Structural Evolution, Mechanical Properties, and Thermal Stability of Multi-Principal TiZrHf(Ta, Y, Cr) Alloy Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
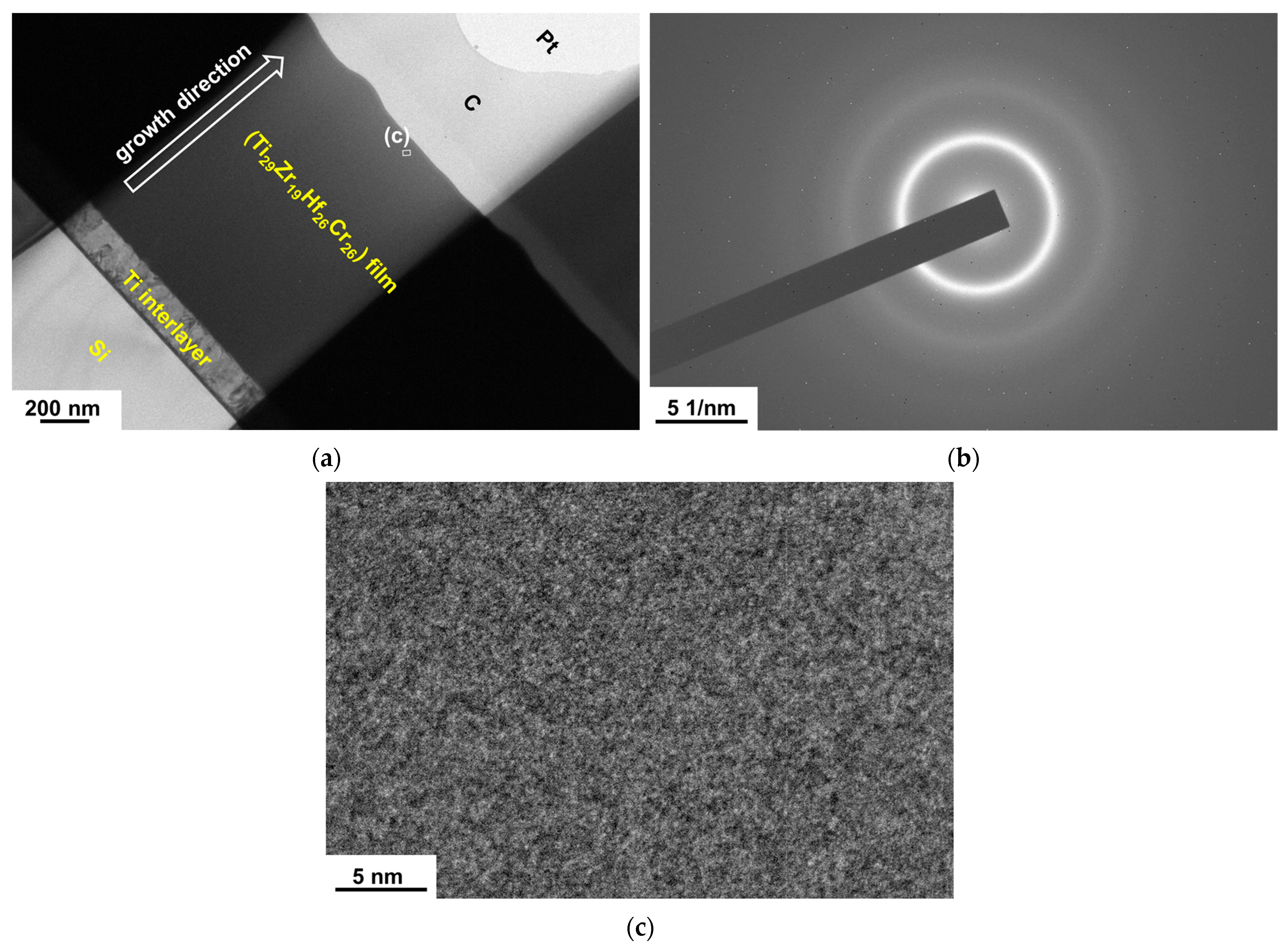
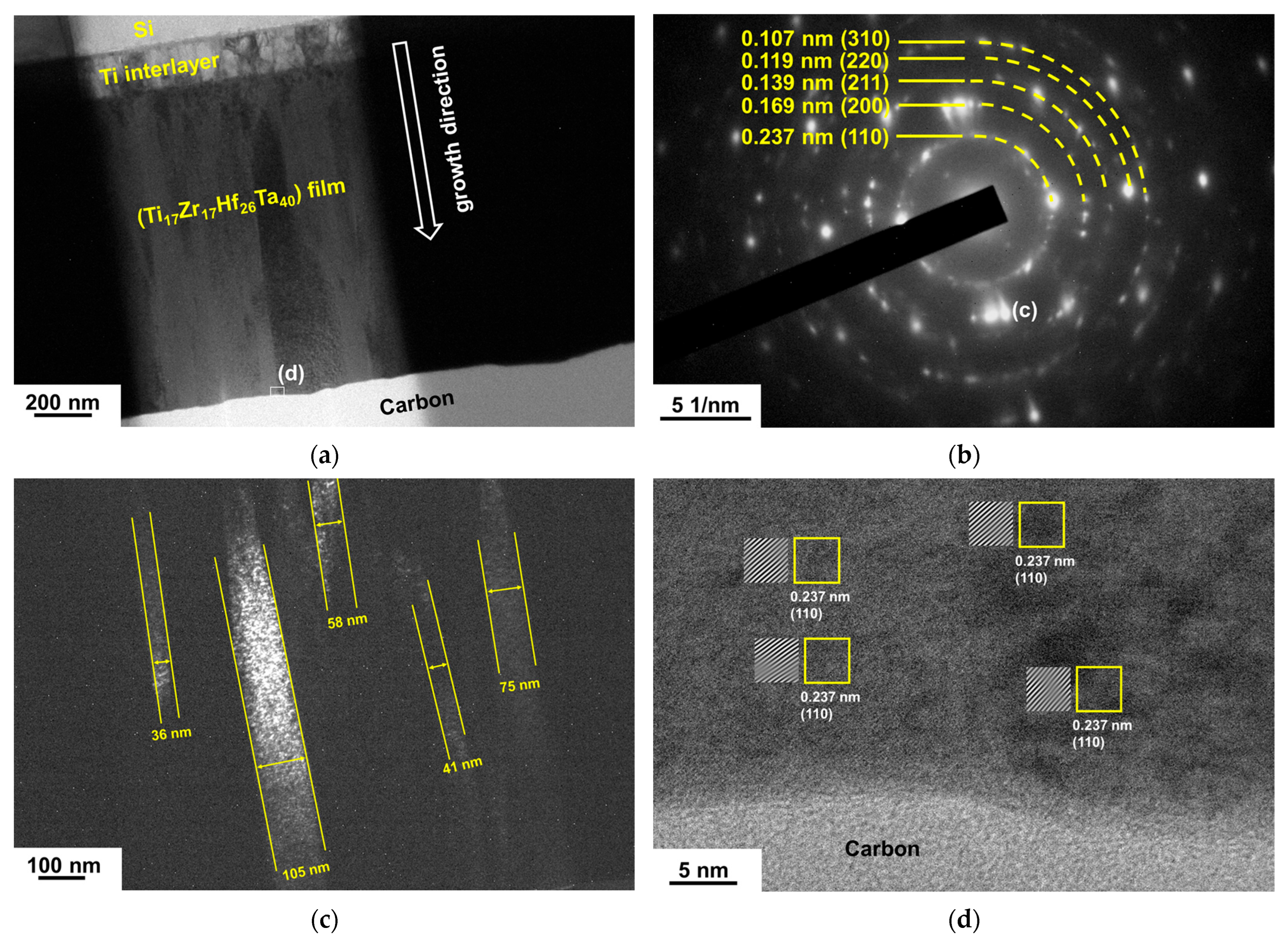
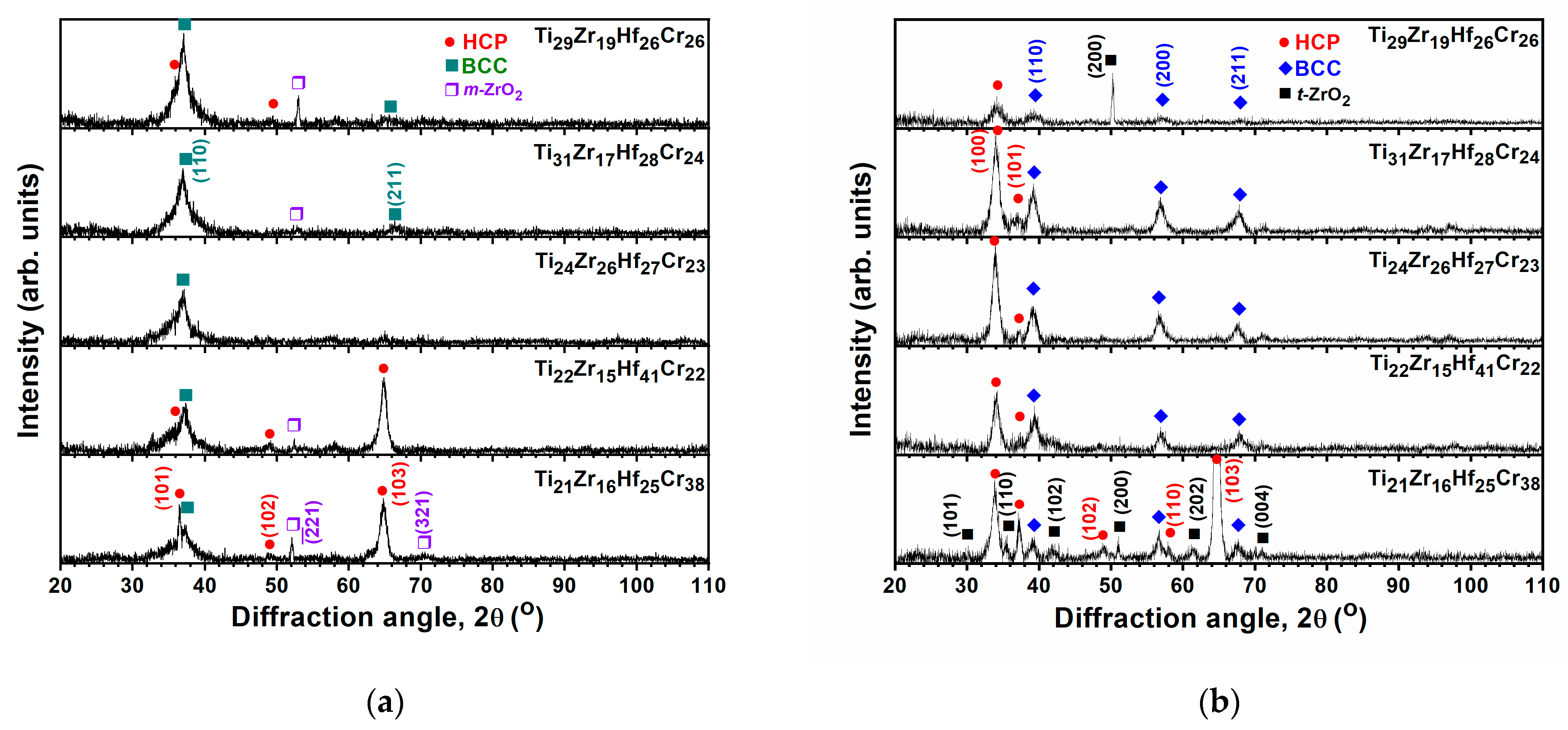
3.1. Chemical Compositions and Phases
3.2. Mechanical and Anticorrosive Properties of TiZrH(Ta, Y, Cr) Films
3.3. Thermal Stability of TiZrHf(Ta, Y, Cr) Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Chun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Tung, C.H.; Lin, S.J.; Yeh, J.W.; Chang, S.Y. Thermodynamic route for self-forming 1.5 nm V-Nb-Mo-Ta-W high-entropy alloy barrier layer: Roles of enthalpy and mixing entropy. Acta Mater. 2020, 199, 107–115. [Google Scholar] [CrossRef]
- Gruber, G.C.; Kirchmair, M.; Wurster, S.; Cordill, M.J.; Franz, R. A new design rule for high entropy alloy diffusion barriers in Cu metallization. J. Alloys Compd. 2023, 953, 170166. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, X.; Yao, W.; Liang, X. Structure and properties of high-entropy amorphous thin films: A review. JOM 2022, 74, 794–807. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; She, H.; Wu, M.; Shu, D.; Dong, A.; Lai, H.; Sun, B. Design of high-ductile medium entropy alloys for dental implants. Mater. Sci. Eng. C 2020, 113, 110959. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible TiZrHfNbTa high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Yanao, K.; Toda, Y.; Ohnuma, I.; Matsunaga, T. Phase stability of Ti-containing high-entropy alloys with a bcc or hcp structure. J. Alloys Compd. 2022, 911, 164849. [Google Scholar] [CrossRef]
- Chen, Y.I.; Chen, Y.J.; Lai, C.Y.; Chang, L.C. Mechanical and Anticorrosive Properties of TiNbTa and TiNbTaZr Films on Ti-6Al-4V Alloy. Coatings 2022, 12, 1985. [Google Scholar] [CrossRef]
- Raabe, D.; Sander, B.; Friák, M.; Ma, D.; Neugebauer, J. Theory-guided bottom-up design of β-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar] [CrossRef]
- Hussein, A.H.; Gepreel, M.A.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti–Nb–Ta base alloys. Mater. Sci. Eng. C 2016, 61, 574–578. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-entropy alloys: Potential candidates for high-temperature applications–an overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B. Effect of the atomic size distribution on glass forming ability of amorphous metallic alloys. Mater. Res. Bull. 2001, 36, 2183–2198. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Janssen, G.C.A.M.; Abdalla, M.M.; van Keulen, F.; Pujada, B.R.; van Venrooy, B. Celebrating the 100th anniversary of the Stoney equation for film stress: Developments from polycrystalline steel strips to single crystal silicon wafers. Thin Solid Films 2009, 517, 1858–1867. [Google Scholar] [CrossRef]
- Braic, M.; Braic, V.; Vladescu, A.; Zoita, C.N.; Balaceanu, M. Solid solution or amorphous phase formation in TiZr-based ternary to quinternary multi-principal-element films. Prog. Nat. Sci. 2014, 24, 305–312. [Google Scholar] [CrossRef]
- Tsai, D.C.; Chang, Z.C.; Kuo, B.H.; Liu, Y.C.; Chen, E.C.; Shieu, F.S. Structural, electro-optical, and mechanical properties of reactively sputtered (TiZrHf)N coatings. Ceram. Int. 2016, 42, 14257–14265. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, K.; Zheng, Y.; Zhou, H.; Wan, Z. Effect of Zr content on structure and mechanical properties of (CrTaNbMoV)Zrx films. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2019, 457, 56–62. [Google Scholar] [CrossRef]
- Ou, T.Y.; Chang, L.C.; Annalakshmi, M.; Lee, J.W.; Chen, Y.I. Effects of nitrogen flow ratio on the mechanical and anticorrosive properties of cosputtered (TiZrHfTa)Nx films. Surf. Coat. Technol. 2024, 477, 130410. [Google Scholar] [CrossRef]
- Ou, T.Y.; Chang, L.C.; Chen, Y.I.; Yu, C.S. Characterization of cosputtered (TiZrHfY)Nx films. Surf. Coatings Technol. 2024, 483, 130815. [Google Scholar] [CrossRef]
- Wang, W.; Yang, K.; Wang, Q.; Dai, P.; Fang, H.; Wu, F.; Guo, Q.; Liaw, P.K.; Hua, N. Novel Ti-Zr-Hf-Nb-Fe refractory high-entropy alloys for potential biomedical applications. J. Alloys Compd. 2022, 906, 164383. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Pang, S.; Liaw, P.K.; Zhang, T. Bio-corrosion behavior and in vitro biocompatibility of equimolar TiZrHfNbTa high-entropy alloy. Intermetallics 2020, 124, 106845. [Google Scholar] [CrossRef]
- Zhou, Q.; Sheikh, S.; Ou, P.; Chen, D.; Hu, Q.; Guo, S. Corrosion behavior of Hf0.5Nb0.5Ta0.5T1.5Zr refractory high-entropy alloy in aqueous chloride solutions. Electrochem. Commun. 2019, 98, 63–68. [Google Scholar] [CrossRef]
- Wang, X.; Hu, T.; Ma, T.; Yang, X.; Zhu, D.; Dong, D.; Xiao, J.; Yang, X. Mechanical, corrosion, and wear properties of TiZrTaNbSn biomedical high-entropy alloys. Coatings 2022, 12, 1795. [Google Scholar] [CrossRef]
- Wei, Z.; Attarilar, S.; Ebrahimi, M.; Li, J. Corrosion and wear behavior of additively manufactured metallic parts in biomedical applications. Metals 2024, 14, 96. [Google Scholar] [CrossRef]
- Kotan, H.; Tekin, M.; Bayath, A.; Bayrak, K.G.; Kocabas, M.; Ayas, E. Effect of in-situ formed oxide and carbide phases on microstructure and corrosion behavior of Zr/Y doped CoCrFeNi high entropy alloys prepared by mechanical alloying and spark plasma sintering. Intermetallics 2023, 162, 107998. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, D.; Wang, K.; Yu, T. Corrosion of laser cladding high-entropy alloy coatings: A review. Coatings 2022, 12, 1669. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W.L. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J. Alloys Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- Yao, J.Q.; Liu, X.W.; Gao, N.; Jiang, Q.H.; Li, N.; Liu, G.; Zhang, W.B.; Fan, Z.T. Phase stability of a ductile single-phase BCC Hf0.5Nb0.5Ta0.5Ti1.5Zr refractory high-entropy alloy. Intermetallics 2018, 98, 79–88. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tong, Y.; Tseng, K.K.; Yeh, J.W.; Poplawsky, J.D.; Wen, J.G.; Gao, M.C.; Kim, G.; Chen, W.; Ren, Y.; et al. Phase transformations of HfNbTaTiZr high-entropy alloy at intermediate temperatures. Scr. Mater. 2019, 158, 50–56. [Google Scholar] [CrossRef]
- Cheng, C.; Feng, R.; Lyu, T.; Zou, Y. Accelerated discovery of (TiZrHf )x(NbTa)1−x high-entropy alloys with superior thermal stability and a new crystallization mechanism. Adv. Mater. 2024, 36, 2403632. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH: New York, NY, USA, 1995. [Google Scholar]
- Rice, R.W.; Wu, C.C.; Boichelt, F. Hardness–Grain-Size Relations in Ceramics. J. Am. Ceram. Soc. 1994, 77, 2539–2553. [Google Scholar] [CrossRef]
- Xu, S.; Yao, Z.; Zhou, J.; Du, M. Effects of post-deposition annealing on the chemical composition, microstructure, optical and mechanical properties of Y2O3 film. Surf. Coat. Technol. 2018, 344, 636–643. [Google Scholar] [CrossRef]
- Goedicke, K.; Liebig, J.S.; Zywitzki, O.; Sahm, H. Influence of process parameters on the structure and the properties of ZrO2 coatings deposited by reactive pulsed magnetron sputtering (PMS). Thin Solid Films 2000, 377–378, 37–42. [Google Scholar] [CrossRef]
- Koski, K.; Hölsä, J.; Juliet, P. Properties of zirconium oxide thin films deposited by pulsed reactive magnetron sputtering. Surf. Coat. Technol. 1999, 120–121, 303–312. [Google Scholar] [CrossRef]
- Zegtoul, H.; Saoula, N.; Azibi, M.; Bait, L.; Madaoui, N.; Khelladi, M.R.; Kechouane, M. Influence of substrate bias voltage on structure, mechanical and corrosion properties of ZrO2 thin films deposited by reactive magnetron sputter deposition. Surf. Coat. Technol. 2020, 393, 125821. [Google Scholar] [CrossRef]









| Sample | Gun 1 | Gun 2 | Gun 3 | Gun 4 | TF 1 (nm) | TI 2 (nm) |
|---|---|---|---|---|---|---|
| PTi 3 | PHf | PZr | ||||
| Ti31Zr33Hf36 | 120 | 80 | 100 | 796 ± 9 | 131 ± 0 | |
| PTi | PHf | PZr | PTa | |||
| Ti24Zr23Hf27Ta26 | 120 | 80 | 120 | 80 | 916 ± 8 | 143 ± 3 |
| Ti30Zr20Hf25Ta25 | 170 | 80 | 120 | 80 | 1058 ± 10 | 135 ± 1 |
| Ti21Zr30Hf25Ta24 | 120 | 80 | 170 | 80 | 795 ± 8 | 181 ± 8 |
| Ti20Zr18Hf38Ta24 | 120 | 130 | 120 | 80 | 923 ± 13 | 163 ± 3 |
| Ti17Zr17Hf26Ta40 | 120 | 80 | 120 | 130 | 685 ± 5 | 102 ± 8 |
| PTi | PHf | PZr | PY | |||
| Ti24Zr23Hf27Y26 | 130 | 80 | 90 | 100 | 1046 ± 7 | 117 ± 1 |
| Ti29Zr21Hf26Y24 | 180 | 80 | 90 | 100 | 1110 ± 10 | 117 ± 6 |
| Ti22Zr30Hf27Y21 | 130 | 80 | 140 | 100 | 1145 ± 3 | 115 ± 7 |
| Ti21Zr19Hf42Y18 | 130 | 130 | 90 | 100 | 1189 ± 6 | 117 ± 6 |
| Ti19Zr18Hf26Y37 | 130 | 80 | 90 | 150 | 1272 ± 2 | 124 ± 1 |
| PTi | PHf | PZr | PCr | |||
| Ti29Zr19Hf26Cr26 | 150 | 90 | 100 | 60 | 1133 ± 7 | 139 ± 9 |
| Ti31Zr17Hf28Cr24 | 200 | 90 | 100 | 60 | 1129 ± 10 | 137 ± 0 |
| Ti24Zr26Hf27Cr23 | 150 | 90 | 150 | 60 | 1119 ± 11 | 90 ± 10 |
| Ti22Zr15Hf41Cr22 | 150 | 140 | 100 | 60 | 1165 ± 5 | 125 ± 2 |
| Ti21Zr16Hf25Cr38 | 150 | 90 | 100 | 110 | 1200 ± 7 | 107 ± 0 |
| Sample | Chemical Composition (at.%) | VEC 1 | δ 2 | ΔHmix 3 | ΔSmix 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | Zr | Hf | Ta/Y/Cr | O | (%) | (kJ/mol) | (J/mol K) | ||
| Ti31Zr33Hf36 | 28.9 ± 0.4 | 31.5 ± 0.6 | 34.5 ± 0.3 | - | 5.1 ± 0.1 | 4.00 | 3.79 | 0 | 9.11 |
| Ti24Zr23Hf27Ta26 | 22.7 ± 0.0 | 21.2 ± 0.4 | 25.9 ± 0.4 | 24.9 ± 0.2 | 5.3 ± 0.4 | 4.26 | 4.70 | 1.82 | 11.50 |
| Ti30Zr20Hf25Ta25 | 28.7 ± 0.3 | 19.1 ± 0.2 | 23.9 ± 0.3 | 23.1 ± 0.3 | 5.2 ± 0.4 | 4.24 | 4.62 | 1.62 | 11.44 |
| Ti21Zr30Hf25Ta24 | 20.2 ± 0.4 | 27.8 ± 0.2 | 23.9 ± 0.1 | 22.5 ± 0.1 | 5.6 ± 0.3 | 4.24 | 4.74 | 1.77 | 11.47 |
| Ti20Zr18Hf38Ta24 | 18.6 ± 0.8 | 16.6 ± 0.5 | 36.1 ± 0.5 | 22.7 ± 0.6 | 6.0 ± 0.4 | 4.24 | 4.54 | 1.82 | 11.12 |
| Ti17Zr17Hf26Ta40 | 15.7 ± 0.8 | 16.5 ± 0.4 | 24.9 ± 0.2 | 37.7 ± 0.4 | 5.2 ± 0.5 | 4.40 | 4.82 | 2.35 | 10.97 |
| Ti24Zr23Hf27Y26 | 22.0 ± 0.4 | 21.3 ± 0.5 | 25.4 ± 0.3 | 24.8 ± 0.7 | 6.5 ± 1.7 | 3.73 | 7.72 | 9.09 | 11.50 |
| Ti29Zr21Hf26Y24 | 27.9 ± 0.5 | 20.0 ± 0.1 | 24.2 ± 0.2 | 23.0 ± 0.2 | 4.9 ± 0.3 | 3.76 | 7.85 | 8.79 | 11.47 |
| Ti22Zr30Hf27Y21 | 20.4 ± 0.9 | 28.3 ± 0.5 | 25.8 ± 0.5 | 20.0 ± 0.8 | 5.5 ± 0.2 | 3.79 | 7.12 | 7.57 | 11.43 |
| Ti21Zr19Hf42Y18 | 19.9 ± 0.4 | 18.4 ± 0.2 | 40.7 ± 1.5 | 17.6 ± 0.9 | 3.4 ± 3.0 | 3.82 | 6.81 | 6.89 | 10.94 |
| Ti19Zr18Hf26Y37 | 18.1 ± 0.5 | 16.5 ± 0.5 | 24.3 ± 0.0 | 34.9 ± 0.3 | 6.2 ± 0.4 | 3.63 | 8.07 | 10.91 | 10.94 |
| Ti29Zr19Hf26Cr26 | 28.4 ± 0.4 | 18.2 ±0.6 | 25.3 ± 0.3 | 25.7 ± 0.4 | 2.4 ± 0.5 | 4.53 | 9.29 | 6.96 | 11.42 |
| Ti31Zr17Hf28Cr24 | 30.2 ± 0.2 | 17.0 ± 0.3 | 27.8 ± 0.5 | 23.3 ± 0.1 | 1.7 ± 0.3 | 4.47 | 8.96 | 6.42 | 11.34 |
| Ti24Zr26Hf27Cr23 | 23.6 ± 0.6 | 25.3 ± 0.4 | 26.3 ± 0.3 | 22.2 ± 0.6 | 2.6 ± 1.2 | 4.46 | 9.10 | 6.61 | 11.51 |
| Ti22Zr15Hf41Cr22 | 21.7 ± 0.3 | 14.1 ± 0.2 | 40.4 ± 0.4 | 21.6 ± 0.3 | 2.2 ± 0.2 | 4.44 | 8.87 | 6.19 | 10.91 |
| Ti21Zr16Hf25Cr38 | 20.5 ± 0.1 | 16.0 ± 0.1 | 25.2 ± 0.0 | 38.3 ± 0.2 | 0.0 ± 0.0 | 4.77 | 10.41 | 8.61 | 11.08 |
| Sample | H 1 (GPa) | E 2 (GPa) | We 3 (%) | σ 4 (GPa) |
|---|---|---|---|---|
| Ti31Zr33Hf36 | 7.4 ± 0.8 | 179 ± 14 | 31 | −0.13 ± 0.00 |
| Ti24Zr23Hf27Ta26 | 4.7 ± 0.6 | 104 ± 9 | 32 | −0.23 ± 0.00 |
| Ti30Zr20Hf25Ta25 | 4.6 ± 0.3 | 100 ± 4 | 28 | 0.00 ± 0.00 |
| Ti21Zr30Hf25Ta24 | 5.0 ± 0.3 | 101 ± 3 | 33 | −0.14 ± 0.24 |
| Ti20Zr18Hf38Ta24 | 5.1 ± 0.5 | 105 ± 6 | 30 | −0.04 ± 0.11 |
| Ti17Zr17Hf26Ta40 | 5.4 ± 0.3 | 114 ± 3 | 30 | −0.14 ± 0.05 |
| Ti24Zr23Hf27Y26 | 6.0 ± 0.6 | 106 ± 6 | 33 | −0.48 ± 0.02 |
| Ti29Zr21Hf26Y24 | 7.4 ± 0.5 | 114 ± 6 | 37 | −0.54 ± 0.01 |
| Ti22Zr30Hf27Y21 | 7.9 ± 0.5 | 131 ± 7 | 36 | −0.41 ± 0.13 |
| Ti21Zr19Hf42Y18 | 7.9 ± 0.5 | 134 ± 9 | 38 | −0.46 ± 0.07 |
| Ti19Zr18Hf26Y37 | 6.1 ± 0.3 | 106 ± 4 | 37 | −0.74 ± 0.04 |
| Ti29Zr19Hf26Cr26 | 6.6 ± 0.1 | 105 ± 3 | 45 | −0.24 ± 0.00 |
| Ti31Zr17Hf28Cr24 | 6.8 ± 0.0 | 104 ± 1 | 44 | −0.27 ± 0.01 |
| Ti24Zr26Hf27Cr23 | 5.3 ± 0.4 | 91 ± 5 | 44 | −0.15 ± 0.00 |
| Ti22Zr15Hf41Cr22 | 6.5 ± 0.0 | 105 ± 1 | 43 | −0.43 ± 0.01 |
| Ti21Zr16Hf25Cr38 | 8.4 ± 0.1 | 113 ± 1 | 48 | −0.28 ± 0.01 |
| Sample | Ecorr 1 (mV) | Icorr 2 (μA/cm2) | Rp 3 (Ω·cm2) | βa 4 (mV) | βc 5 (mV) | Rp Ratio |
|---|---|---|---|---|---|---|
| SUS420 | −345 | 4.826 | 7.7 × 103 | 132.6 | 238.5 | 1.0 |
| Ti31Zr33Hf36 | −354 | 0.338 | 1.9 × 105 | 472.3 | 221.1 | 25.2 |
| Ti24Zr23Hf27Ta26 | −714 | 2.129 | 2.4 × 104 | 169.4 | 360.1 | 3.1 |
| Ti30Zr20Hf25Ta25 | −281 | 1.497 | 1.7 × 104 | 66.3 | 443.2 | 2.2 |
| Ti21Zr30Hf25Ta24 | −388 | 1.330 | 3.4 × 104 | 139.6 | 396.2 | 4.4 |
| Ti20Zr18Hf38Ta24 | −199 | 0.231 | 1.0 × 105 | 65.5 | 366.7 | 13.6 |
| Ti17Zr17Hf26Ta40 | −251 | 0.221 | 1.2 × 105 | 88.4 | 178.2 | 15.1 |
| Ti24Zr23Hf27Y26 | −619 | 6.291 | 2.9 × 103 | 47.4 | 318.8 | 0.4 |
| Ti29Zr21Hf26Y24 | −372 | 0.418 | 6.9 × 104 | 95.3 | 216.9 | 9.0 |
| Ti22Zr30Hf27Y21 | −578 | 18.120 | 3.1 × 103 | 178.2 | 470.9 | 0.4 |
| Ti21Zr19Hf42Y18 | −199 | 15.414 | 1.2 × 103 | 46.1 | 473.2 | 0.2 |
| Ti19Zr18Hf26Y37 | −997 | 0.551 | 8.3 × 104 | 254.3 | 179.2 | 10.8 |
| Ti29Zr19Hf26Cr26 | −225 | 0.269 | 2.0 × 105 | 359.3 | 190.1 | 26.2 |
| Ti31Zr17Hf28Cr24 | −202 | 0.064 | 4.5 × 105 | 101.9 | 184.8 | 58.1 |
| Ti24Zr26Hf27Cr23 | −180 | 0.140 | 3.5 × 105 | 301.6 | 176.7 | 45.1 |
| Ti22Zr15Hf41Cr22 | −243 | 0.319 | 7.8 × 104 | 78.3 | 215.1 | 10.2 |
| Ti21Zr16Hf25Cr38 | −201 | 0.133 | 3.3 × 105 | 318.5 | 146.6 | 42.7 |
| Sample | Hardness (GPa) | Elastic Modulus (GPa) | ||||
|---|---|---|---|---|---|---|
| RT | 500 °C | 700 °C | RT | 500 °C | 700 °C | |
| Ti31Zr33Hf36 | 7.4 ± 0.8 | 11.6 ± 0.2 | 18.8 ± 1.7 | 104 ± 9 | 171 ± 2 | 241 ± 14 |
| Ti24Zr23Hf27Ta26 | 4.7 ± 0.6 | 7.9 ± 0.6 | 12.5 ± 0.4 | 100 ± 4 | 138 ± 6 | 182 ± 3 |
| Ti30Zr20Hf25Ta25 | 4.6 ± 0.3 | 7.4 ± 0.2 | 11.4 ± 1.2 | 101 ± 3 | 131 ± 3 | 196 ± 13 |
| Ti21Zr30Hf25Ta24 | 5.0 ± 0.3 | 6.9 ± 0.3 | 12.2 ± 0.5 | 105 ± 6 | 128 ± 5 | 193 ± 4 |
| Ti20Zr18Hf38Ta24 | 5.1 ± 0.5 | 6.8 ± 0.6 | 11.2 ± 0.4 | 114 ± 3 | 135 ± 8 | 188 ± 5 |
| Ti17Zr17Hf26Ta40 | 5.4 ± 0.3 | 7.5 ± 0.3 | 12.1 ± 0.8 | 106 ± 6 | 144 ± 5 | 192 ± 7 |
| Ti24Zr23Hf27Y26 | 6.0 ± 0.6 | 9.1 ± 0.5 | 10.4 ± 0.6 | 114 ± 6 | 139 ± 3 | 184 ± 9 |
| Ti29Zr21Hf26Y24 | 7.4 ± 0.5 | 8.4 ± 0.4 | 10.8 ± 0.5 | 131 ± 7 | 141 ± 7 | 189 ± 7 |
| Ti22Zr30Hf27Y21 | 7.9 ± 0.5 | 8.7 ± 0.3 | 9.3 ± 0.4 | 134 ± 9 | 132 ± 3 | 172 ± 4 |
| Ti21Zr19Hf42Y18 | 7.9 ± 0.5 | 11.6 ± 0.4 | 8.5 ± 0.4 | 106 ± 4 | 199 ± 2 | 170 ± 4 |
| Ti19Zr18Hf26Y37 | 6.1 ± 0.3 | 9.8 ± 0.7 | 15.8 ± 1.1 | 104 ± 9 | 165 ± 6 | 236 ± 10 |
| Ti29Zr19Hf26Cr26 | 6.6 ± 0.1 | 10.5 ± 0.6 | 16.4 ± 3.1 | 105 ± 3 | 142 ± 6 | 202 ± 23 |
| Ti31Zr17Hf28Cr24 | 6.8 ± 0.0 | 11.1 ± 0.2 | 20.6 ± 0.6 | 104 ± 1 | 152 ± 2 | 241 ± 6 |
| Ti24Zr26Hf27Cr23 | 5.3 ± 0.4 | 9.0 ± 0.7 | 15.0 ± 5.2 | 91 ± 5 | 139 ± 10 | 205 ± 43 |
| Ti22Zr15Hf41Cr22 | 6.5 ± 0.0 | 11.3 ± 0.4 | 19.0 ± 0.4 | 105 ± 1 | 154 ± 2 | 223 ± 3 |
| Ti21Zr16Hf25Cr38 | 8.4 ± 0.1 | 11.6 ± 0.1 | 19.5 ± 0.2 | 113 ± 1 | 154 ± 1 | 234 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-I.; Ou, T.-Y.; Chang, L.-C.; Liao, Y.-Z. Structural Evolution, Mechanical Properties, and Thermal Stability of Multi-Principal TiZrHf(Ta, Y, Cr) Alloy Films. Materials 2025, 18, 3672. https://doi.org/10.3390/ma18153672
Chen Y-I, Ou T-Y, Chang L-C, Liao Y-Z. Structural Evolution, Mechanical Properties, and Thermal Stability of Multi-Principal TiZrHf(Ta, Y, Cr) Alloy Films. Materials. 2025; 18(15):3672. https://doi.org/10.3390/ma18153672
Chicago/Turabian StyleChen, Yung-I, Tzu-Yu Ou, Li-Chun Chang, and Yan-Zhi Liao. 2025. "Structural Evolution, Mechanical Properties, and Thermal Stability of Multi-Principal TiZrHf(Ta, Y, Cr) Alloy Films" Materials 18, no. 15: 3672. https://doi.org/10.3390/ma18153672
APA StyleChen, Y.-I., Ou, T.-Y., Chang, L.-C., & Liao, Y.-Z. (2025). Structural Evolution, Mechanical Properties, and Thermal Stability of Multi-Principal TiZrHf(Ta, Y, Cr) Alloy Films. Materials, 18(15), 3672. https://doi.org/10.3390/ma18153672






