The Impact of Substituting Chalk with Fly Ash in Formulating a Two-Component Polyurethane Adhesive on Its Physicochemical and Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
- 100% chalk—FA0;
- 100% fly ash instead of chalk—FA100;
- 75% fly ash and 25% chalk—FA75;
- 50% fly ash and 50% chalk—FA50;
- 25% fly ash and 75% chalk—FA25;
- 10% fly ash and 90% chalk—FA10.
3. Results and Discussion
3.1. The Influence of Filler on the Viscosity of 2C PU
3.2. The Influence of Filler Type on the Physicochemical and Mechanical Properties of 2C PU
3.3. The Influence of Filler Type on VOC Release and Metal Leachability from 2C PU
3.4. Does the Fly Ash Cease to Be Waste?
3.4.1. The Substance or Object Is Commonly Used for Specific Purposes
3.4.2. A Market or Demand Exists for Such a Substance or Object
3.4.3. The Substance or Object Fulfills the Technical Requirements for Specific Purposes and Meets the Existing Legislation and Standards Applicable to Products
3.4.4. The Use of the Substance or Object Will Not Lead to Overall Adverse Environmental or Human Health Impacts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrie, E.M. Handbook of Adhesive and Sealants, 2nd ed.; The McGraw-Hill Companies: New York, NY, USA, 2007. [Google Scholar]
- Wirpsza, Z. Poliuretany: Chemia, Technologia, Zastosowanie; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1991. [Google Scholar]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2016. [Google Scholar]
- Sikdar, P.; Dip, T.M.; Dhar, A.K.; Bhattacharjee, M.; Hoque, M.S.; Ali, S.B. Polyurethane based multifunctional materials: Emerging paradigm for functional textiles, smart, and biomedical applications. J. Appl. Polym. Sci. 2022, 139, e52832. [Google Scholar] [CrossRef]
- Hinrichsen, G. Polyurethane handbook (2nd ed.). Edited by G. Oertel, Hanser Publishers, Munich 1993, 770, DM 358, ISBN 3-446-17198-3. Acta Polym. 1994, 45, 398. [Google Scholar] [CrossRef]
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; Taylor & Francis Ltd.: Abingdon, UK, 2017. [Google Scholar]
- Dodge, J. Polyurethanes and Polyureas. In Synthetic Methods in Step-Growth Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 197–263. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes, 2nd ed.; Smithers Rapra Technology Ltd.: Shrewsbury, UK, 2016. [Google Scholar]
- Evans, R.M. Polyurethane Sealants: Technology & Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Choe, H.; Lee, J.H.; Kim, J.H. Polyurethane composite foams including CaCO3 fillers for enhanced sound absorption and compression properties. Compos. Sci. Technol. 2020, 194, 108153. [Google Scholar] [CrossRef]
- Boumdouha, N.; Safidine, Z.; Boudiaf, A. A new study of dynamic mechanical analysis and the microstructure of polyurethanefoams filled. Turk. J. Chem. 2022, 46, 814–834. [Google Scholar] [CrossRef]
- Vrsaljko, D.; Blagojević, S.L.; Leskovac, M.; Kovačević, V. Effect of calcium carbonate particle size and surface pretreatment on polyurethane composite Part I: Interface and mechanical properties. Mater. Res. Innov. 2008, 12, 40–46. [Google Scholar] [CrossRef]
- e Sant’Anna, S.S.; de Souza, D.A.; de Araujo, D.M.; Carvalho, C.d.F.; Yoshida, M.I. Physico-chemical analysis of flexible polyurethane foams containing commercial calcium carbonate. Mater. Res. 2008, 11, 433–438. [Google Scholar] [CrossRef]
- Wondu, E.; Lule, Z.C.; Kim, J. Fabrication of Aliphatic Water-Soluble Polyurethane Composites with Silane Treated CaCO3. Polymers 2020, 12, 747. [Google Scholar] [CrossRef]
- Muñoz-Milán, A.B.; Pérez-Limiñana, M.Á.; Arán-Aís, F.; Torró-Palau, A.; Orgilés-Barceló, A.C. Effect of the Amount of Calcium Carbonate as Filler on the Rheological and Adhesion Properties of a Water-Based Polyurethane Dispersion. Macromol. Symp. 2005, 221, 33–42. [Google Scholar] [CrossRef]
- Ciastowicz, Ż.; Pamuła, R.; Białowiec, A. Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review. Materials 2024, 17, 1738. [Google Scholar] [CrossRef]
- European Adhesives and Sealants Market Report-2023–2028 A Quantitative Demand a Quantitative Demand Analysis and Trend Forecast Analysis and Trend Forecast. 2023. Available online: www.feica.eu (accessed on 5 March 2025).
- European Commission. Circular Economy Action Plan; European Community: Brussels, Belgium, 2020; p. 28. [Google Scholar] [CrossRef]
- Pęczek, E.; Pamuła, R.; Białowiec, A. Recycled Waste as Polyurethane Additives or Fillers: Mini-Review. Materials 2024, 17, 1013. [Google Scholar] [CrossRef]
- Kuźnia, M.; Zygmunt-Kowalska, B.; Szajding, A.; Magiera, A.; Stanik, R.; Gude, M. Comparative Study on Selected Properties of Modified Polyurethane Foam with Fly Ash. Int. J. Mol. Sci. 2022, 23, 9725. [Google Scholar] [CrossRef]
- Kuźnia, M.; Magiera, A.; Zygmunt-Kowalska, B.; Kaczorek-Chrobak, K.; Pielichowska, K.; Szatkowski, P.; Benko, A.; Ziąbka, M.; Jerzak, W. Fly ash as an eco-friendly filler for rigid polyurethane foams modification. Materials 2021, 14, 6604. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Yang, K.; Yu, W.; Zhu, F.; Zheng, Q. The Influence of Fly Ash on the Foaming Behavior and Flame Retardancy of Polyurethane Grouting Materials. Polymers 2022, 14, 1113. [Google Scholar] [CrossRef] [PubMed]
- Hadała, B.; Zygmunt-Kowalska, B.; Kuźnia, M.; Szajding, A.; Telejko, T. Thermal insulation properties of rigid polyurethane foam modified with fly ash—A comparative study. Thermochim. Acta 2024, 731, 179659. [Google Scholar] [CrossRef]
- Zakrzewska, P.; Kuźnia, M.; Zygmunt-Kowalska, B.; Magiera, A.; Magdziarz, A. Utilization of Sunflower Husk Ash in the Production of Polyurethane Materials. Energies 2023, 16, 8080. [Google Scholar] [CrossRef]
- Naveen, P.N.E.; Raju, T.D. Evaluation of Mechanical Properties of Coconut Coir Fiber Reinforced Polymer Matrix Composites. J. Nano Res. 2013, 24, 34–45. [Google Scholar] [CrossRef]
- Aminur, A.; Saputra, J.S.D.; Syah, C.Y. Analysis of Tensile and Bending Strength of Coconut Fiber Reinforcement Composite on Quasi Isotropic Laminates Stacking Sequence. J. Metall. Eng. Process. Technol. 2024, 4, 67. [Google Scholar] [CrossRef]
- Hejna, A.; Kosmela, P.; Olszewski, A.; Zedler, Ł.; Formela, K.; Skórczewska, K.; Piasecki, A.; Marć, M.; Barczewski, R.; Barczewski, M. Management of ground tire rubber waste by incorporation into polyurethane-based composite foams. Environ. Sci. Pollut. Res. 2023, 31, 17591–17616. [Google Scholar] [CrossRef]
- Piszczyk, Ł.; Hejna, A.; Formela, K.; Danowska, M.; Strankowski, M. Effect of ground tire rubber on structural, mechanical and thermal properties of flexible polyurethane foams. Iran. Polym. J. 2015, 24, 75–84. [Google Scholar] [CrossRef]
- Yeşilyurt, A. Enhancing sound transmission loss of polyurethane foams using waste soda glass filler. Gümüşhane Üniversitesi Fen Bilim. Enstitüsü Derg. 2024, 14, 1274–1286. [Google Scholar] [CrossRef]
- Naidoo, D.; Onwubu, S.C.; Mokhothu, T.H.; Mdluli, P.S.; Makgobole, M.U.; Mishra, A.K. Effectiveness of Fish Scale-Derived Collagen as an Alternative Filler Material in the Fabrication of Polyurethane Foam Composites. Adv. Polym. Technol. 2024, 2024, 1723927. [Google Scholar] [CrossRef]
- Makowska, S.; Miedzińska, K.; Kairytė, A.; Strzelec, K. Synergistic Reinforcing Effect of Hazelnut Shells and Hydrotalcite on Properties of Rigid Polyurethane Foam Composites. Polymers 2024, 16, 2968. [Google Scholar] [CrossRef]
- Onwubu, S.C.; Naidoo, D.; Obiechefu, Z.; Mokhothu, T.H. Biobased Filler Materials in Polymeric Composites: A Comprehensive Review of Epoxy and Polyurethane Systems. Preprints 2025. [Google Scholar] [CrossRef]
- Trojanowska, D.; Monie, F.; Perotto, G.; Athanassiou, A.; Grignard, B.; Grau, E.; Vidil, T.; Cramail, H.; Detrembleur, C. Valorization of waste biomass for the fabrication of isocyanate-free polyurethane foams. Green Chem. 2024, 26, 8383–8394. [Google Scholar] [CrossRef]
- Mancel, V.; Krilek, J.; Čabalová, I.; Réh, R.; Osvaldová, M.; Darabošová, A. Evaluation of selected mechanical and physical properties of particleboards containing waste plastics. Wood Res. 2024, 69, 169–178. [Google Scholar] [CrossRef]
- Vujović, N.; Alivojvodić, V.; Radovanović, D.; Štulović, M.; Sokić, M.; Kokalj, F. Towards Circularity in Serbian Mining: Unlocking the Potential of Flotation Tailings and Fly Ash. Minerals 2025, 15, 254. [Google Scholar] [CrossRef]
- EN ISO 2811-1:2023; Determination of Density—Part 1: Pycnometer Method. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 3219-2:2021; Rheology Part 2: General Principles of Rotational and Oscillatory Rheometry. International Organization for Standardization: Geneva, Switzerland, 2021.
- EN 12667:2001; Thermal Performance of Building Materials and Products—Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods—Products of High and Medium Thermal Resistance. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- PN-ISO 1928:2020; Solid Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method, and Calculation of Net Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2020.
- EN 1607:2013; Thermal Insulating Products for Building Applications—Determination of Tensile Strength Perpendicular to Faces. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 10365:2022; Adhesives. Designation of Main Failure Patterns. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 37:2024; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2024.
- Dziennik Ustaw Rzeczypospolitej Polskiej. Warszawa, Dnia 1 Września 2015 r. Poz. 1277 Rozporządzenie Ministra Gospodarki z Dnia 16 Lipca 2015 r. w Sprawie Dopuszczania Odpadów do Składowania na Składowiskach. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20150001277/O/D20151277.pdf (accessed on 29 July 2025).
- PN-EN ISO/IEC 17025:2018-02; Ogólne Wymagania Dotyczące Kompetencji Laboratoriów Badawczych i Wzorcujących. Polish Committee for Standardization: Warsaw, Poland, 2018. Available online: https://sklep.pkn.pl/pn-en-iso-iec-17025-2018-02p.html (accessed on 28 July 2025).
- PN-EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Polish Committee for Standardization: Warsaw, Poland, 2009.
- PN-EN ISO 17294-2:2016-11; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. Polish Committee for Standardization: Warsaw, Poland, 2016.
- Šebenik, U.; Krajnc, M. Influence of the soft segment length and content on the synthesis and properties of isocyanate-terminated urethane prepolymers. Int. J. Adhes. Adhes. 2007, 27, 527–535. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Wang, D.; Wang, H.; Chen, G. Rheological Properties of Cement Paste Containing Ground Fly Ash Based on Particle Morphology Analysis. Crystals 2022, 12, 524. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Fillers; ChemTec Publishing: Toronto, ON, Canada, 2021. [Google Scholar]
- Qiao, J.; Wu, G. Tensile properties of fly ash/polyurea composites. J. Mater. Sci. 2011, 46, 3935–3941. [Google Scholar] [CrossRef]
- Usman, M.A.; Adeosun, S.O.; Osifeso, G.O. Optimum Calcium Carbonate Filler Concentration for Flexible Polyurethane Foam Composite. J. Miner. Mater. Charact. Eng. 2012, 11, 311–320. [Google Scholar] [CrossRef]
- Kowalska, B.Z.; Szajding, A.; Zakrzewska, P.; Kuźnia, M.; Stanik, R.; Gude, M. Disposal of rigid polyurethane foam with fly ash as a method to obtain sustainable thermal insulation material. Constr. Build. Mater. 2024, 417, 135329. [Google Scholar] [CrossRef]
- Tlegenov, R.B.; Niyazbekova, R.K.; Jexembayeva, A.E.; Korniejenko, K.; Aruova, L.B.; Aldabergenova, S.S.; Maykonov, A.S. The Effect of Fly Ash Additive on the Thermal Conductivity of Polystyrene Concrete. Buildings 2024, 14, 2850. [Google Scholar] [CrossRef]
- Kustiyah, E.; Roziafanto, A.N.; Amrullah, M.; Priadi, D.; Chalid, M. Effect of Calcium Carbonate Content on the Mechanical and Thermal Properties of Chitosan-Coated Poly(urethane) Foams. Indones. J. Chem. 2022, 22, 827. [Google Scholar] [CrossRef]
- Thiele, L.; Poel, A.T.; Garmann, H.; Gorzny, M.; Sanch, F. Highly Flame-Resistant Polyurethane Adhesives. U.S. Patent US20050080218A1, 14 April 2005. [Google Scholar]
- Lu, S.; Feng, Y.; Zhang, P.; Hong, W.; Chen, Y.; Fan, H.; Yu, D.; Chen, X. Preparation of Flame-Retardant Polyurethane and Its Applications in the Leather Industry. Polymers 2021, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.Y.; Yuhana, N.Y.; Fariz, S.; Otoh, M.Z. The Mechanical and Thermal Properties of Polyurethanes/Precipitated Calcium Carbonate Composites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 943, 012018. [Google Scholar] [CrossRef]
- Sudoł, E.; Kozikowska, E. Mechanical Properties of Polyurethane Adhesive Bonds in a Mineral Wool-Based External Thermal Insulation Composite System for Timber Frame Buildings. Materials 2021, 14, 2527. [Google Scholar] [CrossRef]
- Torro-Palau, A.; Fernandez-Garcia, J.C.; Orgiles-Barcelo, A.C.; Pastor-Blas, M.M.; Martin-Martinez, J.M. Characterization of solvent-based polyurethane adhesives containing sepiolite as a filler. Rheological, mechanical, surface, and adhesion properties. J. Adhes. Sci. Technol. 1997, 11, 247–262. [Google Scholar] [CrossRef]
- De Smet, D.; Uyttendaele, W.; Vanneste, M. Bio-Based 2K PU Coating for Durable Textile Applications. Coatings 2022, 12, 169. [Google Scholar] [CrossRef]
- ISO 13934-1:2013; Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- Zhai, D.; Sun, Q.; Wang, Y.; Li, J. Microstructure and mechanical performance of polyurethane-fly ash composites (PU-FAC) under different effect factors. Case Stud. Constr. Mater. 2024, 21, e03405. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wu, Y.; Zhou, Y.; Guo, J.; Zhu, B. Effects of Temperature on the Mechanical Properties and Microstructure of Polyurethane Composites. Polymers 2022, 14, 4586. [Google Scholar] [CrossRef]
- Stein, S.E.; Ausloos, P.; Clifton, C.L.; Lias, S.G.; Mikaia, A.I.; Tchekhovskoi, D.; Sparkman, O.D.; Zaikin, V.G.; Zhu, D. NIST Standard Reference Database 1A NIST/EPA/NIH Mass Spectral Library (NIST 08) and NIST Mass Spectral Search Program (Version 2.0f) for Use with Microsoft® Windows User’s Guide; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 1987. [Google Scholar]
- Amirav, A.; Neumark, B.; Elkabets, O.; Alon, T. NIST Library Identification Probabilities Are the Highest with Cold EI Mass Spectra. J. Am. Soc. Mass. Spectrom. 2025, 36, 221–228. [Google Scholar] [CrossRef]
- Kozicki, M.; Guzik, K. Comparison of VOC Emissions Produced by Different Types of Adhesives Based on Test Chambers. Materials 2021, 14, 1924. [Google Scholar] [CrossRef]
- Regulation of the Minister of Economy of 16 July 2015 on the Acceptance of Waste for Landfill. Journal of Laws of the Republic of Poland (Dziennik Ustaw) 2015, item 1277. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001277 (accessed on 28 July 2025).
- Wowkonowicz, P.; Bojanowicz-Bablok, A.; Gworek, B. Wykorzystanie Odpadów z Przemysłu Wydobywczego i Hutnictwa w Drogownictwie; Politechnika Koszalińska: Koszalin, Poland, 2018; Volume 20. [Google Scholar]
- Directive 2008/98/EC on Waste (Waste Framework Directive). Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-framework-directive_en?prefLang=pl (accessed on 22 April 2025).

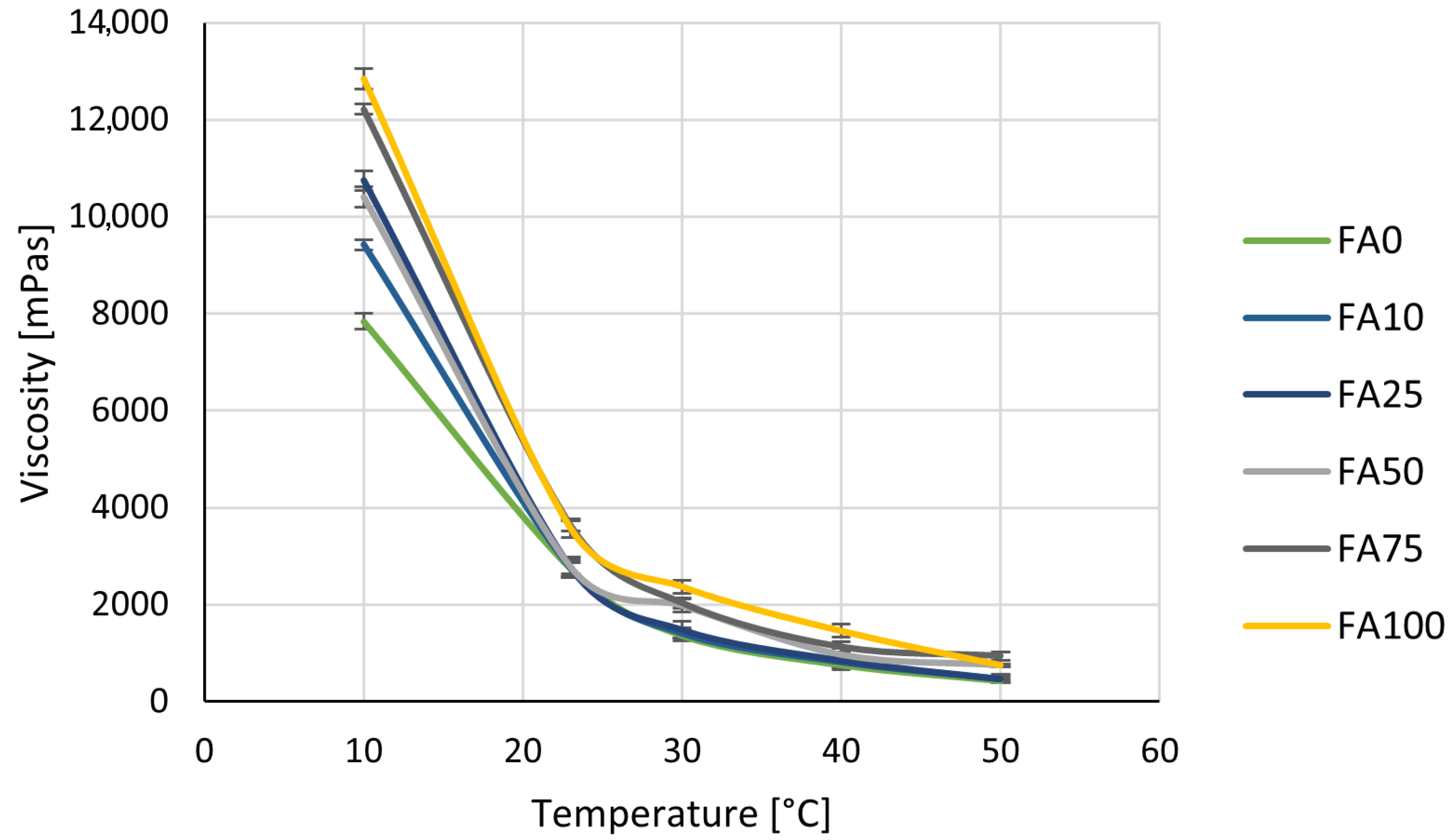

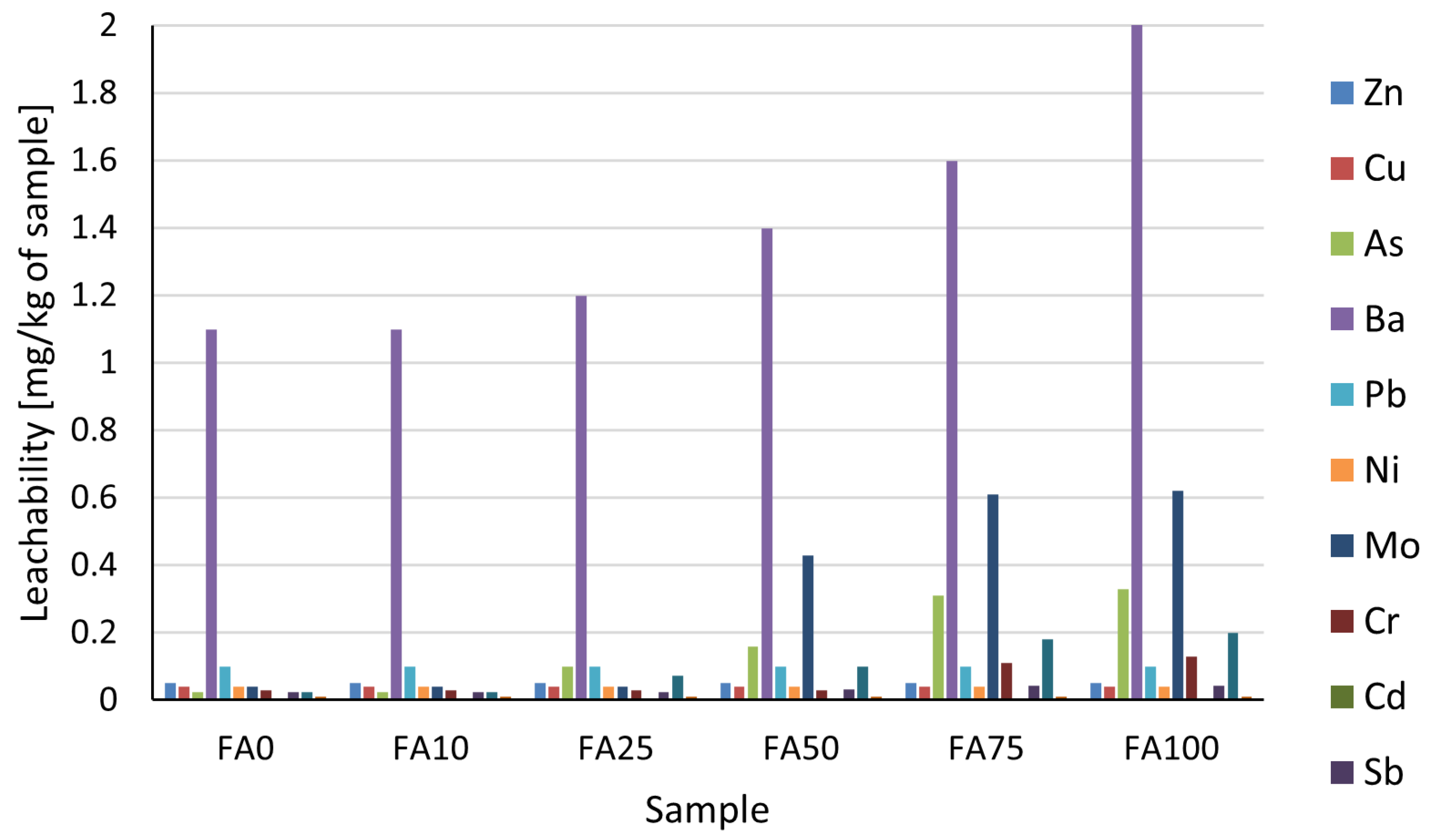
| Parameter | FA0 | FA10 | FA25 | FA50 | FA75 | FA100 |
|---|---|---|---|---|---|---|
| Density [g/cm3] | 1.46 ± 0.01 | 1.45 ± 0.01 | 1.44 ± 0.01 | 1.42 ± 0.01 | 1.39 ± 0.01 | 1.32 ± 0.01 |
| Open time [min] | 70 ± 0 | 70 ± 0 | 70 ± 0 | 70 ± 0 | 70 ± 0 | 70 ± 0 |
| Thermal conductivity [W/m.K] | 0.2960 ± 0.0021 | 0.3277 ± 0.0010 | 0.3355 ± 0.0030 | 0.3361 ± 0.0017 | 0.3814 ± 0.0019 | 0.3936 ± 0.0027 |
| High heating value [J/g] | 18,700 ± 200 | 18,700 ± 300 | 19,300 ± 200 | 20,000 ± 300 | 20,400 ± 300 | 21,700 ± 200 |
| Shore hardness A | 85 ± 2 | 80 ± 3 | 78 ± 3 | 78 ± 3 | 76 ± 2 | 73 ± 4 |
| Parameter: Tensile Strength Perpendicular to Faces | FA0 | FA10 | FA25 | FA50 | FA75 | FA100 | |
|---|---|---|---|---|---|---|---|
| galvanized steel + XPS | Maximum Load [N] | 1150 ± 30 | 1140 ± 30 | 1180 ± 60 | 1160 ± 70 | 1190 ± 30 | 1160 ± 40 |
| Stress at Maximum Load [kPa] | 460 ± 20 | 440 ± 60 | 430 ± 40 | 460 ± 40 | 450 ± 20 | 440 ± 30 | |
| aluminum + XPS | Maximum Load [N] | 1160 ± 30 | 1150 ± 30 | 1180 ± 70 | 1170 ± 40 | 1170 ± 40 | 1100 ± 70 |
| Stress at Maximum Load [kPa] | 460 ± 30 | 500 ± 30 | 440 ± 40 | 430 ± 30 | 460 ± 50 | 460 ± 40 | |
| PARAMETER | TEMP. | FA0 | FA10 | FA25 | FA50 | FA75 | FA100 |
|---|---|---|---|---|---|---|---|
| Maximum Stress [MPa] | T = 10° | 5.61 ± 0.11 | 5.60 ± 0.24 | 5.59 ± 0.20 | 5.50 ± 0.15 | 5.42 ± 0.21 | 5.36 ± 0.06 |
| T = 23° | 5.63 ± 0.16 | 5.61 ± 0.20 | 5.55 ± 0.19 | 5.53 ± 0.22 | 5.40 ± 0.08 | 5.29 ± 0.10 | |
| T = 40° | 5.37 ± 0.12 | 5.31 ± 0.16 | 5.29 ± 0.15 | 4.90 ± 0.19 | 4.86 ± 0.20 | 4.68 ± 0.12 | |
| T = 80° | 4.85 ± 0.10 | 4.81 ± 0.17 | 4.81 ± 0.16 | 4.76 ± 0.20 | 4.43 ± 0.24 | 3.96 ± 0.16 | |
| T = 120° | 3.86 ± 0.01 | 3.71 ± 0.2 | 3.58 ± 0.10 | 3.53 ± 0.18 | 3.43 ± 0.14 | 3.37 ± 0.20 | |
| Stress at Break [MPa] | T = 10° | 4.67 ± 0.10 | 4.67 ± 0.21 | 4.57 ± 0.14 | 4.56 ± 0.24 | 4.48 ± 0.21 | 4.47 ± 0.14 |
| T = 23° | 4.77 ± 0.22 | 4.63 ± 0.20 | 4.57 ± 0.17 | 4.55 ± 0.17 | 4.48 ± 0.20 | 4.48 ± 0.16 | |
| T = 40° | 4.68 ± 0.19 | 4.50 ± 0.19 | 4.30 ± 0.18 | 3.99 ± 0.18 | 3.98 ± 0.18 | 3.94 ± 0.18 | |
| T = 80° | 4.58 ± 0.17 | 4.50 ± 0.16 | 4.29 ± 0.24 | 3.94 ± 0.20 | 3.83 ± 0.09 | 3.81 ± 0.19 | |
| T = 120° | 3.56 ± 0.20 | 3.51 ± 0.13 | 3.51 ± 0.17 | 3.46 ± 0.21 | 3.33 ± 0.05 | 3.35 ± 0.20 | |
| Elongation at Break [%] | T = 10° | 63.20 ± 0.13 | 62.80 ± 0.14 | 62.50 ± 0.14 | 62.30 ± 0.13 | 60.90 ± 0.16 | 59.99 ± 0.25 |
| T = 23° | 62.20 ± 0.14 | 61.23 ± 0.13 | 61.10 ± 0.13 | 61.80 ± 0.14 | 60.60 ± 0.14 | 59.58 ± 0.11 | |
| T = 40° | 61.90 ± 0.15 | 61.30 ± 0.20 | 60.91 ± 0.15 | 60.70 ± 0.21 | 59.40 ± 0.20 | 58.10 ± 0.11 | |
| T = 80° | 44.90 ± 0.16 | 43.60 ± 0.13 | 43.10 ± 0.16 | 42.10 ± 0.20 | 42.90 ± 0.16 | 42.10 ± 0.13 | |
| T = 120° | 40.60 ± 0.08 | 40.20 ± 0.16 | 40.10 ± 0.17 | 39.60 ± 0.15 | 38.30 ± 0.18 | 33.10 ± 0.11 |
| Parameter | DOC | TDS | Sulfates | Fluorides | Chlorides |
|---|---|---|---|---|---|
| Unit | mg/kg s.m. | ||||
| FA0 | 340 | 3200 | <100 | <1.0 | <50 |
| FA10 | 310 | 2360 | <100 | <1.0 | <50 |
| FA25 | 320 | 2460 | 150 | 4.7 | <50 |
| FA50 | 200 | 3220 | 250 | 7.0 | <50 |
| FA75 | 220 | 3780 | 400 | 11 | <50 |
| FA100 | 240 | 4260 | 430 | 13 | <50 |
| acceptable limit | 800 | 60,000 | 2000 | 150 | 1500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pęczek, E.; Pamuła, R.; Ciastowicz, Ż.; Telega, P.; Bobak, Ł.; Białowiec, A. The Impact of Substituting Chalk with Fly Ash in Formulating a Two-Component Polyurethane Adhesive on Its Physicochemical and Mechanical Properties. Materials 2025, 18, 3591. https://doi.org/10.3390/ma18153591
Pęczek E, Pamuła R, Ciastowicz Ż, Telega P, Bobak Ł, Białowiec A. The Impact of Substituting Chalk with Fly Ash in Formulating a Two-Component Polyurethane Adhesive on Its Physicochemical and Mechanical Properties. Materials. 2025; 18(15):3591. https://doi.org/10.3390/ma18153591
Chicago/Turabian StylePęczek, Edyta, Renata Pamuła, Żaneta Ciastowicz, Paweł Telega, Łukasz Bobak, and Andrzej Białowiec. 2025. "The Impact of Substituting Chalk with Fly Ash in Formulating a Two-Component Polyurethane Adhesive on Its Physicochemical and Mechanical Properties" Materials 18, no. 15: 3591. https://doi.org/10.3390/ma18153591
APA StylePęczek, E., Pamuła, R., Ciastowicz, Ż., Telega, P., Bobak, Ł., & Białowiec, A. (2025). The Impact of Substituting Chalk with Fly Ash in Formulating a Two-Component Polyurethane Adhesive on Its Physicochemical and Mechanical Properties. Materials, 18(15), 3591. https://doi.org/10.3390/ma18153591







