Alloy Selection and Manufacturing Technologies for Total Ankle Arthroplasty: A Narrative Review
Abstract
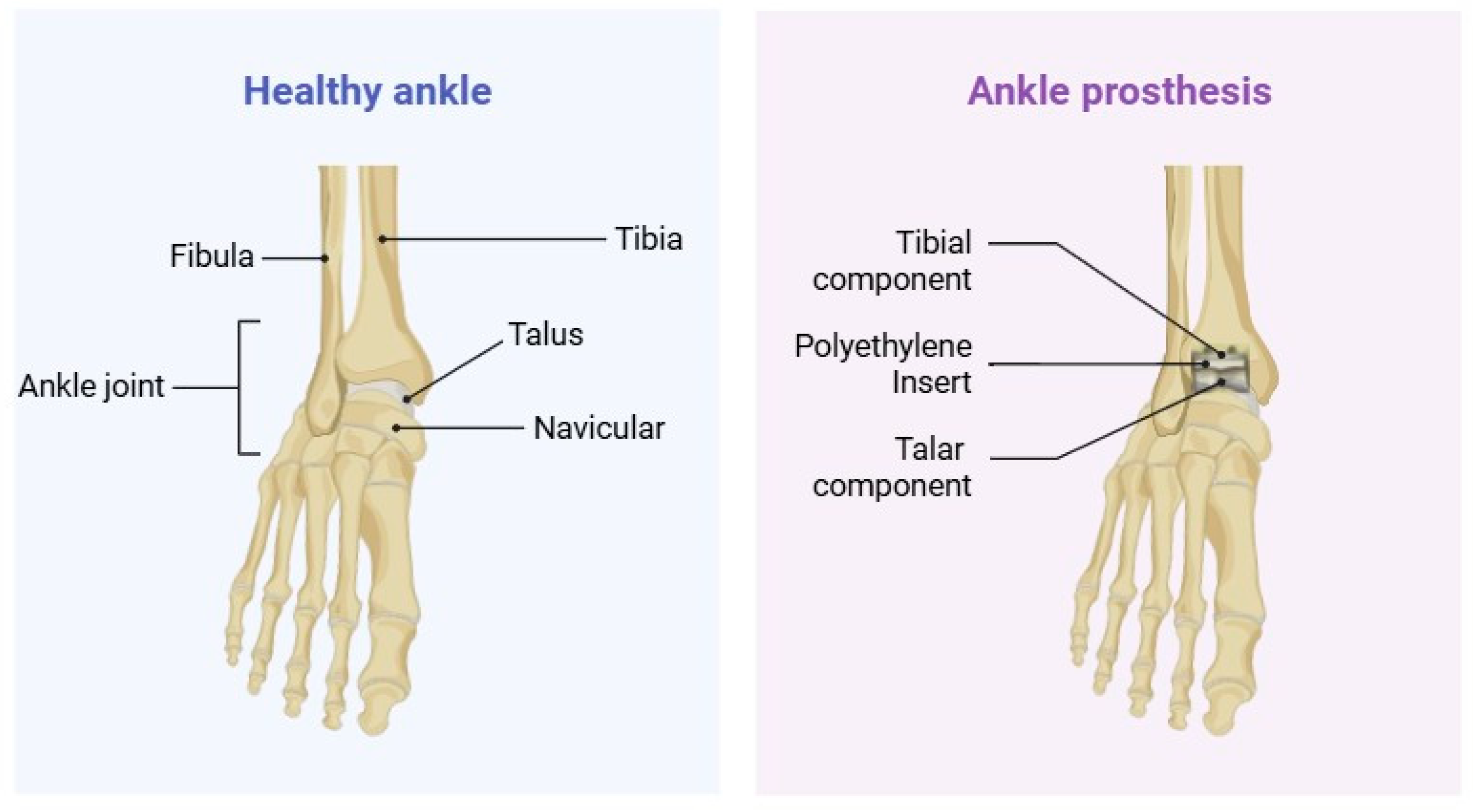
1. Introduction
2. Metallurgical Considerations
2.1. Cobalt-Chromium Alloys
2.2. Titanium Alloys
2.3. Stainless Steel
2.4. Novel Alloy Formulations
2.5. Clinical Outcome Analysis
3. Manufacturing Methodologies
3.1. Traditional Manufacturing Techniques
3.1.1. Casting Processes
3.1.2. Forging Methodologies
3.1.3. CNC Machining
3.1.4. Heat Treatments and Their Effects on Material Properties
3.2. Additive Manufacturing
3.2.1. Electron Beam Melting Processes
3.2.2. Selective Laser Melting Techniques
3.2.3. Advantages for Complex Geometries and Patient-Specific Designs
3.2.4. Material Property Considerations Specific to AM Processes
Microstructural Alterations and Thermal Effects
Anisotropy and Build Orientation Effects
Powder Characteristics and Processing Variables
Residual Stress Management
3.3. Manufacturing in Commercial TAA Products and Clinical Cases
4. Emerging Technologies
4.1. Biodegradable Scaffolds and Hybrid Implants
4.2. Composite Structures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gougoulias, N.E.; Khanna, A.; Maffulli, N. History and Evolution in Total Ankle Arthroplasty. Br. Med. Bull. 2009, 89, 111–151. [Google Scholar] [CrossRef]
- Henne, T.D.; Anderson, J.G. Total Ankle Arthroplasty: A Historical Perspective. Foot Ankle Clin. 2002, 7, 695–702. [Google Scholar] [CrossRef]
- Pugely, A.J.; Lu, X.; Amendola, A.; Callaghan, J.J.; Martin, C.T.; Cram, P. Trends in the Use of Total Ankle Replacement and Ankle Arthrodesis in the United States Medicare Population. Foot Ankle Int. 2013, 35, 207–215. [Google Scholar] [CrossRef]
- Hintermann, B. Total Ankle Arthroplasty: Historical Overview, Current Concepts, and Future Perspectives; Springer Medicine: Berlin/Heidelberg, Germany, 2005; ISBN 9783211212523. [Google Scholar]
- DiDomenico, L.A.; Anania, M.C. Total Ankle Replacements: An Overview. Clin. Podiatr. Med. Surg. 2011, 28, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Younger, A.; Penner, M.; Wing, K. Mobile-Bearing Total Ankle Arthroplasty. Foot Ankle Clin. 2008, 13, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, A.T. Foot and Ankle Surgery: New Frontiers for Translational Advancements. Ann. Transl. Med. 2024, 12, 42. [Google Scholar] [CrossRef]
- Ho, N.C.; Ebramzadeh, E.; Sangiorgio, S.N. Preclinical Biomechanical Testing Models for the Tibiotalar Joint and Its Replacements: A Systematic Review. Foot Ankle Surg. 2020, 26, 14–18. [Google Scholar] [CrossRef]
- Hannon, P. A Brief Review of Current Orthopedic Implant Device Issues: Biomechanics and Biocompatibility. Biol. Eng. Med. 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Azmat, A.; Asrar, S.; Channa, I.A.; Ashfaq, J.; Ali Chandio, I.; Chandio, A.D.; Ali Shar, M.; AlSalhi, M.S.; Devanesan, S. Comparative Study of Biocompatible Titanium Alloys Containing Non-Toxic Elements for Orthopedic Implants. Crystals 2023, 13, 467. [Google Scholar] [CrossRef]
- Haynes, D.R.; Rogers, S.D.; Hay, S.; Pearcy, M.J.; Howie, D.W. The Differences in Toxicity and Release of Bone-Resorbing Mediators Induced by Titanium and Cobalt-Chromium-Alloy Wear Particles. J. Bone Jt. Surg. 1993, 75, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Badell, J.S.; Cottom, J.M. Metal Allergy and the Use of Custom Implants in Primary Total Ankle Replacement. Foot Ankle Surg. Tech. Rep. Cases 2023, 3, 100285. [Google Scholar] [CrossRef]
- Fleming, T.J.; Kavanagh, A.; Duggan, G. The Effect of Melt Temperature on the Mechanical Properties of Cast ASTM F75 CoCrMo Alloy as Explained by Nitrogen and Oxygen Content. J. Mater. Res. Technol. 2020, 9, 9479–9486. [Google Scholar] [CrossRef]
- ASTM F1537-20; Standard Specification for Wrought Cobalt-28Chromium-6Molybdenum Alloys for Surgical Implants (UNS R31537, UNS R31538, and UNS R31539). ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM F799-19; Standard Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Forgings for Surgical Implants (UNS R31537, R31538, R31539). ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM F75-23; Standard Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS R30075). ASTM International: West Conshohocken, PA, USA, 2023.
- Devine, T.M.; Wulff, J. Cast vs. Wrought Cobalt-Chromium Surgical Implant Alloys. J. Biomed. Mater. Res. 1975, 9, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Favaro, G.; Inam, F.; Reece, M.J.; Angadji, A.; Bonfield, W.; Huang, J.; Edirisinghe, M. Cobalt-Based Orthopaedic Alloys: Relationship between Forming Route, Microstructure and Tribological Performance. Mater. Sci. Eng. C 2012, 32, 1222–1229. [Google Scholar] [CrossRef]
- Mani, G.; Porter, D.; Collins, S.; Schatz, T.; Ornberg, A.; Shulfer, R. A Review on Manufacturing Processes of Cobalt-Chromium Alloy Implants and Its Impact on Corrosion Resistance and Biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35431. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.B.; Ouyang, J.Y.; Noori, M.; Altabey, W.A. A Review Study on Total Ankle Replacement. Appl. Sci. 2023, 13, 535. [Google Scholar] [CrossRef]
- Conti, M.C.; Andreas, K.; Pierre Schembri, W.; Buhagiar, J. Biocompatibility and Characterization of a Kolsterised® Medical Grade Cobalt-Chromium-Molybdenum Alloy. Biomatter 2014, 4, e27713. [Google Scholar] [CrossRef]
- ASTM F1472-20a; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400). ASTM International: West Conshohocken, PA, USA, 2023.
- Mandil, G.; Le, V.T.; Paris, H.; Suard, M. Building New Entities from Existing Titanium Part by Electron Beam Melting: Microstructures and Mechanical Properties. Int. J. Adv. Manuf. Technol. 2016, 85, 1835–1846. [Google Scholar] [CrossRef]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based Alloys and Composites for Orthopedic Implants Applications: A Comprehensive Review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Haston, S.; Langton, D.; Townshend, D.; Bhalekar, R.; Joyce, T. Metal Debris Release Is Commonly Seen from Explanted Total Ankle Replacements. J. Mech. Behav. Biomed. Mater. 2023, 144, 105932. [Google Scholar] [CrossRef]
- Kasai, Y.; Iida, R.; Uchida, A. Metal Concentrations in the Serum and Hair of Patients with Titanium Alloy Spinal Implants. Spine 2003, 28, 1320–1326. [Google Scholar] [CrossRef]
- Catalani, S.; Stea, S.; Beraudi, A.; Gilberti, M.E.; Bordini, B.; Toni, A.; Apostoli, P. Vanadium Release in Whole Blood, Serum and Urine of Patients Implanted with a Titanium Alloy Hip Prosthesis. Clin. Toxicol. 2013, 51, 550–556. [Google Scholar] [CrossRef]
- Shelly, S.; Liraz Zaltsman, S.; Ben-Gal, O.; Dayan, A.; Ganmore, I.; Shemesh, C.; Atrakchi, D.; Garra, S.; Ravid, O.; Rand, D.; et al. Potential Neurotoxicity of Titanium Implants: Prospective, in-Vivo and in-Vitro Study. Biomaterials 2021, 276, 121039. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium Ionic Species from Degradation of Ti-6Al-4V Metallic Implants: In Vitro Cytotoxicity and Speciation Evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.; Xiong, J.; Hodgson, P.; Wen, C. Cytotoxicity of Titanium and Titanium Alloying Elements. J. Dent. Res. 2010, 89, 493–497. [Google Scholar] [CrossRef]
- Badhe, R.V.; Akinfosile, O.; Bijukumar, D.; Barba, M.; Mathew, M.T. Systemic Toxicity Eliciting Metal Ion Levels from Metallic Implants and Orthopedic Devices—A Mini Review. Toxicol. Lett. 2021, 350, 213–224. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential Release of in Vivo Trace Metals from Metallic Medical Implants in the Human Body: From Ions to Nanoparticles—A Systematic Analytical Review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- ASTM F138-19; Standard Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Bar and Wire for Surgical Implants (UNS S31673). ASTM International: West Conshohocken, PA, USA, 2020.
- Mahajan, A.; Sandeep, D.; Kalyanasundaram, D. Surface Alteration of Cobalt-Chromium and Duplex Stainless Steel Alloys for Biomedical Applications: A Concise Review. Mater. Manuf. Process. 2023, 38, 260–270. [Google Scholar] [CrossRef]
- Joshi, G.R.; Naveen, B.M. Comparative Study of Stainless Steel and Titanium Limited Contact-Dynamic Compression Plate Application in the Fractures of Radius and Ulna. Med. J. Dr. D.Y. Patil Vidyapeeth 2019, 12, 256–261. [Google Scholar]
- Badell, J.S.; Cottom, J.M.; Verdoni, T. Approach to Patients with Metal Allergies in Foot and Ankle Surgery. J. Foot Ankle Surg. 2025, 64, 302–308. [Google Scholar] [CrossRef]
- Ummethala, R.; Karamched, P.S.; Rathinavelu, S.; Singh, N.; Aggarwal, A.; Sun, K.; Ivanov, E.; Kollo, L.; Okulov, I.; Eckert, J.; et al. Selective Laser Melting of High-Strength, Low-Modulus Ti–35Nb–7Zr–5Ta Alloy. Materialia 2020, 14, 100941. [Google Scholar] [CrossRef]
- Hagihara, K.; Nakano, T.; Maki, H.; Umakoshi, Y.; Niinomi, M. Isotropic Plasticity of β-Type Ti-29Nb-13Ta-4.6Zr Alloy Single Crystals for the Development of Single Crystalline β-Ti Implants. Sci. Rep. 2016, 6, 29779. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Li, X.; Zhang, X.; Gong, X.; Zhu, Y.; Ren, Z.; Zhang, B.; Cheng, J. Biocompatibility and Osseointegration Properties of a Novel High Strength and Low Modulus β-Ti10Mo6Zr4Sn3Nb Alloy. Front. Bioeng. Biotechnol. 2023, 11, 1127929. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.M.; Schneider, S.G.; Neto, C.M. Study of Nontoxic Aluminum and Vanadium-Free Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2004, 24, 679–682. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, D.; Guo, L.; Lin, J.; Li, Y.; Wen, C. Spinodal Zr–Nb Alloys with Ultrahigh Elastic Admissible Strain and Low Magnetic Susceptibility for Orthopedic Applications. Acta Biomater. 2024, 184, 444–460. [Google Scholar] [CrossRef]
- Mitra, K.; Anastasio, A.T.; Wu, K.A.; Abar, B.; Schweitzer, K.M.; Parekh, S.G.; Easley, M.E.; Adams, S.B. Outcomes of Cobalt-Chrome 3D-Printed Total Talus Replacement with and without Combined Total Ankle Replacement. Foot Ankle Surg. 2025, 31, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Abar, B.; Allen, N.B.; Gall, K.; Adams, S.B. Clinical Outcomes of 3D Printed Titanium Cage Implantation for Foot and Ankle Surgery. Foot Ankle Orthop. 2020, 5, 2473011420S00088. [Google Scholar] [CrossRef]
- Kanzaki, N.; Chinzei, N.; Yamamoto, T.; Yamashita, T.; Ibaraki, K.; Kuroda, R. Clinical Outcomes of Total Ankle Arthroplasty with Total Talar Prosthesis. Foot Ankle Int. 2019, 40, 948–954. [Google Scholar] [CrossRef]
- Mesinkovska, N.A.; Tellez, A.; Molina, L.; Honari, G.; Sood, A.; Barsoum, W.; Taylor, J.S. The Effect of Patch Testing on Surgical Practices and Outcomes in Orthopedic Patients with Metal Implants. Arch. Dermatol. 2012, 148, 687–693. [Google Scholar] [CrossRef]
- Belvedere, C.; Siegler, S.; Fortunato, A.; Caravaggi, P.; Liverani, E.; Durante, S.; Ensini, A.; Konow, T.; Leardini, A. New Comprehensive Procedure for Custom-Made Total Ankle Replacements: Medical Imaging, Joint Modeling, Prosthesis Design, and 3D Printing. J. Orthop. Res. 2019, 37, 760–768. [Google Scholar] [CrossRef]
- Mukhtarkhanov, M.; Perveen, A.; Talamona, D. Application of Stereolithography Based 3D Printing Technology in Investment Casting. Micromachines 2020, 11, 946. [Google Scholar] [CrossRef]
- Kaiser, R.; Williamson, K.; O’Brien, C.; Ramirez-Garcia, S.; Browne, D.J. The Influence of Cooling Conditions on Grain Size, Secondary Phase Precipitates and Mechanical Properties of Biomedical Alloy Specimens Produced by Investment Casting. J. Mech. Behav. Biomed. Mater. 2013, 24, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Mori, M.; Chiba, A. Effects of Carbon Concentration on Microstructure and Mechanical Properties of As-Cast Nickel-Free Co–28Cr–9W-Based Dental Alloys. Mater. Sci. Eng. C 2014, 40, 127–134. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2024, 17, 114. [Google Scholar] [CrossRef]
- Campanella, D.; Buffa, G.; El Hassanin, A.; Squillace, A.; Gagliardi, F.; Filice, L.; Fratini, L. Mechanical and Microstructural Characterization of Titanium Gr.5 Parts Produced by Different Manufacturing Routes. Int. J. Adv. Manuf. Technol. 2022, 122, 741–759. [Google Scholar] [CrossRef]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A Comprehensive Review on Metallic Implant Biomaterials and Their Subtractive Manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef]
- Crawford, H.V.; Unwin, P.S.; Walker, P.S. The CADCAM Contribution to Customized Orthopaedic Implants. Proc. Inst. Mech. Eng. H 1992, 206, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.R.; Davis, W.H.; Cohen, B.E.; Jones, C.P.; Ellington, J.K.; Anderson, R.B. Radiographic Outcomes of Preoperative CT Scan–Derived Patient-Specific Total Ankle Arthroplasty. Foot Ankle Int. 2015, 36, 1163–1169. [Google Scholar] [CrossRef]
- Hinh, C.M.; Chong, A.C.M.; Bierman, B.R.; Uglem, T.P. Computed Tomography Derived Patient-Specific Instrumentation Total Ankle Arthroplasty Survivorship Outcomes. J. Foot Ankle Surg. 2023, 62, 338–346. [Google Scholar] [CrossRef]
- García-Martínez, E.; Miguel, V.; Martínez-Martínez, A.; Manjabacas, M.C.; Coello, J. Sustainable Lubrication Methods for the Machining of Titanium Alloys: An Overview. Materials 2019, 12, 3852. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, S.; Gan, Y.; Zhang, S.; Guo, S.; Lin, J.; Lin, J. Microstructure, Mechanical Property and Metal Release of As-SLM CoCrW Alloy under Different Solution Treatment Conditions. J. Mech. Behav. Biomed. Mater. 2016, 55, 179–190. [Google Scholar] [CrossRef]
- Yu, J.; Yin, Z.; Huang, Z.; Zhao, S.; Huang, H.; Yu, K.; Zhou, R.; Xiao, H. Effect of Aging Treatment on Microstructural Evolution and Mechanical Properties of the Electron Beam Cold Hearth Melting Ti-6Al-4V Alloy. Materials 2022, 15, 7122. [Google Scholar] [CrossRef]
- Jaber, H.; Kónya, J.; Kulcsár, K.; Kovács, T. Effects of Annealing and Solution Treatments on the Microstructure and Mechanical Properties of Ti6Al4V Manufactured by Selective Laser Melting. Materials 2022, 15, 1978. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, R.N.; El-Hadad, S.; Nofal, A. Influence of Heat Treatment Processes on Microstructure Evolution, Tensile and Tribological Properties of Ti6Al4V Alloy. Sci. Rep. 2023, 13, 11292. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Zhang, Y.; Bose, S. Recent Developments in Metal Additive Manufacturing. Curr. Opin. Chem. Eng. 2020, 28, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kara, L.B.; Nie, Z.; Simpson, T.W.; Whitefoot, K.S. Is Additive Manufacturing an Environmentally and Economically Preferred Alternative for Mass Production? Env. Sci. Technol. 2023, 57, 6373–6386. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and Electron-Beam Powder-Bed Additive Manufacturing of Metallic Implants: A Review on Processes, Materials and Designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, A.; Jolic, M.; Hryha, E.; Shah, F.A. Complex Geometry and Integrated Macro-Porosity: Clinical Applications of Electron Beam Melting to Fabricate Bespoke Bone-Anchored Implants. Acta Biomater. 2023, 156, 125–145. [Google Scholar] [CrossRef]
- Tamayo, J.A.; Riascos, M.; Vargas, C.A.; Baena, L.M. Additive Manufacturing of Ti6Al4V Alloy via Electron Beam Melting for the Development of Implants for the Biomedical Industry. Heliyon 2021, 7, e06892. [Google Scholar] [CrossRef]
- Galati, M.; Minetola, P.; Rizza, G. Surface Roughness Characterisation and Analysis of the Electron Beam Melting (EBM) Process. Materials 2019, 12, 2211. [Google Scholar] [CrossRef]
- Tebianian, M.; Aghaie, S.; Jafari, N.S.R.; Hosseini, S.R.E.; Pereira, A.B.; Fernandes, F.A.O.; Farbakhti, M.; Chen, C.; Huo, Y. A Review of the Metal Additive Manufacturing Processes. Materials 2023, 16, 7514. [Google Scholar] [CrossRef]
- Wang, C.; Lei, Y.; Li, C. Achieving an Excellent Strength and Ductility Balance in Additive Manufactured Ti-6Al-4V Alloy through Multi-Step High-to-Low-Temperature Heat Treatment. Materials 2023, 16, 6947. [Google Scholar] [CrossRef]
- Ali, H.; Ghadbeigi, H.; Mumtaz, K. Processing Parameter Effects on Residual Stress and Mechanical Properties of Selective Laser Melted Ti6Al4V. J. Mater. Eng. Perform. 2018, 27, 4059–4068. [Google Scholar] [CrossRef]
- Ginestra, P.; Ferraro, R.M.; Zohar-Hauber, K.; Abeni, A.; Giliani, S.; Ceretti, E. Selective Laser Melting and Electron Beam Melting of Ti6Al4V for Orthopedic Applications: A Comparative Study on the Applied Building Direction. Materials 2020, 13, 5584. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piattelli, A. Direct Laser Metal Sintering as a New Approach to Fabrication of an Isoelastic Functionally Graded Material for Manufacture of Porous Titanium Dental Implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Gopal, V.; Gupta, S.K.; Nilawar, S.; Manivasagam, G.; Suwas, S.; Chatterjee, K. Anisotropy of Additively Manufactured Co–28Cr–6Mo Influences Mechanical Properties and Biomedical Performance. ACS Appl. Mater. Interfaces 2022, 14, 21906–21915. [Google Scholar] [CrossRef]
- Jin, B.; Wang, Q.; Zhao, L.; Pan, A.; Ding, X.; Gao, W.; Song, Y.; Zhang, X. A Review of Additive Manufacturing Techniques and Post-Processing for High-Temperature Titanium Alloys. Metals 2023, 13, 1327. [Google Scholar] [CrossRef]
- Diniță, A.; Neacșa, A.; Portoacă, A.I.; Tănase, M.; Ilinca, C.N.; Ramadan, I.N. Additive Manufacturing Post-Processing Treatments, a Review with Emphasis on Mechanical Characteristics. Materials 2023, 16, 4610. [Google Scholar] [CrossRef]
- Dejene, N.D.; Lemu, H.G.; Gutema, E.M. Critical Review of Comparative Study of Selective Laser Melting and Investment Casting for Thin-Walled Parts. Materials 2023, 16, 7346. [Google Scholar] [CrossRef]
- Gross, C.E.; Palanca, A.A.; DeOrio, J.K. Design Rationale for Total Ankle Arthroplasty Systems: An Update. J. Am. Acad. Orthop. Surg. 2018, 26, 353–359. [Google Scholar] [CrossRef]
- Gross, C.E.; Hsu, A.R.; Scott, D.J.; Palanca, A. Design Rational for Total Ankle Arthroplasty: An Update. J. Am. Acad. Orthop. Surg. 2024, 33, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Grove, K.; Wei, A.; Lee, J.; Akkouch, A. Ankle and Foot Arthroplasty and Prosthesis: A Review on the Current and Upcoming State of Designs and Manufacturing. Micromachines 2023, 14, 2081. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Shafiq, M.; Abbas, N.; Sajjad, U.; Hamid, K. Advances in Biodegradable Materials: Degradation Mechanisms, Mechanical Properties, and Biocompatibility for Orthopedic Applications. Heliyon 2024, 10, e32713. [Google Scholar] [CrossRef]
- Noh, J.H.; Roh, Y.H.; Yang, B.G.; Kim, S.W.; Lee, J.S.; Oh, M.K. Outcomes of Operative Treatment of Unstable Ankle Fractures: A Comparison of Metallic and Biodegradable Implants. J. Bone Jt. Surg. 2012, 94, e166. [Google Scholar] [CrossRef]
- Filli, L.; Luechinger, R.; Frauenfelder, T.; Beck, S.; Guggenberger, R.; Farshad-Amacker, N.; Andreisek, G. Metal-Induced Artifacts in Computed Tomography and Magnetic Resonance Imaging: Comparison of a Biodegradable Magnesium Alloy versus Titanium and Stainless Steel Controls. Skelet. Radiol. 2015, 44, 849–856. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Han, Y.; Zhang, Z.; Qu, X.; Zhuang, Y.; Wu, Q.; Zheng, Y.; Dai, K. In Vitro and in Vivo Studies of Zn-Mn Biodegradable Metals Designed for Orthopedic Applications. Acta Biomater. 2020, 108, 358–372. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Yuan, Q.; Liu, L.; Li, H.; Xiong, L.; Guo, X.; Yan, Y.; Yu, K.; Dai, Y.; et al. In Vitro and in Vivo Assessment of the Effect of Biodegradable Magnesium Alloys on Osteogenesis. Acta Biomater. 2022, 141, 454–465. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Li, H.; Liu, Y.; Bai, X.; Chau, W.; Zheng, Y.; Qin, L. Magnesium Alloy Based Interference Screw Developed for ACL Reconstruction Attenuates Peri-Tunnel Bone Loss in Rabbits. Biomaterials 2018, 157, 86–97. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, M.; Backman, L.J.; Chen, J. Effects of Zinc, Magnesium, and Iron Ions on Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 2321–2335. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Agrawal, A. Micro-Hydroxyapatite Reinforced Ti-Based Composite with Tailored Characteristics to Minimize Stress-Shielding Impact in Bio-Implant Applications. J. Mech. Behav. Biomed. Mater. 2023, 142, 105852. [Google Scholar] [CrossRef] [PubMed]
- Sadlik, J.; Kosińska, E.; Bańkosz, M.; Tomala, A.; Bruzda, G.; Jampilek, J.; Sobczak-Kupiec, A. Gradient Titanium Alloy with Bioactive Hydroxyapatite Porous Structures for Potential Biomedical Applications. Materials 2024, 17, 5511. [Google Scholar] [CrossRef] [PubMed]

| Alloy Type | Composition | Mechanical Properties | Biological Response | Primary Applications |
|---|---|---|---|---|
| Cobalt-Chromium (CoCr) | Co (58.71–68%), Cr (27–30%), Mo (5–7%), trace elements [13,19] | High strength (UTS 665–2482 MPa) High elastic modulus (200–300 GPa) Excellent wear resistance [19] |
|
|
| Titanium (Ti6Al4V) | Ti (88.085–91%), Al (5.5–6.75%), V (3.5–4.5%), trace elements [23,24] | Moderate strength (UTS 860–1173 MPa) Low elastic modulus (101–113 GPa) Poor tribological properties [24] |
|
|
| Stainless Steel (316L) | Fe (59.485–64.335%), Cr (17–19%), Mo (2.25–3%), Ni (13–15%), trace elements [19] | Moderate strength (UTS 490 MPa) High elastic modulus (190 GPa) Susceptible to pitting corrosion [34] |
|
|
| β-titanium alloys | Ti with Nb, Zr, Ta, Mo, Sn (various compositions) [37,38,39] | Ti-35Nb-7Zr-5Ta: UTS 630 MPa, E-modulus 81 GPa Ti-29Nb-13Ta-4.6Zr: E-modulus 65 GPa Ti10Mo6Zr4Sn3Nb: UTS 970 MPa, E-modulus 50 GPa [37,38,39] |
|
|
| Zirconium-Based Alloys | Zr with Nb (various compositions) [41] | Aged Zr50Nb50: UTS 1338 MPa, E-modulus 66 GPa [41] |
|
|
| Manufacturing Method | Key Advantages | Limitations | Applications |
|---|---|---|---|
| Investment casting |
|
|
|
| Forging |
|
|
|
| CNC Machining |
|
|
|
| Electron Beam Melting (EBM) |
|
|
|
| Selective Laser Melting (SLM) |
|
|
|
| Direct Metal Laser Sintering (DMLS) |
|
|
|
| Key Innovation | Description | Potential Benefits | Current Status |
|---|---|---|---|
| Biodegradable Materials | Magnesium, iron, and zinc-based alloys that provide temporary mechanical support with controlled in vivo degradation and replacement by natural tissue |
|
|
| Hybrid Implant Designs | Engineering approach combining biodegradable components with permanent traditional materials to leverage advantages of both systems |
|
|
| Metal Matrix Composites (MMCs) | Titanium-ceramic composites with dispersed ceramic particles (e.g., hydroxyapatite, alumina) in a metallic matrix |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, K.; Movva, A.K.; Sohn, M.O.; Tennyson, J.M.; Talaski, G.M.; Adams, S.B.; Anastasio, A.T. Alloy Selection and Manufacturing Technologies for Total Ankle Arthroplasty: A Narrative Review. Materials 2025, 18, 3770. https://doi.org/10.3390/ma18163770
Mitra K, Movva AK, Sohn MO, Tennyson JM, Talaski GM, Adams SB, Anastasio AT. Alloy Selection and Manufacturing Technologies for Total Ankle Arthroplasty: A Narrative Review. Materials. 2025; 18(16):3770. https://doi.org/10.3390/ma18163770
Chicago/Turabian StyleMitra, Kishen, Arun K. Movva, Michael O. Sohn, Joshua M. Tennyson, Grayson M. Talaski, Samuel B. Adams, and Albert T. Anastasio. 2025. "Alloy Selection and Manufacturing Technologies for Total Ankle Arthroplasty: A Narrative Review" Materials 18, no. 16: 3770. https://doi.org/10.3390/ma18163770
APA StyleMitra, K., Movva, A. K., Sohn, M. O., Tennyson, J. M., Talaski, G. M., Adams, S. B., & Anastasio, A. T. (2025). Alloy Selection and Manufacturing Technologies for Total Ankle Arthroplasty: A Narrative Review. Materials, 18(16), 3770. https://doi.org/10.3390/ma18163770









