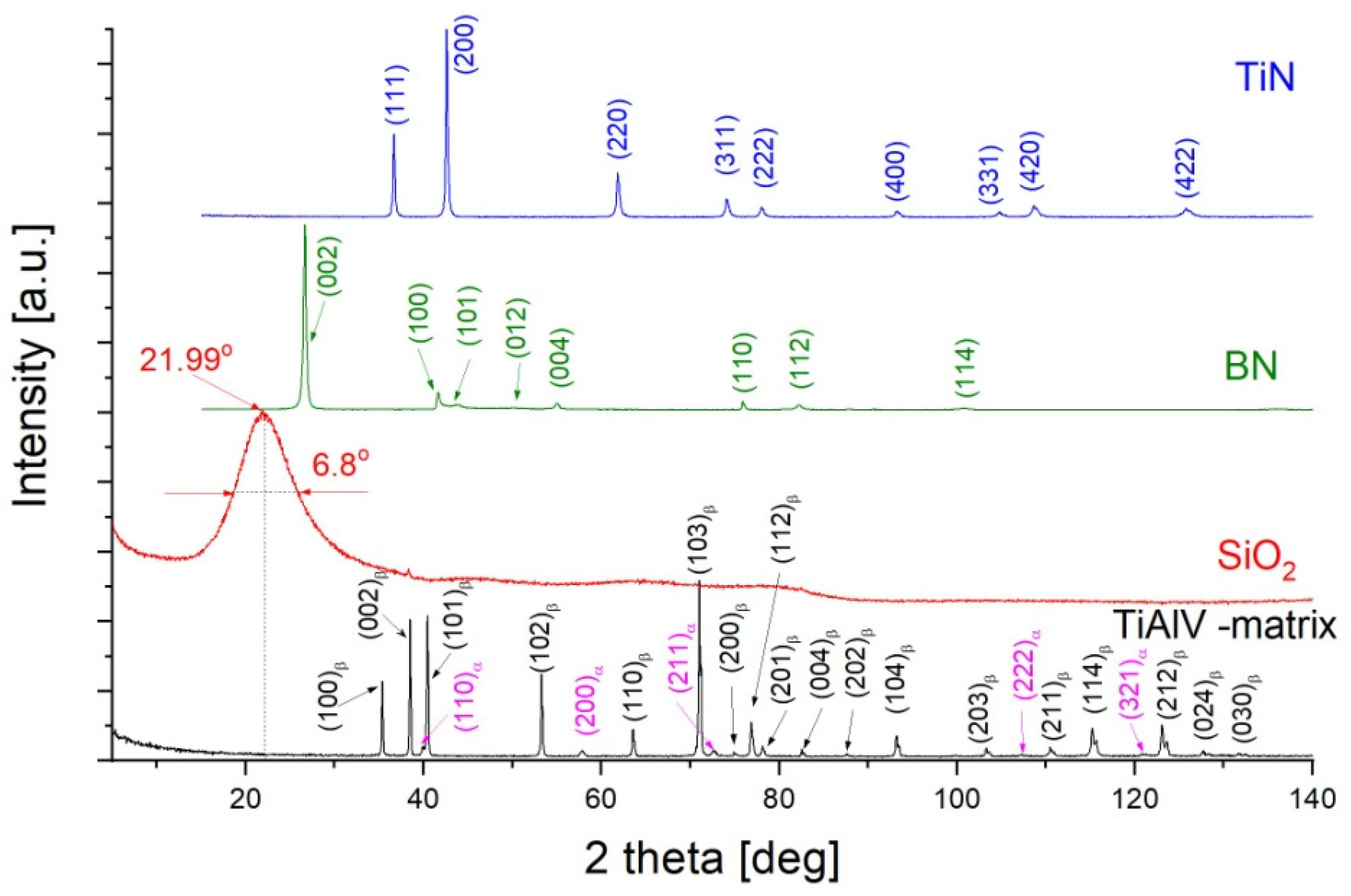


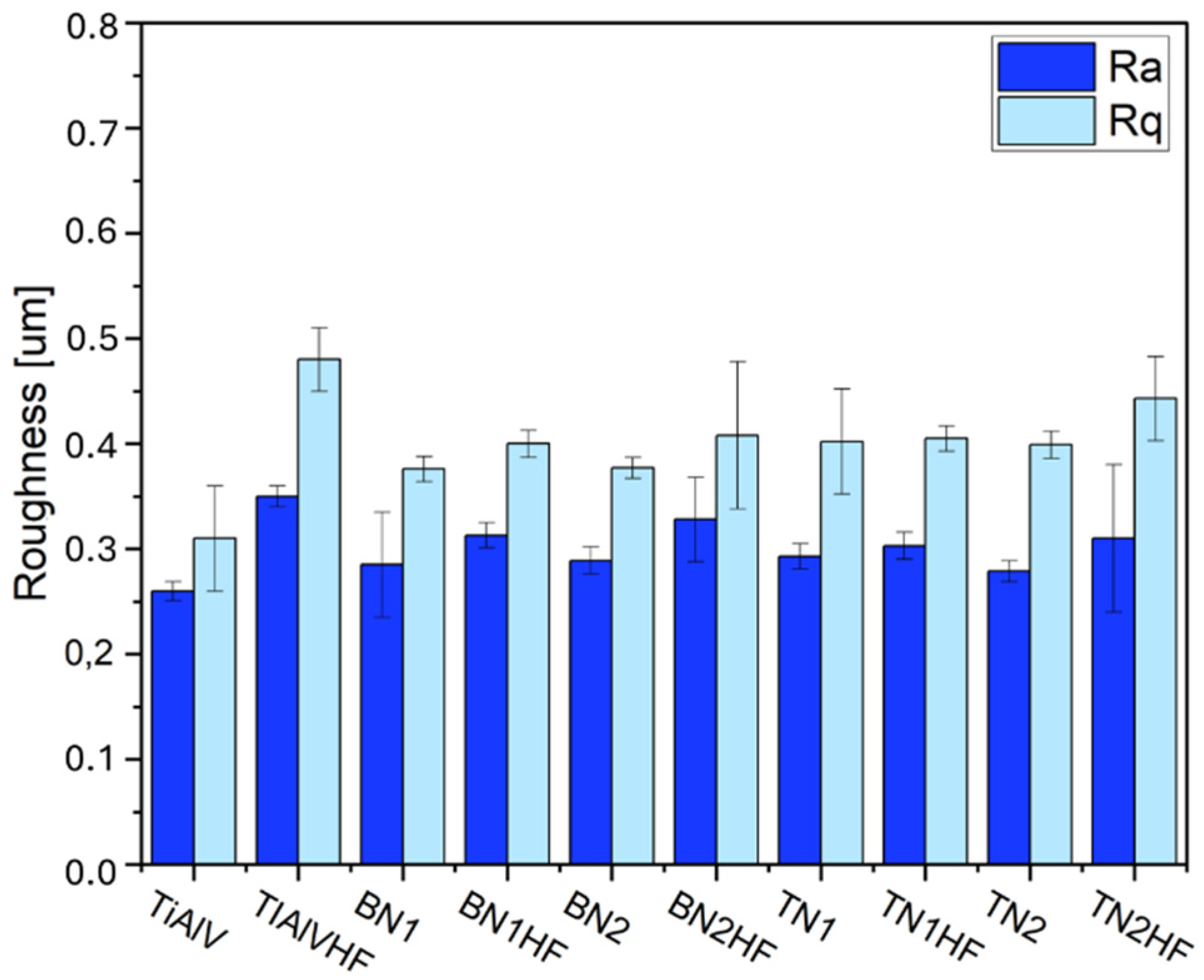

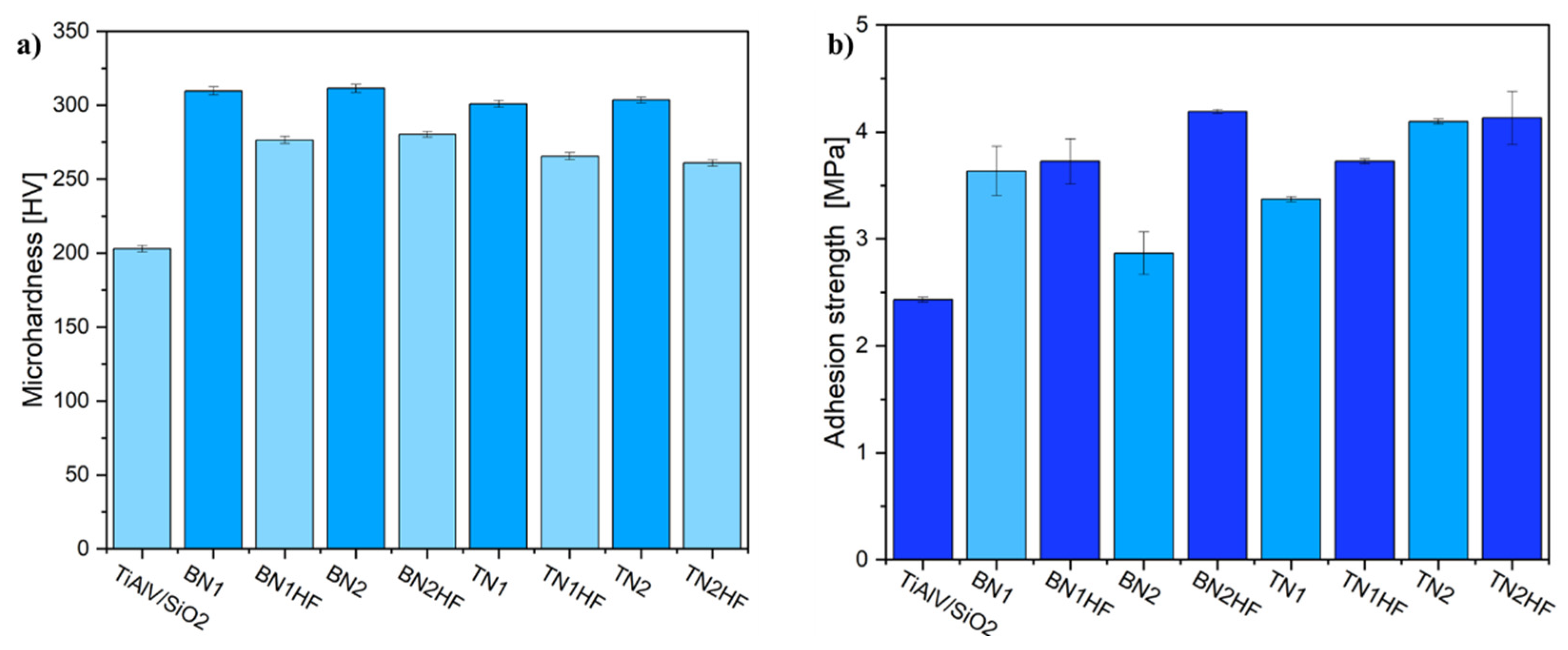

| Sol 1 | Sol 2 | Withdrawal Speed | Sample Surface Preparation | Nomenclature of Samples |
|---|---|---|---|---|
| GTSi | GTBN | 1 | non-etched | BN1 |
| GTBN | 1 | etched | BN1HF | |
| GTBN | 50 | non-etched | BN2 | |
| GTBN | 50 | etched | BN2HF | |
| GTTN | 1 | non-etched | TN1 | |
| GTTN | 1 | etched | TN1HF | |
| GTTN | 50 | non-etched | TN2 | |
| GTTN | 50 | etched | TN2HF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matysiak, K.; Biegun-Żurowska, M.; Cholewa-Kowalska, K.; Goryczka, T.; Zając, W.; Ziąbka, M. Double-Layer Sol–Gel Modifications on Titanium Alloy Substrates—Physicochemical Properties Evaluation. Materials 2025, 18, 3857. https://doi.org/10.3390/ma18163857
Matysiak K, Biegun-Żurowska M, Cholewa-Kowalska K, Goryczka T, Zając W, Ziąbka M. Double-Layer Sol–Gel Modifications on Titanium Alloy Substrates—Physicochemical Properties Evaluation. Materials. 2025; 18(16):3857. https://doi.org/10.3390/ma18163857
Chicago/Turabian StyleMatysiak, Katarzyna, Maria Biegun-Żurowska, Katarzyna Cholewa-Kowalska, Tomasz Goryczka, Wojciech Zając, and Magdalena Ziąbka. 2025. "Double-Layer Sol–Gel Modifications on Titanium Alloy Substrates—Physicochemical Properties Evaluation" Materials 18, no. 16: 3857. https://doi.org/10.3390/ma18163857
APA StyleMatysiak, K., Biegun-Żurowska, M., Cholewa-Kowalska, K., Goryczka, T., Zając, W., & Ziąbka, M. (2025). Double-Layer Sol–Gel Modifications on Titanium Alloy Substrates—Physicochemical Properties Evaluation. Materials, 18(16), 3857. https://doi.org/10.3390/ma18163857








