The Biocorrosion of a Rare Earth Magnesium Alloy in Artificial Seawater Containing Chlorella vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Algal Culture
2.3. Electrochemical Measurements
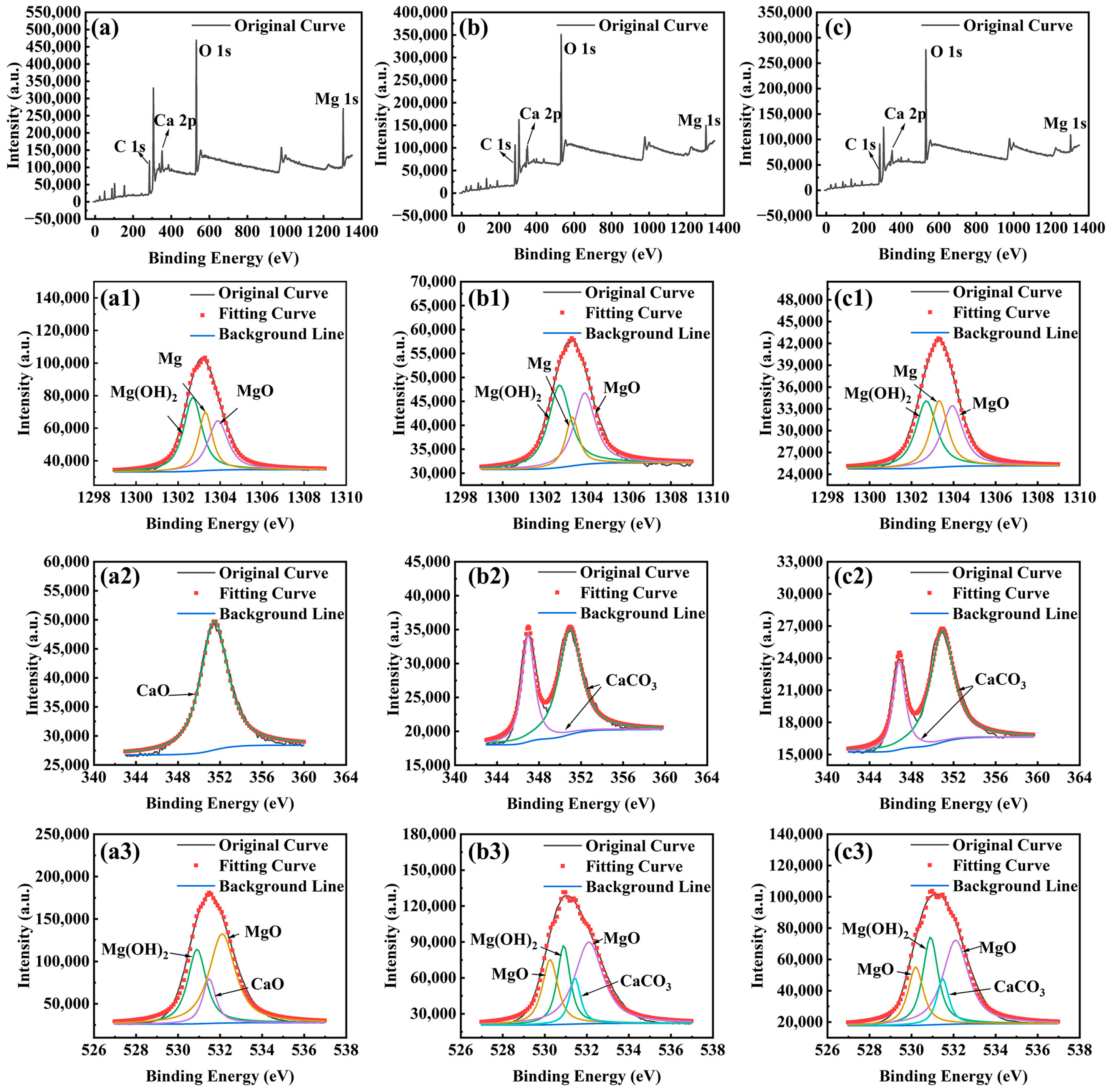
2.4. Surface Characterization
3. Results and Discussion
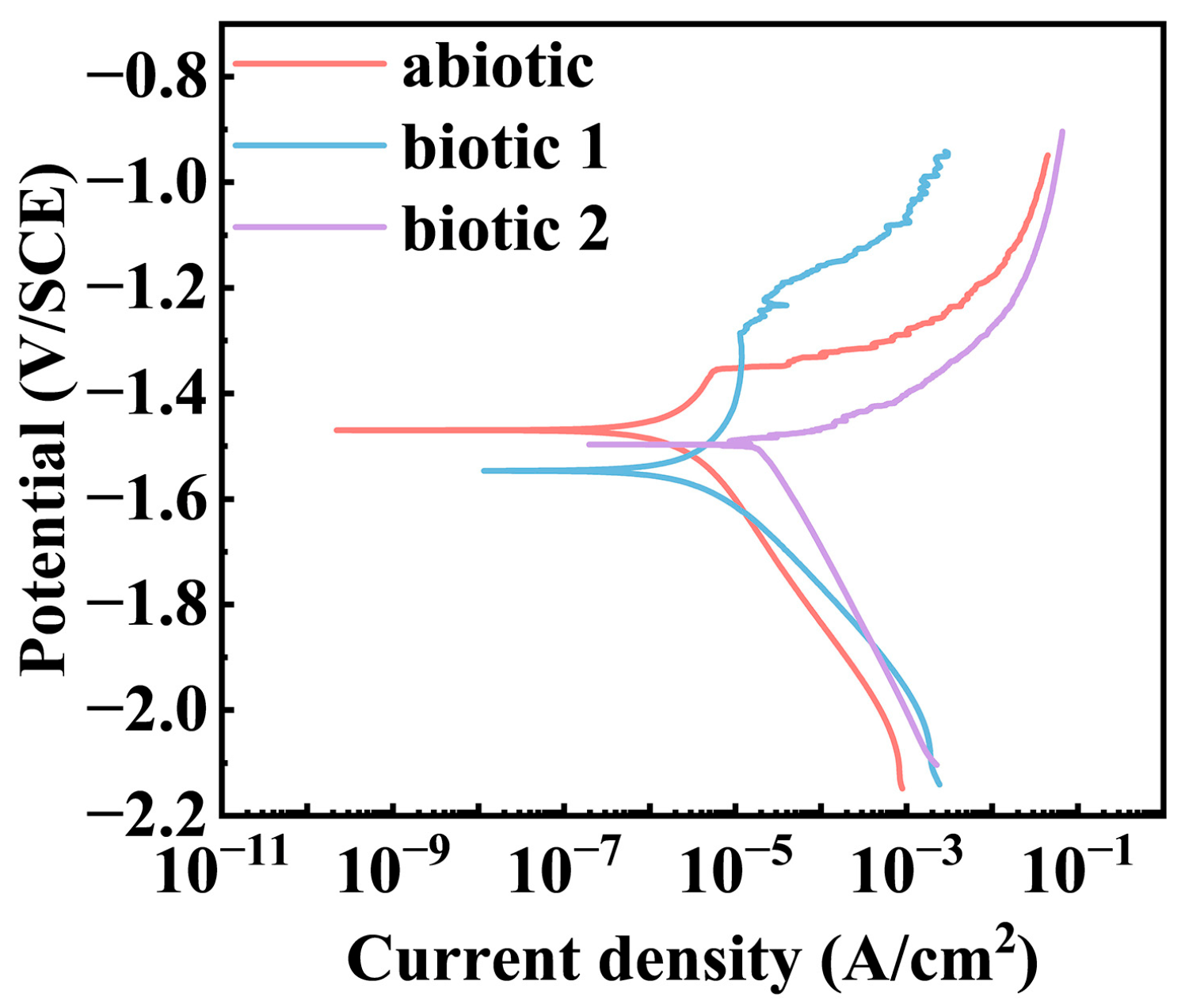
3.1. Electrochemical Results
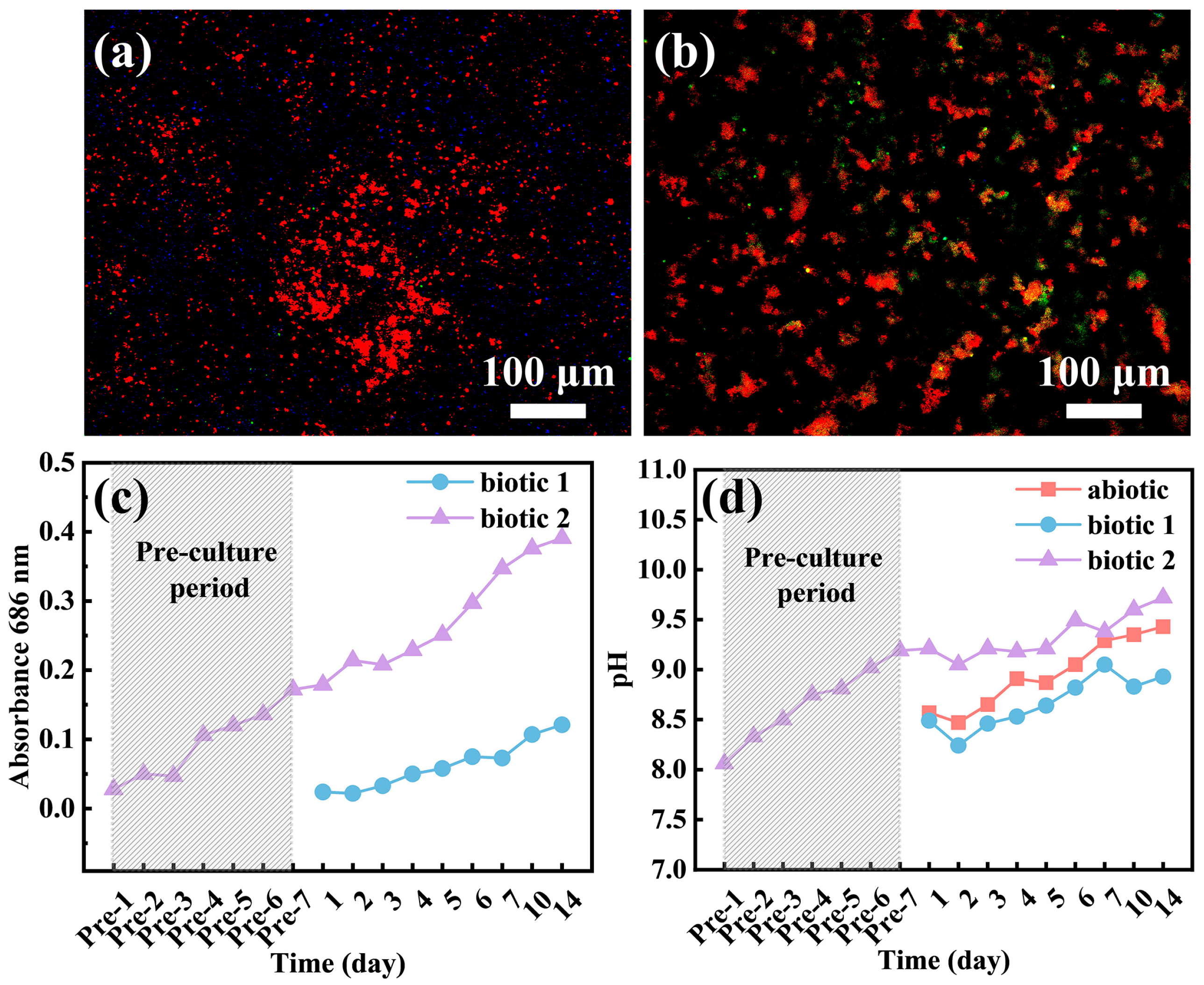
3.2. Biofilm and Corrosion Product Film
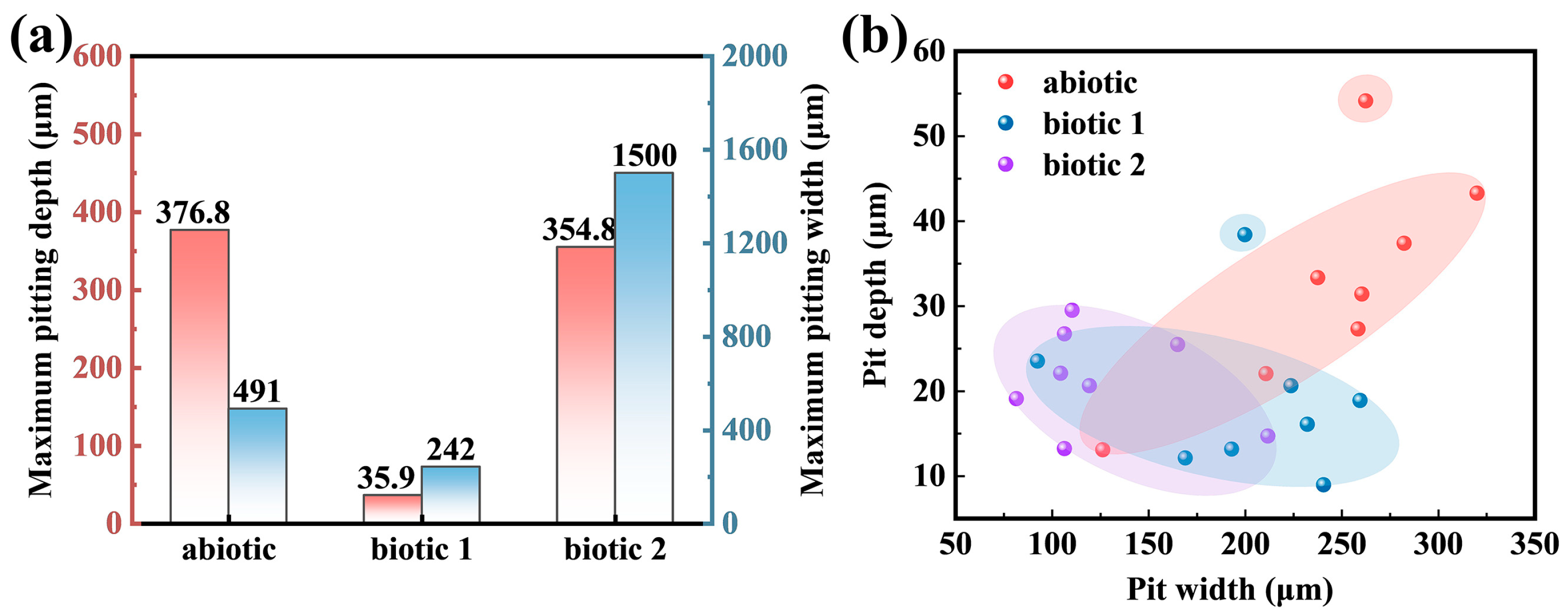
3.3. Corrosion Damage
3.4. Adhesion of C. vulgaris
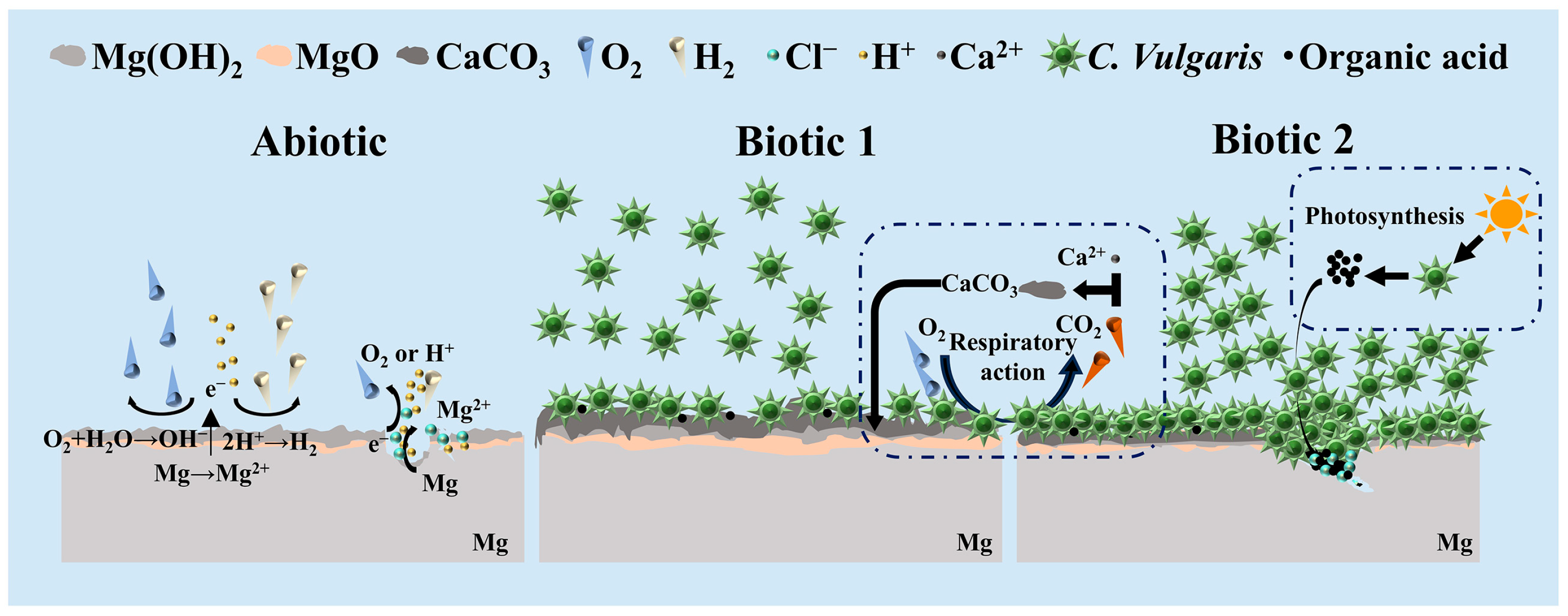
3.5. Corrosion Mechanism of C. vulgaris on Mg-3Nd-2Gd-Zn-Zr Alloy
4. Conclusions
- (1)
- Although Mg alloys exhibit unique antibacterial properties in the medical field, C. vulgaris can adhere to the surface of the Mg alloy to form a biofilm, significantly influencing its corrosion process.
- (2)
- Severe corrosion penetration can occur on the Mg alloy in the abiotic solution. Differently, in the biotic solution containing the low concentration of C. vulgaris cells, biomineralization occurs, leading to CaCO3 deposition on the Mg alloy surface, which to a certain degree inhibits the localized corrosion.
- (3)
- In the pre-cultured biotic solution, the high concentration of C. vulgaris cells on the alloy surface may generate substantial organic acids through photosynthesis, significantly accelerating the local rupture of surface film at some sites. However, in the other areas, the surface film is intact, exhibiting good corrosion resistance compared to the films formed in the abiotic and biotic solutions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, Y.; Xu, H.; Pan, J.; Zhang, R.; Zhang, P.; Zhang, C.; Jin, Y.; Zhang, Z. Microstructure and corrosion performance of AlCoCrFeNi2.1 high-entropy alloy coating on AZ31B magnesium alloy via friction stir processing. Appl. Surf. Sci. 2025, 706, 163566. [Google Scholar] [CrossRef]
- Huang, R.; Dong, Z.; Zhou, Y.; Ye, T.; Liu, Z.; Tang, D. Reinforced adhesion by in-situ growth converse chemical layers between substrates and electrosprayed organic coatings to improve anti-corrosion of magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2025, 723, 137419. [Google Scholar] [CrossRef]
- Wang, T.; Li, P.; Guo, Y.; Xu, Y.; Kou, W.; Li, G.; Lian, J. Enhanced corrosion resistance of calcium carbonate coatings on magnesium alloy via simple stearic acid treatment. J. Magnes. Alloys 2025, 13, 1602–1616. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Tang, Y.; Li, W.; Zhang, S.; Wang, H.; Ran, X. Effect of USRP on corrosion properties of Mg–Y-Nd-Gd-Zr magnesium alloy. Mater. Chem. Phys. 2025, 344, 131042. [Google Scholar] [CrossRef]
- Huang, Z.; Yong, Q.; Xie, Z.-H. Stearic acid modified porous nickel-based coating on magnesium alloy AZ31 for high superhydrophobicity and corrosion resistance. Corros. Commun. 2023, 10, 38–47. [Google Scholar] [CrossRef]
- Song, G.-L.; Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Wang, M.; Sun, C.; Wang, X.; Li, J.; Li, J. Corrosion behavior of AZ91 magnesium alloys in harsh marine atmospheric environment in South China Sea. J. Mater. Res. Technol. 2025, 35, 2477–2486. [Google Scholar] [CrossRef]
- Chenghui, Y.; Xin, Z.; Shuliu, W.; Junhang, C.; Hao, Z.; Qiang, Y.; Kui, X. Corrosion behavior and mechanism of Cr and Cu alloy weathering steel in simulated marine atmospheric environment. J. Mater. Res. Technol. 2025, 35, 3098–3106. [Google Scholar] [CrossRef]
- Dong, Y.; Song, G.-L.; Xu, Y.; Zheng, D. Bio-inhibitive effect of an algal symbiotic bacterium on corrosion of magnesium in marine environment. J. Magnes. Alloys 2023, 11, 4603–4618. [Google Scholar] [CrossRef]
- Xiang, L.; Li, F.; Wu, X.; Zhang, F.; Tao, J.; Wang, M.; Lei, W.; Ran, X.; Wang, H. Variation of Corrosion Characteristics and Tensile Performances of WE43 Alloy Under Marine Atmospheric Environment. Materials 2024, 17, 5353. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.; Wang, Y.; Wang, X.; Gao, H. Dynamic Marine Atmospheric Corrosion Behavior of AZ91 Mg Alloy Sailing from Yellow Sea to Western Pacific Ocean. Materials 2024, 17, 2294. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Ma, A.; Jiang, J.; Liu, H.; Cheng, Z.; Gu, Y. Tailoring the corrosion behavior and mechanism of AZ31 magnesium alloys by different Ca contents for marine application. Corros. Sci. 2021, 192, 109842. [Google Scholar] [CrossRef]
- Song, G.L.; Yang, M.Y. Strategy of galvanic corrosion mitigation for Mg alloys. Front. Mater. 2025, 12, 1582380. [Google Scholar] [CrossRef]
- Yang, M.; Song, G.-L. A hot-dip self-passive zinc alloy coating to inhibit the galvanic corrosion of a Mg alloy. Corros. Sci. 2025, 255, 113111. [Google Scholar] [CrossRef]
- Jaume, J.; Mercier, D.; Seyeux, A.; Semetey, V.; Zanna, S.; Marcus, P. Dual role of marine bacteria Pseudoalteromonas NCIMB 2021 in corrosion of mild steel in artificial seawater. Corros. Sci. 2025, 255, 113076. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, J.; Hou, Q.; Zhang, C.; Wang, K.; Duan, J.; Chen, X.; Hou, B. Study on the Effect of Different Cathodic Protection Potentials on the Growth of Mixed Bacteria and Cathodic Protection Efficiency. Chemistry 2025, 7, 54. [Google Scholar] [CrossRef]
- Li, G.; Du, M. Influence of bio-corrosion film formed on X80 steel in sulfate-reducing bacteria-containing seawater on hydrogen-induced stress corrosion cracking. Int. J. Hydrogen Energy 2025, 113, 12–25. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, R.; Wang, C.; Li, R.; Huang, J.; Dong, J.; Xu, D. Influence of Shewanella algae and calcium-magnesium deposit layer on the corrosion mechanism of X80 carbon steel in marine environment. Mater. Des. 2025, 254, 114017. [Google Scholar] [CrossRef]
- Pu, J.; Huang, Y.; Cai, F.; Xin, Y.; Wang, Y.; Hou, X.; Lu, D.; Wang, X. Effect of algae adhesion on the corrosion and hydrogen entry of AISI 4135 high-strength steel in the marine environment. J. Mater. Res. Technol. 2025, 35, 3763–3773. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, Y.; Li, Y.; Lu, B.; Huang, H.; Li, X.; Duan, J. Hydrocarbon-degrading Shewanella algae shows oxidative deterioration corrosion at the aluminium alloy & coating interface. Corros. Sci. 2024, 234, 112072. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Wang, X.; Xu, Y.; Cai, F. Effects of oyster as macrofouling organism on corrosion mechanisms of a high-strength low-alloy steel. Corros. Sci. 2022, 207, 110580. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Wan, H.; Wang, G.; Wu, H.; Chen, X.; Liu, H. Localized corrosion and brittle fracture of X80 carbon steel under tensile stress induced by sulfate reducing bacteria. Electrochim. Acta 2024, 497, 144598. [Google Scholar] [CrossRef]
- Sun, M.Q.; Yang, J.; Wang, Z.B.; Zheng, Y.G. Effect of coexistence of sulfate reducing bacteria and nitrate reducing bacteria on the under-deposit corrosion of carbon steel. Corros. Sci. 2024, 231, 111958. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Song, Y.; Liu, W.; Zhang, J.; Li, N.; Dong, K.; Cai, Y.; Han, E.-H. The respective roles of sulfate-reducing bacteria (SRB) and iron-oxidizing bacteria (IOB) in the mixed microbial corrosion process of carbon steel pipelines. Corros. Sci. 2024, 240, 112479. [Google Scholar] [CrossRef]
- Liu, X.-M.; Wang, Z.-X.; Ai, L.-Z.; Fan, Y.-Q.; Wang, G.-Q.; Gu, T.-Y.; Wang, F.-H.; Xu, D.-K.; Li, Z. Microbial corrosion of 316L stainless steel stents by gut Lactobacillus plantarum via extracellular electron transfer. J. Mater. Sci. Technol. 2026, 243, 283–293. [Google Scholar] [CrossRef]
- Zhou, E.; Li, F.; Zhang, D.; Xu, D.; Li, Z.; Jia, R.; Jin, Y.; Song, H.; Li, H.; Wang, Q.; et al. Direct microbial electron uptake as a mechanism for stainless steel corrosion in aerobic environments. Water Res. 2022, 219, 118553. [Google Scholar] [CrossRef]
- Song, D.; Zou, J.; Sun, L.; Zhang, Y.; Zhang, J.; Liang, X.; Zhang, S.; Li, Y.; Li, H.; Xi, B.; et al. Enhanced the SRB corrosion resistance of 316L stainless steel via adjusting the addition of Cu and Ce elements. Vacuum 2024, 224, 113183. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Du, M.; Sun, M.; Ma, L. Study on mechanism underlying the acceleration of pitting corrosion of B30 copper–nickel alloy by sulfate-reducing bacteria in seawater. Sci. Total Environ. 2024, 928, 172645. [Google Scholar] [CrossRef]
- Qian, H.; Wang, T.; Xu, P.; Feng, Z.; Lei, B.; Zhang, P.; Guo, H.; Meng, G. Sulfate reducing bacteria corrosion of a 90/10 Cu-Ni alloy coupled to an Al sacrificial anode. Bioelectrochemistry 2025, 163, 108892. [Google Scholar] [CrossRef]
- Pu, Y.; Chen, S.; Hou, Y.; Hou, S.; Feng, F.; Guo, Z.; Zhu, C.; Cheng, Y.F. Unlocking the effect of interfacial microstructure and Desulfovibrio vulgaris on corrosion characteristics in copper-nickel alloy welded joint. Corros. Sci. 2024, 230, 111947. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, J.; Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Sun, C. Mechanistic diversity between nitrate and nitrite on biocorrosion of X80 pipeline steel caused by Desulfovibrio desulfurican and Pseudomonas stutzeri. Corros. Sci. 2022, 207, 110573. [Google Scholar] [CrossRef]
- Wu, T.; Yan, M.; Yu, L.; Zhao, H.; Sun, C.; Yin, F.; Ke, W. Stress corrosion of pipeline steel under disbonded coating in a SRB-containing environment. Corros. Sci. 2019, 157, 518–530. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, B.; Xu, J.; Yu, C.; Sun, C. The role of hydrogen gas in SRB-induced degradation of X80 pipeline steel in hydrogen-blending environments. Electrochim. Acta 2025, 509, 145301. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Jabbar, K.A.; Rasool, K.; Pandey, R.P.; Sliem, M.H.; Helal, M.; Samara, A.; Abdullah, A.M.; Mahmoud, K.A. Controlling the biocorrosion of sulfate-reducing bacteria (SRB) on carbon steel using ZnO/chitosan nanocomposite as an eco-friendly biocide. Corros. Sci. 2019, 148, 397–406. [Google Scholar] [CrossRef]
- He, L.-J.; Qiu, Z.-H.; Li, S.-Q.; Yan, H.; Lin, C.-G.; Zeng, R.-C. An S2--responsive coating on 70Cu-30Ni alloy for suppressing microbiologically influenced corrosion induced by sulfate-reducing bacteria. Appl. Surf. Sci. 2025, 684, 161914. [Google Scholar] [CrossRef]
- Li, C.; Wei, B.; Cai, Z.; Xu, J.; Yu, C.; Sun, C.; Xu, D.; Wang, F. Synthesis and synergistic effects of antibacterial and corrosion inhibition based on concentrated hydrosols of silver nanoparticles. Corros. Sci. 2024, 233, 112102. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Illiyanasafa, N.; Jaya, D.E.C.; Darmokoesoemo, H.; Putra, N.R. Utilization of the microalga Chlorella vulgaris for mercury bioremediation from wastewater and biomass production. Sustain. Chem. Pharm. 2024, 37, 101346. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Yang, H. The Role of Antibacterial Metallic Elements in Simultaneously Improving the Corrosion Resistance and Antibacterial Activity of Magnesium Alloys. Mater. Des. 2021, 198, 109350. [Google Scholar] [CrossRef]
- Dobroň, P.; Drozdenko, D.; Loose, P.; Trinh, H.C. From microtubes to cardiovascular stents: A complex characterization of microstructure and mechanical performance of Mg-10Dy-1Nd-1Zn-0.2Zr alloy. Mater. Sci. Eng. A 2025, 933, 148274. [Google Scholar] [CrossRef]
- Feng, H.; Wang, G.; Jin, W.; Zhang, X.; Huang, Y.; Gao, A.; Wu, H.; Wu, G.; Chu, P.K. Systematic Study of Inherent Antibacterial Properties of Magnesium-based Biomaterials. ACS Appl. Mater. Interfaces 2016, 8, 9662–9673. [Google Scholar] [CrossRef]
- Fu, Q.; Song, G.-L.; Yao, X. Biofouling and corrosion of magnesium alloys WE43 and AM60 by Chlorella vulgaris in artificial seawater. Corros. Sci. 2025, 250, 112884. [Google Scholar] [CrossRef]
- Wei, B.; Pang, J.; Xu, J.; Sun, C.; Zhang, H.; Wang, Z.; Yu, C.; Ke, W. Microbiologically influenced corrosion of TiZrNb medium-entropy alloys by Desulfovibrio desulfuricans. J. Alloy. Compd. 2021, 875, 160020. [Google Scholar] [CrossRef]
- Song, G.-L.; Liu, M. The effect of Mg alloy substrate on “electroless” E-coating performance. Corros. Sci. 2011, 53, 3500–3508. [Google Scholar] [CrossRef]

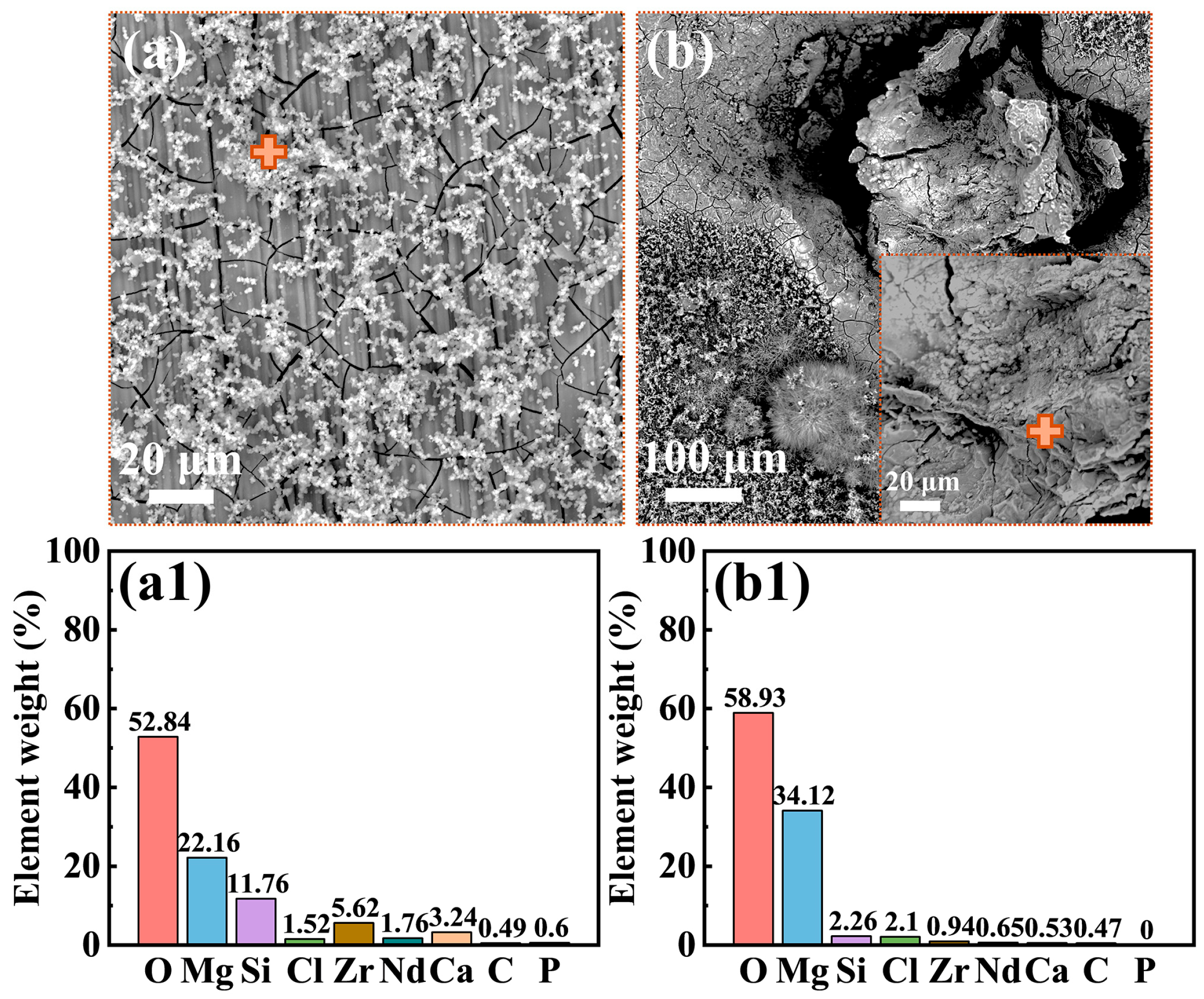
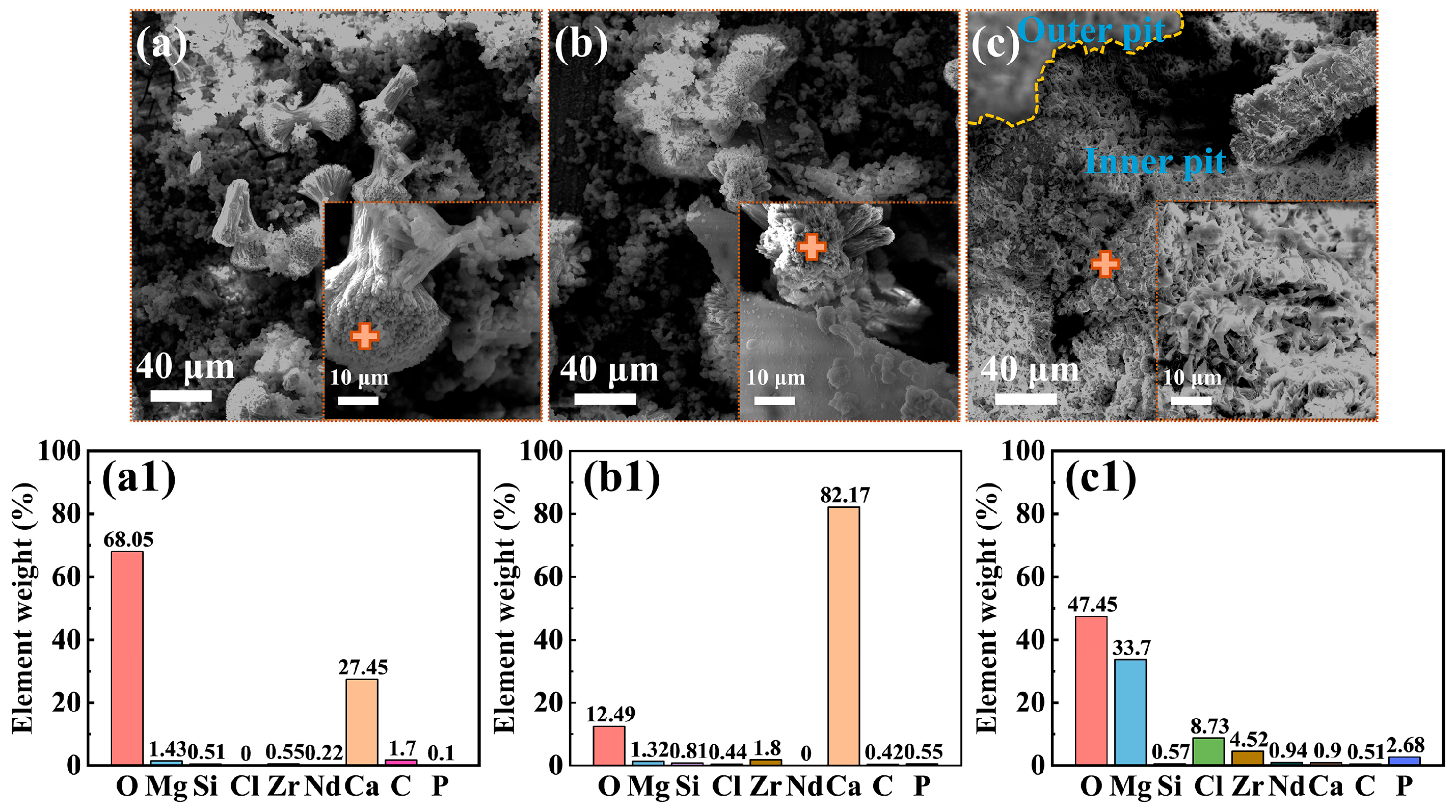
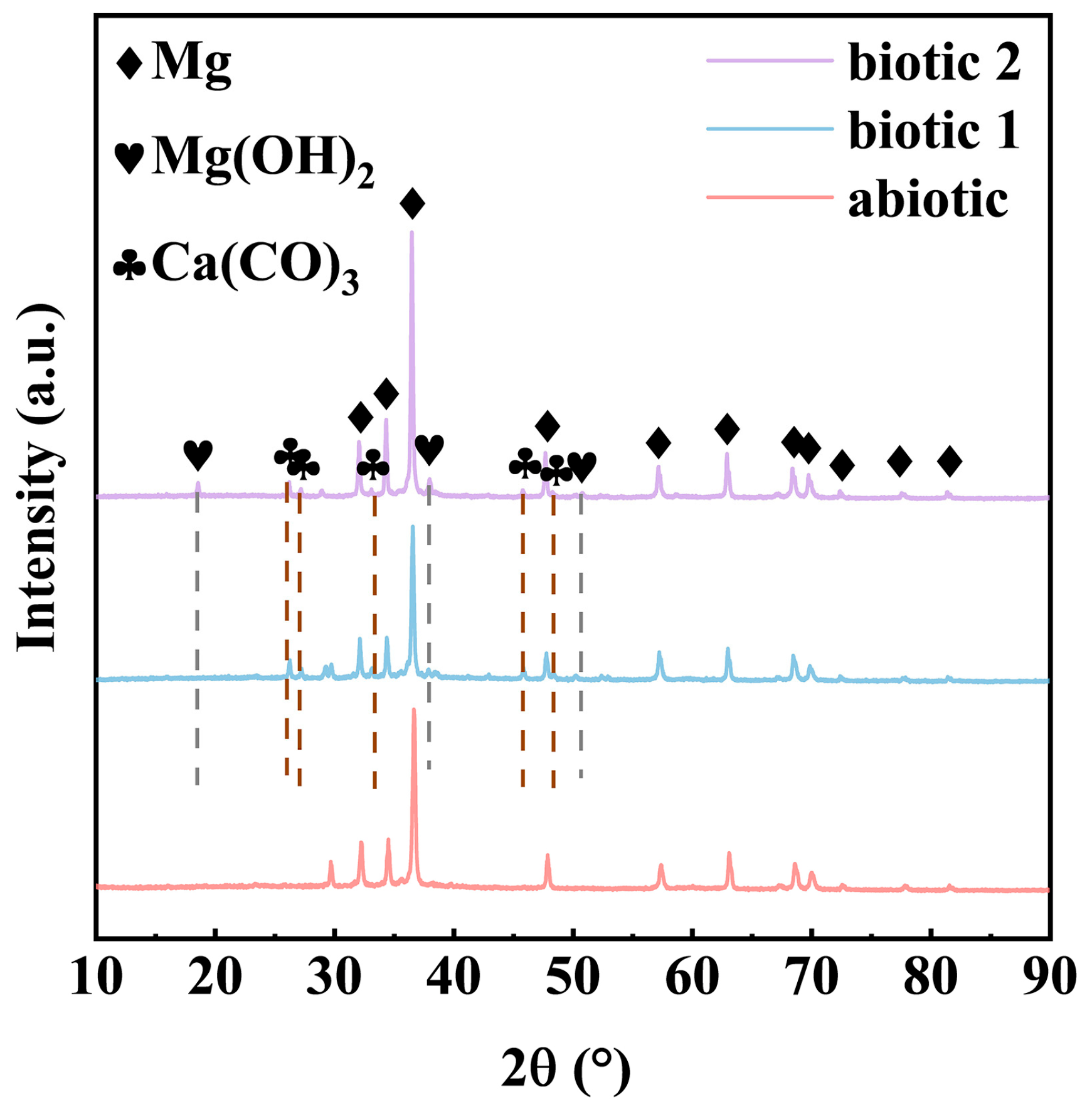

| Element | Nd | Gd | Zr | Y | Zn | Si | P |
|---|---|---|---|---|---|---|---|
| wt.% | 3.782 | 2.131 | 0.501 | 0.197 | 0.154 | 0.026 | 0.019 |
| Element | Sn | Al | Fe | Mn | S | Mg | Others |
| wt.% | 0.009 | 0.009 | 0.005 | 0.005 | 0.005 | balance | <0.001 |
| Media | Time (Day) | Rs (Ω·cm2) | Qf × 10−5 (S·cm−2·sn) | n1 | Rf (Ω·cm2) | Qdl × 10−5 (S·cm−2·sn) | n2 | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| abiotic | 1 | 11.05 | 1.81 | 0.90 | 1881 | 94.61 | 0.82 | 1240 |
| 3 | 19.29 | 2.52 | 0.86 | 3742 | 58.15 | 1.00 | 1852 | |
| 5 | 15.20 | 0.46 | 0.77 | 20.87 | 1.73 | 0.87 | 2552 | |
| 7 | 21.77 | 0.34 | 0.81 | 28.84 | 1.26 | 0.91 | 2205 | |
| 10 | 27.72 | 0.28 | 0.81 | 41.42 | 1.08 | 0.93 | 2952 | |
| 14 | 28.29 | 0.33 | 0.76 | 56.56 | 1.14 | 0.90 | 2575 | |
| biotic 1 | 1 | 10.53 | 1.71 | 0.91 | 2595 | 93.84 | 0.87 | 1288 |
| 3 | 15.18 | 2.48 | 0.87 | 2354 | 122.40 | 1.00 | 852.8 | |
| 5 | 20.43 | 2.86 | 0.84 | 4159 | 108.40 | 1.00 | 1646 | |
| 7 | 24.84 | 3.00 | 0.81 | 6620 | 107.40 | 1.00 | 2037 | |
| 10 | 20.72 | 2.57 | 0.84 | 5034 | 136.40 | 1.00 | 1841 | |
| 14 | 18.78 | 0.30 | 0.90 | 15.46 | 1.17 | 0.92 | 2799 | |
| biotic 2 | 1 | 10.13 | 1.89 | 0.87 | 4602 | 42.50 | 1.00 | 2197 |
| 3 | 8.87 | 1.16 | 0.79 | 16.29 | 0.79 | 0.93 | 4247 | |
| 5 | 12.25 | 0.44 | 0.89 | 21.62 | 1.04 | 0.92 | 3355 | |
| 7 | 10.86 | 0.42 | 0.86 | 20.75 | 1.17 | 0.93 | 3154 | |
| 10 | 11.48 | 0.44 | 0.85 | 19.70 | 1.38 | 0.92 | 1919 | |
| 14 | 12.22 | 0.50 | 0.83 | 21.28 | 1.49 | 0.93 | 616 |
| Media | Ecorr (V vs. SCE) | icorr (μA/cm2) |
|---|---|---|
| abiotic | −1.45 | 2.29 |
| biotic 1 | −1.58 | 7.14 |
| biotic 2 | −1.52 | 26.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Fu, Q.; Song, G.-L.; Wang, K. The Biocorrosion of a Rare Earth Magnesium Alloy in Artificial Seawater Containing Chlorella vulgaris. Materials 2025, 18, 3698. https://doi.org/10.3390/ma18153698
Yao X, Fu Q, Song G-L, Wang K. The Biocorrosion of a Rare Earth Magnesium Alloy in Artificial Seawater Containing Chlorella vulgaris. Materials. 2025; 18(15):3698. https://doi.org/10.3390/ma18153698
Chicago/Turabian StyleYao, Xinran, Qi Fu, Guang-Ling Song, and Kai Wang. 2025. "The Biocorrosion of a Rare Earth Magnesium Alloy in Artificial Seawater Containing Chlorella vulgaris" Materials 18, no. 15: 3698. https://doi.org/10.3390/ma18153698
APA StyleYao, X., Fu, Q., Song, G.-L., & Wang, K. (2025). The Biocorrosion of a Rare Earth Magnesium Alloy in Artificial Seawater Containing Chlorella vulgaris. Materials, 18(15), 3698. https://doi.org/10.3390/ma18153698






