Inside the Framework: Structural Exploration of Mesoporous Silicas MCM-41, SBA-15, and SBA-16
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mesoporous Silicas
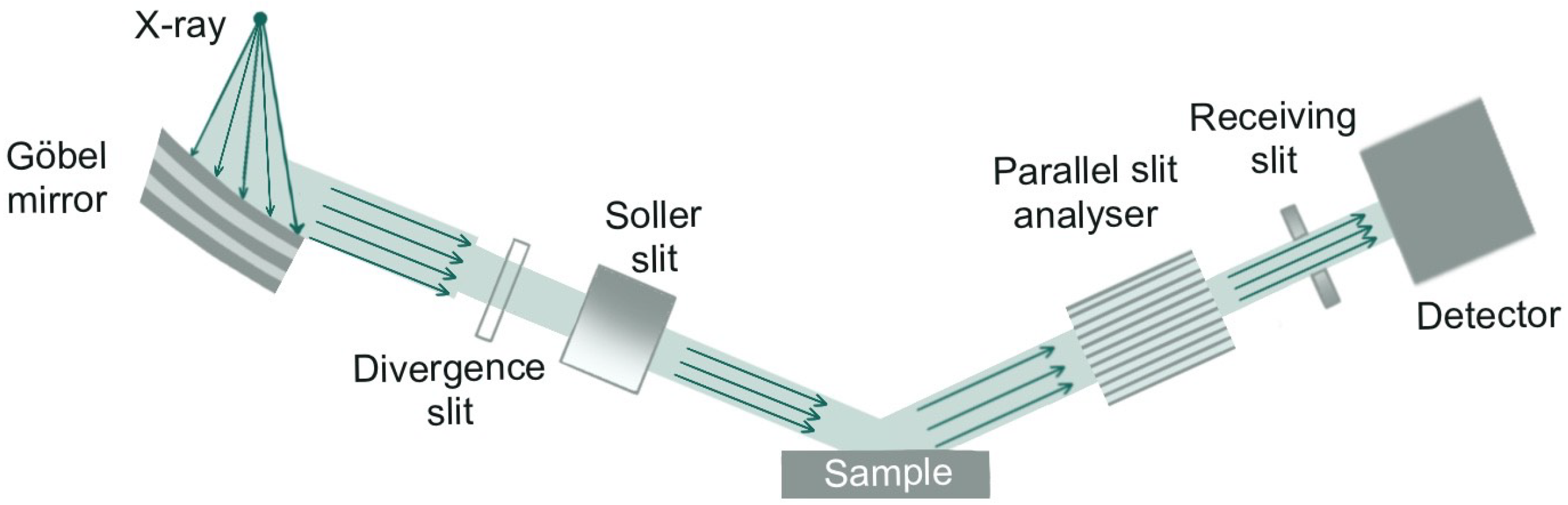
2.3. Characterization
3. Results
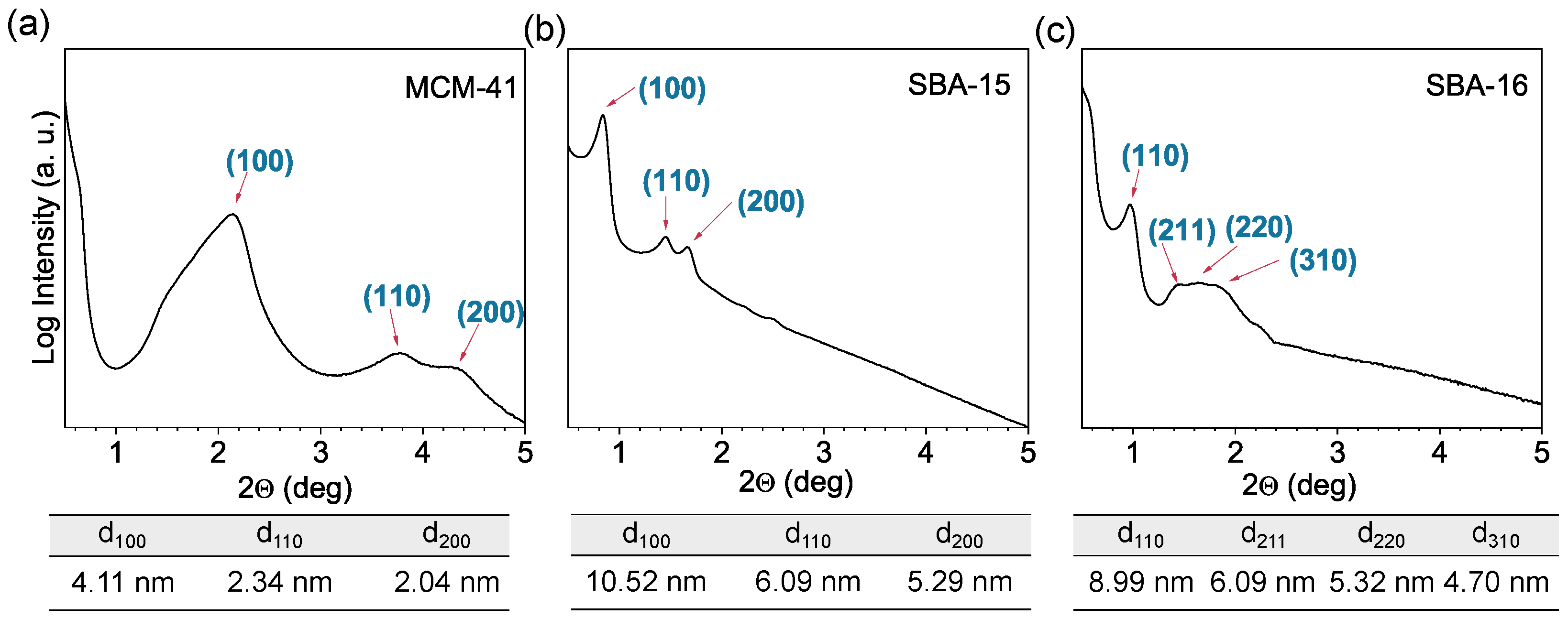
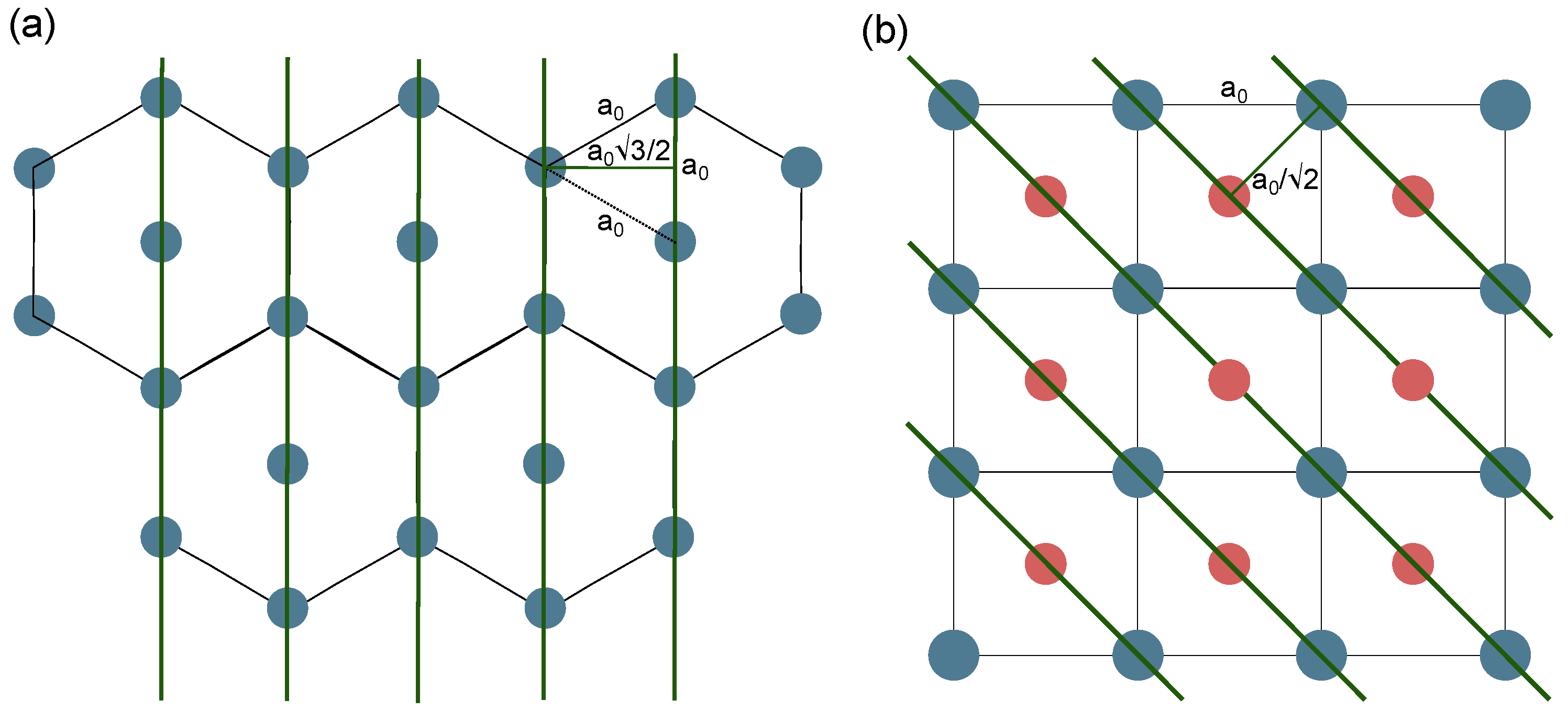
3.1. X-Ray Diffraction Analysis
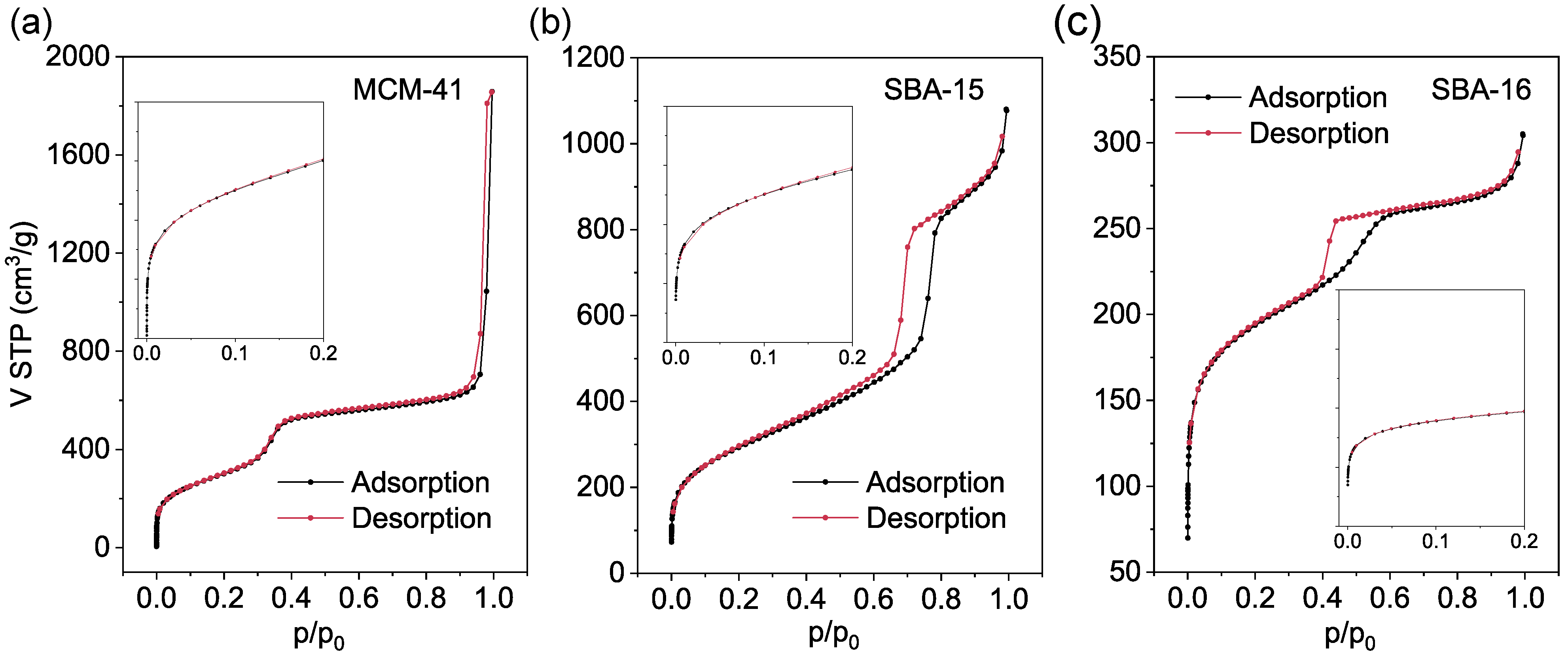
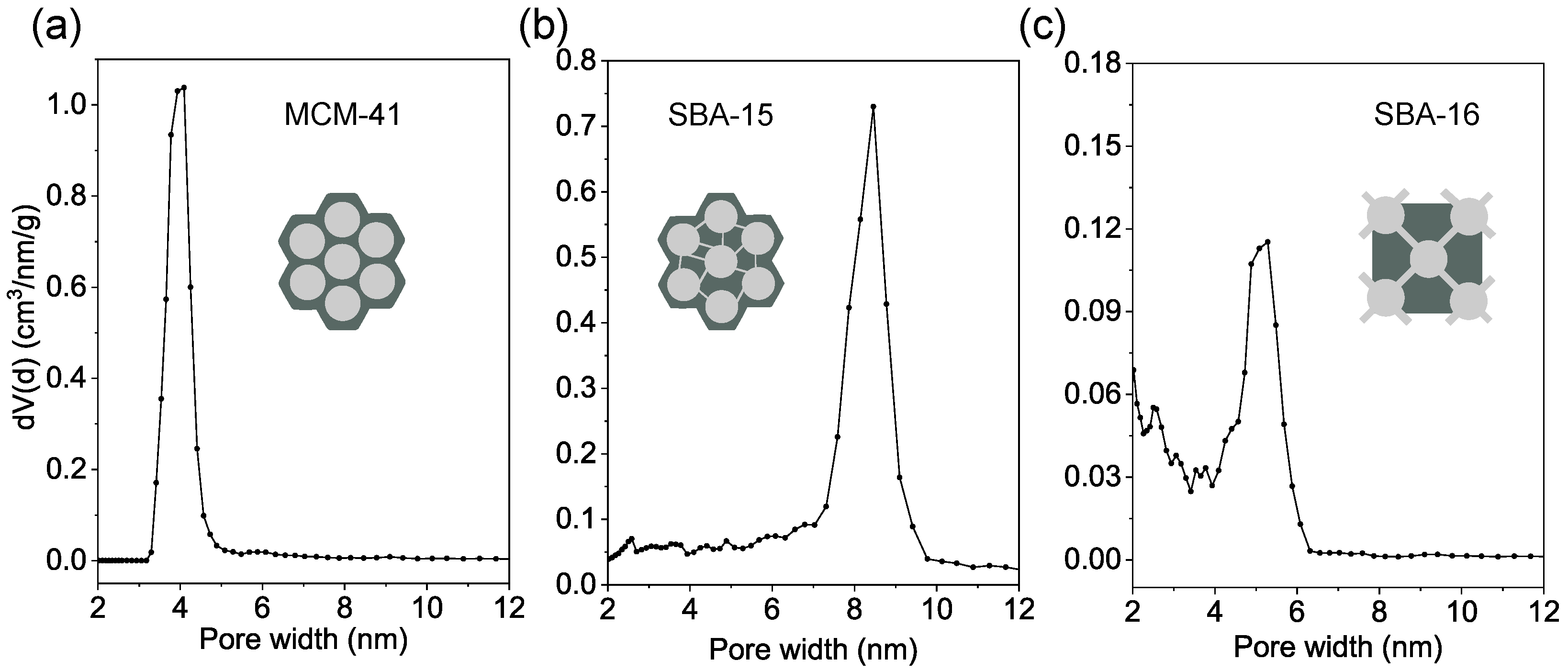
3.2. Nitrogen Physisorption Analysis
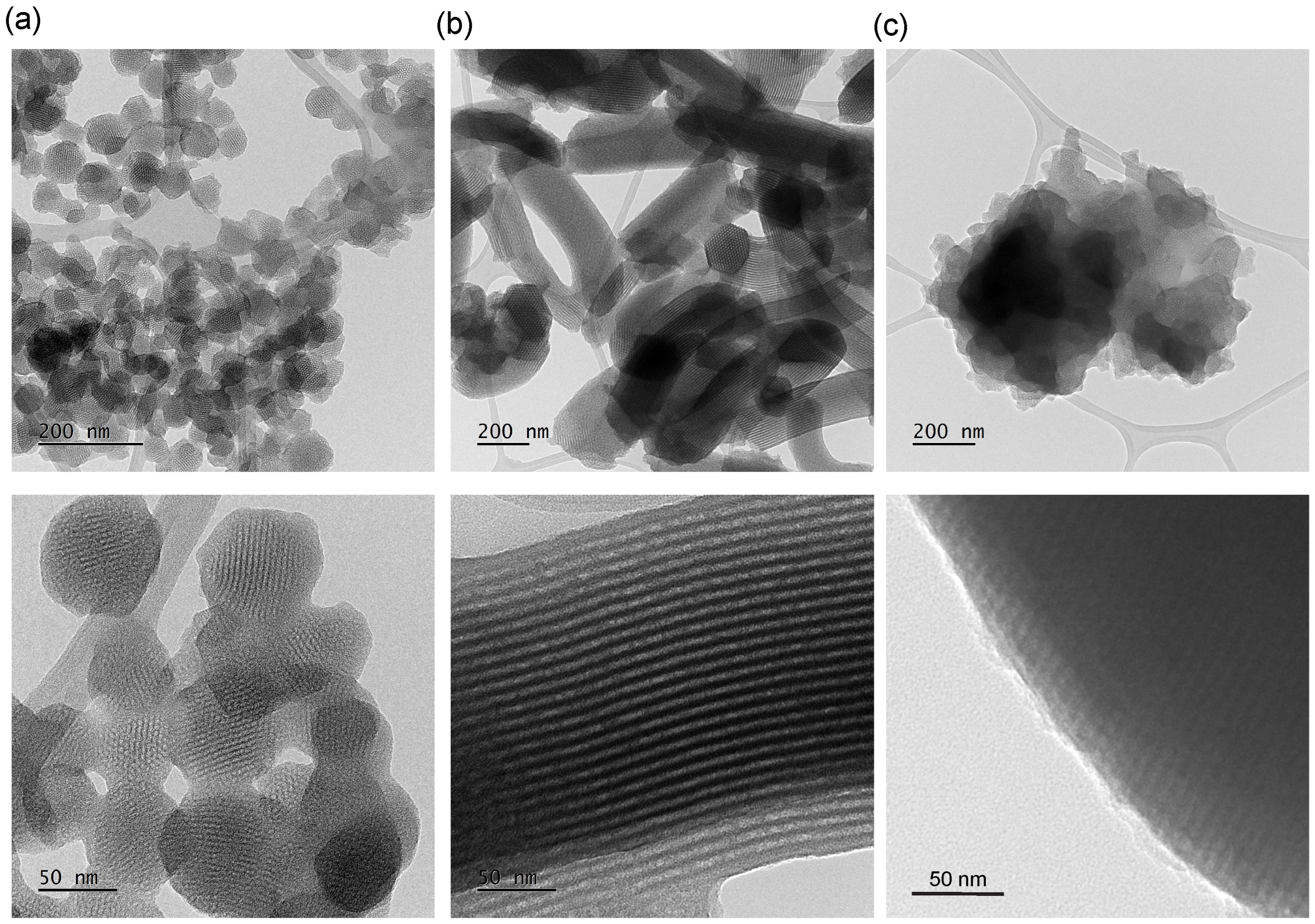
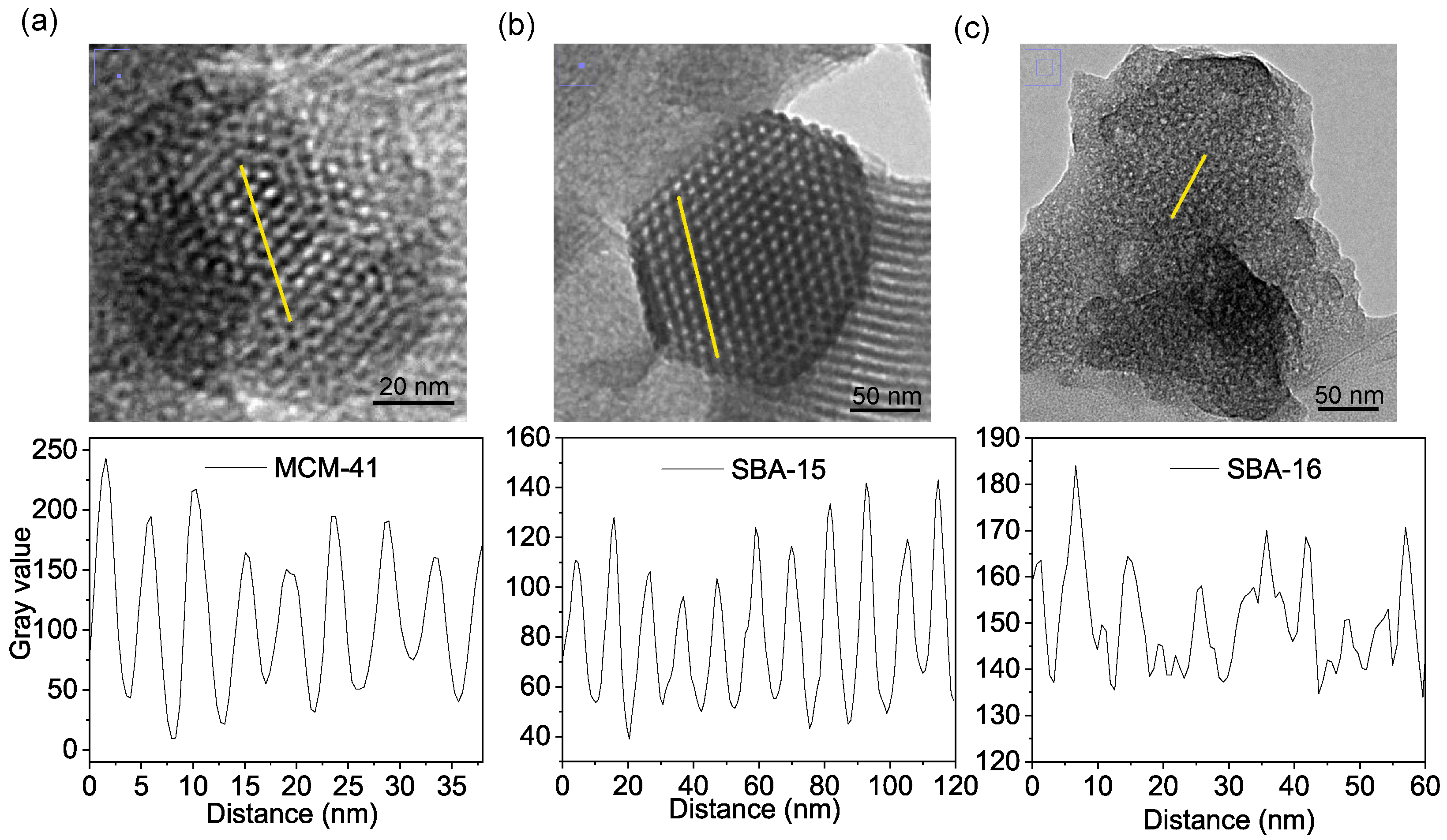
3.3. Transmission Electron Microscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller method (equation) |
| BJH | Barret–Joyner–Halenda method |
| CTAB | Cetrimonium bromide |
| EDS | Energy dispersive spectroscopy |
| F127 | Pluronic F127 |
| GM | Göbel Mirror |
| HCl | Hydrochloric acid |
| IUPAC | International Union of Pure and Applied Chamistry |
| LAXRD | Low-Angle X-ray Diffraction |
| MCM-41 | Mobil Composition of Matter No. 41 |
| Mw | Molecular weight |
| NaOH | Sodium hydroxide |
| NLDFT | Non-Local Density Functional Theory method |
| OMS | Ordered Mesoporous Silica |
| P123 | Pluronic P123 |
| SAXS | Small-Angle X-ray Scattering |
| SBA-15 | Santa Barbara Amorphous No. 15 |
| SBA-16 | Santa Barbara Amorphous No. 16 |
| SBET | BET specific surface area |
| STP | Standard temperature and pressure |
| TEM | Transmission electron microscope |
| TEOS | Tetraethyl orthosilicate |
| XRD | X-ray diffraction |
References
- Pu, T.; Zhang, W.; Zhu, M. Engineering heterogeneous catalysis with strong metal–support interactions: Characterization, theory and manipulation. Angew. Chem. Int. Ed. 2023, 62, e202212278. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrogen Energy 2022, 47, 41596–41620. [Google Scholar] [CrossRef]
- Adamek, M.; Pastukh, O.; Laskowska, M.; Karczmarska, A.; Laskowski, Ł. Nanostructures as the Substrate for Single-Molecule Magnet Deposition. Int. J. Mol. Sci. 2023, 25, 52. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Gafari, S.; Kazemzad, M. Concepts, fabrication and applications of MOF thin films in optoelectronics: A review. Appl. Mater. Today 2024, 38, 102153. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Huang, Z.; Yang, J. A new class of electronic devices based on flexible porous substrates. Adv. Sci. 2022, 9, 2105084. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Garalleh, H.A.; Alalawi, A.; Al-Sarayreh, E.; Pugazhendhi, A. Carbon nanomaterials: Types, synthesis strategies and their application as drug delivery system for cancer therapy. Biochem. Eng. J. 2023, 192, 108828. [Google Scholar] [CrossRef]
- Bagheri, B.; Surwase, S.S.; Lee, S.S.; Park, H.; Rad, Z.F.; Trevaskis, N.L.; Kim, Y.C. Carbon-based nanostructures for cancer therapy and drug delivery applications. J. Mater. Chem. B 2022, 10, 9944–9967. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.; Gan, F.; Qin, P.; Shao, Z. Recent advances of single-atom catalysts for peroxymonosulfate-based advanced oxidation processes aimed at environmental remediation. Curr. Opin. Chem. Eng. 2023, 41, 100928. [Google Scholar] [CrossRef]
- Priya, A.; Muruganandam, M.; Suresh, S. Bio-derived carbon-based materials for sustainable environmental remediation and wastewater treatment. Chemosphere 2024, 362, 142731. [Google Scholar] [CrossRef]
- Grisolia, A.; Dell’Olio, G.; Spadafora, A.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. Hybrid polymer-silica nanostructured materials for environmental remediation. Molecules 2023, 28, 5105. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Shi, J.; Lu, X.; Liu, Q.; Liu, Y.; Zhu, T.; Wang, D.; Yang, Q. Incorporating metal–organic frameworks into substrates for environmental applications. Chem. Eng. J. 2022, 446, 136866. [Google Scholar] [CrossRef]
- Laskowska, M.; Pastukh, O.; Fedorchuk, A.; Schabikowski, M.; Kowalczyk, P.; Zalasiński, M.; Laskowski, Ł. Nanostructured Silica with Anchoring Units: The 2D Solid Solvent for Molecules and Metal Ions. Int. J. Mol. Sci. 2020, 21, 8137. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Nowak, A.; Dulski, M.; Weigl, P.; Blochowicz, T.; Laskowski, Ł. Spherical Silica Functionalized by 2-Naphthalene Methanol Luminophores as a Phosphorescence Sensor. Int. J. Mol. Sci. 2021, 22, 13289. [Google Scholar] [CrossRef]
- Laskowska, M.; Kowalczyk, P.; Karczmarska, A.; Kramkowski, K.; Wrzosek, K.; Laskowski, Ł. A novel biocidal nanocomposite: Spherical silica with silver ions anchored at the surface. Int. J. Mol. Sci. 2022, 24, 545. [Google Scholar] [CrossRef]
- Laskowski, L.; Kityk, I.; Konieczny, P.; Pastukh, O.; Schabikowski, M.; Laskowska, M. The separation of the Mn12 single-molecule magnets onto spherical silica nanoparticles. Nanomaterials 2019, 9, 764. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, Z.; Li, M.; Zhang, H.; Li, T.; Qin, X.; Li, S.; Wu, C.; You, F.; Liao, X.; et al. Mesoporous silica nanoparticles-based nanoplatforms: Basic construction, current state, and emerging applications in anticancer therapeutics. Adv. Healthc. Mater. 2023, 12, 2201884. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Vila, N.; Schabikowski, M.; Walcarius, A. Mesoporous silica-based materials for electronics-oriented applications. Molecules 2019, 24, 2395. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Wang, C.; Zhang, W.; Ma, B.; Deng, Y.; Li, W.; Zhao, D. Interfacial assembly and applications of functional mesoporous materials. Chem. Rev. 2021, 121, 14349–14429. [Google Scholar] [CrossRef]
- Wang, J.; Vilà, N.; Walcarius, A. Mesoporous Silica-Based. Nanomater. Sustain. Energy Appl. 2023, 8, 160. [Google Scholar]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized silica nanoparticles: Classification, synthetic approaches and recent advances in adsorption applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique properties of surface-functionalized nanoparticles for bio-application: Functionalization mechanisms and importance in application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Bałanda, M.; Fitta, M.; Dulski, M.; Zubko, M.; Pawlik, P.; Laskowski, Ł. Magnetic behaviour of Mn12-stearate single-molecule magnets immobilized inside SBA-15 mesoporous silica matrix. J. Magn. Magn. Mater. 2019, 478, 20–27. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How advancing are mesoporous silica nanoparticles? A comprehensive review of the literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Kong, X.P.; Zhang, B.H.; Wang, J. Multiple roles of mesoporous silica in safe pesticide application by nanotechnology: A review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption study of surface and structural properties of MCM-41 materials of different pore sizes. J. Phys. Chem. B 1997, 101, 583–589. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Lelong, G.; Saboungi, M.L. Recent progress in the synthesis and selected applications of MCM-41: A short review. J. Exp. Nanosci. 2006, 1, 375–395. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Shakeri, M.; Khatami Shal, Z.; Van Der Voort, P. An overview of the challenges and progress of synthesis, characterization and applications of plugged SBA-15 materials for heterogeneous catalysis. Materials 2021, 14, 5082. [Google Scholar] [CrossRef]
- Galindres, D.M.; Cifuentes, D.; Tinoco, L.E.; Murillo-Acevedo, Y.; Rodrigo, M.M.; Ribeiro, A.C.; Esteso, M.A. A Review of the Application of Resorcinarenes and SBA-15 in Drug Delivery. Processes 2022, 10, 684. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Dash, S.; Ghorai, S.; Pal, S.; Sarkar, A. SBA-16: Application for the removal of neutral, cationic, and anionic dyes from aqueous medium. J. Environ. Chem. Eng. 2016, 4, 157–166. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Pope, C.G. X-ray diffraction and the Bragg equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
- Zhu, X.; Birringer, R.; Herr, U.; Gleiter, H. X-ray diffraction studies of the structure of nanometer-sized crystalline materials. Phys. Rev. B 1987, 35, 9085. [Google Scholar] [CrossRef]
- Sinkó, K.; Torma, V.; Kovács, A. SAXS investigation of porous nanostructures. J. Non-Cryst. Solids 2008, 354, 5466–5474. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzałka, M.; Skibińska, M.; Pikus, S. Small-angle X-ray scattering (SAXS) studies of the structure of mesoporous silicas. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 411, 72–77. [Google Scholar] [CrossRef]
- Beurer, A.K.; Dieterich, S.; Solodenko, H.; Kaya, E.; Merdanoǧlu, N.; Schmitz, G.; Traa, Y.; Bruckner, J.R. Comparative study of lattice parameter and pore size of ordered mesoporous silica materials using physisorption, SAXS measurements and transmission electron microscopy. Microporous Mesoporous Mater. 2023, 354, 112508. [Google Scholar] [CrossRef]
- Harrington, G.F.; Santiso, J. Back-to-Basics tutorial: X-ray diffraction of thin films. J. Electroceram. 2021, 47, 141–163. [Google Scholar] [CrossRef]
- Schlumberger, C.; Scherdel, C.; Kriesten, M.; Leicht, P.; Keilbach, A.; Ehmann, H.; Kotnik, P.; Reichenauer, G.; Thommes, M. Reliable surface area determination of powders and meso/macroporous materials: Small-angle X-ray scattering and gas physisorption. Microporous Mesoporous Mater. 2022, 329, 111554. [Google Scholar] [CrossRef]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Int. Union Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ustinov, E.A.; Do, D. Comparison of nitrogen adsorption at 77 K on non-porous silica and pore wall of MCM-41 materials by means of density functional theory. J. Colloid Interface Sci. 2006, 297, 480–488. [Google Scholar] [CrossRef]
- Díaz, I.; Alfredsson, V.; Sakamoto, Y. Transmission electron microscopy in formation and growth of ordered mesoporous materials. Curr. Opin. Colloid Interface Sci. 2006, 11, 302–307. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Zu, S.Z.; Mao, L.J.; Sayari, A.; Han, B.H. Facile synthesis route to monodispersed platelet-like SBA-15 silica. J. Porous Mater. 2012, 19, 745–749. [Google Scholar] [CrossRef]
- Gobin, O.C.; Wan, Y.; Zhao, D.; Kleitz, F.; Kaliaguine, S. Mesostructured silica SBA-16 with tailored intrawall porosity part 1: Synthesis and characterization. J. Phys. Chem. C 2007, 111, 3053–3058. [Google Scholar] [CrossRef]
- Blender Foundation. Blender–Free and Open 3D Creation Software. Available online: https://www.blender.org (accessed on 20 June 2025).
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Chytil, S.; Haugland, L.; Blekkan, E.A. On the mechanical stability of mesoporous silica SBA-15. Microporous Mesoporous Mater. 2008, 111, 134–142. [Google Scholar] [CrossRef]
- Kim, T.W.; Ryoo, R.; Kruk, M.; Gierszal, K.P.; Jaroniec, M.; Kamiya, S.; Terasaki, O. Tailoring the pore structure of SBA-16 silica molecular sieve through the use of copolymer blends and control of synthesis temperature and time. J. Phys. Chem. B 2004, 108, 11480–11489. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Li, Q.; Stucky, G.D. Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mater. 2000, 12, 275–279. [Google Scholar] [CrossRef]
- Sing, K.S. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76, 3–11. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, N. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Juárez, J.M.; Téllez, J.F.; Bercero, M.Á.L.; Moyano, E.L.; Gómez Costa, M.B. Synthesis of MCM-41 and SBA-15 from rice husk silica and their carbon replicas for hydrogen adsorption. J. Chem. Technol. Biotechnol. 2025, 100, 1609–1617. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Esparza, J.M.; Campero, A.; Cordero, S.; Kornhauser, I.; Rojas, F. On comparing BJH and NLDFT pore-size distributions determined from N2 sorption on SBA-15 substrata. Phys. Chem. Chem. Phys. 2003, 5, 1859–1866. [Google Scholar] [CrossRef]
- Lim, M.H.; Blanford, C.F.; Stein, A. Synthesis and characterization of a reactive vinyl-functionalized MCM-41: Probing the internal pore structure by a bromination reaction. J. Am. Chem. Soc. 1997, 119, 4090–4091. [Google Scholar] [CrossRef]
- Idris, S.A.; Davidson, C.M.; McManamon, C.; Morris, M.A.; Anderson, P.; Gibson, L.T. Large pore diameter MCM-41 and its application for lead removal from aqueous media. J. Hazard. Mater. 2011, 185, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voort, P.; Benjelloun, M.; Vansant, E.F. Rationalization of the synthesis of SBA-16: Controlling the micro-and mesoporosity. J. Phys. Chem. B 2002, 106, 9027–9032. [Google Scholar] [CrossRef]
- Wang, S.; Salim, O.; Piri, M. The effects of pore shape and geometry on the storage of CO2 in mesoporous media. Mater. Today Sustain. 2025, 29, 101076. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Density functional theory model of adsorption on amorphous and microporous silica materials. Langmuir 2006, 22, 11171–11179. [Google Scholar] [CrossRef] [PubMed]
- Bergna, H.E. Colloid Chemistry of Silica: An Overview; American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
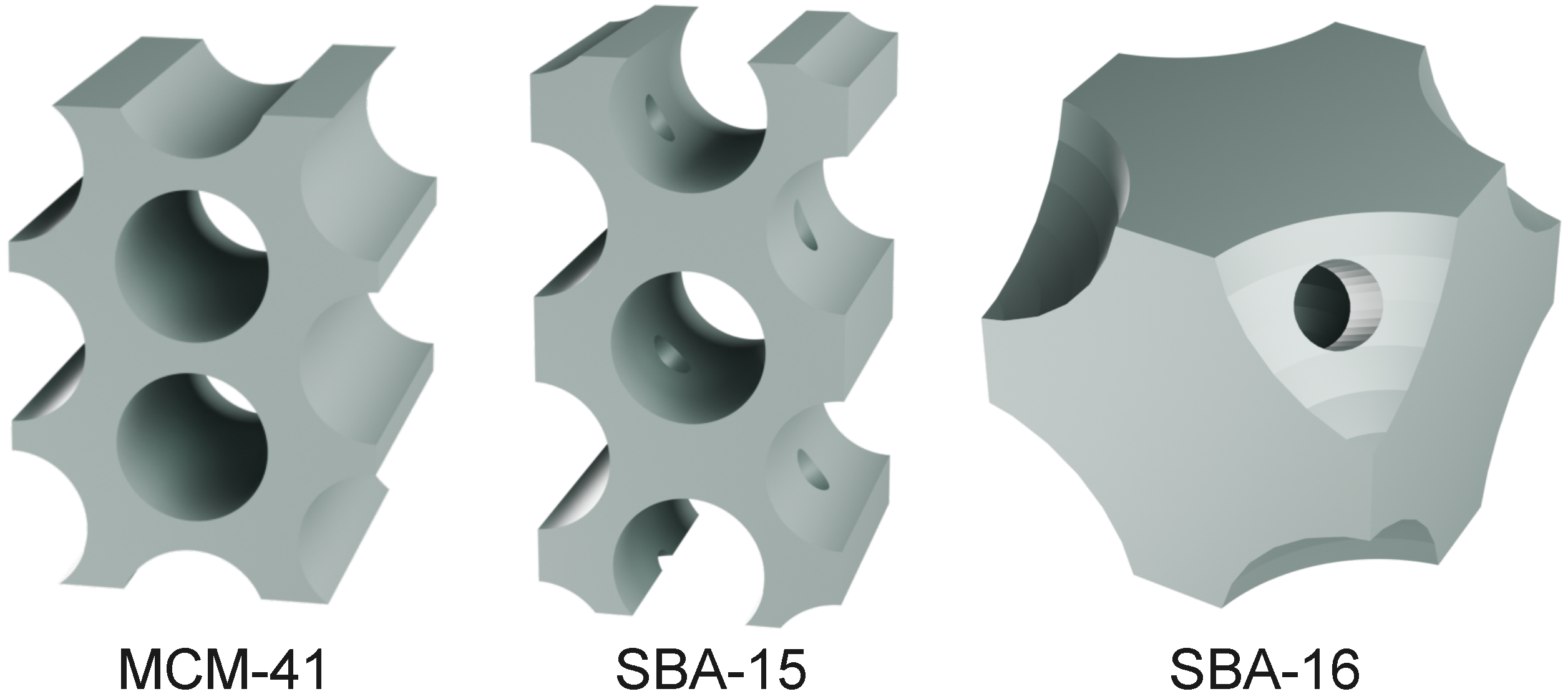
| Sample | Space Group | Pore | Interconnectivity | Reference | ||
|---|---|---|---|---|---|---|
| Shape | Arrangement | Size (nm) | ||||
| MCM-41 | P6mm | cylindrical | 2D hexagonal | 2–6.5 | – | [49] |
| SBA-15 | P6mm | cylindrical | 2D hexagonal | 5–30 | 1D channels | [50] |
| SBA-16 | Imm | spherical | 3D cubic | 4–9 | 3D interconnected | [51] |
| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Pore Width (nm) | Wall Thickness (nm) | ||
|---|---|---|---|---|---|---|
| Total Volume | Micropore Volume | Mesopore Volume | ||||
| MCM-41 | 1137 | 1.79 | - | 1.79 | 4.0 | 0.85 |
| SBA-15 | 1056 | 1.67 | 0.14 | 1.53 | 8.5 | 3.78 |
| SBA-16 | 715 | 0.47 | - | 0.47 | 5.1 | 7.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczmarska, A.; Laskowska, W.; Stróż, D.; Pawlik, K. Inside the Framework: Structural Exploration of Mesoporous Silicas MCM-41, SBA-15, and SBA-16. Materials 2025, 18, 3597. https://doi.org/10.3390/ma18153597
Karczmarska A, Laskowska W, Stróż D, Pawlik K. Inside the Framework: Structural Exploration of Mesoporous Silicas MCM-41, SBA-15, and SBA-16. Materials. 2025; 18(15):3597. https://doi.org/10.3390/ma18153597
Chicago/Turabian StyleKarczmarska, Agnieszka, Wiktoria Laskowska, Danuta Stróż, and Katarzyna Pawlik. 2025. "Inside the Framework: Structural Exploration of Mesoporous Silicas MCM-41, SBA-15, and SBA-16" Materials 18, no. 15: 3597. https://doi.org/10.3390/ma18153597
APA StyleKarczmarska, A., Laskowska, W., Stróż, D., & Pawlik, K. (2025). Inside the Framework: Structural Exploration of Mesoporous Silicas MCM-41, SBA-15, and SBA-16. Materials, 18(15), 3597. https://doi.org/10.3390/ma18153597







