The Fabrication of Porous Al2O3 Ceramics with Ultra-High Mechanical Strength and Oil Conductivity via Reaction Bonding and the Addition of Pore-Forming Agents
Abstract
1. Introduction
2. Experimental Procedure
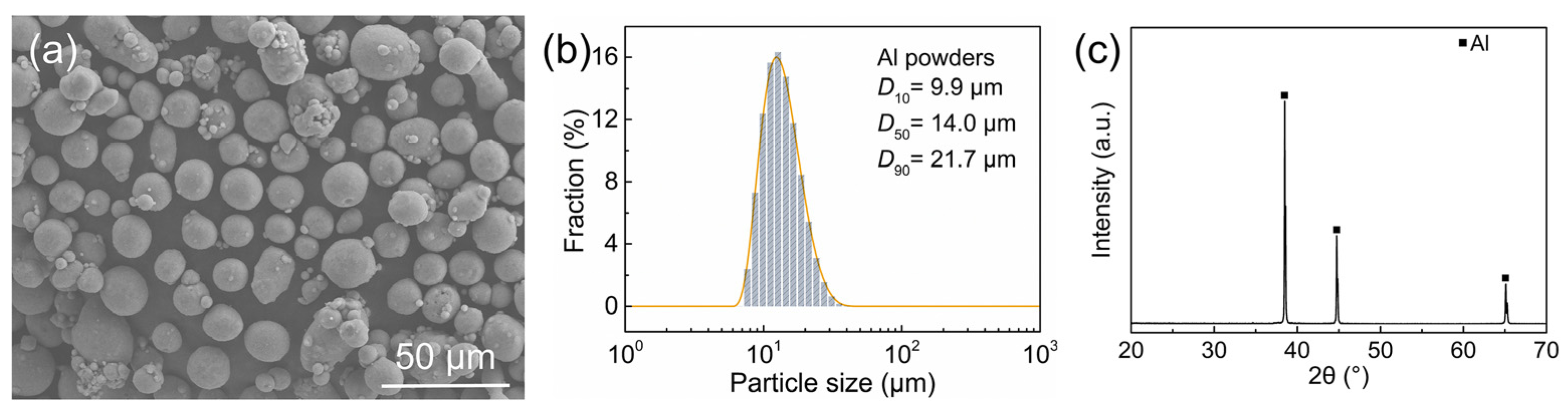
2.1. Raw Materials
2.2. Porous Ceramic Preparation
2.3. Characterization
3. Results and Discussion
3.1. Tunable Pore Structures and Optimized Comprehensive Properties
3.1.1. Effect of PMMA Microbead Addition Amount
3.1.2. Effect of PMMA Microbead Particle Size
3.1.3. Effect of PMMA Microbead Particle Gradation
3.2. Enhancement Mechanisms of Mechanical Strength and Oil Conductivity
3.2.1. Enhancement Mechanisms of Mechanical Strength
3.2.2. Oil Conductivity Enhancement Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Li, D.; Ru, J.; Yang, M.; Lu, L.; Lu, L.; Wu, J.; Huang, Z.; Xie, Y.; Gao, N. A numerical study on capillary-evaporation behavior of porous wick in electronic cigarettes. Sci. Rep. 2021, 11, 10348. [Google Scholar] [CrossRef]
- Hai, O.; Xiao, X.; Xie, Q.; Ren, Q.; Wu, X.; Pei, M.; Zheng, P. Preparation of three-dimensionally linked pore-like porous atomized ceramics with high oil and water absorption rates. J. Eur. Ceram. Soc. 2023, 43, 4530–4540. [Google Scholar] [CrossRef]
- Zhu, D.-Q.; Yang, R.; Chen, S.-Y.; He, Z.-Z.; Lin, X.-W.; Zhou, Z.-F.; Chen, B. Experimental study on the boiling behavior and film evolution of e-liquid on the surface of porous ceramic in e-cigarette. Appl. Therm. Eng. 2024, 236, 121694. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, Y.; Xia, Z.; Yu, H.; Yang, J.; Wang, X. High-strength, 3D interconnected alumina ceramic foams with high porosity comparable to aerogels. Ceram. Int. 2023, 49, 39070–39075. [Google Scholar] [CrossRef]
- Pandey, V.; Panda, S.K.; Singh, V.K. Alumina dissolution process to fabricate bimodal pore architecture alumina with superior green and sintered properties. J. Am. Ceram. Soc. 2023, 106, 6425–6440. [Google Scholar] [CrossRef]
- Huang, X.; Feng, G.; Mu, J.; Li, Y.; Xie, W.; Wu, F.; Guo, Z.; Xu, Y.; Wang, Z.; Jiang, F. Effects of Sodium Sources on Nonaqueous Precipitation Synthesis of β″-Al2O3 and Formation Mechanism of Uniform Ionic Channels. Langmuir 2025, 41, 2044–2052. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, W.; Liu, J.; Li, C.; Zhang, Q.; Miao, L.; Wu, Q. Synthesis and luminescence properties of Al2O3@YAG: Ce core–shell yellow phosphor for white LED application. Ceram. Int. 2018, 44, 8435–8439. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Yan, S.; Zhang, Z.; Huo, W.; Zhang, X.; Yang, J. Optimal design on the mechanical and thermal properties of porous alumina ceramics based on fractal dimension analysis. Int. J. Appl. Ceram. Technol. 2017, 15, 643–652. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.T.; Hanim, M.A.A.; Norkhairunnisa, M.; Sobri, S.; Calin, R. Investigating the effect of porosity level and pore former type on the mechanical and corrosion resistance properties of agro-waste shaped porous alumina ceramics. Ceram. Int. 2017, 43, 8743–8754. [Google Scholar] [CrossRef]
- Vemoori, R.; Bejugama, S.; Khanra, A.K. Fabrication and characterization of alumina and zirconia-toughened alumina porous structures. Ceram. Int. 2023, 49, 21708–21715. [Google Scholar] [CrossRef]
- Schelm, K.; Fey, T.; Dammler, K.; Betke, U.; Scheffler, M. Hierarchical-porous ceramic foams by a combination of replica and freeze technique. Adv. Eng. Mater. 2019, 21, 1801362. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Li, Y.; Xiang, R.; Luo, H.; Zhou, Z.; Zhang, Z.; Guo, W. Preparation of novel reticulated prickly porous ceramics with mullite whiskers. J. Eur. Ceram. Soc. 2021, 41, 864–870. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Z.; Zhang, X.; Huo, W.; Liu, J.; Yan, S.; Yang, J. Design and formulation of polyurethane foam used for porous alumina ceramics. J. Polym. Res. 2018, 25, 136. [Google Scholar] [CrossRef]
- Liu, J.; Ren, B.; Wang, Y.; Lu, Y.; Wang, L.; Chen, Y.; Yang, J.; Huang, Y. Hierarchical porous ceramics with 3D reticular architecture and efficient flow-through filtration towards high-temperature particulate matter capture. Chem. Eng. J. 2019, 362, 504–512. [Google Scholar] [CrossRef]
- Dang, W.; Wang, W.; Wu, P.; Li, F.; Zhao, K.; Tang, Y. Freeze-cast porous Al2O3 ceramics strengthened by up to 80% ceramics fibers. Ceram. Int. 2022, 48, 9835–9841. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, S.; Wu, C.; Miao, Q.; Zhao, K. Freeze cast fabrication of porous ceramics using tert-butyl alcohol-water crystals as template. J. Eur. Ceram. Soc. 2016, 36, 1513–1518. [Google Scholar] [CrossRef]
- Ahmad, J.; Tariq, M.I.; Ahmad, R.; ul-Hassan, S.M.; Mehmood, M.; Khan, A.F.; Waseem, S.; Mehboob, S.; Tanvir, M.T. Formation of porous α-alumina from ammonium aluminum carbonate hydroxide whiskers. Ceram. Int. 2019, 45, 4645–4652. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.; Guo, A.; Zhang, Y.; Ren, S.; Sui, G.; Du, H. Synthesis and properties of porous alumina ceramics with inter-locked plate-like structure through the tert-butyl alcohol-based gel-casting method. J. Alloys Compd. 2017, 694, 1045–1053. [Google Scholar] [CrossRef]
- Zou, Y.; Li, C.-H.; Liu, J.-A.; Wu, J.-M.; Hu, L.; Gui, R.-F.; Shi, Y.-S. Towards fabrication of high-performance Al2O3 ceramics by indirect selective laser sintering based on particle packing optimization. Ceram. Int. 2019, 45, 12654–12662. [Google Scholar] [CrossRef]
- Liu, S.S.; Li, M.; Wu, J.M.; Chen, A.N.; Shi, Y.S.; Li, C.H. Preparation of high-porosity Al2O3 ceramic foams via selective laser sintering of Al2O3 poly-hollow microspheres. Ceram. Int. 2020, 46, 4240–4247. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, A.; Yang, T.; Gao, S.; Liu, S.; Jiang, H.; Shi, Y.; Hu, C. Ultra-lightweight ceramic scaffolds with simultaneous improvement of pore interconnectivity and mechanical strength. J. Mater. Sci. Technol. 2023, 137, 247–258. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, H.; Yang, S.; Guo, Y.; Li, N.; Zhou, H.; Cao, Y. Frozen slurry-based laminated object manufacturing to fabricate porous ceramic with oriented lamellar structure. J. Eur. Ceram. Soc. 2018, 38, 4014–4019. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, X.; Tervoort, E.; Gantenbein, S.; Yang, J.; Studart, A.R. Ultrastrong hierarchical porous materials via colloidal assembly and oxidation of metal particles. Adv. Funct. Mater. 2020, 30, 2003550. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, H.; Chen, A.; Yang, T.; Gao, S.; Liu, S. Near-zero-shrinkage Al2O3 ceramic foams with coral-like and hollow-sphere structures via selective laser sintering and reaction bonding. J. Eur. Ceram. Soc. 2021, 41, 239–246. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, A.; Yang, T.; Gao, S.; Liu, S.; Guo, B.; Jiang, H.; Shi, Y.; Yan, C. Microstructure evolution and mechanical properties of Al2O3 foams via laser powder bed fusion from Al particles. Adv. Powder Mater. 2023, 2, 100135. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, M.; Rong, Y.; Zhang, Y.; Li, Y.; Wang, X.; Yang, J. A novel process to high-strength and controlled-shrinkage Al2O3 foams with open-cell by Al powder hollowing technology. J. Am. Ceram. Soc. 2023, 106, 3954–3963. [Google Scholar] [CrossRef]
- Li, H.; Xia, Z.; Rong, Y.; Dong, Y.; Qiao, J.; Yang, J. Alumina ceramic foams with an open hierarchical pore structure prepared by oxidation hollowing of aluminum. Int. J. Appl. Ceram. Technol. 2025, 22, e15049. [Google Scholar] [CrossRef]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef]
- Tianou, H.; Wang, W.; Yang, X.; Cao, Z.; Kuang, Q.; Wang, Z.; Shan, Z.; Jin, M.; Yin, Y. Inflating hollow nanocrystals through a repeated Kirkendall cavitation process. Nat. Commun. 2017, 8, 1261. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, J. Preparation and properties of porous diatomite ceramics by pore-forming method. Mater. Rep. 2022, 36, 21070121. (In Chinese) [Google Scholar]
- Dong, Y.; Jiang, H.; Chen, A.; Yang, T.; Zou, T.; Xu, D. Porous Al2O3 ceramics with spontaneously formed pores and enhanced strength prepared by indirect selective laser sintering combined with reaction bonding. Ceram. Int. 2020, 46, 15159–15166. [Google Scholar] [CrossRef]
- Tığlı, A.; Çağın, T. A case study on metal-ceramic interfaces: Wetting of alumina by molten aluminum. Mater. Sci. Forum 2018, 915, 185–189. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Yang, G.; Guan, R.; Zhen, H.; Ou, K.; Fang, J.; Li, D.S.; Fu, Q.; Sun, Y. Tunable size of hierarchically porous alumina ceramics based on DIW 3D printing supramolecular gel. ACS Appl Mater Interfaces 2022, 14, 10998–11005. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, S.; Chen, G.; Li, K.; Fei, Z.; Mummery, P.; Yang, Z. High-strength, multifunctional and 3D printable mullite-based porous ceramics with a controllable shell-pore structure. Adv. Powder Mater. 2024, 3, 100153. [Google Scholar] [CrossRef]
- Grebenyuk, Y.; Zhang, H.X.; Wilhelm, M.; Rezwan, K.; Dreyer, M.E. Wicking into porous polymer-derived ceramic monoliths fabricated by freeze-casting. J. Eur. Ceram. Soc. 2017, 37, 1993–2000. [Google Scholar] [CrossRef]
- Roger, J.; Avenel, M.; Lapuyade, L. Characterization of SiC ceramics with complex porosity by capillary infiltration: Part A—Filling by hexadecane at 20 °C. J. Eur. Ceram. Soc. 2020, 40, 1859–1868. [Google Scholar] [CrossRef]
- Cai, J.; Jin, T.; Kou, J.; Zou, S.; Xiao, J.; Meng, Q. Lucas–Washburn equation-based modeling of capillary-driven flow in porous systems. Langmuir 2021, 37, 1623–1636. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Yang, X.; Li, H.; Xia, Z.; Yang, J. The Fabrication of Porous Al2O3 Ceramics with Ultra-High Mechanical Strength and Oil Conductivity via Reaction Bonding and the Addition of Pore-Forming Agents. Materials 2025, 18, 3574. https://doi.org/10.3390/ma18153574
Dong Y, Yang X, Li H, Xia Z, Yang J. The Fabrication of Porous Al2O3 Ceramics with Ultra-High Mechanical Strength and Oil Conductivity via Reaction Bonding and the Addition of Pore-Forming Agents. Materials. 2025; 18(15):3574. https://doi.org/10.3390/ma18153574
Chicago/Turabian StyleDong, Ye, Xiaonan Yang, Hao Li, Zun Xia, and Jinlong Yang. 2025. "The Fabrication of Porous Al2O3 Ceramics with Ultra-High Mechanical Strength and Oil Conductivity via Reaction Bonding and the Addition of Pore-Forming Agents" Materials 18, no. 15: 3574. https://doi.org/10.3390/ma18153574
APA StyleDong, Y., Yang, X., Li, H., Xia, Z., & Yang, J. (2025). The Fabrication of Porous Al2O3 Ceramics with Ultra-High Mechanical Strength and Oil Conductivity via Reaction Bonding and the Addition of Pore-Forming Agents. Materials, 18(15), 3574. https://doi.org/10.3390/ma18153574




