Biocompatible Thermoplastics in Additive Manufacturing of Bone Defect Fillers: State-of-the-Art and Future Prospects
Abstract
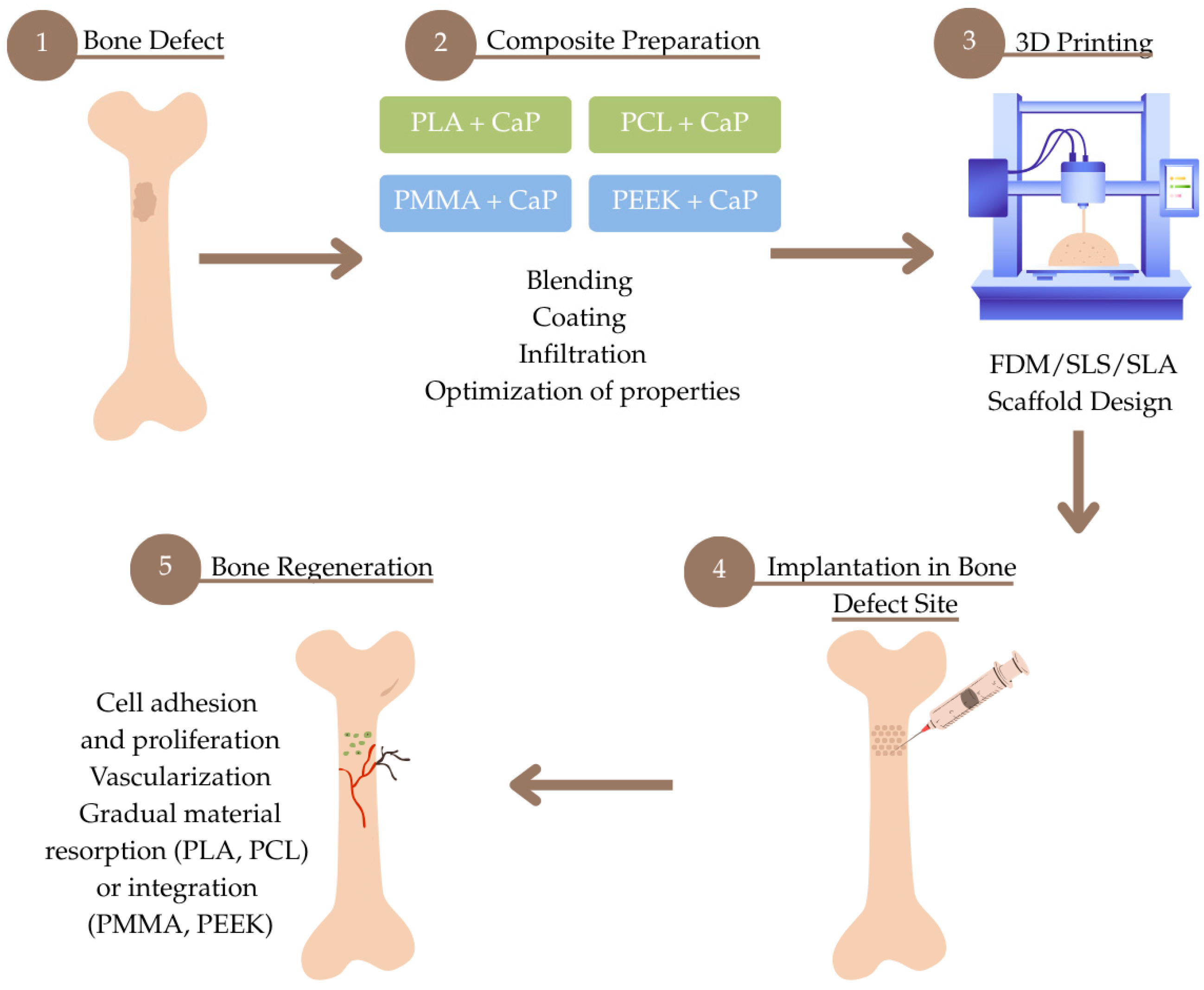
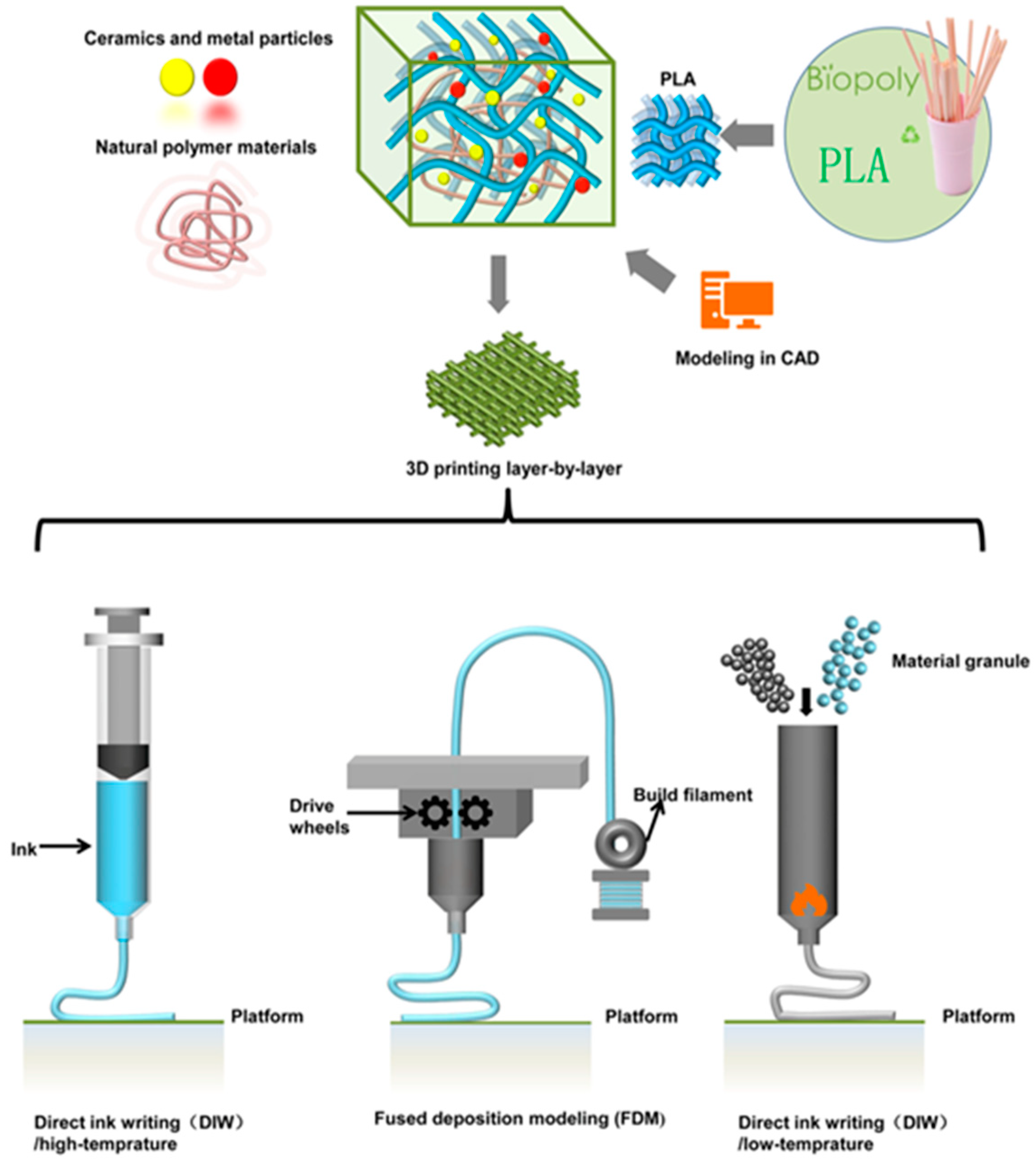
1. Introduction
2. Overview of Biocompatible Thermoplastic Materials
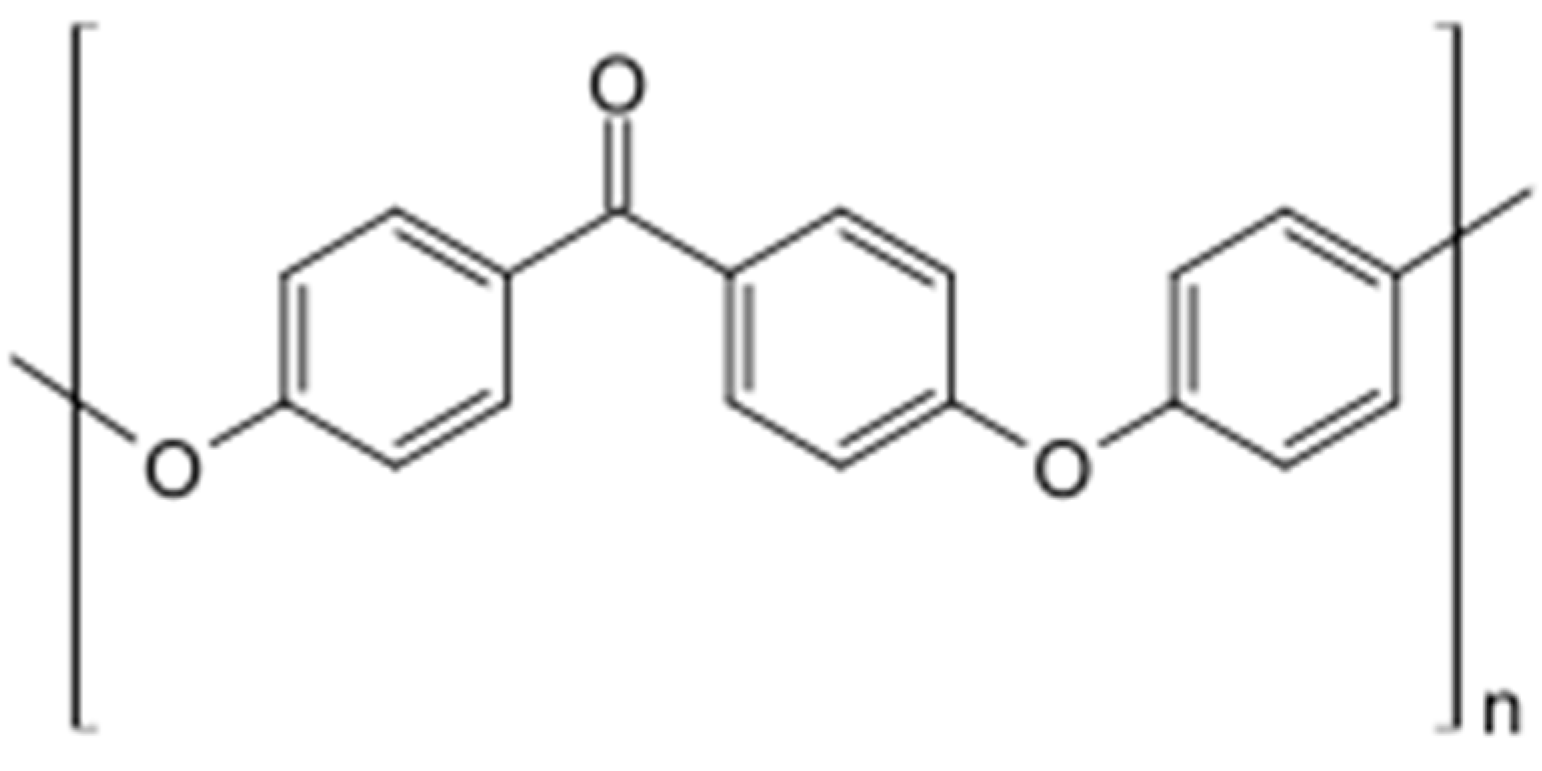
2.1. PEEK (Polyetheretherketone) in Bone Defect Bimaterials
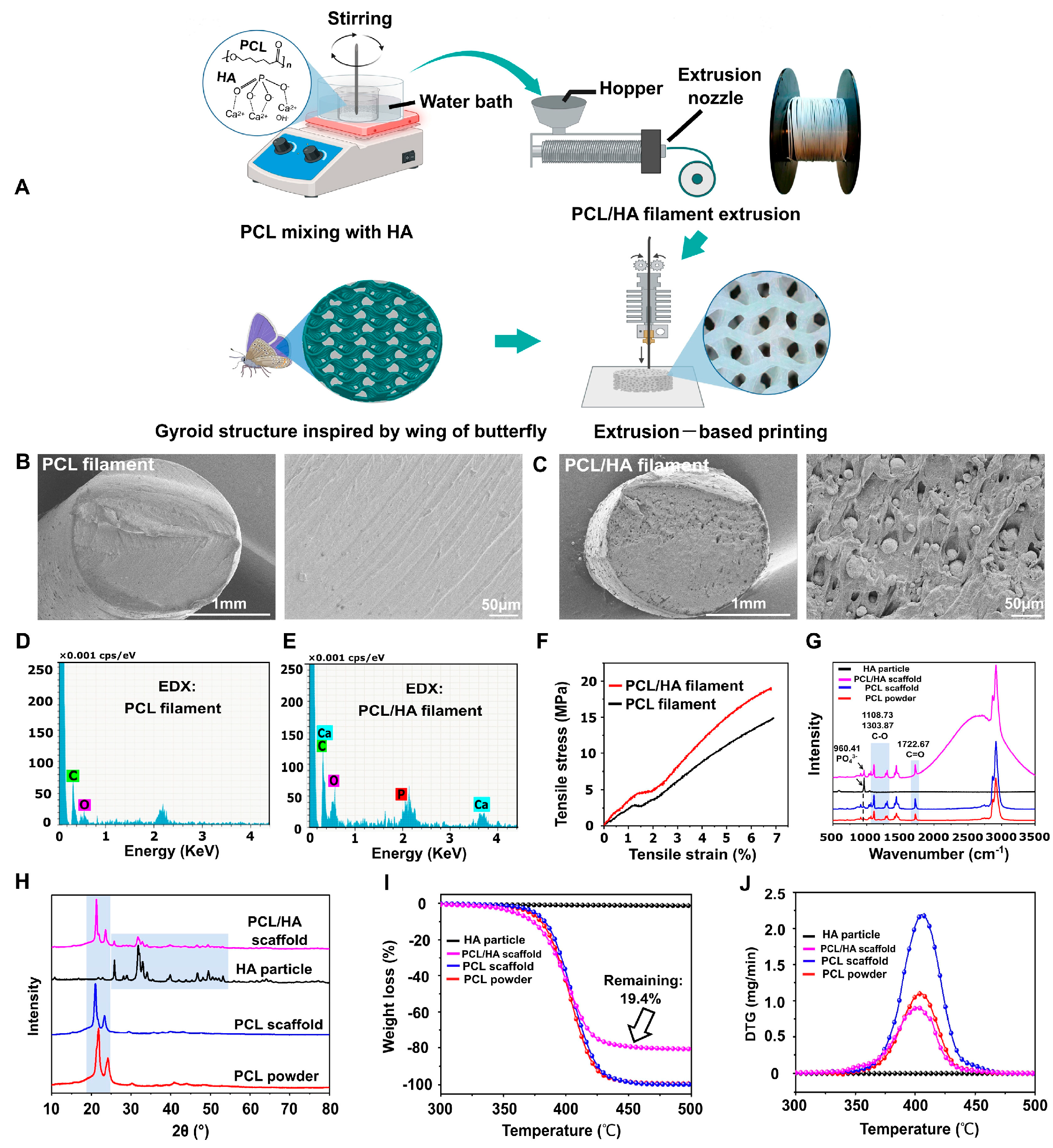
2.2. PCL (Polycaprolactone-Lactide) in Bone Defect Bimaterials
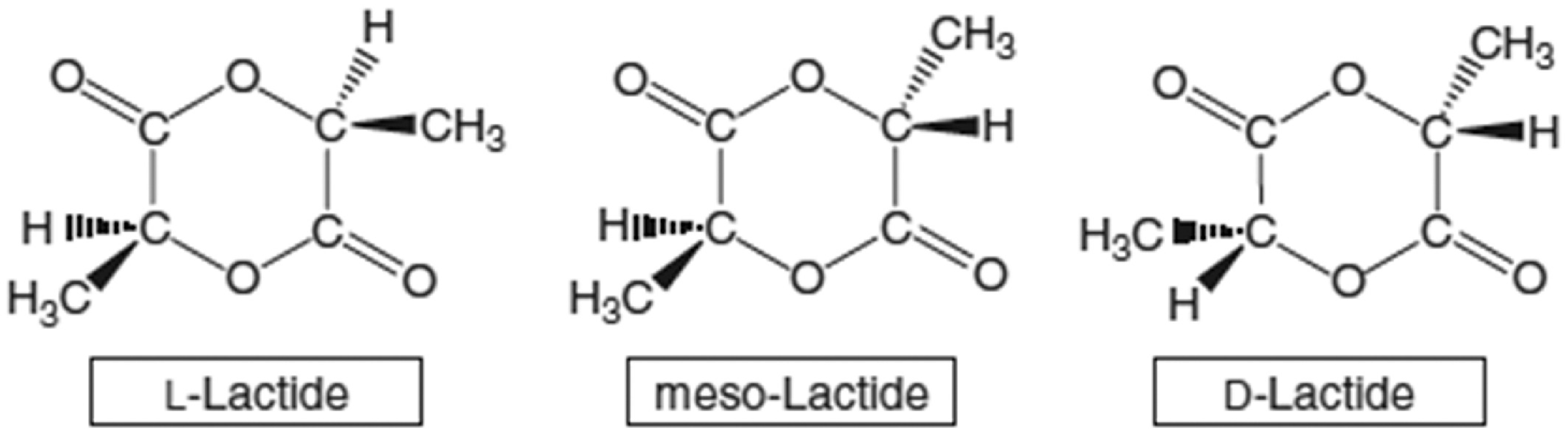
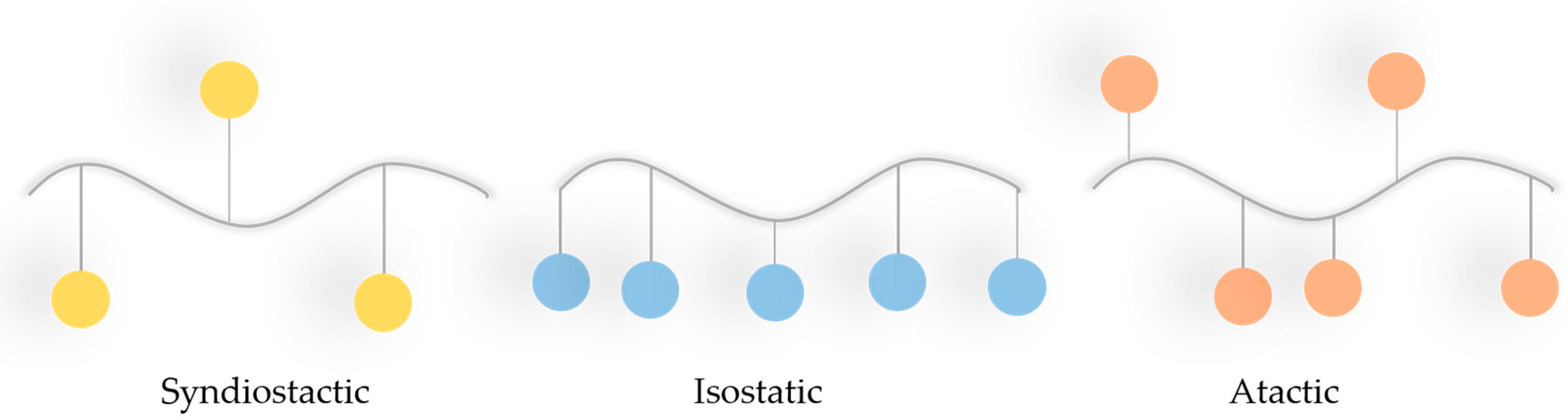
2.3. PLA (Polylactic Acid) in Bone Defect Bimaterials
2.4. PMMA (Polymethyl Methacrylate) in Bone Defect Bimaterials
2.5. Summary
3. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEEK | Multidisciplinary Digital Publishing Institute |
| PLA | Directory of open access journals |
| PCL | Three-letter acronym |
| PMMA | Linear dichroism |
| HAp | Hydroxyapatite |
| FDM | Fused deposition modeling |
| β-TCP/TCP | Tricalcium phosphate |
| BG | Bioactive glass |
| SMPs | Shape memory polymers |
| FDA | Food and Drug Administration |
| ROP | Ring-opening polymerization |
References
- Verrier, S.; Alini, M.; Alsberg, E.; Buchman, S.R.; Kelly, D.; Laschke, M.W.; Menger, M.D.; Murphy, W.L.; Stegemann, J.P.; Schütz, M.; et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur. Cells Mater. 2016, 32, 87–110. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Lasanianos, N.G.; Kanakaris, N.K.; Giannoudis, P.V. Current management of long bone large segmental defects. Orthop. Trauma 2010, 24, 149–163. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 4–10. [Google Scholar] [CrossRef]
- Wang, X.; Jia, C.; Wu, H.; Luo, F.; Hou, T.; Li, G.; Lin, S.; Xie, Z. Activated allograft combined with induced menbrane technique for the reconstruction of infected segmental bone defects. Sci. Rep. 2024, 14, 12587. [Google Scholar] [CrossRef]
- Azi, M.L.; Aprato, A.; Santi, I.; Junior, M.K.; Masse, A.; Joeris, A. Autologous bone graft in the treatment of post-traumatic bone defects: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2016, 17, 465. [Google Scholar] [CrossRef]
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y.J. A review on 3D printed bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Zhang, M. Technical development and application of supercritical CO2 foaming technology in PCL foam production. Sci. Rep. 2024, 14, 6825. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Schatkoski, V.M.; de Menezes, B.R.C.; Pereira, R.M.; Ribas, R.G.; Freitas, A.D.S.M.D.; Lemes, A.P.; Fernandes, M.H.F.V.; Thim, G.P. Recent progress on polymer scaffolds production: Methods, main results, advantages and disadvantages. Express Polym. Lett. 2022, 16, 197–219. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Moczulska-Heljak, M.; Gradys, A.; Sajkiewicz, P. Advances in 3D printing for tissue engineering. Materials 2021, 14, 3149. [Google Scholar] [CrossRef]
- Ahmed, Y.; Alshami, A.S.; Al-Goraee, A.; Obeng, C.P.; Kennedy, R.; Abdelaziz, H.; Striker, R. Enhancing Bone Scaffold Fabrication: A Comparative Study of Manual Casting and Automated 3D Bioprinting. Ann. Biomed. Eng. 2025. [Google Scholar] [CrossRef]
- Zhou, J.; See, C.W.; Sreenivasamurthy, S.; Zhu, D. Customized Additive Manufacturing in Bone Scaffolds—The Gateway to Precise Bone Defect Treatment. Research 2023, 6, 0239. [Google Scholar] [CrossRef]
- Zhao, X.; Li, N.; Zhang, Z.; Hong, J.; Zhang, X.; Hao, Y.; Wang, J.; Xie, Q.; Zhang, Y.; Li, H.; et al. Beyond hype: Unveiling the Real challenges in clinical translation of 3D printed bone scaffolds and the fresh prospects of bioprinted organoids. J. Nanobiotechnol. 2024, 22, 500. [Google Scholar] [CrossRef]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. Biomed. Eng. Online 2020, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, T.; Choong, P.F.; Wallace, G.G.; O’connell, C.D. The regulatory challenge of 3D bioprinting. Regen. Med. 2023, 18, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Le Ferrand, H.; Athanasiou, C.E. A Materials Perspective on the Design of Damage-Resilient Bone Implants Through Additive/Advanced Manufacturing. J. Am. Ceram. Soc. 2020, 72, 1195–1210. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Mousa, H.M.; Abdel-Jaber, G.T.; Barhoum, A.; Abdal-hay, A. Innovative designs of 3D scaffolds for bone tissue regeneration: Understanding principles and addressing challenges. Eur. Polym. J. 2024, 215, 113251. [Google Scholar] [CrossRef]
- Aftab, M.; Ikram, S.; Ullah, M.; Khan, N.; Naeem, M.; Khan, M.A.; Bakhtiyor o’g’li, R.B.; Qizi, K.S.S.; Erkinjon Ugli, O.O.; Abdurasulovna, B.M.; et al. Recent Trends and Future Directions in 3D Printing of Biocompatible Polymers. J. Manuf. Mater. Process. 2025, 9, 129. [Google Scholar] [CrossRef]
- Valino, A.D.; Dizon, J.R.C.; Jr, A.H.E.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Roskies, M.; Jordan, J.O.; Fang, D.; Abdallah, M.N.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J. Biomater. Appl. 2016, 31, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Golbang, A.; Jindal, S.; Dixon, D.; McIlhagger, A.; Harkin-Jones, E.; Crawford, D.; Mancuso, E. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J. Mech. Behav. Biomed. Mater. 2021, 121, 104601. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Delille, R.; Lesueur, D.; Potier, P.; Drazetic, P.; Markiewicz, E. Experimental study of the bone behaviour of the human skull bone for the development of a physical head model. Int. J. Crashworthiness 2007, 12, 101–108. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef]
- Honigmann, P.; Sharma, N.; Schumacher, R.; Rueegg, J.; Haefeli, M.; Thieringer, F. In-Hospital 3D Printed Scaphoid Prosthesis Using Medical-Grade Polyetheretherketone (PEEK) Biomaterial. BioMed Res. Int. 2021, 2021, 1301028. [Google Scholar] [CrossRef]
- Walsh, W.R.; Bertollo, N.; Christou, C.; Schaffner, D.; Mobbs, R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015, 15, 1041–1049. [Google Scholar] [CrossRef]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-hydroxyapatite-coated PEEK implants: A pilot study in rabbit bone. J. Biomed. Mater. Res. Part A 2013, 101A, 465–471. [Google Scholar] [CrossRef]
- Durham, J.W.; Montelongo, S.A.; Ong, J.L.; Guda, T.; Allen, M.J.; Rabiei, A. Hydroxyapatite coating on PEEK implants: Biomechanical and histological study in a rabbit model. Mater. Sci. Eng. C 2016, 68, 723–731. [Google Scholar] [CrossRef]
- Rinaldi, M.; Cecchini, F.; Pigliaru, L.; Ghidini, T.; Lumaca, F.; Nanni, F. Additive manufacturing of polyether ether ketone (Peek) for space applications: A nanosat polymeric structure. Polymers 2021, 23, 11. [Google Scholar] [CrossRef]
- Sharma, N.; Aghlmandi, S.; Cao, S.; Kunz, C.; Honigmann, P.; Thieringer, F.M. Quality characteristics and clinical relevance of in-house 3D-printed customized polyetheretherketone (PEEK) implants for craniofacial reconstruction. J. Clin. Med. 2020, 9, 2818. [Google Scholar] [CrossRef]
- Rodzeń, K.; Sharma, P.K.; McIlhagger, A.; Mokhtari, M.; Dave, F.; Tormey, D.; Sherlock, R.; Meenan, B.J.; Boyd, A. The direct 3D printing of functional PEEK/hydroxyapatite composites via a fused filament fabrication approach. Polymers 2021, 13, 545. [Google Scholar] [CrossRef]
- Rodzeń, K.; McIvor, M.J.; Sharma, P.K.; Acheson, J.G.; McIlhagger, A.; Mokhtari, M.; McFerran, A.; Ward, J.; Meenan, B.J.; Boyd, A.R. The surface characterisation of fused filament fabricated (Fff) 3d printed peek/hydroxyapatite composites. Polymers 2021, 13, 3117. [Google Scholar] [CrossRef]
- Manzoor, F.; Golbang, A.; Dixon, D.; Mancuso, E.; Azhar, U.; Manolakis, I.; Crawford, D.; McIlhagger, A.; Harkin-Jones, E. 3D Printed Strontium and Zinc Doped Hydroxyapatite Loaded PEEK for Craniomaxillofacial Implants. Polymers 2022, 14, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zheng, J.; Hui, Y.; Li, D. Mechanical Properties of 3D-Printed PEEK/HA Composite Filaments. Polymers 2022, 14, 4293. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kang, J.; Sun, C.; Yang, C.; Wang, L.; Li, D. Effects of printing path and material components on mechanical properties of 3D-printed polyether-ether-ketone/hydroxyapatite composites. J. Mech. Behav. Biomed. Mater. 2021, 118, 104475. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zeng, B.; Shen, D.; Deng, J.; Zhong, L.; Hu, H.; Wang, X.; Li, H.; Xu, L.; Deng, Y. Biomechanical and osteointegration study of 3D-printed porous PEEK hydroxyapatite-coated scaffolds. J. Biomater. Sci. Polym. Ed. 2023, 34, 435–448. [Google Scholar] [CrossRef]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Yu, S.; Hariram, K.P.; Kumar, R.; Cheang, P.; Aik, K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials 2005, 26, 2343–2352. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef]
- Molinar-Díaz, J.; Parsons, A.J.; Ahmed, I.; Warrior, N.A.; Harper, L.T. Poly-Ether-Ether-Ketone (PEEK) Biomaterials and Composites: Challenges, Progress, and Opportunities. Polym. Rev. 2025, 65, 527–565. [Google Scholar] [CrossRef]
- Minami, Y.; Matsuyama, N.; Takeichi, Y.; Watanabe, R.; Mathew, S.; Nakajima, Y. Depolymerization of robust polyetheretherketone to regenerate monomer units using sulfur reagents. Commun. Chem. 2023, 6, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Atanase, L.I.; Salhi, S.; Cucoveica, O.; Ponjavic, M.; Nikodinovic-Runic, J.; Delaite, C. Biodegradability Assessment of Polyester Copolymers Based on Poly(ethylene adipate) and Poly(ε-caprolactone). Polymers 2022, 14, 3736. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.J.; Harrison, K.L. The effect of molecular weight on the crystallization kinetics of polycaprolactone. Polym. Adv. Technol. 2006, 17, 474–478. [Google Scholar] [CrossRef]
- Höglund, A.; Hakkarainen, M.; Albertsson, A.C. Degradation profile of poly(ε;-caprolactone)-the influence of macroscopic and macromolecular biomaterial design. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 1041–1046. [Google Scholar] [CrossRef]
- Oh, Y.R.; Jang, Y.A.; Song, J.K.; Eom, G.T. Efficient enzymatic depolymerization of polycaprolactone into 6-hydroxyhexanoic acid by optimizing reaction conditions and microbial conversion of 6-hydroxyhexanoic acid into adipic acid for eco-friendly upcycling of polycaprolactone. Biochem. Eng. J. 2022, 185, 108504. [Google Scholar] [CrossRef]
- Asaduzzaman, F.; Salmon, S. Controllable Water-Triggered Degradation of PCL Solution-Blown Nanofibrous Webs Made Possible by Lipase Enzyme Entrapment. Fibers 2023, 11, 49. [Google Scholar] [CrossRef]
- Ntrivala, M.A.; Pitsavas, A.C.; Lazaridou, K.; Baziakou, Z.; Karavasili, D.; Papadimitriou, M.; Ntagkopoulou, C.; Balla, E.; Bikiaris, D.N. Polycaprolactone (PCL): The biodegradable polyester shaping the future of material –a review on synthesis, properties, biodegradation, applications and future perspectives. Eur. Polym. J. 2025, 234, 114033. [Google Scholar] [CrossRef]
- Fernández, J.; Meaurio, E.; Chaos, A.; Etxeberria, A.; Alonso-Varona, A.; Sarasua, J.R. Synthesis and characterization of poly (l-lactide/ε-caprolactone) statistical copolymers with well resolved chain microstructures. Polymer 2013, 54, 2621–2631. [Google Scholar] [CrossRef]
- Chen, H.Y.; Fang, H.J.; Chen, Y.J.; Hsu, S.C.N.; Lai, Y.C.; Ou, H.W.; Peng, W.T.; Chang, Y.J.; Tsou, Y.C.; Wu, T.Y.; et al. In situ formation of Sn (IV) catalyst with increased activity in ε-caprolactone and L-lactide polymerization using stannous(II) 2-ethylhexanoate. J. Polym. Sci. Part A. Polym. Chem. 2012, 50, 3286–3294. [Google Scholar] [CrossRef]
- Deshpande, M.V.; Girase, A.; King, M.W. Degradation of Poly(ε-caprolactone) Resorbable Multifilament Yarn under Physiological Conditions. Polymers 2023, 15, 3819. [Google Scholar] [CrossRef]
- Basak, S.; Dangate, M.S.; Samy, S. Oil- and water-resistant paper coatings: A review. Prog. Org. Coat. 2024, 186, 107938. [Google Scholar] [CrossRef]
- Aoyama, T.; Uto, K.; Shimizu, H.; Ebara, M.; Kitagawa, T.; Tachibana, H.; Suzuki, K.; Kodaira, T. Development of a new poly-ε-caprolactone with low melting point for creating a thermoset mask used in radiation therapy. Sci. Rep. 2021, 11, 20409. [Google Scholar] [CrossRef]
- Chen, T.; Cai, T.; Jin, Q.; Ji, J. Design and fabrication of functional polycaprolactone. E-Polymers 2015, 15, 3–13. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Zheng, Y.; Yuan, W.; Zhou, J.; Yu, C.; Bao, Y.; Shan, G.; Pan, P. Hierarchical ordering and multilayer structure of poly(ϵ-caprolactone) end-functionalized by a liquid crystalline unit: Role of polymer crystallization. Polym. Chem. 2021, 12, 4175–4183. [Google Scholar] [CrossRef]
- Cheung, E.; Alberti, C.; Bycinskij, S.; Enthaler, S. Zinc-Catalyzed Chemical Recycling of Poly(ϵ-caprolactone) Applying Transesterification Reactions. ChemistrySelect 2021, 6, 8063–8067. [Google Scholar] [CrossRef]
- Cai, C.; Ma, J.; Liang, X.; Zhang, S.; Zhang, H.; Zhang, C.; Zhang, S. An efficient “depolymerization–polymerization” closed-loop recycling strategy for selective degradation of polycaprolactone. Polym. Chem. 2025, 16, 1568–1577. [Google Scholar] [CrossRef]
- Dong, B.; Guo, J.; Xu, G.; Hou, H.; Yang, R.; Guo, X.; Wang, Q. Trash into treasure: Chemical upcycling of poly(ɛ-caprolactone) waste plastic to plasticizer with excellent plasticizing performance and migration resistance. Sustain. Mater. Technol. 2024, 39, e00823. [Google Scholar] [CrossRef]
- Kumar Kalita, N.; Hazarika, D.; Srivastava, R.K.; Hakkarainen, M. Faster biodegradable and chemically recyclable polycaprolactone with embedded enzymes: Revealing new insights into degradation kinetics. Chem. Eng. J. 2024, 496, 153982. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Lee, W.-K.; Hsu, J.-T.; Shih, J.-Y.; Ma, T.-L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef]
- Emadi, H.; Baghani, M.; Masoudi Rad, M.; Hoomehr, B.; Baniassadi, M.; Lotfian, S. 3D-Printed Polycaprolactone-Based Containing Calcium Zirconium Silicate: Bioactive Scaffold for Accelerating Bone Regeneration. Polymers 2024, 16, 1389. [Google Scholar] [CrossRef]
- Da Cunha, D.A.L.V.; Inforçatti Neto, P.; Micocci, K.C.; Bellani, C.F.; Selistre-De-Araujo, H.S.; Silveira, Z.C.; Branciforti, M.C. Fabrication and characterization of scaffolds of poly(ϵ-caprolactone)/biosilicate® biocomposites prepared by generative manufacturing process. Int. J. Biomater. 2019, 2019, 2131467. [Google Scholar] [CrossRef]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive calcium phosphate-based composites for bone regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Wang, F.Z.; Liu, S.; Gao, M.; Yu, Y.; Zhang, W.B.; Li, H.; Peng, X. 3D-Printed Polycaprolactone/Hydroxyapatite Bionic Scaffold for Bone Regeneration. Polymers 2025, 17, 858. [Google Scholar] [CrossRef]
- Cao, C.; Huang, P.; Prasopthum, A.; Parsons, A.J.; Ai, F.; Yang, J. Characterisation of bone regeneration in 3D printed ductile PCL/PEG/hydroxyapatite scaffolds with high ceramic microparticle concentrations. Biomater. Sci. 2022, 10, 138–152. [Google Scholar] [CrossRef]
- Laubach, M.; Herath, B.; Bock, N.; Suresh, S.; Saifzadeh, S.; Dargaville, B.L.; McGovern, J.; Wille, M.L.; Hutmacher, D.W.; Medeiros Savi, F. In vivo characterization of 3D-printed polycaprolactone-hydroxyapatite scaffolds with Voronoi design to advance the concept of scaffold-guided bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1272348. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Suresh, N.; Shanmugavadivu, A.; Selvamurugan, N. Chitosan–exosome synergy: Advanced cell-free scaffold approaches for bone tissue engineering. Int. J. Biol. Macromol. 2025, 304, 140753. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Chen, L.; He, X.; Weng, Y.; Zhang, X.; Yang, D.P.; Yu, H. 3D printing of fish-scale derived hydroxyapatite/chitosan/PCL scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2024, 274, 133172. [Google Scholar] [CrossRef]
- Niu, Y.; Song, F.; Shan, R.; Zhao, Q.; Chen, Z.; Wang, N.; Xie, W. Large artificial bone from 3D printed polycaprolactone/β-tricalcium phosphate (3D PCL/β-TCP) effectively promoting MC3T3-E1 cell adhesion, proliferation, and new bone formation. Eur. Polym. J. 2024, 113046. [Google Scholar] [CrossRef]
- Ghezzi, B.; Matera, B.; Meglioli, M.; Rossi, F.; Duraccio, D.; Faga, M.G.; Zappettini, A.; Macaluso, G.M.; Lumetti, S. Composite PCL Scaffold With 70% β-TCP as Suitable Structure for Bone Replacement. Int. Dent. J. 2024, 74, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Helaehil, J.V.; Huang, B.; Bartolo, P.; Santamaria-JR, M.; Caetano, G.F. Bone regeneration: The influence of composite HA/TCP scaffolds and electrical stimulation on TGF/BMP and RANK/RANKL/OPG pathways. Injury 2025, 56, 112158. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Song, T.; Cao, Z.; Wang, T. 3D printed polycaprolactone/β-tricalcium phosphate/carbon nanotube composite –Physical properties and biocompatibility. Heliyon 2024, 10, e26071. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D printed PCL/β-TCP cross-scale scaffold with high-precision fiber for providing cell growth and forming bones in the pores. Mater. Sci. Eng. C 2021, 127, 112197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Kim, Y.H.; Hong, J.; Kim, W.K.; Jin, S.; Kang, B.J. Sustained BMP-2 delivery via alginate microbeads and polydopamine-coated 3D-Printed PCL/β-TCP scaffold enhances bone regeneration in long bone segmental defects. J. Orthop. Transl. 2024, 49, 11–22. [Google Scholar] [CrossRef]
- dos Santos, V.I.; Merlini, C.; Aragones, Á.; Cesca, K.; Fredel, M.C. Influence of calcium phosphates incorporation into poly(lactic-co-glycolic acid) electrospun membranes for guided bone regeneration. Polym. Degrad. Stab. 2020, 179, 109253. [Google Scholar] [CrossRef]
- Tyona, M.D.; Itodo, A. Effects of surface modification (surface treatment) on friction and surface abrasion of ceramic composites. Surf. Modif. Funct. Ceram. Compos. 2023, 91–114. [Google Scholar] [CrossRef]
- Stamnitz, S.; Krawczenko, A.; Szałaj, U.; Górecka, Ż.; Antończyk, A.; Kiełbowicz, Z.; Święszkowski, W.; Łojkowski, W.; Klimczak, A. Osteogenic Potential of Sheep Mesenchymal Stem Cells Preconditioned with BMP-2 and FGF-2 and Seeded on an nHAP-Coated PCL/HAP/β-TCP Scaffold. Cells 2022, 11, 3446. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yoon, I.A.; Woo, S.H.; Song, D.R.; Kim, K.Y.; Kim, S.J.; Jeong, W.S.; Choi, J.W. Complications arising from clinical application of composite polycaprolactone/bioactive glass ceramic implants for craniofacial reconstruction: A prospective study. J. Cranio-Maxillofac. Surg. 2022, 50, 863–872. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Ramezani Dana, H.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2025, 107, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.F.; Bellin, K.; Behl, M.; Lendlein, A. Demonstrating the influence of water on shape-memory polymer networks based on poly[(rac-lactide)-co-glycolide] segments in vitro. Int. J. Artif. Organs 2011, 34, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Neffe, A.T.; Steuer, S.; Lendlein, A. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J. Control. Release 2009, 138, 243–250. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- McKeen, L.W. Plastics Used in Medical Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780323221696. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices-A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of PLA. J. Polym. Environ. 2002, 9, 63–83. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Zaidi, S.; Bougarech, A.; Abid, M.; Abid, S.; Silvestre, A.J.D.; Sousa, A.F. Highly Flexible Poly(1,12-dodecylene 5,5′-isopropylidene-bis(ethyl 2-furoate)): A Promising Biobased Polyester Derived from a Renewable Cost-Effective Bisfuranic Precursor and a Long-Chain Aliphatic Spacer. Molecules 2023, 28, 4124. [Google Scholar] [CrossRef]
- Sui, T.; Salvati, E.; Zhang, H.; Nyaza, K.; Senatov, F.S.; Salimon, A.I.; Korsunsky, A.M. Probing the complex thermo-mechanical properties of a 3D-printed polylactide-hydroxyapatite composite using in situ synchrotron X-ray scattering. J. Adv. Res. 2019, 16, 113–122. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.d.N.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the degradation during melt processing of pla/biosilicate® composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef]
- Majchrowicz, A.; Roguska, A.; Krawczyńska, A.; Lewandowska, M.; Martí-Muñoz, J.; Engel, E.; Castano, O. In vitro evaluation of degradable electrospun polylactic acid/bioactive calcium phosphate ormoglass scaffolds. Arch. Civ. Mech. Eng. 2020, 20, 50. [Google Scholar] [CrossRef]
- Birgani, Z.T.; van Blitterswijk, C.A.; Habibovic, P. Monolithic calcium phosphate/poly(Lactic acid) composite versus calcium phosphate-coated poly(lactic acid) for support of osteogenic differentiation of human mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2016, 27, 54. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Mattoso, L.H.C. Development of three-dimensional printing filaments based on poly(lactic acid)/hydroxyapatite composites with potential for tissue engineering. J. Compos. Mater. 2021, 55, 2289–2300. [Google Scholar] [CrossRef]
- Tcacencu, I.; Rodrigues, N.; Alharbi, N.; Benning, M.; Toumpaniari, S.; Mancuso, E.; Marshall, M.; Bretcanu, O.; Birch, M.; McCaskie, A.; et al. Osseointegration of porous apatite-wollastonite and poly(lactic acid) composite structures created using 3D printing techniques. Mater. Sci. Eng. C 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Wu, D.; Spanou, A.; Diez-Escudero, A.; Persson, C. 3D-printed PLA/HA composite structures as synthetic trabecular bone: A feasibility study using fused deposition modeling. J. Mech. Behav. Biomed. Mater. 2020, 103, 6–7. [Google Scholar] [CrossRef]
- Söhling, N.; Al Zoghool, S.; Schätzlein, E.; Neijhoft, J.; Oliveira, K.M.C.; Leppik, L.; Ritz, U.; Dörsam, E.; Frank, J.; Marzi, I.; et al. In vitro Evaluation of a 20% Bioglass-Containing 3D printable PLA Composite for Bone Tissue Engineering. Int. J. Bioprint. 2022, 8, 65–81. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater. Today Proc. 2019, 29, 228–232. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Guo, F.; Wang, E.; Yang, Y.; Mao, Y.; Liu, C.; Bu, W.; Li, P.; Zhao, L.; Jin, Q.; Liu, B.; et al. A natural biomineral for enhancing the biomineralization and cell response of 3D printed polylactic acid bone scaffolds. Int. J. Biol. Macromol. 2023, 242, 124728. [Google Scholar] [CrossRef]
- Guo, W.; Liu, C.; Bu, W.; Yang, Y.; Guo, F.; Li, J.; Wang, E.; Mao, Y.; Mai, H.; You, H.; et al. 3D printing of polylactic acid/boron nitride bone scaffolds: Mechanical properties, biomineralization ability and cell responses. Ceram. Int. 2023, 49, 25886–25898. [Google Scholar] [CrossRef]
- Alonso-Fernández, I.; Haugen, H.J.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Use of 3D-printed polylactic acid/bioceramic composite scaffolds for bone tissue engineering in preclinical in vivo studies: A systematic review. Acta Biomater. 2023, 168, 1–21. [Google Scholar] [CrossRef]
- Pcl, P.L.A.; Batio, H.; Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based onPLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar]
- Pietrzykowska, E.; Romelczyk-Baishya, B.; Wojnarowicz, J.; Sokolova, M.; Szlazak, K.; Swieszkowski, W.; Locs, J.; Lojkowski, W. Preparation of a ceramic matrix composite made of hydroxyapatite nanoparticles and polylactic acid by consolidation of composite granules. Nanomaterials 2020, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hogan, K.A.; Cai, C.; Rieth, S. Human health effects of biphenyl: Key findings and scientific issues. Environ. Health Perspect. 2016, 124, 703–712. [Google Scholar] [CrossRef]
- Figueroa Romero, G.; Maldonado, S.R.; Arciniaga, L.F.; Gonzales, D.A.; Villalobos, E.B.; Potter, B.G.; Muralidharan, K.; Loy, D.A.; Szivek, J.A.; Margolis, D.S. Polymer-ceramic composites for fused deposition modeling of biomimetic bone scaffolds. Results Eng. 2024, 23, 102407. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and mechanical properties of PLA/HA composites: A study of in vitro degradation. Mater. Sci. Eng. C 2006, 26, 1289–1295. [Google Scholar] [CrossRef]
- Lou, C.W.; Yao, C.H.; Chen, Y.S.; Hsieh, T.C.; Lin, J.H.; Hsing, W.H. Manufacturing and Properties of PLA Absorbable Surgical Suture. Text. Res. J. 2008, 78, 958–965. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Hu, X.; Lin, Z.; He, J.; Zhou, M.; Yang, S.; Wang, Y.; Li, K. Recent progress in 3D printing degradable polylactic acid-based bone repair scaffold for the application of cancellous bone defect. Med. Comm. Biomater. Appl. 2022, 1, e14. [Google Scholar] [CrossRef]
- Subramaniyan, M.; Karuppan, S.; Helaili, S.; Ahmad, I. Structural, mechanical, and in-vitro characterization of hydroxyapatite loaded PLA composites. J. Mol. Struct. 2024, 1306, 137862. [Google Scholar] [CrossRef]
- Yerli, H.K.; Cava, K.; Aslan, M. Characterisation of 3d printed hydroxyapitate powder (HAp) filled polylactic acid (PLA) composites. Int. J. 3D Print. Technol. Digit. Ind. 2022, 6, 540–547. [Google Scholar] [CrossRef]
- Chapter, H.B. Material Processing of PLA-HAp-CS-Based Thermoplastic Composite Through Fused Deposition Modeling for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2025; ISBN 9783030139513. [Google Scholar]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Poleunis, C.; Debecker, D.P.; Giebeler, L.; Oswald, S.; Makshina, E.; Sels, B.F. Titania-Silica Catalysts for Lactide Production from Renewable Alkyl Lactates: Structure-Activity Relations. ACS Catal. 2018, 8, 8130–8139. [Google Scholar] [CrossRef]
- Ghadamyari, M.; Chaemchuen, S.; Zhou, K.; Dusselier, M.; Sels, B.F.; Mousavi, B.; Verpoort, F. One-step synthesis of stereo-pure L,L lactide from L-lactic acid. Catal. Commun. 2018, 114, 33–36. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, E.; Briassoulis, D. Degradation behaviour of poly(lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Crops Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2021, 31, 2006967. [Google Scholar] [CrossRef]
- Wróblewska, M.; Łasica, P.A.; Cylwik-rokicka, D. Zastosowanie żywic akrylowych w leczeniu bezzębia z wykorzystaniem technologii cyfrowej–przegląd piśmiennictwa Część 1–CAD / CAM Use of acrylic resins in digital technology in the treatment of edentia. Prosthodontics 2022, 72, 265–271. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Peyton, F.A. History of resins in dentistry. Dent. Clin. N. Am. 1975, 19, 211–222. [Google Scholar] [CrossRef]
- DiMaio, F.R. The science of bone cement: A historical review. Orthopedics 2002, 25, 1399. [Google Scholar] [CrossRef]
- Ezrin, M. Plastics Failure Guide: Cause and Prevention; SPE books; Hanser Publishers: Cincinnati, OH, USA, 1996; ISBN 9781569901847. [Google Scholar]
- Ibraheem, H.A.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Hashim, H.; Kariuki, B.M. Investigation of the Impact of Chemical Modifications on the Photostability of Polymethyl Methacrylate. Int. J. Polym. Sci. 2024, 2024, 3354280. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Harper, C.; Petrie, E. Plastics Materials and Processes: A Concise Encyclopedia; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 9780471456032. [Google Scholar]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook, 2nd ed.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-31235-4. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 212, 2299. [Google Scholar]
- Deb, S. Polymers in dentistry. Proc. Inst. Mech. Eng. Part H 1998, 212, 453–464. [Google Scholar] [CrossRef]
- Alla, R.K.; Swamy, K.N.; Vyas, R.; Konakanchi, A. Conventional and Contemporary polymers for the fabrication of denture prosthesis: Part I–Overview, composition and properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Marin, E.; Mukai, M.; Boschetto, F.; Sunthar, T.P.M.; Adachi, T.; Zhu, W.; Rondinella, A.; Lanzutti, A.; Kanamura, N.; Yamamoto, T.; et al. Production of antibacterial PMMA-based composites through stereolithography. Mater. Today Commun. 2022, 32, 103943. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Liu, X.; Chen, H.; Huang, Y.; Li, A.; Pu, Y.; Guo, L. Study on Mechanical Properties, Optical Properties, Cytotoxicity of TiO2-HAP Nanoparticles-Modified PMMA and Photodynamically Assisted Antibacterial Activity Against Candida Albicans in Vitro. Int. J. Nanomed. 2025, 20, 2695–2709. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. Tio2 and PEEK reinforced 3d printing pmma composite resin for dental denture base applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef]
- Tontowi, A.E.; Mada, U.G.; Kuswanto, D.; Sihaloho, R.I.; Mada, U.G.; Sosiati, H. Composite of [HA/PMMA] for 3D-printer material application. AIP Conf. Proc. 2016, 1755, 150020. [Google Scholar] [CrossRef]
- Esmi, A.; Jahani, Y.; Yousefi, A.A.; Zandi, M. PMMA-CNT-HAp nanocomposites optimized for 3D-printing applications. Mater. Res. Express 2019, 6, 85405. [Google Scholar] [CrossRef]
- Burcea, A.; Bănățeanu, A.-M.; Poalelungi, C.V.; Forna, N.; Cumpătă, C.N. Enhanced Properties and Multifaceted Applications of Polymethyl Methacrylate (Pmma) in Modern Medicine and Dentistry. Rom. J. Oral Rehabil. 2024, 16, 108–123. [Google Scholar] [CrossRef]
- Achilias, D.S. Chemical recycling of poly(methyl methacrylate) by pyrolysis. Potential use of the liquid fraction as a raw material for the reproduction of the polymer. Eur. Polym. J. 2007, 43, 2564–2575. [Google Scholar] [CrossRef]
- Sponchioni, M.; Altinok, S. Seven-Poly (methyl methacrylate): Market trends and recycling. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2022; Volume 60, pp. 269–287. [Google Scholar]
- Achilias, D.S.; Andriotis, L.; Koutsidis, I.A.; Louka, A.D.; Nianias, N.; Siafaka, P.; Tsagkalias, I.; Tsintzou, G. Recent Advances in the Chemical Recycling of Polymers (PP, PS, LDPE, HDPE, PVC, PC, Nylon, PMMA). Mater. Recycl. Trends Perspect. 2012, 1–6. [Google Scholar] [CrossRef]
- Khangkham, S. Catalytic Degradation of Poly(methyl methacrylate) by Zeolites and Regeneration of Used Zeolites via Ozonation. Ph.D. Thesis, Institut National Polytechnique de Toulouse—INPT, Toulouse, France, 2012; pp. 1–170. [Google Scholar]
- Esmizadeh, E.; Khalili, S.; Vahidifar, A.; Naderi, G.; Dubois, C. Waste Polymethyl Methacrylate (PMMA): Recycling and high-yield monomer recovery. Handb. Ecomater. 2019, 4, 2977–3009. [Google Scholar] [CrossRef]
- Wijeyatunga, S.K.; Derr, K.M.; Maladeniya, C.P.; Sauceda-Oloño, P.Y.; Tennyson, A.G.; Smith, R.C. Upcycling waste PMMA to durable composites via a transesterification-inverse vulcanization process. J. Polym. Sci. 2024, 62, 554–563. [Google Scholar] [CrossRef]
- Tilton, M.; Jacobs, E.; Overdorff, R.; Astudillo Potes, M.; Lu, L.; Manogharan, G. Biomechanical behavior of PMMA 3D printed biomimetic scaffolds: Effects of physiologically relevant environment. J. Mech. Behav. Biomed. Mater. 2023, 138, 105612. [Google Scholar] [CrossRef]
- Van der Vegt, A.K.; Govaert, L.E. Polymeren: Van Keten Tot Kunststof; Eindhoven University of Technology: Eindhoven, The Netherlands, 2003; ISBN 9789071301483. [Google Scholar]
- Shrivastava, S.P.; Dable, R.; Raj, A.N.; Mutneja, P.; Srivastava, S.B.; Haque, M.; Ali, R.W. Comparison of Mechanical Properties of PEEK and PMMA: An In Vitro Study. J. Contemp. Dent. Pract. 2021, 22, 179–183. [Google Scholar] [CrossRef]
- Bathala, L.; Majeti, V.; Rachuri, N.; Singh, N.; Gedela, S. The Role of Polyether Ether Ketone (Peek) in Dentistry–A Review. J. Med. Life 2019, 12, 5–9. [Google Scholar] [CrossRef]
- Nukala, S.G.; Kong, I.; Patel, V.I.; Kakarla, A.B.; Kong, W.; Buddrick, O. Development of Biodegradable Composites Using Polycaprolactone and Bamboo Powder. Polymers 2022, 14, 4169. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Slavković, V.; Hanželič, B.; Plesec, V.; Milenković, S.; Harih, G. Thermo-Mechanical Behavior and Strain Rate Sensitivity of 3D-Printed Polylactic Acid (PLA) below Glass Transition Temperature (Tg). Polymers 2024, 16, 1526. [Google Scholar] [CrossRef] [PubMed]
- Khammassi, S.; Tarfaoui, M.; Škrlová, K.; Měřínská, D.; Plachá, D.; Erchiqui, F. Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior. J. Compos. Sci. 2022, 6, 112. [Google Scholar] [CrossRef]
- Choudhury, S.; Joshi, A.; Agrawal, A.; Nain, A.; Bagde, A.; Patel, A.; Syed, Z.Q.; Asthana, S.; Chatterjee, K. NIR-Responsive Deployable and Self-Fitting 4D-Printed Bone Tissue Scaffold. ACS Appl. Mater. Interfaces 2024, 16, 49135–49147. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Joshi, A.; Dasgupta, D.; Ghosh, A.; Asthana, S.; Chatterjee, K. 4D printed biocompatible magnetic nanocomposites toward deployable constructs. Mater. Adv. 2024, 5, 3345–3356. [Google Scholar] [CrossRef]


| PROPERTY | VALUE | UNIT |
|---|---|---|
| Elastic modulus (GPa) | 2.6 | GPa |
| Tensile strength | 55 | MPa |
| Vickers Hardness | 20 | VHN |
| Elongation | 1–2 | % |
| Glass transition temperature | 125 | °C |
| Flexural strength | 90 | MPa |
| Property | PEEK | PCL | PLA | PMMA |
|---|---|---|---|---|
| Elastic modulus [GPa] | 2–3 | 0.21–0.44 | 3.5 | 2.6 |
| Tensile strength [MPa] | 70–100 | 4–785 | 59 | 55 |
| Vickers Hardness [VHN] | 24 | 7.2 | 25 | 20 |
| Elongation [%] | 50 | 20–1000 | 7 | 1–2 |
| Glass transition temperature [°C] | 143 | (−65)– (−60) | 57–60 | 125 |
| Flexural strength [MPa] | 140–170 | 12 | 106 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słota, D.; Niziołek, K.; Kosińska, E.; Sadlik, J.; Sobczak-Kupiec, A. Biocompatible Thermoplastics in Additive Manufacturing of Bone Defect Fillers: State-of-the-Art and Future Prospects. Materials 2025, 18, 3723. https://doi.org/10.3390/ma18163723
Słota D, Niziołek K, Kosińska E, Sadlik J, Sobczak-Kupiec A. Biocompatible Thermoplastics in Additive Manufacturing of Bone Defect Fillers: State-of-the-Art and Future Prospects. Materials. 2025; 18(16):3723. https://doi.org/10.3390/ma18163723
Chicago/Turabian StyleSłota, Dagmara, Karina Niziołek, Edyta Kosińska, Julia Sadlik, and Agnieszka Sobczak-Kupiec. 2025. "Biocompatible Thermoplastics in Additive Manufacturing of Bone Defect Fillers: State-of-the-Art and Future Prospects" Materials 18, no. 16: 3723. https://doi.org/10.3390/ma18163723
APA StyleSłota, D., Niziołek, K., Kosińska, E., Sadlik, J., & Sobczak-Kupiec, A. (2025). Biocompatible Thermoplastics in Additive Manufacturing of Bone Defect Fillers: State-of-the-Art and Future Prospects. Materials, 18(16), 3723. https://doi.org/10.3390/ma18163723









