Lignin/Epoxidized Natural Rubber Compounds Based on Wet Mixing: Impact of Epoxidation Degree on the Interface of Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Performance Testing and Characterization
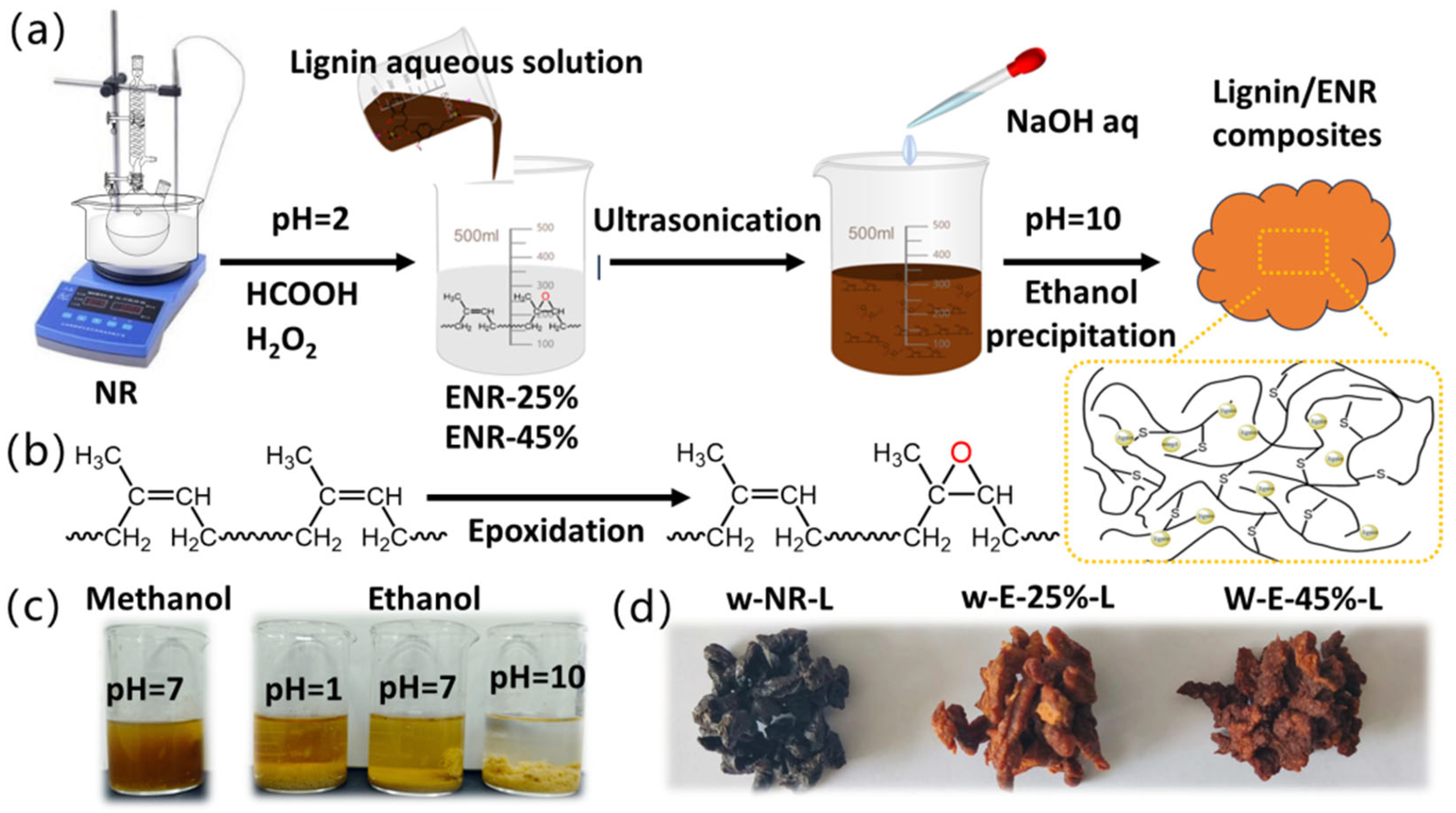
2.3. Preparation of ENR/Lignin Compounds
3. Results and Discussion
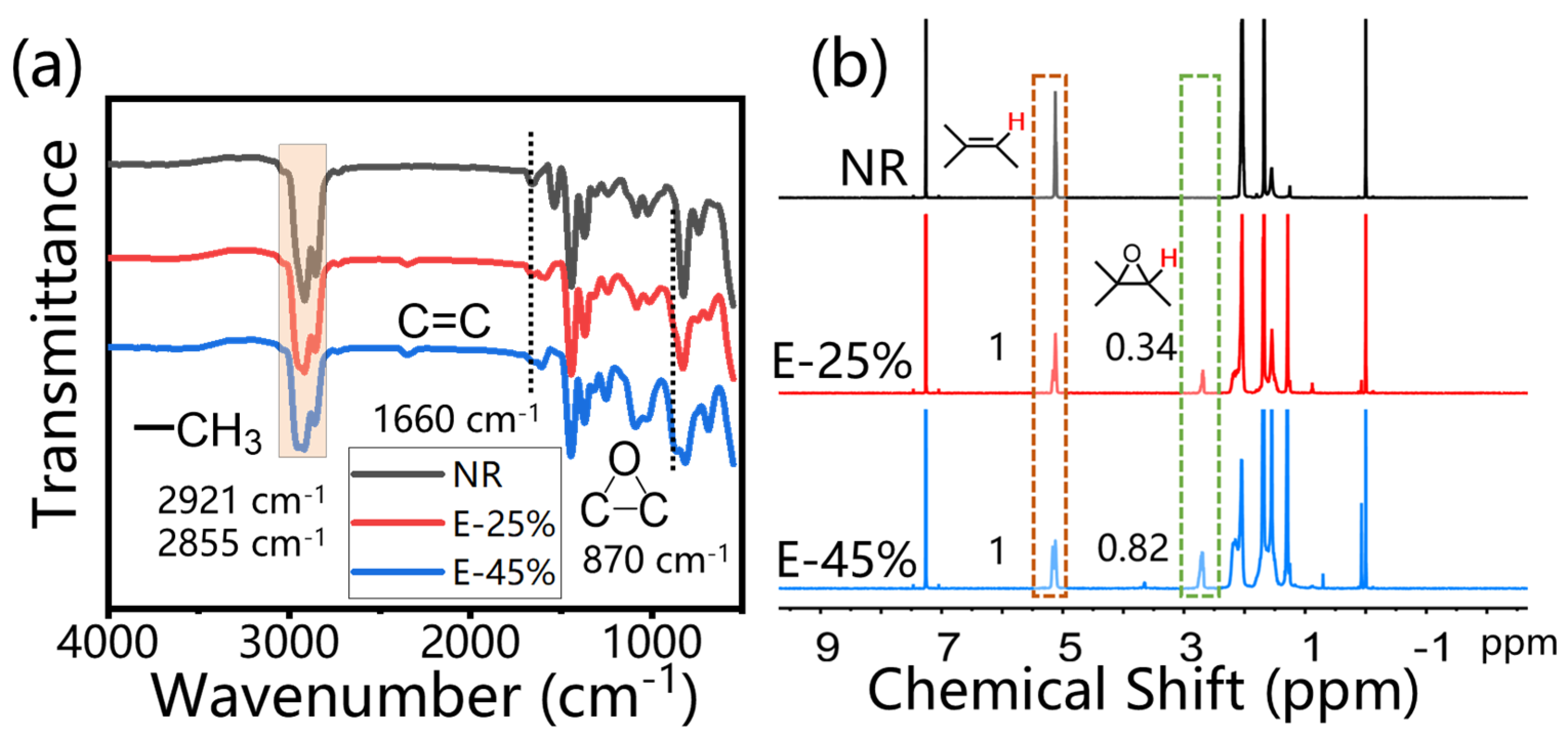
3.1. Characterization of Epoxidized Natural Rubber
3.2. Preparation of ENR/Lignin Compounds via Wet Mixing
3.3. Curing Characteristic Curve of ENR/Lignin Compounds
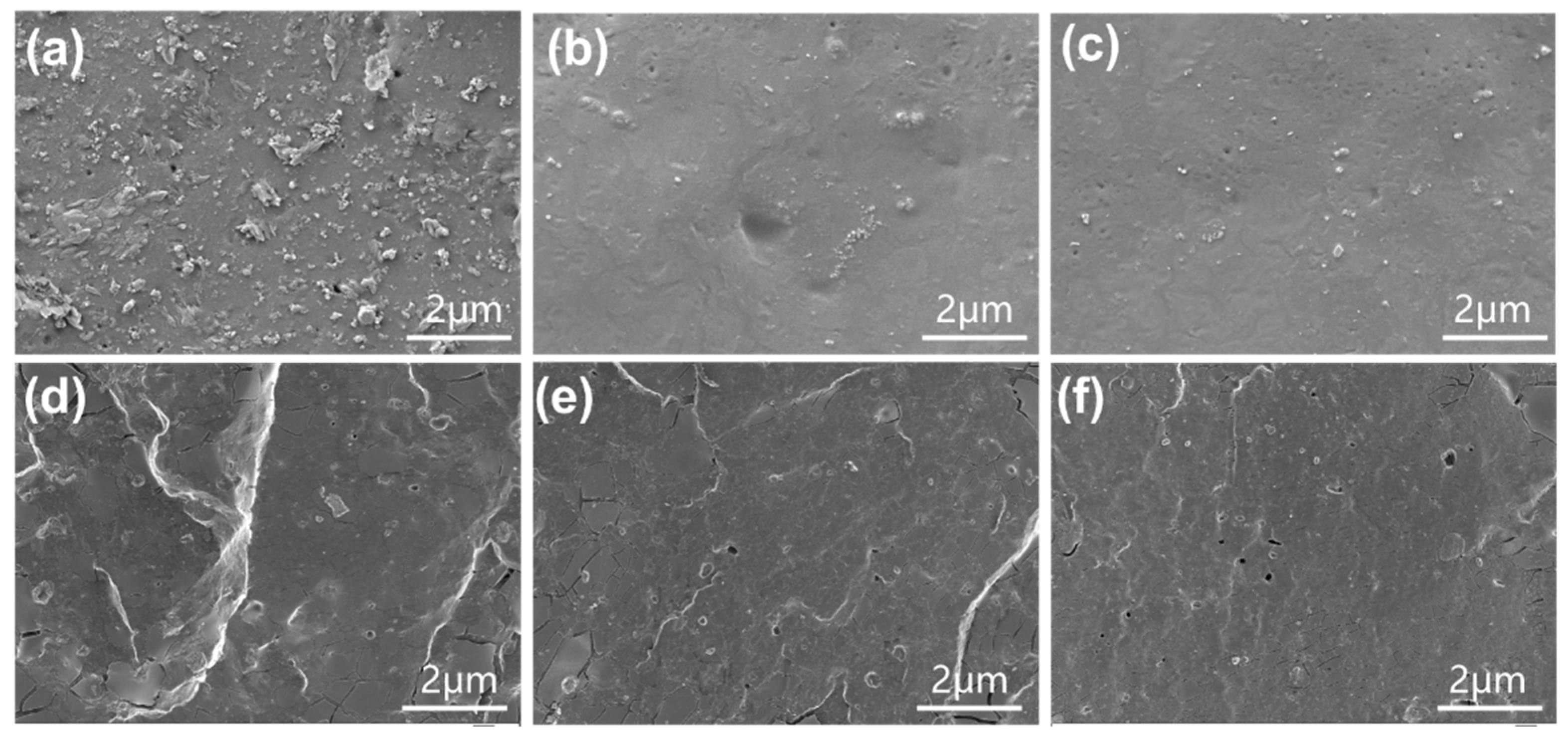
3.4. SEM Analysis of ENR/Lignin Compounds
3.5. Mechanical Property Characterization of ENR/Lignin Compounds
3.6. Dynamic Thermomechanical Characterization of ENR/Lignin Compounds
3.7. Characterization of Thermal Stability of ENR/Lignin Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Jiang, Y.; Wang, S.; Zhang, Y. Influence of a Novel Coupling Agent on the Performance of Recovered Carbon Black Filled Natural Rubber. Compos. Part B Eng. 2023, 255, 110614. [Google Scholar] [CrossRef]
- Yamashita, S.; Takahashi, S. Molecular Mechanisms of Natural Rubber Biosynthesis. Annu. Rev. Biochem. 2020, 89, 821–851. [Google Scholar] [CrossRef]
- Martinez, J.M.M. Natural Rubber by a Rubber Man. Mater. Today 2006, 9, 55. [Google Scholar] [CrossRef]
- Bawn, C.E.H. The Chemistry and Physics of Rubber-like Substances. Polymer 1964, 5, 311. [Google Scholar] [CrossRef]
- Mai, T.; Yasui, T.; Tanaka, R.; Masunaga, H.; Kabe, T.; Tsunoda, K.; Sakurai, S.; Urayama, K. Unraveling Non-Uniform Strain-Induced Crystallization Near a Crack Tip in Natural Rubber. Adv. Sci. 2024, 11, 2307741. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Y.; Pan, X.; Meng, F.; You, J.; Wang, Z. Preparation and Properties of Modified Porous Starch/Carbon Black/Natural Rubber Composites. Compos. Part B Eng. 2019, 156, 1–7. [Google Scholar] [CrossRef]
- Dwivedi, C.; Manjare, S.; Rajan, S.K. Recycling of Waste Tire by Pyrolysis to Recover Carbon Black: Alternative & Environment-Friendly Reinforcing Filler for Natural Rubber Compounds. Compos. Part B Eng. 2020, 200, 108346. [Google Scholar]
- Saengdee, L.; Phinyocheep, P.; Daniel, P. Chemical Modification of Natural Rubber in Latex Stage for Improved Thermal, Oil, Ozone and Mechanical Properties. J. Polym. Res. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Mei, J.; Liu, W.; Huang, J.; Qiu, X. Lignin-Reinforced Ethylene-Propylene-Diene Copolymer Elastomer via Hydrogen Bonding Interactions. Macromol. Mater. Eng. 2019, 304, 1800689. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Chen, L.; Zhuang, J. Lignocellulosic Fibre Mediated Rubber Composites: An Overview. Compos. Part B Eng. 2015, 76, 180–191. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Pongwisuthiruchte, A.; Potiyaraj, P. Recent Advances of Natural Fibers Based Green Rubber Composites: Properties, Current Status, and Future Perspectives. J. Appl. Polym. Sci. 2021, 138, 50866. [Google Scholar] [CrossRef]
- Kazemi, H.; Mighri, F.; Park, K.W.; Frikha, S.; Rodrigue, D. Hybrid Nanocellulose/Carbon Nanotube/Natural Rubber Nanocomposites with a Continuous Three-dimensional Conductive Network. Polym. Compos. 2022, 43, 2362–2374. [Google Scholar] [CrossRef]
- Noguchi, T.; Bamba, Y.; Miura, T.; Iwamoto, R.; Endo, M.; Isogai, A. Cellulose-Nanofiber-Reinforced Rubber Composites with Resorcinol Resin Prepared by Elastic Kneading. Macromol. Mater. Eng. 2021, 306, 2100483. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Xiao, H.; Shen, T.; Tan, Z.; Zhuang, W.; Xi, Y.; Ji, X.; Zhu, C.; Ying, H. Design of a Lignin-Based Versatile Bioreinforcement for High-Performance Natural Rubber Composites. ACS Sustain. Chem. Eng. 2022, 10, 8031–8042. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, B.Z.; Yuan, J.S.; Yuan, Y.J. Creative Biological Lignin Conversion Routes toward Lignin Valorization. Trends Biotechnol. 2022, 40, 1550–1566. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and Valorization of Cellulose, Lignin and Lignocellulose Using Ionic Liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional Lignin-Based Nanocomposites and Nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef]
- Ikeda, Y.; Phakkeeree, T.; Junkong, P.; Yokohama, H.; Phinyocheep, P.; Kitano, R.; Kato, A. Reinforcing Biofiller “Lignin” for High Performance Green Natural Rubber Nanocomposites. RSC Adv. 2017, 7, 5222–5231. [Google Scholar] [CrossRef]
- Barana, D.; Ali, S.D.; Salanti, A.; Orlandi, M.; Castellani, L.; Hanel, T.; Zoia, L. Influence of Lignin Features on Thermal Stability and Mechanical Properties of Natural Rubber Compounds. ACS Sustain. Chem. Eng. 2016, 4, 5258–5267. [Google Scholar] [CrossRef]
- Xue, B.; Wang, X.; Yu, L.; Di, B.; Chen, Z.; Zhu, Y.; Liu, X. Self-Assembled Lignin-Silica Hybrid Material Derived from Rice Husks as the Sustainable Reinforcing Fillers for Natural Rubber. Int. J. Biol. Macromol. 2020, 145, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.P.; Gupta, A.; Muthuraj, R.; Mekonnen, T.H. Bioresourced Fillers for Rubber Composite Sustainability: Current Development and Future Opportunities. Green Chem. 2021, 23, 5337–5378. [Google Scholar] [CrossRef]
- Parvathy, G.; Sethulekshmi, A.S.; Jayan, J.S.; Raman, A.; Saritha, A. Lignin Based Nano-Composites: Synthesis and Applications. Process Saf. Environ. Prot. 2021, 145, 395–410. [Google Scholar]
- Hosseinmardi, A.; Amiralian, N.; Hayati, A.N.; Martin, D.J.; Annamalai, P.K. Toughening of Natural Rubber Nanocomposites by the Incorporation of Nanoscale Lignin Combined with an Industrially Relevant Leaching Process. Ind. Crops Prod. 2021, 159, 113063. [Google Scholar] [CrossRef]
- Qiu, J.; Yuan, S.; Xiao, H.; Liu, J.; Shen, T.; Tan, Z.; Zhuang, W.; Ying, H.; Li, M.; Zhu, C. Study on Lignin Amination for Lignin/SiO2 Nano-Hybrids towards Sustainable Natural Rubber Composites. Int. J. Biol. Macromol. 2023, 233, 123547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shen, H.; Bi, X.; Wang, Z.; Yao, M.; Wu, Y.; Zhang, L.; Yu, P. A Green Dual-Phase Carbon-Silica Nanohybrid Derived from Black Liquor Lignin for Reinforcing Styrene-Butadiene Rubber. Compos. Sci. Technol. 2022, 230, 109775. [Google Scholar] [CrossRef]
- Burfield, D.R.; Lim, K.; Law, K. Epoxidation of Natural Rubber Latices: Methods of Preparation and Properties of Modified Rubbers. J. Appl. Polym. Sci. 1984, 29, 1661–1673. [Google Scholar] [CrossRef]
- Singh, M.; Mohd Rasdi, F.R. Colloidal Properties of Epoxidized Natural Rubber Latex Prepared via Membrane Separation Technology. J. Rubber Res. 2019, 22, 153–167. [Google Scholar] [CrossRef]
- Gan, L.H.; Ng, S.C. Kinetic Studies of the Performic Acid Epoxidation of Natural Rubber Latex Stabilized by Cationic Surfactant. Eur. Polym. J. 1986, 22, 573–576. [Google Scholar] [CrossRef]
- Pongdong, W.; Nakason, C.; Kummerlöwe, C.; Vennemann, N. Influence of Filler from a Renewable Resource and Silane Coupling Agent on the Properties of Epoxidized Natural Rubber Vulcanizates. J. Chem. 2015, 2015, 796459. [Google Scholar] [CrossRef]
- Jiang, C.; He, H.; Yao, X.; Yu, P.; Zhou, L.; Jia, D. In Situ Dispersion and Compatibilization of Lignin/Epoxidized Natural Rubber Composites: Reactivity, Morphology and Property. J. Appl. Polym. Sci. 2015, 132, 42044. [Google Scholar] [CrossRef]
- Datta, J.; Parcheta, P. A Comparative Study on Selective Properties of Kraft Lignin–Natural Rubber Composites Containing Different Plasticizers. Iran. Polym. J. 2017, 26, 453–466. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Huang, J.; Yang, D.; Qiu, X. Bioinspired Engineering towards Tailoring Advanced Lignin/Rubber Elastomers. Polymers 2018, 10, 1033. [Google Scholar] [CrossRef]
- Mohamad Aini, N.; Othman, N.; Hussin, M.; Sahakaro, K.; Hayeemasae, N. Hydroxymethylation-Modified Lignin and Its Effectiveness as a Filler in Rubber Composites. Processes 2019, 7, 315. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Lignin as Alternative Reinforcing Filler in the Rubber Industry: A Review. Front. Mater. 2020, 6, 329. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Barana, D.; Orlandi, M.; Zoia, L.; Castellani, L.; Hanel, T.; Bolck, C.; Gosselink, R. Lignin Based Functional Additives for Natural Rubber. ACS Sustain. Chem. Eng. 2018, 6, 11843–11852. [Google Scholar] [CrossRef]
- Rolere, S.; Cartault, M.; Sainte-Beuve, J.; Bonfils, F. A Rheological Method Exploiting Cole-Cole Plot Allows Gel Quantification in Natural Rubber. Polym. Test. 2017, 61, 378–385. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Aziz, A.K.C.; Rahim, R.A.; Ghani, R.A.; Kamaruddin, A.N. Properties of Epoxidized Natural Rubber Tread Compound: The Hybrid Reinforcing Effect of Silica and Silane System. Polym. Polym. Compos. 2016, 24, 775–782. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A.; Osvald, A.; Krajčovičová, J. Role of Lignin Filler in Stabilization of Natural Rubber–Based Composites. J. Appl. Polym. Sci. 2007, 103, 1226–1231. [Google Scholar] [CrossRef]
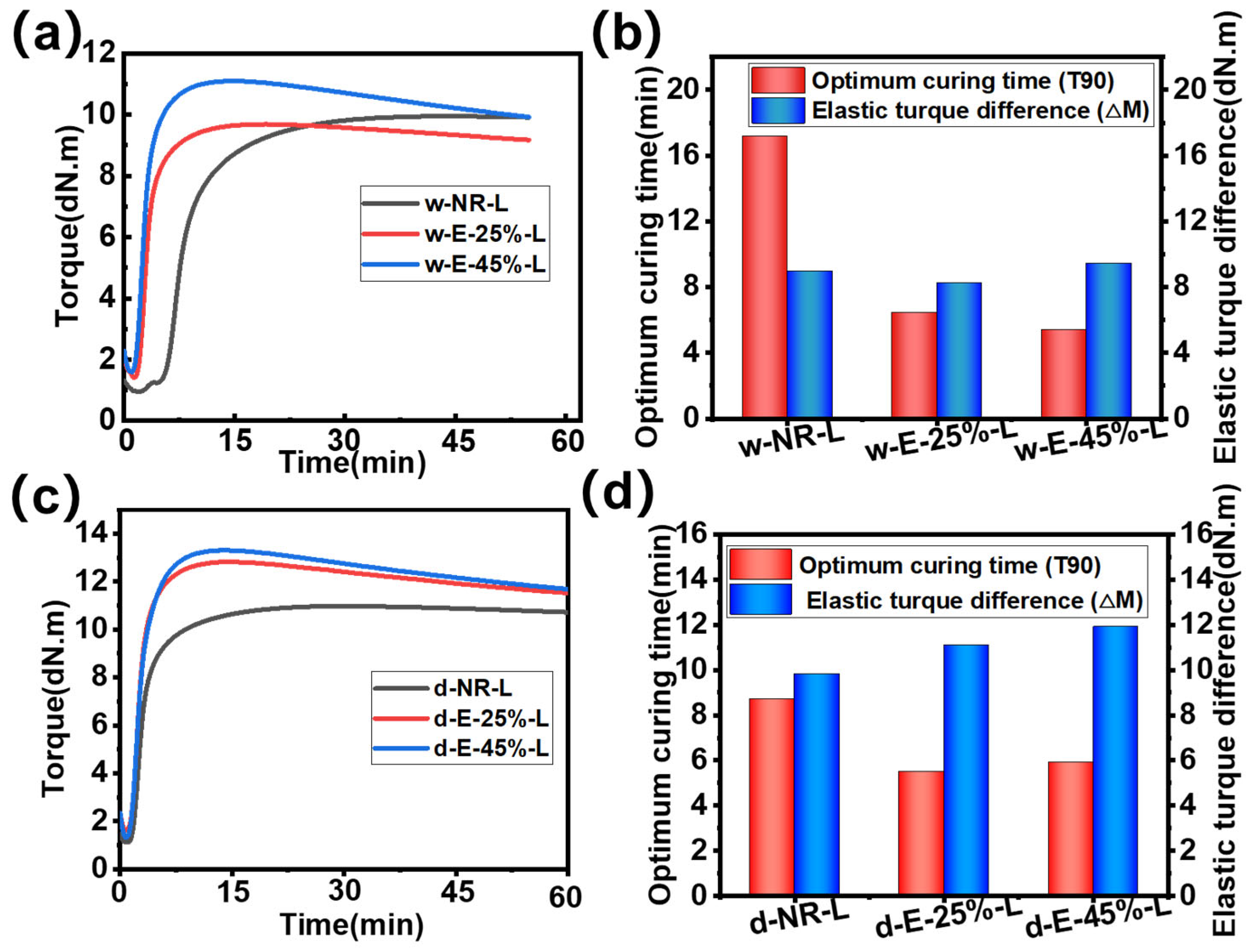



| Samples | T10 (min) | T90 (min) | ML (dN·m) | MH (dN·m) | △M (MH-ML) (dN·m) |
|---|---|---|---|---|---|
| w-NR-L | 5.0 | 17.2 | 1.0 | 10.0 | 9.0 |
| w-E-25%-L | 2.3 | 6.4 | 1.4 | 9.7 | 8.3 |
| w-E-45%-L | 1.9 | 5.4 | 1.6 | 11.1 | 9.5 |
| d-NR-L | 1.6 | 8.7 | 1.2 | 10.9 | 9.3 |
| d-E-25%-L | 1.7 | 5.7 | 1.7 | 12.8 | 11.1 |
| d-E-45%-L | 2.0 | 6.0 | 1.4 | 13.3 | 11.9 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Stress at 100% (MPa) | Stress at 300% (MPa) |
|---|---|---|---|---|
| w-NR-L | 27.5 ± 0.6 | 598.8 ± 19.3 | 1.0 ± 0.02 | 2.2 ± 0.03 |
| w-E-25%-L | 36.2 ± 0.5 | 703.3 ± 25.4 | 1.0 ± 0.03 | 2.1 ± 0.06 |
| w-E-45%-L | 35.0 ± 0.7 | 644.5 ± 17.6 | 1.1 ± 0.04 | 2.5 ± 0.03 |
| d-NR-L | 22.9 ± 0.6 | 554.7 ± 19.1 | 0.9 ± 0.05 | 1.9 ± 0.03 |
| d-E-25%-L | 21.1 ± 1.3 | 531.2 ± 32.5 | 1.3 ± 0.04 | 2.3 ± 0.02 |
| d-E-45%-L | 19.2 ± 1.6 | 505.2 ± 38.6 | 1.2 ± 0.03 | 2.3 ± 0.05 |
| Samples | Decomposition Temperature (°C) |
|---|---|
| w-NR-L | 375.2 |
| w-E-25%-L | 385.0 |
| w-E-45%-L | 389.3 |
| d-NR-L | 373.9 |
| d-E-25%-L | 389.1 |
| d-E-45%-L | 390.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Yue, D. Lignin/Epoxidized Natural Rubber Compounds Based on Wet Mixing: Impact of Epoxidation Degree on the Interface of Compounds. Materials 2025, 18, 3736. https://doi.org/10.3390/ma18163736
Zheng H, Yue D. Lignin/Epoxidized Natural Rubber Compounds Based on Wet Mixing: Impact of Epoxidation Degree on the Interface of Compounds. Materials. 2025; 18(16):3736. https://doi.org/10.3390/ma18163736
Chicago/Turabian StyleZheng, Hongbing, and Dongmei Yue. 2025. "Lignin/Epoxidized Natural Rubber Compounds Based on Wet Mixing: Impact of Epoxidation Degree on the Interface of Compounds" Materials 18, no. 16: 3736. https://doi.org/10.3390/ma18163736
APA StyleZheng, H., & Yue, D. (2025). Lignin/Epoxidized Natural Rubber Compounds Based on Wet Mixing: Impact of Epoxidation Degree on the Interface of Compounds. Materials, 18(16), 3736. https://doi.org/10.3390/ma18163736





