In Vitro Bioactivity and Cytotoxicity Assessment of Two Root Canal Sealers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sealer Extracts
2.2. Cell Isolation and Culture Conditions
2.3. Cryopreservation and Thawing of Cells
2.4. MTT Assay
2.5. Live/Dead Cell Staining
2.6. Immunocytochemistry
2.7. Scratch Wound Healing Assay
2.8. RT-qPCR for Gene Expression
2.9. Statistical Analysis
3. Results
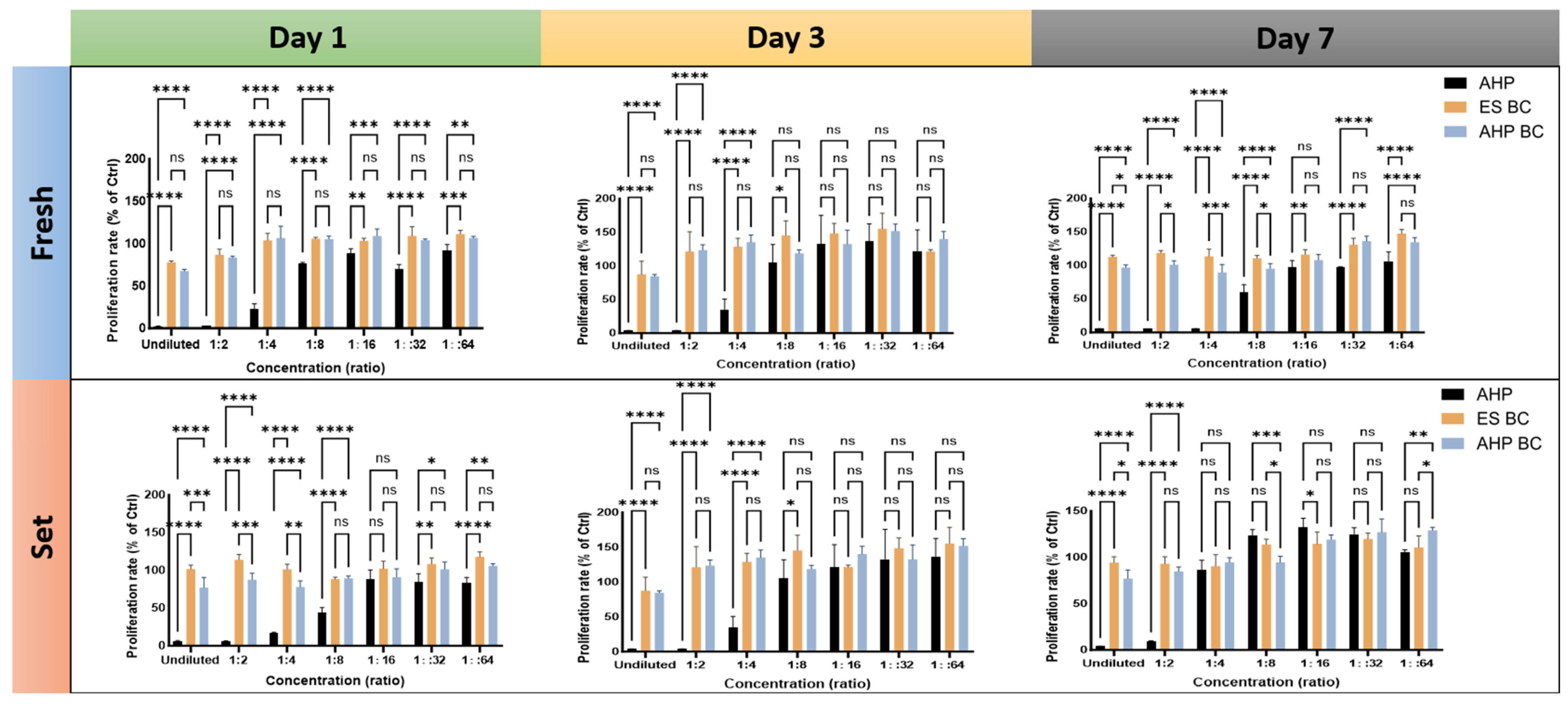
3.1. Cell Proliferation Rates
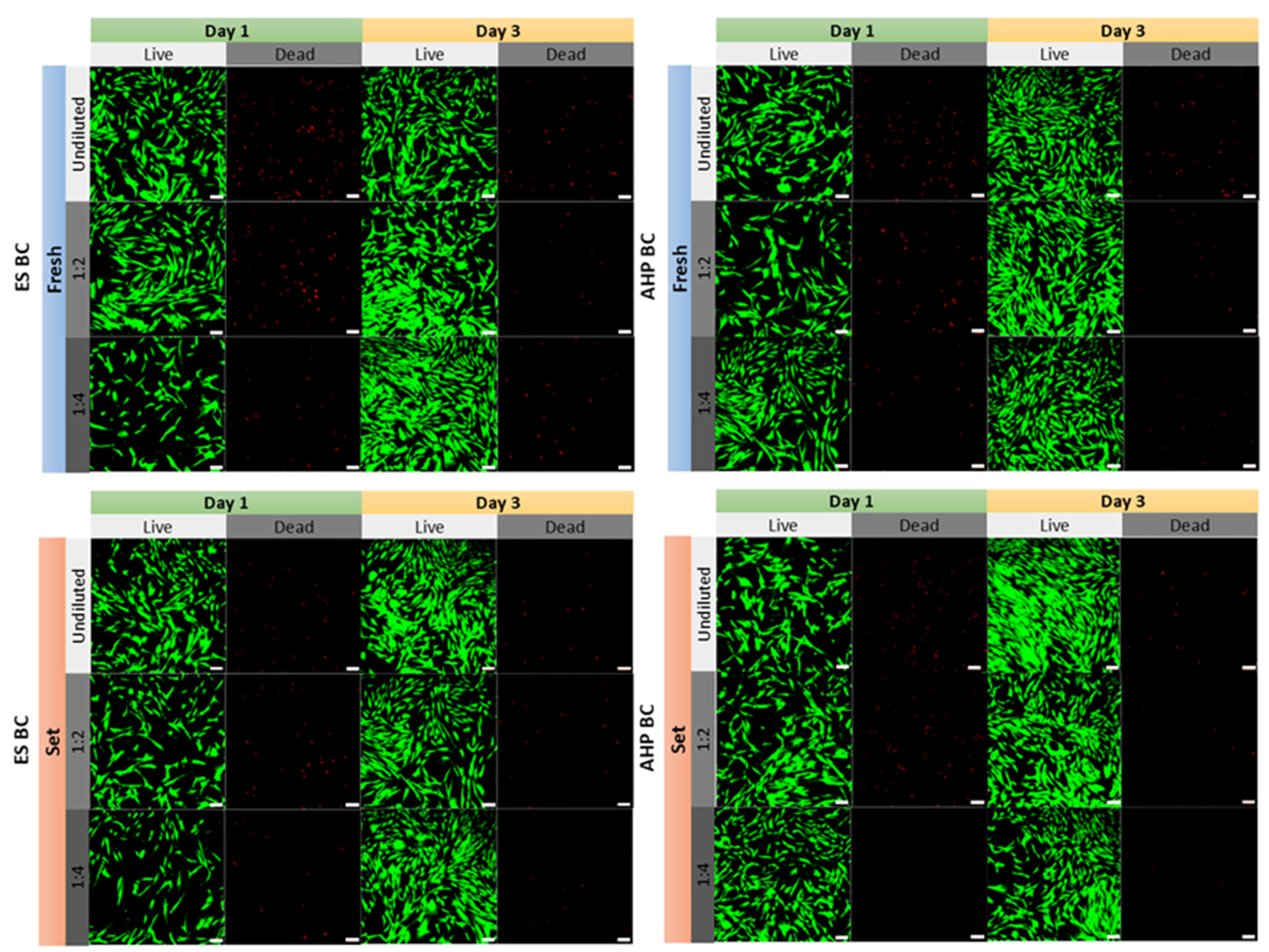
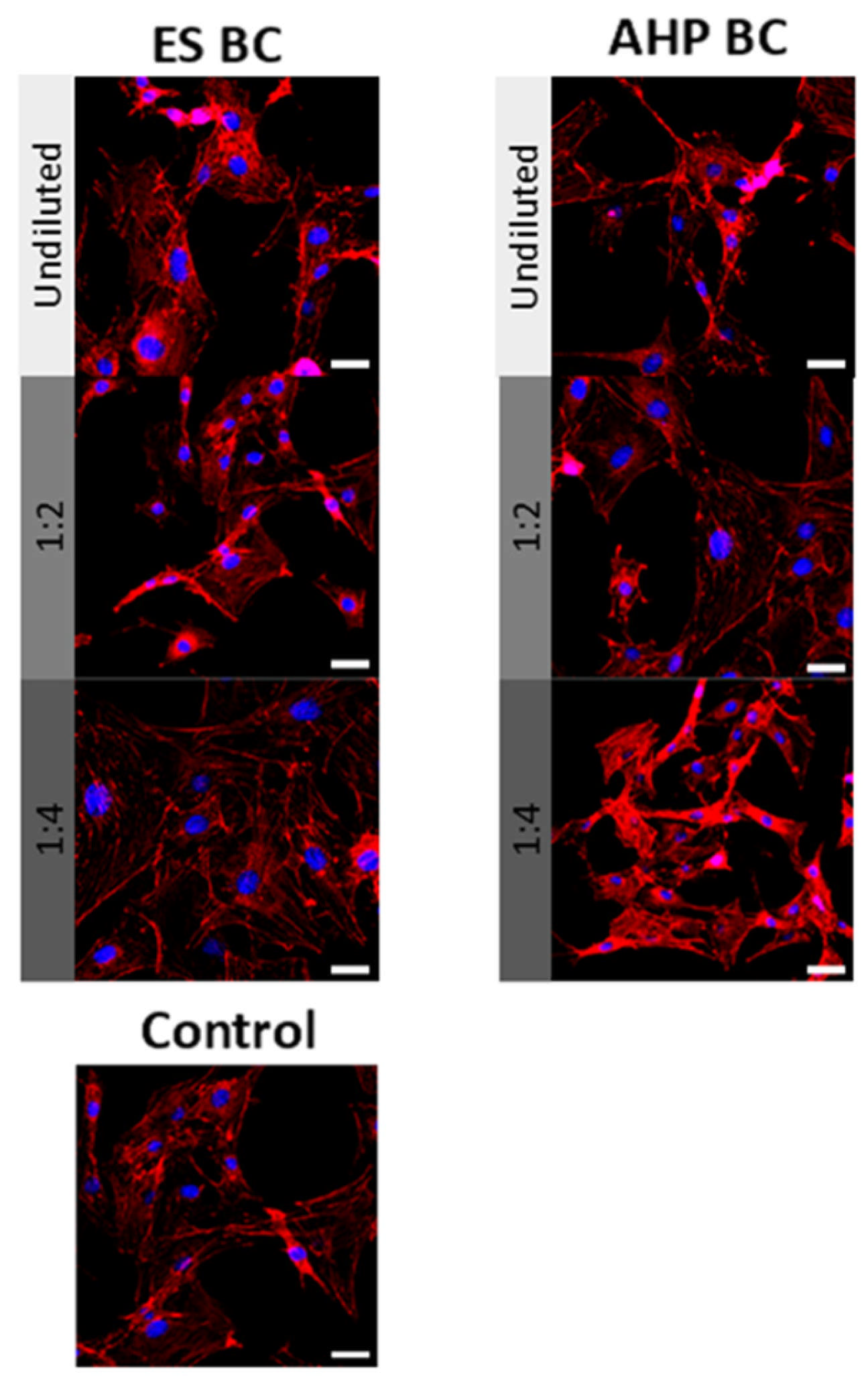
3.2. Cell Viability and Morphology
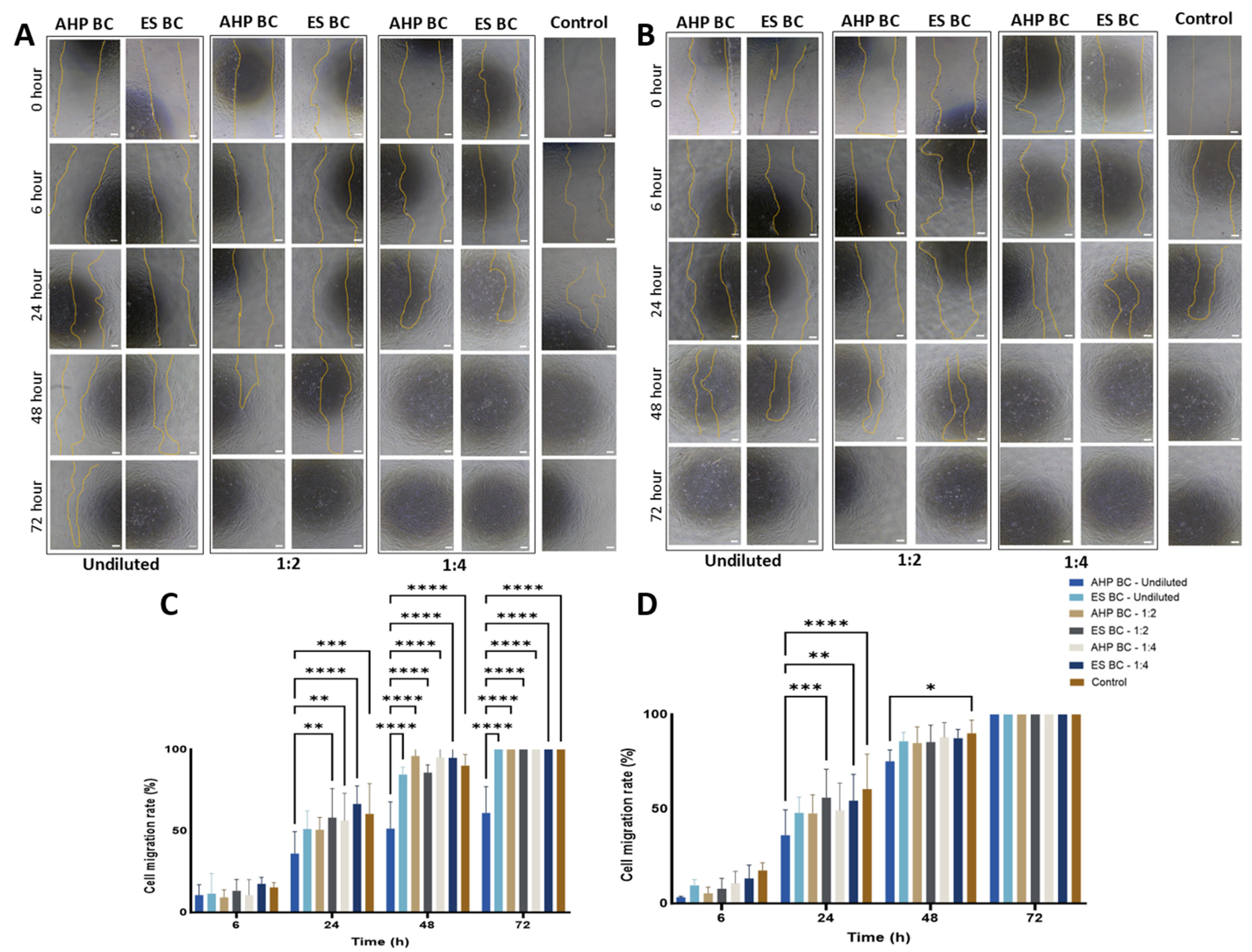
3.3. Wound Healing and Cell Migration
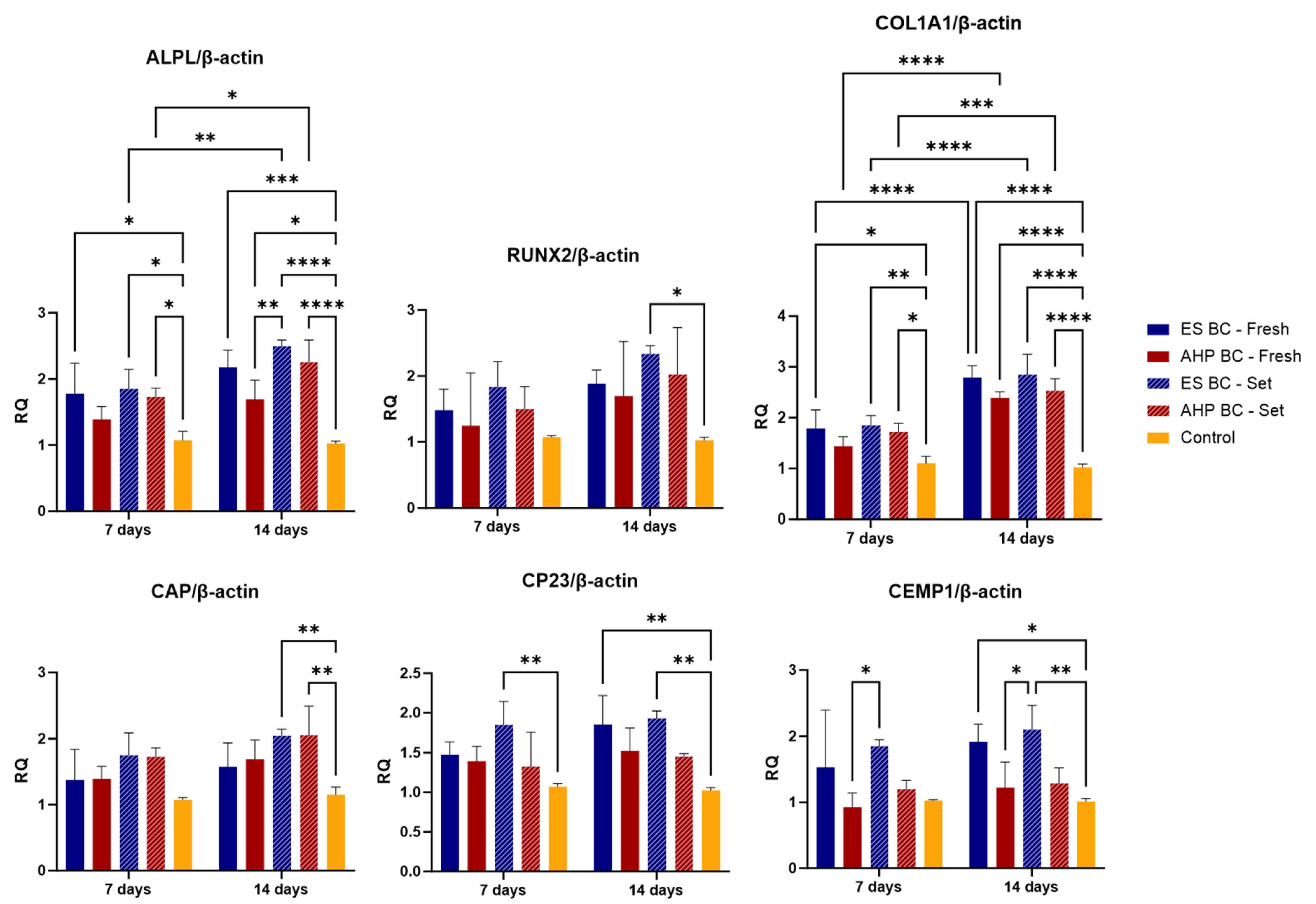
3.4. Gene Expression of Osteogenic and Cementogenic Markers
4. Discussion
4.1. Cytotoxicity and Biocompatibility
4.2. Wound Healing and Cell Migration
4.3. Osteogenic and Cementogenic Differentiation
4.4. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Siqueira Junior, J.F.; Rôças, I.d.N.; Marceliano-Alves, M.F.; Pérez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral Res. 2018, 32 (Suppl. S1), e65. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.A.; Aksel, H.; Zhuang, T.; Mashtare, T.; Babu, J.P.; Huang, G.T.-J. Efficacy of 4 Irrigation Protocols in Killing Bacteria Colonized in Dentinal Tubules Examined by a Novel Confocal Laser Scanning Microscope Analysis. J. Endod. 2016, 42, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.; Acevedo-Jake, A.M.; Griffith, A.; Kadincesme, N.; Dabek, K.; Hindi, D.; Kim, K.K.; Kobayashi, Y.; Shimizu, E.; Kumar, V. Cells and material-based strategies for regenerative endodontics. Bioact. Mater. 2022, 14, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef]
- Huang, F.M.; Tai, K.W.; Chou, M.Y.; Chang, Y.C. Cytotoxicity of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int. Endod. J. 2002, 35, 153–158. [Google Scholar] [CrossRef]
- Kaur, A.; Shah, N.; Logani, A.; Mishra, N. Biotoxicity of commonly used root canal sealers: A meta-analysis. J. Conserv. Dent. 2015, 18, 83–88. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel bioactive and therapeutic root canal sealers with antibacterial and remineralization properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Gaudin, A.; Peters, O.A. A critical analysis of research methods and experimental models to study biocompatibility of endodontic materials. Int. Endod. J. 2022, 55, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Guerrero-Gironés, J.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Melo, M. Biological interactions between calcium silicate-based endodontic biomaterials and periodontal ligament stem cells: A systematic review of in vitro studies. Int. Endod. J. 2021, 54, 2025–2043. [Google Scholar] [CrossRef]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material Without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef]
- Maru, V.; Dixit, U.; Patil, R.S.B.; Parekh, R. Cytotoxicity and bioactivity of mineral trioxide aggregate and bioactive endodontic type cements: A systematic review. Int. J. Clin. Pediatr. Dent. 2021, 14, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Grossman, L.I.; Oliet, S.; Del Rio, C.E. Endodontic Practice, 11th ed.; Lea & Febiger: Philadelphia, PA, USA, 1988. [Google Scholar]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef]
- Vouzara, T.; Dimosiari, G.; Koulaouzidou, E.A.; Economides, N. Cytotoxicity of a New Calcium Silicate Endodontic Sealer. J. Endod. 2018, 44, 849–852. [Google Scholar] [CrossRef]
- Civjan, S.; Brauer, G.M. Physical Properties of Cements, Based on Zinc Oxide, Hydrogenated Rosin, o-Ethoxybenzoic Acid, and Eugenol. J. Dent. Res. 1964, 43, 281–299. [Google Scholar] [CrossRef]
- Whitworth, J.M.; Boursin, E.M. Dissolution of root canal sealer cements in volatile solvents. Int. Endod. J. 2000, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Patni, P.M.; Chandak, M.; Jain, P.; Patni, M.J.; Jain, S.; Mishra, P.; Jain, V. Stereomicroscopic Evaluation of Sealing Ability of Four Different Root Canal Sealers- An invitro Study. J. Clin. Diagn. Res. 2016, 10, ZC37–ZC39. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Lombardini, M.; Colombo, M.; Dagna, A.; Saino, E.; Arciola, C.R.; Visai, L. Antibacterial effects of six endodontic sealers. Int. J. Artif. Organs 2011, 34, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Cintra, L.T.A.; Benetti, F.; de Azevedo Queiroz, Í.O.; Ferreira, L.L.; Massunari, L.; Bueno, C.R.E.; de Oliveira, S.H.P.; Gomes-Filho, J.E. Evaluation of the Cytotoxicity and Biocompatibility of New Resin Epoxy–based Endodontic Sealer Containing Calcium Hydroxide. J. Endod. 2017, 43, 2088–2092. [Google Scholar] [CrossRef]
- Poggio, C.; Arciola, C.R.; Dagna, A.; Colombo, M.; Bianchi, S.; Visai, L. Solubility of Root Canal Sealers: A Comparative Study. Int. J. Artif. Organ. 2010, 33, 676–681. [Google Scholar] [CrossRef]
- Neelakantan, P.M.D.S.; Grotra, D.B.D.S.; Sharma, S.B.D.S. Retreatability of 2 Mineral Trioxide Aggregate–based Root Canal Sealers: A Cone-beam Computed Tomography Analysis. J. Endod. 2013, 39, 893–896. [Google Scholar] [CrossRef]
- Teoh, Y.-Y.; Athanassiadis, B.; Walsh, L.J. Sealing Ability of Alkaline Endodontic Cements versus Resin Cements. Materials 2017, 10, 1228. [Google Scholar] [CrossRef]
- Leonardo, M.R.; Silva, L.A.; Utrilla, L.S.; Assed, S.; Ether, S.S. Calcium hydroxide root canal sealers—histopathologic evaluation of apical and periapical repair after endodontic treatment. J. Endod. 1997, 23, 428–432. [Google Scholar] [CrossRef]
- EN ISO 10993-5:2009/A11:2025; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Gaudin, A.; Tolar, M.; Peters, O.A. Cytokine production and cytotoxicity of calcium silicate–based sealers in 2-and 3-dimensional cell culture models. J. Endod. 2020, 46, 818–826. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.M.; Jung, I.H.; Kim, J.C.; Choi, S.H.; Cho, K.S.; Kim, C.S. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Saygili, G.; Saygili, S.; Tuglu, I.; Capar, I.D. In vitro cytotoxicity of guttaflow bioseal, guttaflow 2, AH-Plus and MTA fillapex. Iran. Endod. J. 2017, 12, 354–359. [Google Scholar] [PubMed]
- Rodríguez-Lozano, F.J.; Collado-González, M.; Tomás-Catalá, C.J.; García-Bernal, D.; López, S.; Oñate-Sánchez, R.E.; Moraleda, J.M.; Murcia, L. GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent. Mater. 2019, 35, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Sielker, S.; Hanisch, M.R.; Libricht, V.; Schäfer, E.; Dammaschke, T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS ONE 2018, 13, e0194467. [Google Scholar] [CrossRef]
- Mann, A.; Zeng, Y.; Kirkpatrick, T.; van der Hoeven, R.; Silva, R.; Letra, A.; de Souza, L.C. Evaluation of the Physicochemical and Biological Properties of EndoSequence BC Sealer HiFlow. J. Endod. 2022, 48, 123–131. [Google Scholar] [CrossRef]
- Zordan-Bronzel, C.L.; Tanomaru-Filho, M.; Rodrigues, E.M.; Chávez-Andrade, G.M.; Faria, G.; Guerreiro-Tanomaru, J.M. Cytocompatibility, bioactive potential and antimicrobial activity of an experimental calcium silicate-based endodontic sealer. Int. Endod. J. 2019, 52, 979–986. [Google Scholar] [CrossRef]
- Park, M.G.; Kim, I.R.; Kim, H.J.; Kwak, S.W.; Kim, H.C. Physicochemical properties and cytocompatibility of newly developed calcium silicate-based sealers. Aust. Endod. J. 2021, 47, 512–519. [Google Scholar] [CrossRef]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting Properties and Cytotoxicity Evaluation of a Premixed Bioceramic Root Canal Sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef]
- Chang, S.W.; Gaudin, A.; Tolar, M.; Oh, S.; Moon, S.-Y.; Peters, O.A. Physicochemical and biological properties of four calcium silicate-based endodontic cements. J. Dent. Sci. 2022, 17, 1586–1594. [Google Scholar] [CrossRef]
- Sanz, J.L.; López-García, S.; Rodríguez-Lozano, F.J.; Melo, M.; Lozano, A.; Llena, C.; Forner, L. Cytocompatibility and bioactive potential of AH Plus Bioceramic Sealer: An in vitro study. Int. Endod. J. 2022, 55, 1066–1080. [Google Scholar] [CrossRef]
- Baik, H.S.; Park, J.; Lee, K.J.; Chung, C. Local application of periodontal ligament stromal cells promotes soft tissue regeneration. Oral Dis. 2014, 20, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Kapila, Y.; Lotz, J.; Kapila, S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Giachelli, C.M.; Somerman, M.J. Immobilization of alkaline phosphatase on microporous nanofibrous fibrin scaffolds for bone tissue engineering. Biomaterials 2009, 30, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, S.; Bender, I.B.; Kaufman, I.J.; Moodnik, R. Alkaline phosphatase in reparative dentinogenesis. Oral Surg. Oral Med. Oral Pathol. 1962, 15, 859–866. [Google Scholar] [CrossRef]
- Camilleri, S.; McDonald, F. RUNX2 and dental development. Eur. J. Oral Sci. 2006, 114, 361–373. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol. 2000 2015, 67, 211–233. [Google Scholar] [CrossRef]
- Pitaru, S.; Narayanan, S.A.; Olson, S.; Savion, N.; Hekmati, H.; Alt, I.; Metzger, Z. Specific cementum attachment protein enhances selectively the attachment and migration of periodontal cells to root surfaces. J. Periodontal Res. 1995, 30, 360–368. [Google Scholar] [CrossRef]
- Alvarez-Pérez, M.A.; Narayanan, S.; Zeichner-David, M.; Rodríguez Carmona, B.; Arzate, H. Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 2006, 38, 409–419. [Google Scholar] [CrossRef]
- Watson, T.F.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef]
| Material | Manufacturer | Composition | Lot Number |
|---|---|---|---|
| EndoSequence BC Sealer (ES BC) | Brasseler USA, One Brasseler Blvd, Savannah, GA 31419, USA | Zirconium oxide, tricalcium silicate, dicalcium silicate, calcium hydroxide, thickening agents | 23050701 |
| AH Plus Bioceramic Sealer (AHP BC) | Dentsply Sirona, Charlotte, NC, USA | Zirconium dioxide, tricalcium silicate, dimethyl sulfoxide, lithium carbonate, thickening agent | 2309000531 |
| AH Plus Jet (AHP) | Dentsply Sirona, Bensheim, Germany | Bisphenol-A epoxy resin, bisphenol-F epoxy resin, calcium tungstate, zirconium oxide, aerosil, iron oxide pigments, amines, silicone oil | 2203000322 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size (bp) | Reference |
|---|---|---|---|---|
| RUNX2 | CATCTAATGACACCACCAGGC | GCCTACAAAGGTGGGGTTTGA | ~150 | Origene (Cat# HP225916) |
| ALPL | TCAGAAGCTCAACACCAACG | GTCAGGGACCTGGGCATT | ~120 | Qiagen (QuantiTect® Primer Assay, Qiagen, Hilden, Germany) |
| COL1A1 | TGACCTCAAGATGTGCCACT | ACCAGACATGCCTCTTGTCC | ~140 | Qiagen (QuantiTect Primer Assay) |
| CAP | CCTGGCTCACCTTCTACGAC | CCTCAAGCAAGGCAAATGTC | ~140 | OriGene Technologies Inc., Rockville, MD, USA (Product Code: HP234663) |
| CP23 | GGCGATGCTCAACCTCTAACC | GATACCCACCTCTGCCTTGA | ~130 | OriGene Technologies Inc. (Product Code: HP218292) |
| CEMP1 | CCATCCTATCTCTTTGGACCTGG | CCTTGCTTACAGGTGCTGTCCT | ~140 | OriGene (Cat# HP203762) |
| β-actin | ATTGCCGACAGGATGCAGA | GAGTACTTGCGCTCAGGAGGA | ~150 | Housekeeping gene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Hosseinpour, S.; Wen, J.; Peters, O.A. In Vitro Bioactivity and Cytotoxicity Assessment of Two Root Canal Sealers. Materials 2025, 18, 3717. https://doi.org/10.3390/ma18153717
Ye Y, Hosseinpour S, Wen J, Peters OA. In Vitro Bioactivity and Cytotoxicity Assessment of Two Root Canal Sealers. Materials. 2025; 18(15):3717. https://doi.org/10.3390/ma18153717
Chicago/Turabian StyleYe, Yicheng, Sepanta Hosseinpour, Juan Wen, and Ove A. Peters. 2025. "In Vitro Bioactivity and Cytotoxicity Assessment of Two Root Canal Sealers" Materials 18, no. 15: 3717. https://doi.org/10.3390/ma18153717
APA StyleYe, Y., Hosseinpour, S., Wen, J., & Peters, O. A. (2025). In Vitro Bioactivity and Cytotoxicity Assessment of Two Root Canal Sealers. Materials, 18(15), 3717. https://doi.org/10.3390/ma18153717








