Mechanical and Microstructural Properties of Alkali-Activated Biomass Fly Ash and Diatomite Blends
Abstract
1. Introduction
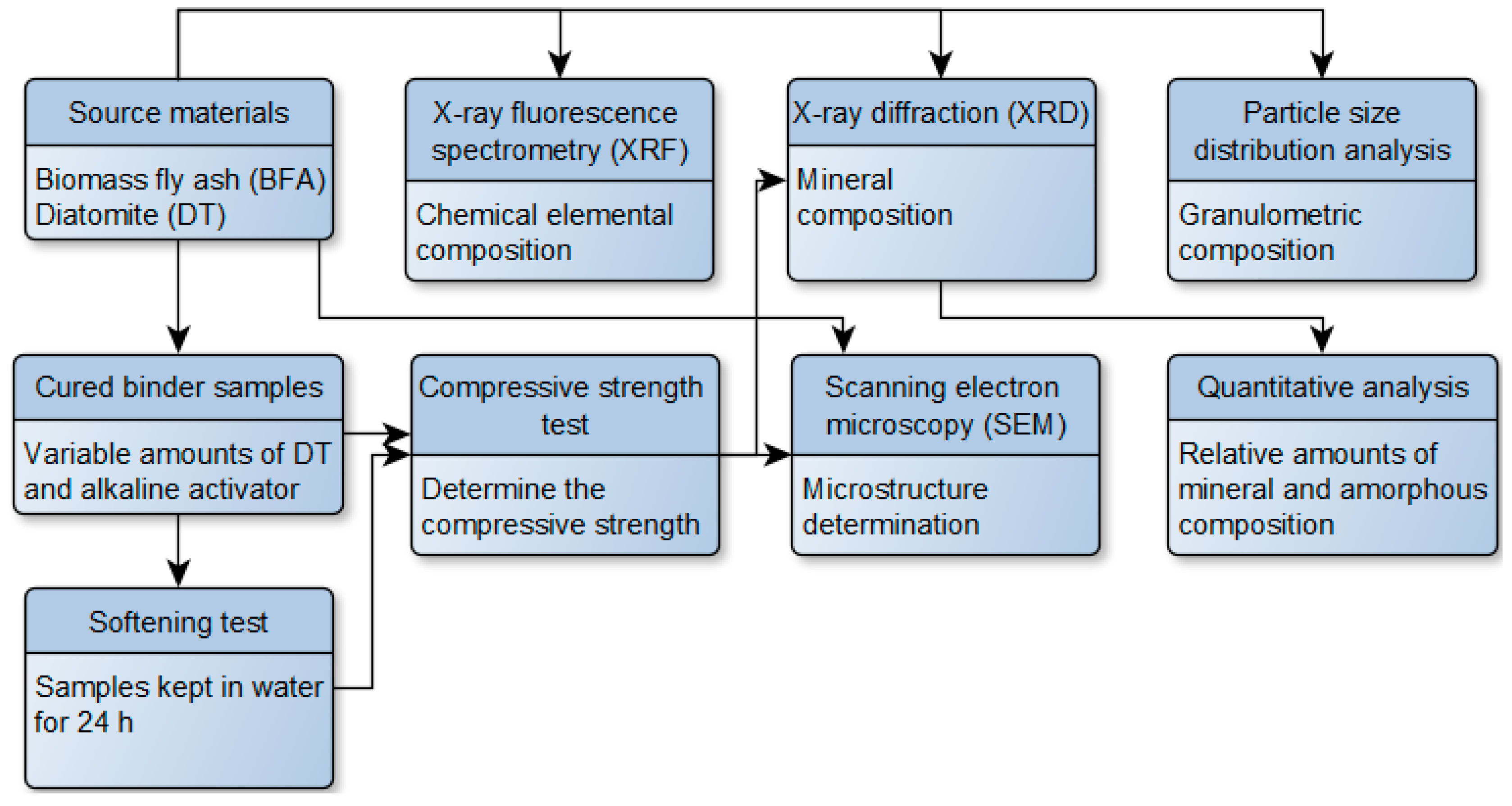
2. Materials and Methods
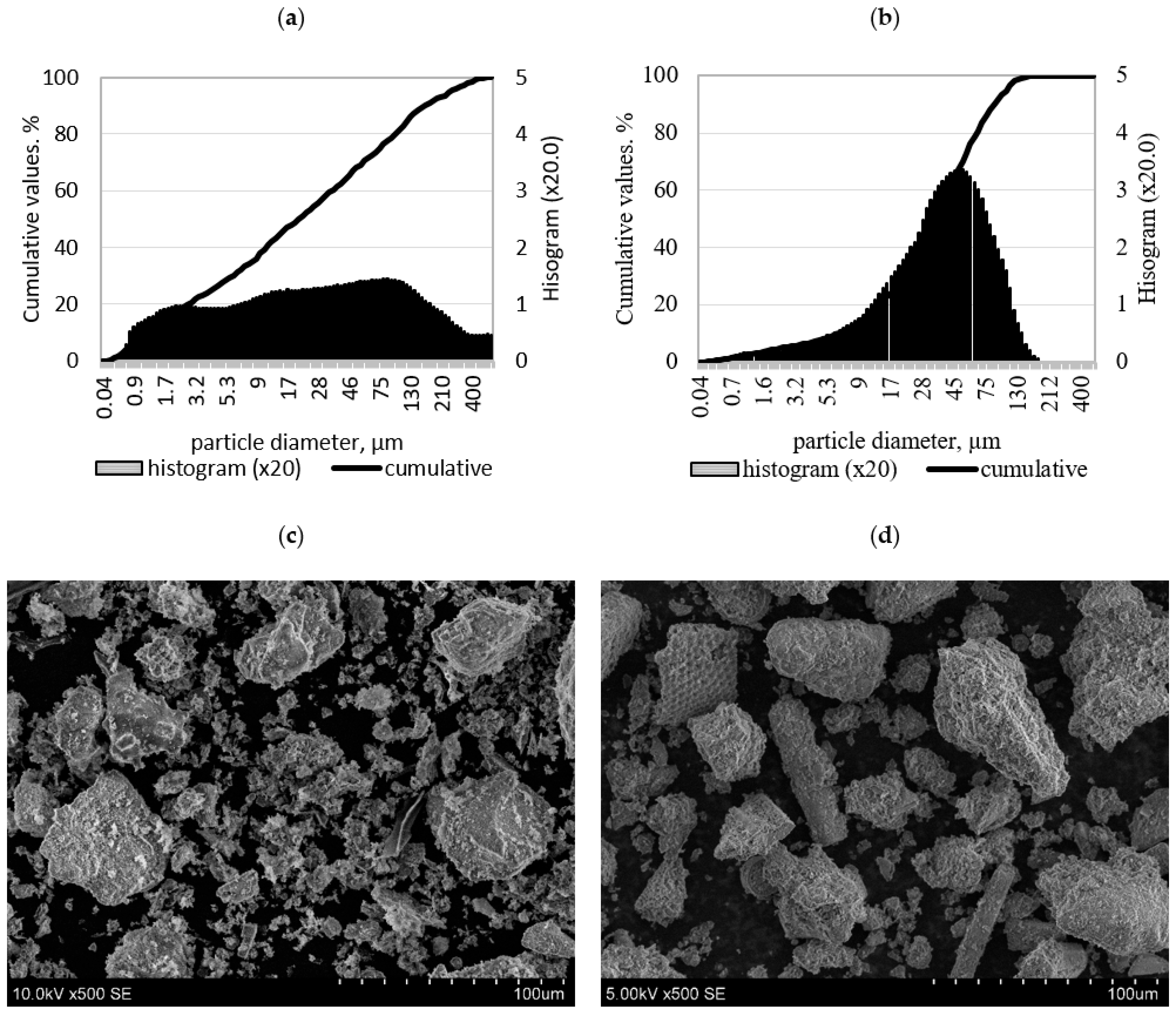
2.1. Materials
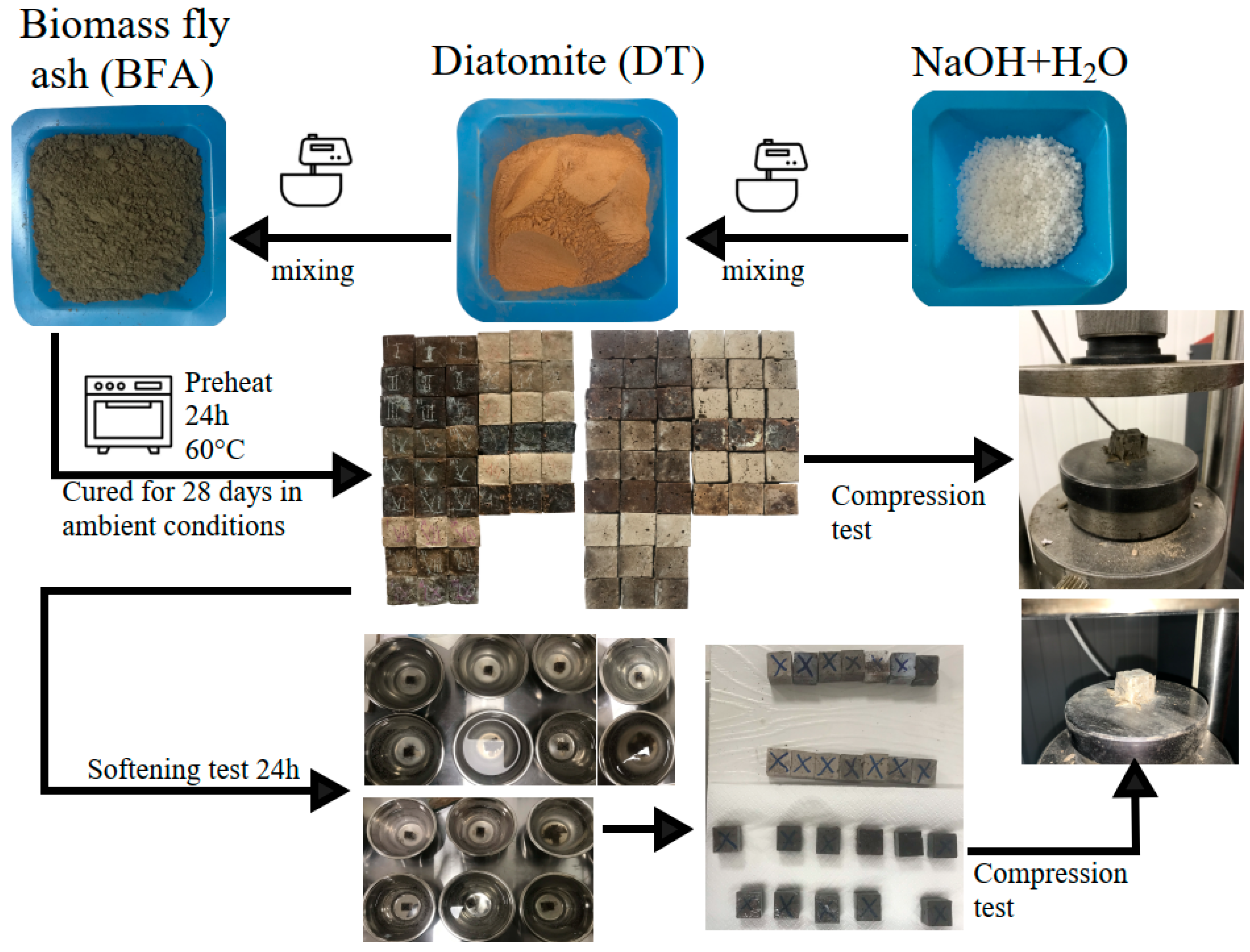
2.2. Preparation and Test of the Samples
2.3. Experimental Techniques
3. Results and Discussion
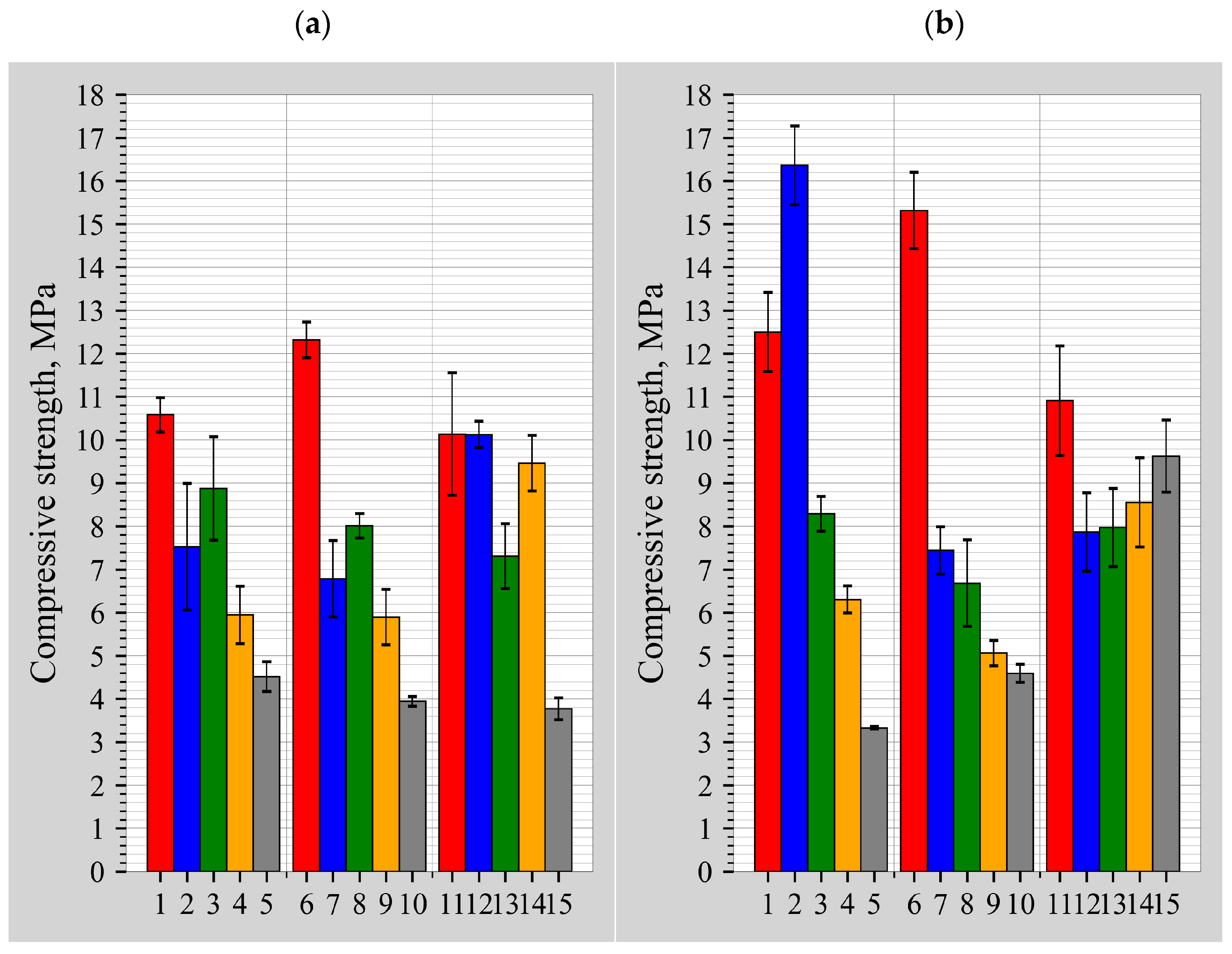
3.1. Influence of DT Content on the Compression Strength Values
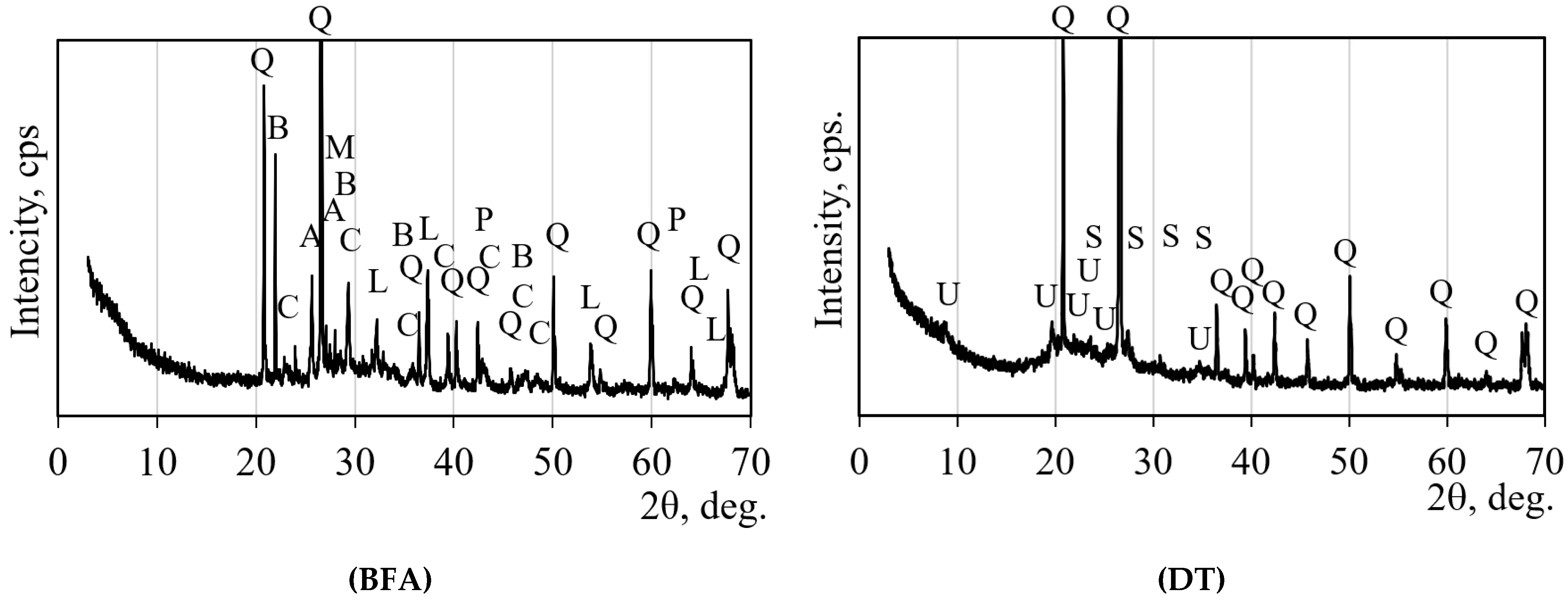
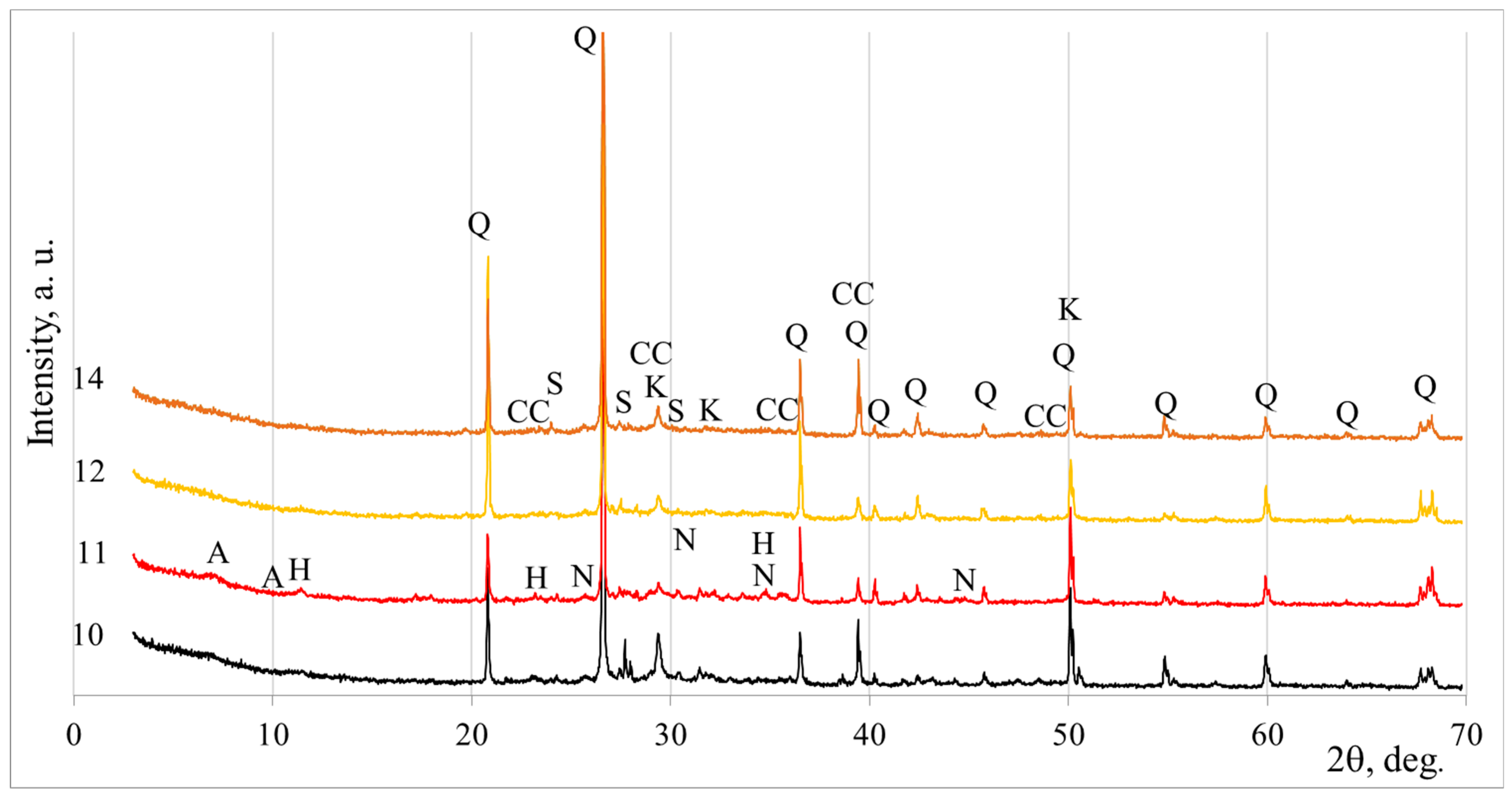
3.2. Mineral Composition According to XRD
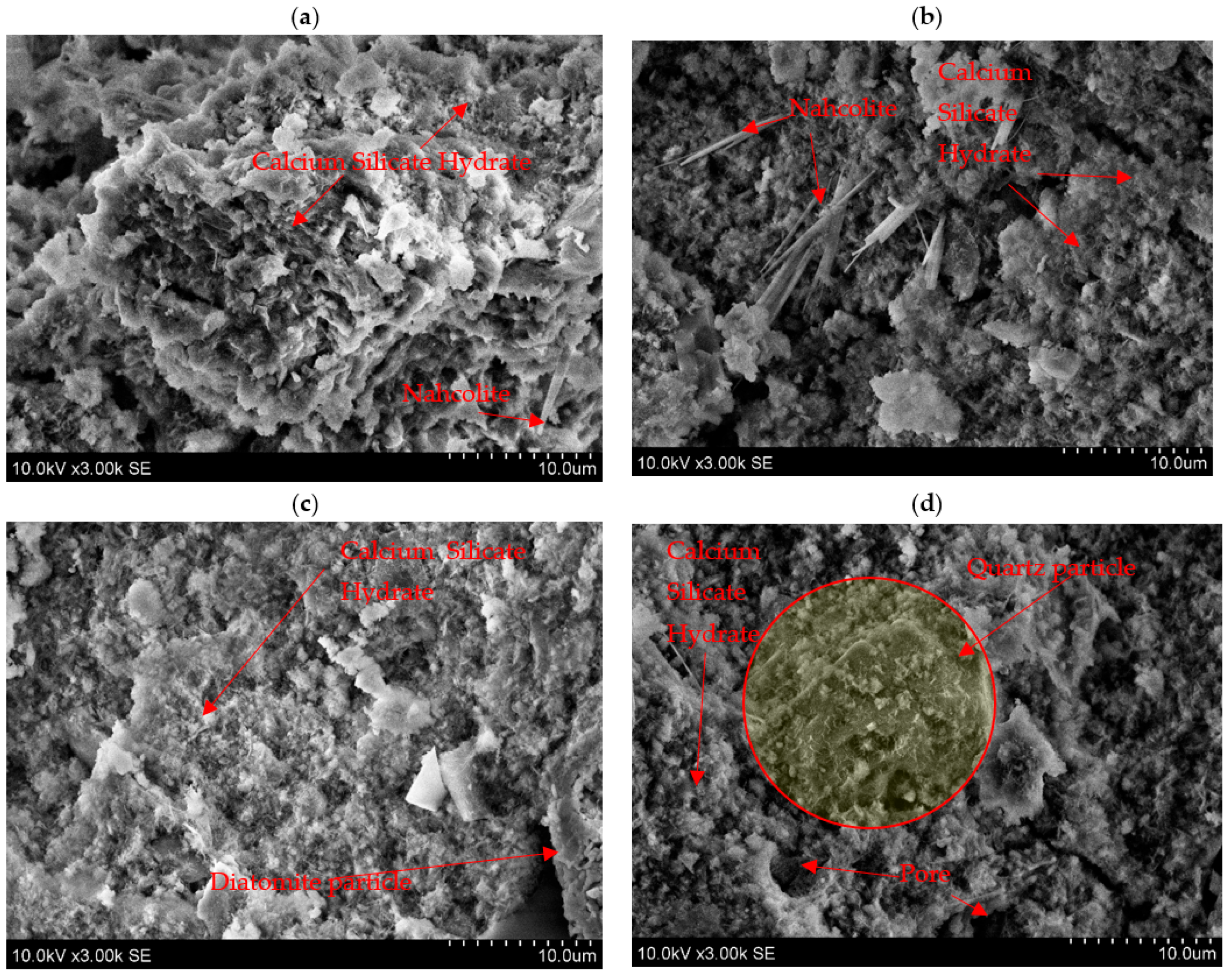
3.3. Morphology of Alkali-Activated Biomass Fly Ash and Diatomite Blends
| Precursor | Alkaline Activator | Curing Conditions | Maximal Compressive Strength, MPa | Reference |
|---|---|---|---|---|
| Biomass fly ash from a biomass power plant | Na2CO3 and Na2SiO3 | ambient temperature for 28 days | 3.08 | [1] |
| High-carbon biomass ash | Na2CO3 and Na2SiO3 | ambient temperature for 28 days | 3.50 | [6] |
| Rice husk and bark ash | NaOH and Na2SiO3 solution | ambient temperature for 28 days; ambient temperature for 27 days with 60 °C for 24 h | 51.00; 56.00 | [4] |
| Metakaolin and biomass wood ash | NaOH and Na2SiO3 solution | ambient temperature for 28 days | 83.00 | [9] |
| Coal fly ash and biomass wood ash | NaOH and Na2SiO3 solution | ambient temperature for 28 days | 50.38 (mortar) | [11] |
| Glass powder and wood biomass ash. | NaOH solution | ambient temperature for 28 days | 19.62 (after 14 days) | [12] |
| Coal fly ash and diatomite | NaOH solution | heat curing at 75 °C for 24 h | 38.40 (mortar) | [18] |
| Calcined diatomite and biomass wood fly ash. | NaOH solution | 20 °C for 27 days with 60 °C for 24 h | 16.40 | This study |
4. Conclusions
- The maximum average compressive strength after 7 days ranged from 10.1 to 12.3 MPa, and after 28 days, it ranged between 15.3 and 16.4 MPa. The highest compressive strength was obtained at the lowest or near-lowest activator level (5 mol/L), irrespective of the DT content for the samples cured for 7 days. The low amount of alkalinity required can be explained by the fact that the Ca2+ cations present in BFA act as an activator. The strength of the binders cured for 28 days was dependent on the amount of DT: the best results were obtained at DT contents of 10 and 30%.
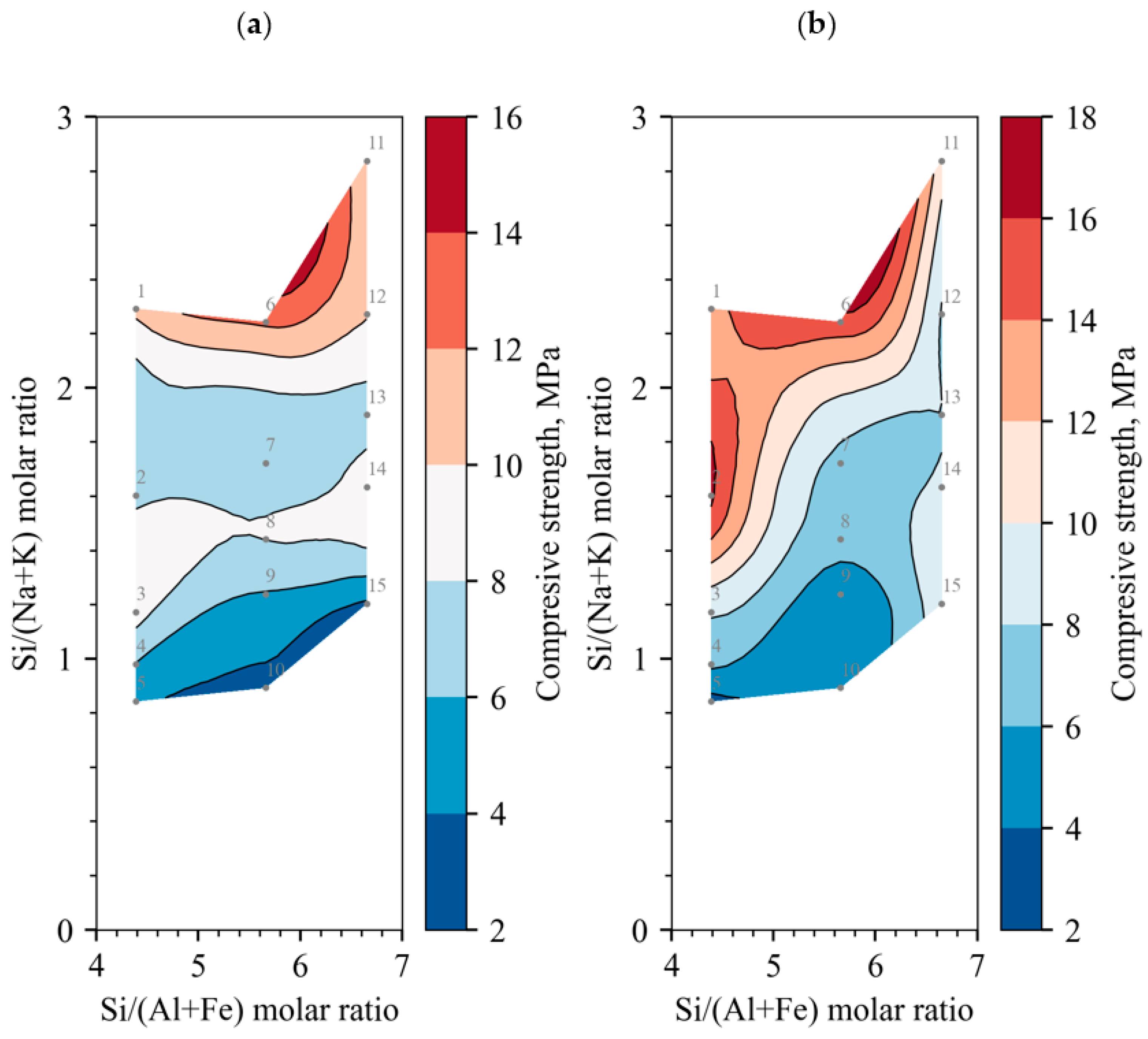
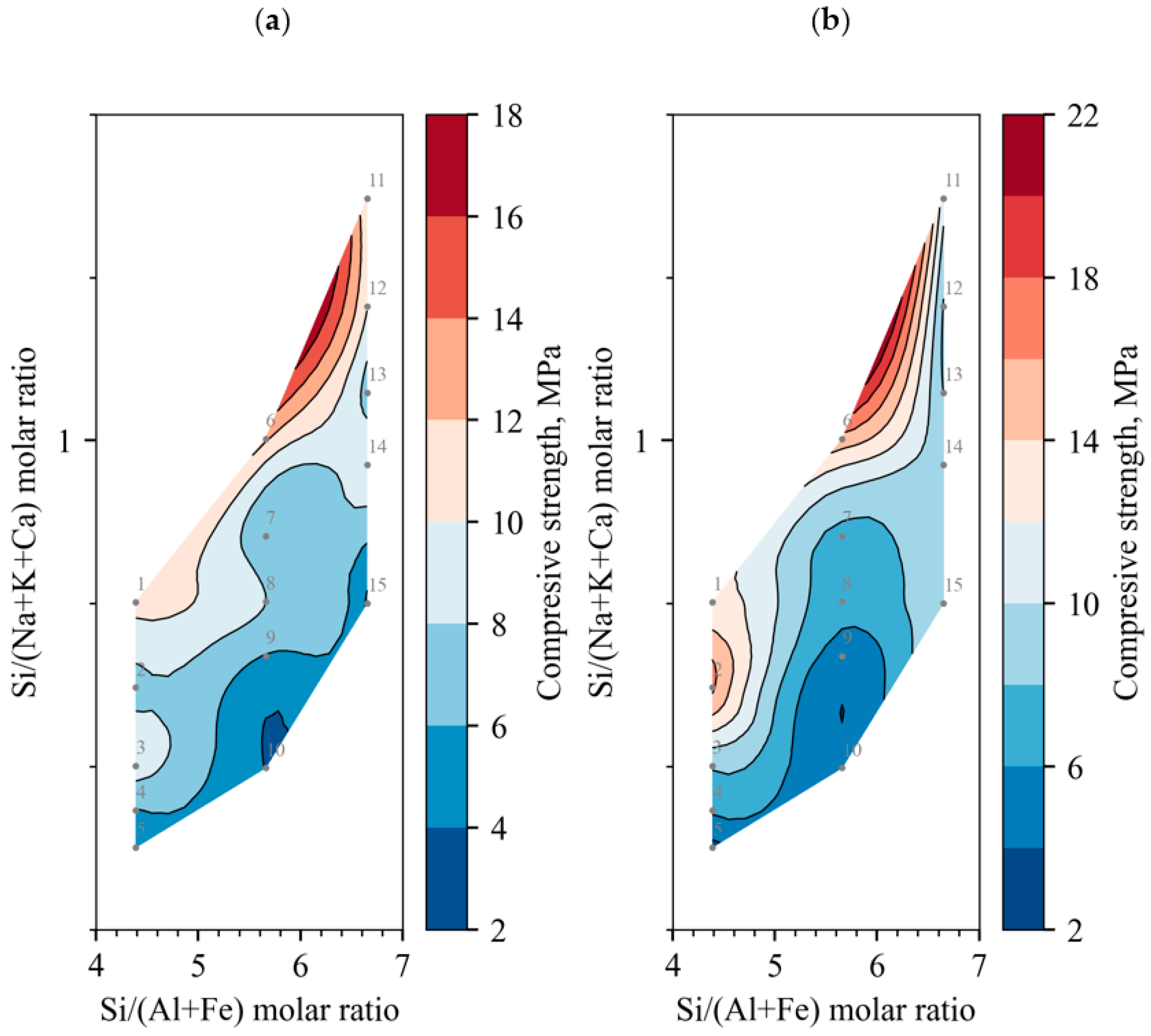
- From the analysis of the dependence of the compressive strength on the molar ratios of chemical elements, it was observed that the maximum average compressive strength was not obtained at the optimal Si/(Na + K) molar ratio of the alkali-activated binder, which was ~2. When Ca moles are evaluated, the ratio decreases to 1.2; in order to achieve the optimal ratio, it is necessary to reduce the amount of either Na or Ca. This can be achieved only by changing the main binder component from BFA to DT. Another tendency for the compressive strength to increase is observed when the Si/(Al + Fe) molar ratio decreases. In order to achieve the optimal composition ratio (~2), it is necessary to increase the amount of Al and Fe; therefore, it is necessary to use an additional raw material in the composition in which Al or Fe predominates.
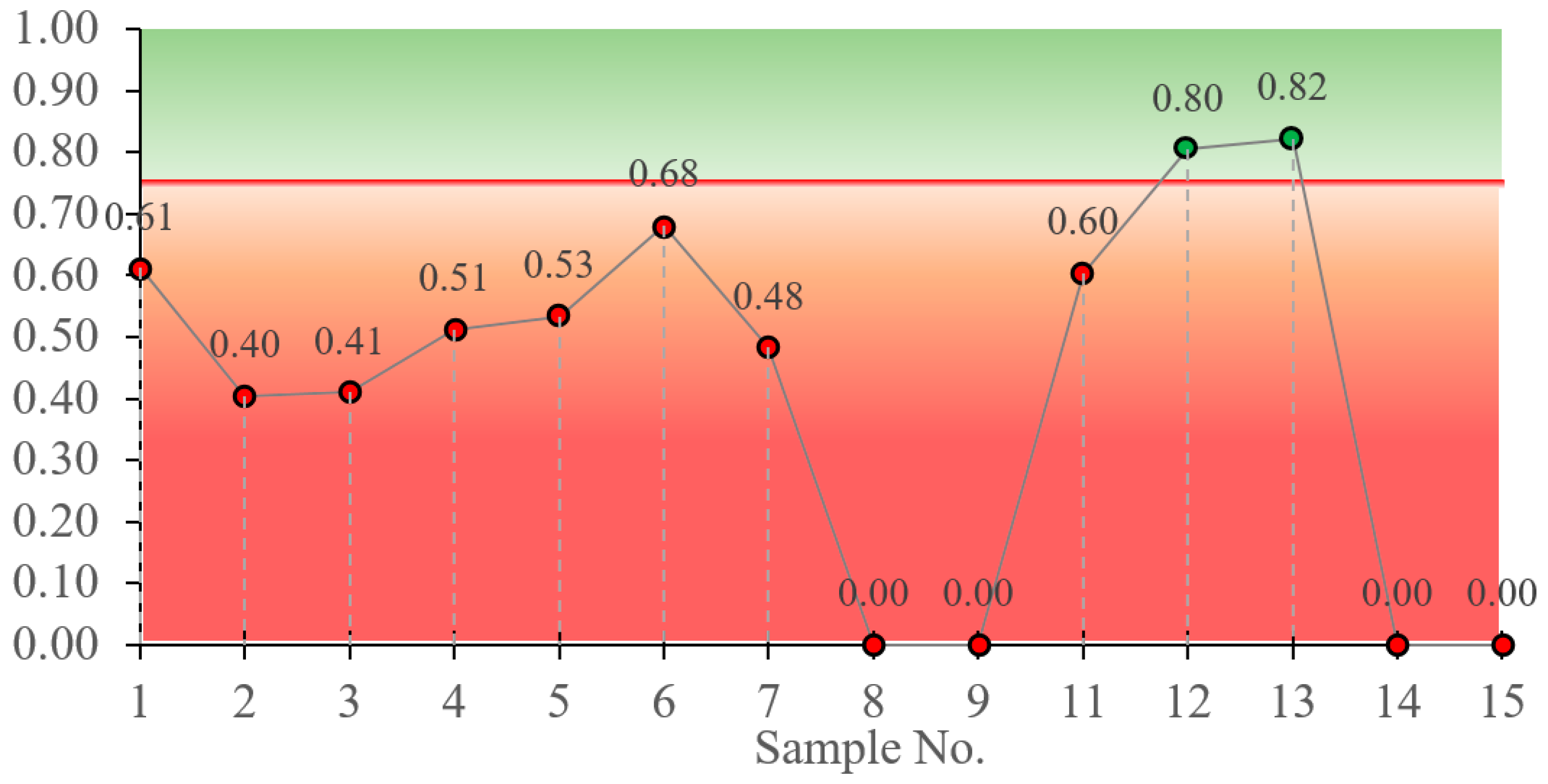
- The performed softening test also repeatedly confirms that the main binder component should be changed from BFA to DT, since samples containing less than 30% DT are unsuitable. Even for samples containing 30% DT, only one composition had a result at the borderline, within the error range.
- XRD analysis showed quartz and calcite from aluminosilicate precursors such as DT and BFA. Both of these compounds are non-reactive, so samples with a higher alkali content contain a certain amount of nahcolite. Despite the non-reactive compounds, calcium silicate hydrate, hydrotalcite, and calcium aluminium silicate hydrate (zeolite A type) formed after geopolymerisation. These additional geopolymerisation products are closely related to higher mechanical properties.
- SEM analysis confirmed the XRD results and showed that DT additives (10 and 30 wt%) improved the microstructure of alkali-activated BFA, which is closely related to compressive strength values.
- Although the practical application of the alkali-activated concrete produced from BFA and DT in this work is rather limited, such concrete could be used for artificial aggregates or small architectural elements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Pundienė, I.; Pranckevičienė, J.; Kligys, M.; Girskas, G.; Korjakins, A. A review of biomass wood ash in alkali-activated materials: Treatment, application, and outlook. J. Compos. Sci. 2024, 8, 161. [Google Scholar] [CrossRef]
- Martínez-García, R.; Jagadesh, P.; Zaid, O.; Șerbănoiu, A.A.; Fraile-Fernández, F.J.; de Prado-Gil, J.; Qaidi, S.M.A.; Grădinaru, C.M. The present state of the use of waste wood ash as an eco-efficient construction material: A review. Materials 2022, 15, 5349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, P.; Zhang, Z. Application of silica-rich biomass ash solid waste in geopolymer preparation: A review. Constr. Build. Mater. 2022, 356, 129142. [Google Scholar] [CrossRef]
- Songpiriyakij, S.; Kubprasit, T.; Jaturapitakkul, C.; Chindaprasirt, P. Compressive strength and degree of reaction of biomass-and fly ash-based geopolymer. Constr. Build. Mater. 2010, 24, 236–240. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gascó, C.; Morales, M.M.; Suárez-Navarro, J.A.; Zamorano, M.; Puertas, F. Olive biomass ash as an alternative activator in geopolymer formation: A study of strength, radiology and leaching behaviour. Cem. Concr. Compos. 2019, 104, 103384. [Google Scholar] [CrossRef]
- Zhu, C.J.; Pundienė, I.; Pranckevičienė, J.; Kligys, M.; Korjakins, A.; Vitola, L. Influence of alkaline activator solution ratio on the properties of biomass fly ash-based alkali-activated materials. J. Phys. Conf. Ser. 2023, 2423, 012033. [Google Scholar] [CrossRef]
- Guo, K.; Dong, H.; Zhang, J.; Zhang, L.; Li, Z. Experimental study of alkali-activated cementitious materials using thermally activated red mud: Effect of the Si/Al ratio on fresh and mechanical properties. Buildings 2025, 15, 565. [Google Scholar] [CrossRef]
- Li, Z.; Dong, H.; Zhao, X.; Wang, K.; Gao, X. Utilisation of Bayer red mud for high-performance geopolymer: Competitive roles of different activators. Case Stud. Constr. Mater. 2025, 23, e05047. [Google Scholar] [CrossRef]
- Silvestro, L.; Scolaro, T.P.; Ruviaro, A.S.; dos Santos Lima, G.T.; Gleize, P.J.P.; Pelisser, F. Use of biomass wood ash to produce sustainable geopolymeric pastes. Constr. Build. Mater. 2023, 370, 130641. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.A.; Vásquez-García, S.R.; Israde-Alcántara, I.; Flores-Ramirez, N.; Rico, J.L.; Mohammed, M.S. Cleaner production of one-part white geopolymer cement using pre-treated wood biomass ash and diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- Bijeljić, J.; Ristić, N.; Grdić, D.; Pavlović, M. Possibilities of Biomass Wood Ash Usage in Geopolymer Mixtures. Teh. Vjesn. 2023, 30, 52–60. [Google Scholar] [CrossRef]
- Silva, G.J.B.; Santana, V.P.; Wójcik, M. Investigation on mechanical and microstructural properties of alkali-activated materials made of wood biomass ash and glass powder. Powder Technol. 2021, 377, 900–912. [Google Scholar] [CrossRef]
- İlkentapar, S.; Özsoy, A. Investigation of mechanical properties, high-temperature resistance and microstructural properties of diatomite-containing geopolymer mortars. Arab. J. Geosci. 2022, 15, 502. [Google Scholar] [CrossRef]
- Sinsiri, T.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. The effects of replacement fly ash with diatomite in geopolymer mortar. Comput. Concr. 2012, 9, 427–437. [Google Scholar] [CrossRef]
- Özsoy, A.; Örklemez, E.; İlkentapar, S. Effect of addition diatomite powder on mechanical strength, elevated temperature resistance and microstructural properties of industrial waste fly ash-based geopolymer. J. Mater. Cycles Waste Manag. 2023, 25, 2338–2349. [Google Scholar] [CrossRef]
- Nykiel, M.; Korniejenko, K.; Setlak, K.; Melnychuk, M.; Polivoda, N.; Kozub, B.; Hebdowska-Krupa, M.; Łach, M. The influence of diatomite addition on the properties of geopolymers based on fly ash and metakaolin. Materials 2024, 17, 2399. [Google Scholar] [CrossRef]
- Pławecka, K.; Bąk, A.; Hebdowska-Krupa, M.; Łach, M. The use of calcined diatomite as an additive to geopolymeric materials. Mater. Proc. 2023, 13, 28. [Google Scholar] [CrossRef]
- Al-kroom, H.; Elshimy, A.S.; Abd Elrahman, M.; Abadel, A.A.; Alghamdi, H.; Seliem, M.K.; Abdel-Gawwad, H.A. A comparative study on the role of metakaolin and diatomite in the performance of eco-friendly dolomite waste–based alkali-activated binder. Case Stud. Constr. Mater. 2023, 19, e02562. [Google Scholar] [CrossRef]
- Liang, G.; Yao, W. Effect of diatomite on the reaction kinetics, early-age chemical shrinkage and microstructure of alkali-activated slag cements. Constr. Build. Mater. 2023, 376, 131026. [Google Scholar] [CrossRef]
- Liao, S.; Xu, H.; Wu, L.; Zhao, Z.; Ma, K. Strength formation mechanism and microstructural evolution of low-grade diatomite-based cementitious materials. Constr. Build. Mater. 2024, 431, 136588. [Google Scholar] [CrossRef]
- Ilkentapar, S.; Örklemez, E.; Durak, U.; Gülçimen, S.; Bayram, S.; Uzal, N.; Uzal, B.; Karahan, O.; Atis, C.D. Evaluation of diatomite substitute with thermal power plant waste fly ash in sustainable geopolymer through life cycle assessment. J. Mater. Cycles Waste Manag. 2025, 27, 1418–1435. [Google Scholar] [CrossRef]
- Bagci, C.; Kutyla, G.P.; Kriven, W.M. Fully reacted high strength geopolymer made with diatomite as a fumed silica alternative. Ceram. Int. 2017, 43, 14784–14790. [Google Scholar] [CrossRef]
- Brudny, K.; Łach, M.; Bąk, A.; Pławecka, K.; Korniejenko, K. Application of diatomite as a substitute for fly ash in foamed geopolymers. J. Phys. Conf. Ser. 2023, 2423, 012028. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y. The evaluation of the heavy metal leaching behavior of MSWI-FA added alkali-activated materials bricks by using different leaching test methods. Int. J. Environ. Res. Public Health 2019, 16, 1151. [Google Scholar] [CrossRef]
- Bouzar, B.; Mamindy-Pajany, Y. Immobilization study of As, Cr, Mo, Pb, Sb, Se and Zn in geopolymer matrix: Application to shooting range soil and biomass fly ash. Int. J. Environ. Sci. Technol. 2023, 20, 11891–11912. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N. Mechanisms of heavy metal immobilisation using geopolymerisation techniques–a review. J. Adv. Concr. Technol. 2018, 16, 124–135. [Google Scholar] [CrossRef]
- Zarębska, K.; Szczurowski, J.; Muszyńska, J.; Baran, P. Geopolymer materials from fly ash—A sustainable approach to hazardous waste management. Materials 2024, 17, 3515. [Google Scholar] [CrossRef]
- Statkauskas, M.; Vaičiukynienė, D.; Grinys, A.; Dvořák, K. Effect of elevated temperature on mechanical properties of ceramic brick and metakaolin waste-based geopolymer mortar. Constr. Build. Mater. 2025, 470, 140431. [Google Scholar] [CrossRef]
- Vasile, R.; Aliona, V.; Igor, P. Composition of mineral phases of the Ghidirim diatomite. Chem. J. Mold. 2007, 2, 63–66. [Google Scholar] [CrossRef]
- EN 13279–2; Determination of Strength. European Committee for Standardization: Bruxelles, Belgium, 2016.
- Rowles, M.; O’connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Chen, X.; Sutrisno, A.; Struble, L.J. Effects of calcium on setting mechanism of metakaolin-based geopolymer. J. Am. Ceram. Soc. 2018, 101, 957–968. [Google Scholar] [CrossRef]
- Chen, K.; Lin, W.T.; Liu, W. Effect of NaOH concentration on properties and microstructure of a novel reactive ultra-fine fly ash geopolymer. Adv. Powder Technol. 2021, 32, 2929–2939. [Google Scholar] [CrossRef]
- Li, Z.; Ikeda, K. Compositions and microstructures of carbonated geopolymers with different precursors. Materials 2024, 17, 1491. [Google Scholar] [CrossRef] [PubMed]
- Azar, P.; Patapy, C.; Samson, G.; Cussigh, F.; Frouin, L.; Cyr, M. Effect of natural and accelerated carbonation on microstructure and pH of sodium carbonate alkali-activated slag. Cem. Concr. Res. 2024, 181, 107525. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Ramli, M.; Matthews, J.C. Production of geopolymer mortar system containing high calcium biomass wood ash as a partial substitution to fly ash: An early age evaluation. Compos. Part B Eng. 2019, 174, 106941. [Google Scholar] [CrossRef]
- Bae, S.J.; Park, S.; Lee, H.K. Role of Al in the crystal growth of alkali-activated fly ash and slag under a hydrothermal condition. Constr. Build. Mater. 2020, 239, 117842. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Bradić, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Jiang, H.; Bi, M.; Gao, W.; Gan, B.; Zhang, D.; Zhang, Q. Inhibition of aluminum dust explosion by NaHCO3 with different particle size distributions. J. Hazard. Mater. 2018, 344, 902–912. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Toprak, M.U.; Uygunoğlu, T. Durability and microstructure characteristics of alkali activated coal bottom ash geopolymer cement. J. Clean. Prod. 2014, 81, 211–217. [Google Scholar] [CrossRef]
| SiO2 | CaO | K2O | Al2O3 | MgO | SO3 | P2O5 | Fe2O3 | Cl | Na2O | MnO | TiO2 | BaO | ZnO | SrO | ZrO2 | Other/LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFA | 32.99 | 32.63 | 7.48 | 5.91 | 5.57 | 5.38 | 4.26 | 2.87 | 0.681 | 0.672 | 0.613 | 0.495 | 0.185 | 0.126 | 0.044 | 0.042 | 0.052/0.042 |
| DT | 84.5 | 0.37 | 1.83 | 7.69 | 1.43 | 0.03 | - | 3.16 | - | 0.34 | - | - | - | - | - | - | 0.65 |
| Symbol | COD Number | Mineral Name | Formula | Quantity, % | Crystalline Phase, % |
|---|---|---|---|---|---|
| BFA | |||||
| Q | 901–0146 | Quartz | SiO2 | 77.64 | 78 |
| C | 901–6705 | Calcite | CaCO3 | 5.97 | |
| B | 901–5087 | Cristobalite | SiO2 | 8.16 | |
| L | 101–1094 | Lime | CaO | 3.19 | |
| M | 900–0701 | Microcline | KAlSi3O8 | 1.55 | |
| P | 900–0492 | Periclase | MgO | 1.10 | |
| A | 412–4074 | α-quartz | SiO2 | 2.39 | |
| Amorphous (between 20 and 43 deg) | 22 | ||||
| DT | |||||
| Q | 901–0146 | Quartz | SiO2 | 91.80 | 69.8 |
| U | 901–2886 | Muscovite | Ca0.011K0.776 Na0.181Al2.726Fe0.03 Mg0.02Si3.15Ti0.02O11 | 6.00 | |
| S | 153–5821 | Low sanidine ferroalumosilicate | Fe0.28KAl0.72Si3O8 | 2.20 | |
| Amorphous (between 14.8 and 36 deg) | 30.2 | ||||
| Sample No. | BFA Weight, % | DT Weight, % | Weight Ratio NaOH/(BFA + DT) | NaOH Solution Concentration, mol/L | Weight Ratio Total Water/(BFA + DT) |
|---|---|---|---|---|---|
| 1 | 100 | 10 | 0.05 | 3.4 | 0.35 |
| 2 | 0.09 | 6.3 | 0.35 | ||
| 3 | 0.14 | 10.8 | 0.32 | ||
| 4 | 0.17 | 13.7 | 0.32 | ||
| 5 | 0.21 | 16.5 | 0.31 | ||
| 6 | 100 | 30 | 0.08 | 5.5 | 0.37 |
| 7 | 0.12 | 8.3 | 0.35 | ||
| 8 | 0.15 | 10.5 | 0.35 | ||
| 9 | 0.18 | 12.6 | 0.35 | ||
| 10 | 0.26 | 17.9 | 0.37 | ||
| 11 | 100 | 50 | 0.07 | 4.4 | 0.42 |
| 12 | 0.10 | 6.5 | 0.39 | ||
| 13 | 0.13 | 8.2 | 0.39 | ||
| 14 | 0.15 | 9.9 | 0.38 | ||
| 15 | 0.22 | 14.2 | 0.39 |
| Symbol | PDF-2 Number | Mineral Name | Formula |
|---|---|---|---|
| Sample No. 1 | |||
| Q | 85–797 | Quartz | SiO2 |
| CC | 81–2027 | Calcite | CaCO3 |
| K | 33–306 | Calcium Silicate Hydrate | Ca1.5SiO3.5∙xH2O |
| N | 1–909 | Nahcolite | NaHCO3 |
| Sample No. 2 | |||
| Q | 85–797 | Quartz | SiO2 |
| K | 33–306 | Calcium Silicate Hydrate | Ca1.5SiO3.5∙xH2O |
| CC | 81–2027 | Calcite | CaCO3 |
| N | 1–909 | Nahcolite | NaHCO3 |
| A | 76–1507 | Calcium aluminium silicate hydrate (zeolite A) | Ca5.57 Al12.3Si12O49.2 H2.34 |
| H | 22–700 | Hydrotalcite | Mg6Al2CO3(OH)16∙4H2O |
| Sample No. 6 | |||
| Q | 85–797 | Quartz | SiO2 |
| K | 33–306 | Calcium Silicate Hydrate | Ca1.5SiO3.5∙xH2O |
| CC | 81–2027 | Calcite | CaCO3 |
| Sample No. 11 | |||
| Q | 85–797 | Quartz | SiO2 |
| K | 33–306 | Calcium Silicate Hydrate | Ca1.5SiO3.5∙xH2O |
| CC | 81–2027 | Calcite | CaCO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žurinskas, D.; Vaičiukynienė, D. Mechanical and Microstructural Properties of Alkali-Activated Biomass Fly Ash and Diatomite Blends. Materials 2025, 18, 3807. https://doi.org/10.3390/ma18163807
Žurinskas D, Vaičiukynienė D. Mechanical and Microstructural Properties of Alkali-Activated Biomass Fly Ash and Diatomite Blends. Materials. 2025; 18(16):3807. https://doi.org/10.3390/ma18163807
Chicago/Turabian StyleŽurinskas, Darius, and Danutė Vaičiukynienė. 2025. "Mechanical and Microstructural Properties of Alkali-Activated Biomass Fly Ash and Diatomite Blends" Materials 18, no. 16: 3807. https://doi.org/10.3390/ma18163807
APA StyleŽurinskas, D., & Vaičiukynienė, D. (2025). Mechanical and Microstructural Properties of Alkali-Activated Biomass Fly Ash and Diatomite Blends. Materials, 18(16), 3807. https://doi.org/10.3390/ma18163807







