1. Introduction
Heat treatment represents a key engineering method for optimizing the microstructure, and consequently the mechanical and corrosion properties of structural steels. Among the various heat treatment techniques, annealing holds a prominent position due to its ability to control phase balance, grain size and shape, and homogenize the material’s structure [
1,
2]. Depending on process parameters, such as temperature, holding time, and cooling rate, annealing can significantly influence the distribution of pearlite and ferrite as well as the presence of carbides or residual stresses, ultimately determining the material’s behavior in service conditions [
3].
In industrial practice, particularly in the production of automotive and mechanical components, the ability to tailor microstructural features through thermal processing directly contributes to increased component reliability and service life. The impact of microstructure on corrosion performance has received growing attention, as it has been demonstrated that corrosion resistance is not solely governed by surface chemistry or alloy composition, but is also strongly influenced by features such as grain boundary density, phase morphology, and the distribution of inclusions or carbides.
Recent studies emphasize that microstructural tailoring via annealing, particularly the adjustment of ferrite-pearlite morphology, carbide precipitation, and grain boundary characteristics, can lead to improved surface passivation and lower corrosion rates in chloride-rich environments.
In [
4], the authors investigated the effect of annealing on the microstructure and corrosion behavior of a low-alloy AISI 5140 steel in a 3.5% NaCl solution, comparing the as-received and annealed conditions. In [
5], it was shown that spheroidized steel, especially when combined with Cu microalloying, significantly improved the corrosion resistance by reducing micro-galvanic coupling between ferrite and cementite in a chloride-containing environment. In [
6], the authors demonstrated that spheroidized steel exhibited a slower corrosion rate during long-term immersion, which was attributed to the weaker micro-galvanic effect caused by the reduced accumulation of cathodic cementite.
In [
7], a high-strength low-alloy (HSLA) steel was examined under various heat treatment conditions. The study found that a high-temperature tempered martensitic microstructure provided superior corrosion resistance (evaluated via 5% NaCl salt-spray tests) compared with other conditions, highlighting the role of tempering, particularly stress-relief and carbide precipitation, in improving corrosion performance.
In [
8], four plain-carbon steels (with 0% to ~100% pearlite) were studied under various annealing and normalizing conditions. The findings showed that pearlite refinement through normalizing (air cooling) significantly lowered the corrosion rates in 3.5% NaCl by reducing interlamellar spacing and pearlite colony size, whereas coarse pearlite produced via slow furnace cooling was more susceptible to galvanic corrosion between ferrite and cementite.
20MnCr5 is a low-alloy case-hardening steel that is frequently used for gears, shafts, and bearing parts. In industrial practice, it is routinely subjected to a sequence of thermal processes: most commonly carburizing at 900–950 °C, followed by quenching and tempering at low temperatures (200–220 °C) to produce a hard, wear-resistant surface; normalization (880–900 °C, air cooling) or full annealing (830–860 °C, slow furnace cooling) to refine equiaxed grains and relieve stresses; spheroidizing annealing (700–740 °C, prolonged holding and slow cooling) to improve machinability; and occasional stress relieving treatments (650–700 °C) to remove residual stresses from previous machining. These heat treatments allow for the independent tuning of surface hardness, core toughness, and corrosion resistance. Due to its service in high-contact and high-load conditions, the precise control of both the surface and core properties is critical for reliable performance and a long service life.
Recent studies have shown that specific annealing and normalizing treatments can induce significant microstructural changes in 20MnCr5 including the development of refined equiaxed grain structures, the spheroidization of carbides, and adjustment in the ferrite-pearlite phase balance [
9]. Such microstructural tailoring has been shown to enhance surface passivation and significantly reduce the corrosion rates in aggressive chloride-containing environments such as 3.5% NaCl solutions [
10].
Moreover, carburizing and subsequent tempering treatments influence the distribution and morphology of carbides, which in turn affect both the mechanical and electrochemical behavior of the steel [
11]. Carburizing at optimized temperatures (around 900–950 °C) refines the surface microstructure by promoting a fine martensitic grain structure and reducing retained austenite, improving hardness and wear resistance without compromising corrosion performance [
12]. Tempering treatments (typically low-temperature tempers around 200 °C for case-hardened 20MnCr5) relieve residual stresses from quenching and precipitate finely dispersed carbides, which benefits both corrosion resistance and toughness. The tempering-induced carbide precipitation is distributed in a uniform, globular form [
11], and the reduction in internal stresses helps mitigate crack initiation sites. These changes lead to improved impact toughness and fatigue performance of the hardened case without sacrificing its corrosion behavior [
9].
Taken together, these findings highlight that controlled heat treatment strategies aimed at microstructural engineering are essential to optimize the multi-functional performance of 20MnCr5 steel components in demanding industrial applications. By tailoring microstructure through processes such as annealing, normalizing, carburizing, and tempering, engineers can achieve a favorable combination of high surface hardness, core toughness, and enhanced corrosion resistance [
10,
12,
13,
14,
15,
16,
17,
18,
19], ensuring a long service life for 20MnCr5-based parts in aggressive service conditions.
Modern electrochemical characterization methods enable a detailed analysis of the influence of certain heat treatment processes on corrosion behavior. Correlation analyses between microstructural features and electrochemical parameters, such as open circuit potential (OCP), linear polarization resistance (LPR), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PDP), are increasingly employed as powerful tools to relate the grain structure, phase balance, and residual stress state to corrosion performance in chloride-containing environments [
4,
5,
6,
7,
8,
10,
12,
13,
14,
15,
16,
17,
18,
19].
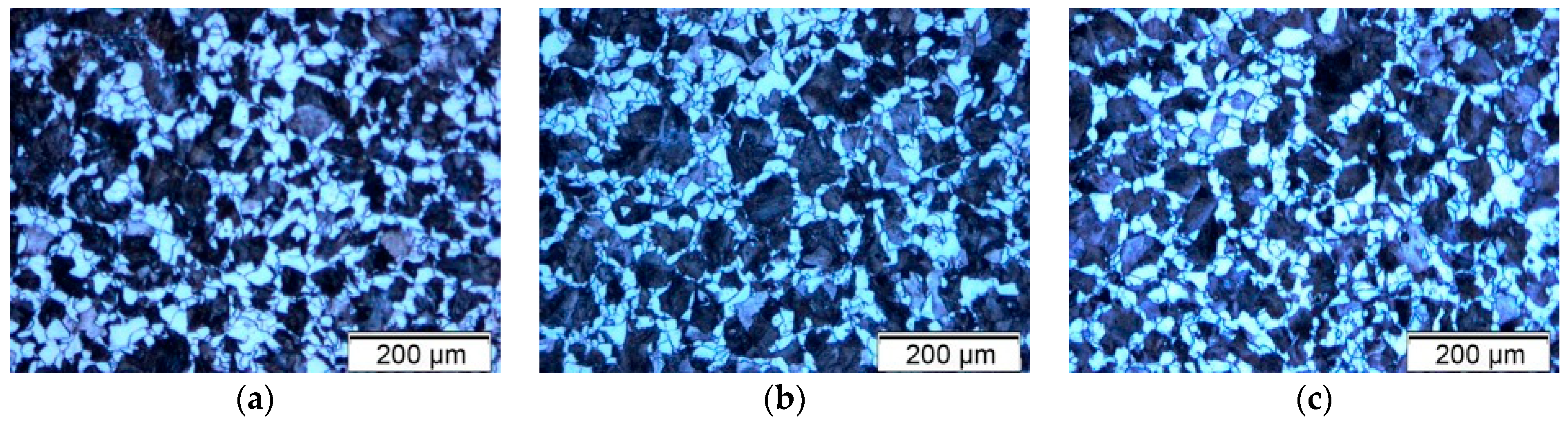
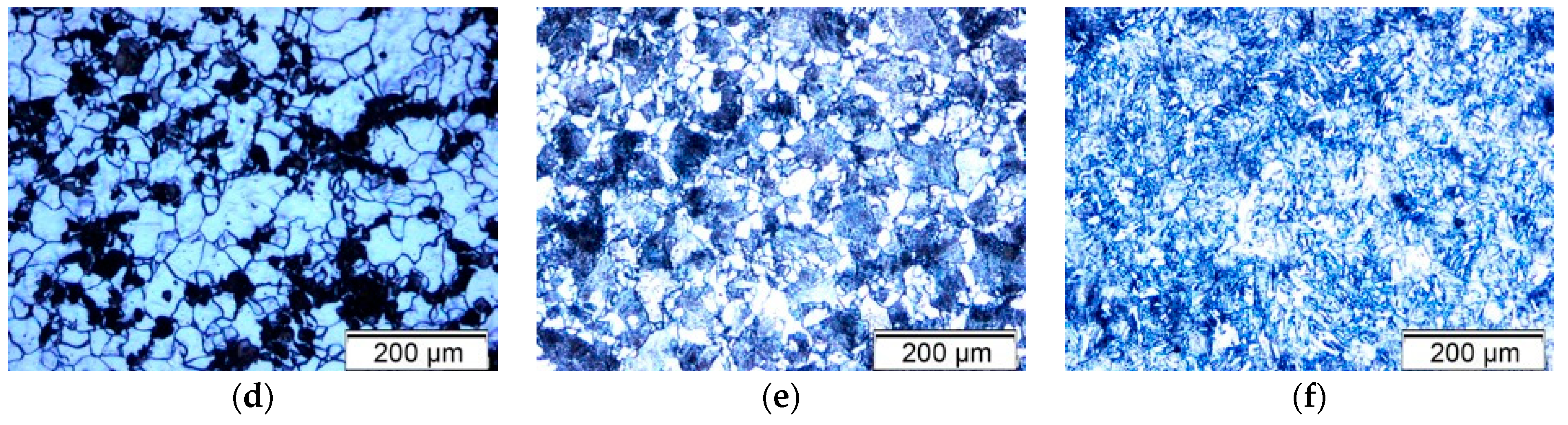
This study investigated the effect of different annealing regimes on the microstructure and corrosion resistance of 20MnCr5 steel in a 3.5% NaCl solution. The applied annealing treatments included normalizing, stress relieving, soft annealing, full annealing, spheroidizing annealing, and transformation annealing. Normalizing (N) was performed to refine the austenitic grain size, improve machinability, and reduce distortion after subsequent heat treatment [
3]. Stress relieving (+SR) was used to reduce residual stresses induced by prior machining or deformation without significantly altering the microstructure. Soft annealing (A) aims to improve the machinability by reducing hardness and homogenizing the structure. Full annealing (FA) was applied to achieve the maximum softness and ductility by producing a coarse, equiaxed ferrite-pearlite microstructure. Spheroidizing annealing (AC) promotes the formation of globular carbides to enhance formability and improve machinability, especially before cold forming. Transformation annealing (+FP) was used to obtain a uniform and fine microstructure by transforming the phases through controlled heating and cooling cycles.
The findings provide valuable insights into how annealing parameters can be tailored to optimize both the structural integrity and durability in corrosive environments. Such knowledge is particularly relevant for the design of components exposed to marine or industrial chloride conditions, where microstructural engineering plays a critical role in long-term performance and material reliability.
2. Materials and Methods
2.1. Material
The material used in this study was case-hardening 20MnCr5 steel, characterized by a low carbon content and good hardenability. It was supplied in the form of factory-produced bars with a diameter of 16 mm.
The average chemical composition of the 20MnCr5 steel was determined using a LECO GDS 500 (LECO Corporation, St. Joseph, MI, USA) a optical glow discharge spectrometer, based on the mean values obtained from five individual measurements. The detailed chemical composition is shown in
Table 1.
2.2. Treatment
From the original 20MnCr5 steel bar, six disc-shaped samples with a thickness of 4 mm were cut for the purpose of thermal processing. Each sample was subjected to a different annealing heat treatment in order to obtain a range of microstructures and corresponding properties. The applied annealing treatments and their parameters are listed in
Table 2.
Specifically, the samples underwent the following treatments: Sample 1—normalizing (N), Sample 2—stress relieving (+SR), Sample 3—soft annealing (A), Sample 4—full annealing (FA), Sample 5—spheroidizing annealing (AC), and Sample 6—transformation annealing (+FP).
The selection of heat treatment parameters (such as heating temperature, holding time, and cooling rate) was based on the technical datasheet for 20MnCr5 steel, ensuring that each regime was industrially relevant and appropriate for achieving the intended microstructural modifications.
The applied annealing heat treatments were designed to produce a range of microstructures in 20MnCr5 steel by controlling the heating temperatures, holding times, and cooling rates. According to the technical datasheet, the Ac1 and Ac3 transformation temperatures for 20MnCr5 steel are approximately 730 °C and 830 °C, respectively. Ac1 is the lower critical temperature, marking the onset of transformation from pearlite to austenite, while Ac3 is the upper critical temperature, at which the transformation from ferrite to austenite is complete, resulting in a fully austenitic structure. The temperature range between Ac1 and Ac3 defines the intercritical region, where partial transformation to austenite occurs.
These critical transformation temperatures served as the basis for determining the thermal regimes. Normalization, full annealing, and transformation annealing involved heating the samples above the Ac3 temperature to achieve complete austenitization. In contrast, stress relieving, soft annealing, and spheroidizing annealing were carried out at subcritical temperatures, typically below the Ac1 threshold.
2.3. Hardness Measurements
The hardness of the samples after heat treatment was measured to verify the effectiveness and correctness of the applied thermal processes. Since each annealing treatment was expected to result in a specific microstructural transformation—reflected in the characteristic hardness values—the measured hardness was compared with the expected values specified in the steel’s technical datasheet. This comparison served as an indirect confirmation that the targeted microstructures had been achieved.
The measurements were performed using a ZHU 187.5 (Zwick Roell Group, Ulm, Germany) universal hardness tester, applying the Rockwell B (HRB) scale, with a steel carbide ball indenter of 1.588 mm in diameter. Consistency between the measured and expected hardness values indicated that the selected heat treatment parameters (temperature, holding time, and cooling rate) were appropriately executed for each sample.
2.4. Microstructural and Phase Analysis
The microstructural characterization of 20MnCr5 steel was performed both in the as-received normalized condition and after the applied annealing heat treatments. Metallographic preparation was carried out using standard grinding and polishing procedures on a STRUERS LABOPOL system (Struers; Ballerup, Denmark). The initial grinding was performed on a resin-bonded MD-Piano 220 diamond disc (Struers), followed by polishing on an MD-Allegro composite disc (Struers) with diamond suspensions of decreasing particle size. The final polishing steps were conducted using an MD-Dac cloth (Struers) with DiaPro Dac (Struers) 3 µm and 1 µm diamond suspensions, and an MD-Chem cloth (Struers) with an OP-U NonDry colloidal silica suspension (Struers).
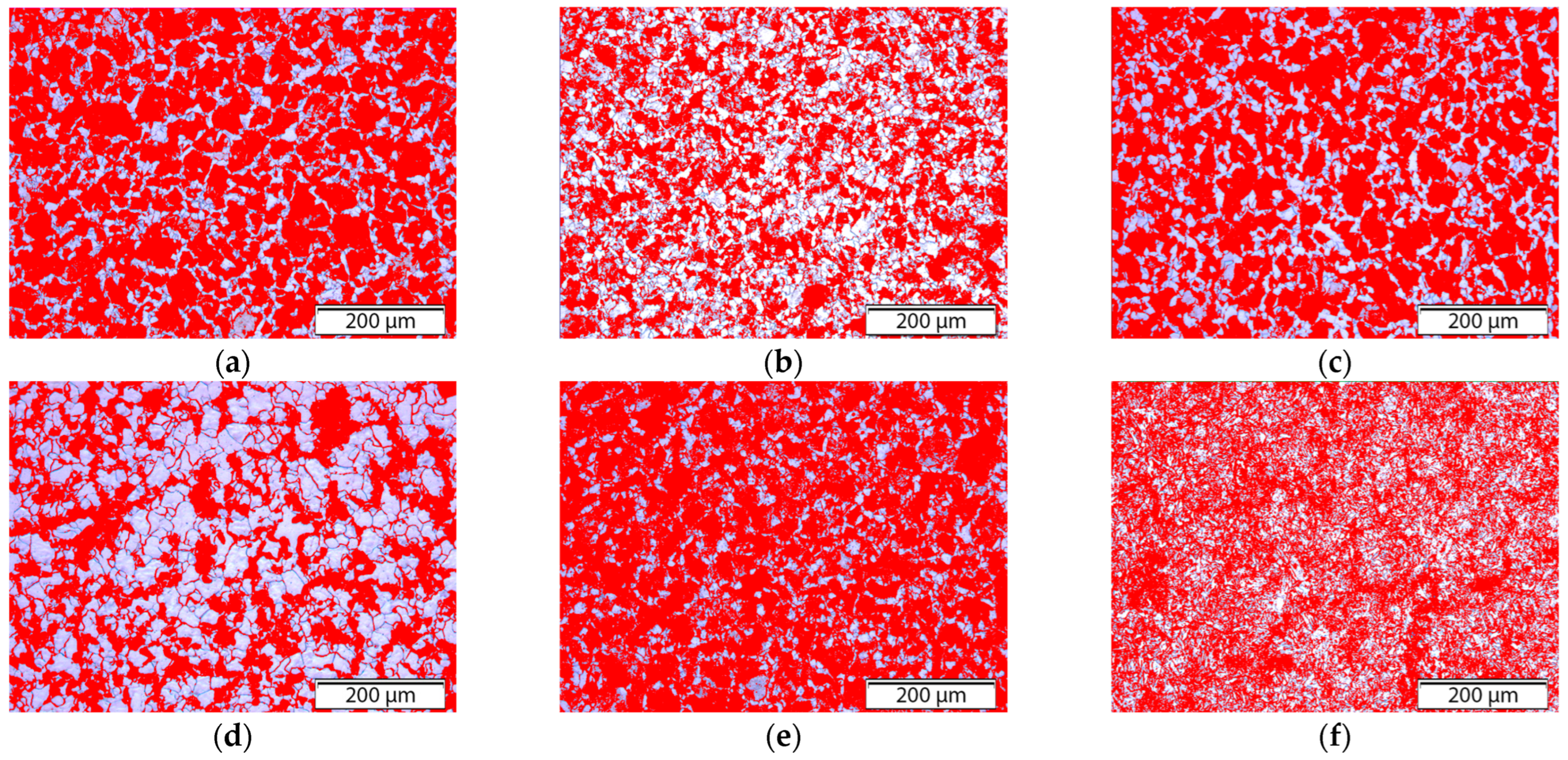
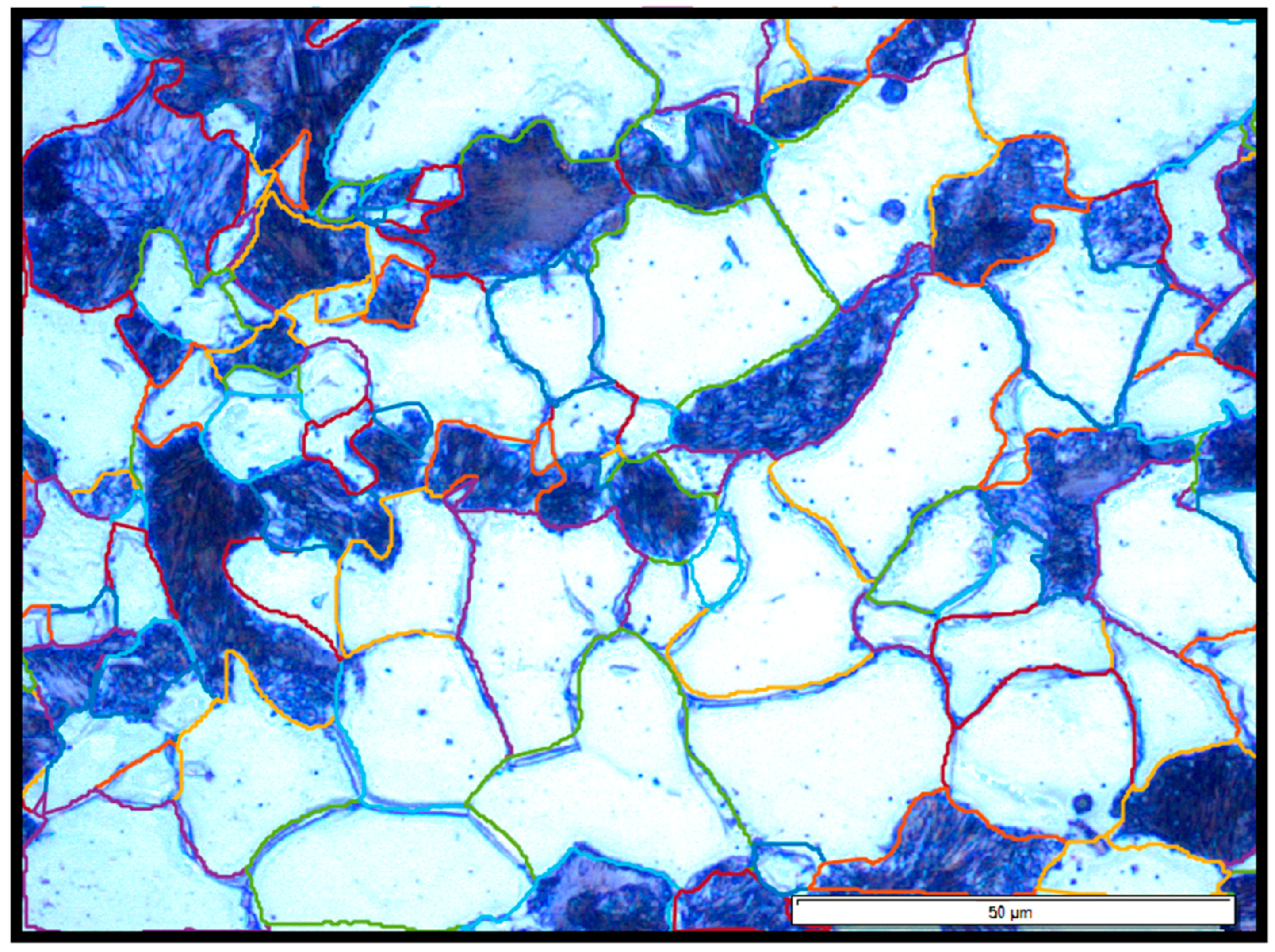
Etching was performed using a 3% Nital solution for 10–20 s to reveal the microstructural features. The samples were examined using an optical microscope (BX-51, Olympus Corporation, Tokyo, Japan) at various magnifications. Phase distribution analysis and quantification of the pearlite and ferrite phases were performed using Olympus Stream Essentials software (Olympus BX51 microscope). Grain size and morphology were further analyzed using MATLAB® (R2022a, MathWorks, Natick, MA, USA) with custom image analysis scripts, enabling the extraction of parameters such as maximum and minimum grain diameter, equivalent diameter, area, perimeter, and circularity for both the ferrite and pearlite phases.
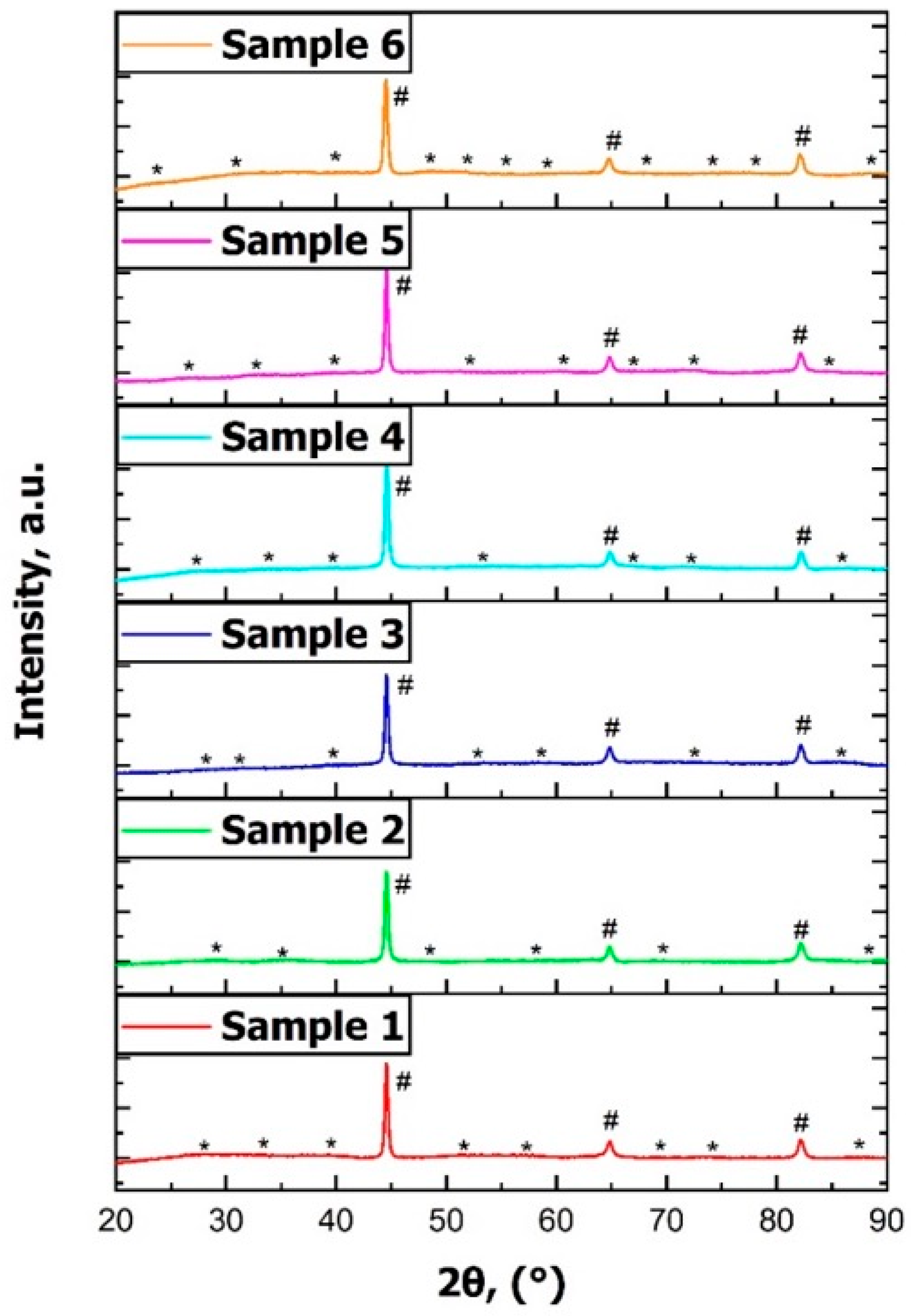
In addition, X-ray diffraction (XRD) analysis was performed on all samples using a PANalytical X’Pert Pro MPD diffractometer (Panalytical B.V. (currently: Malvern Panalytical Ltd.), Almelo, The Netherlands) equipped with a copper anode X-ray tube (Kα Cu = 0.154 nm) and a Ni filter to suppress Kβ radiation to confirm the phase composition and assess possible differences in cementite distribution related to the applied cooling rates. The XRD patterns were collected in the 2θ range from 10° to 110° with a recording step of 0.05°, and the identified peaks were indexed according to the standard diffraction data for α-Fe and Fe3C phases.
2.5. Electrochemical Testing
Electrochemical measurements were carried out using a conventional three-electrode configuration connected to an Interface 1010E potentiostat (Gamry Instruments, Warminster, PA, USA) [
20,
21]. As the electrolyte, a 3.5 wt.% aqueous NaCl solution was used and maintained at ambient temperature [
22,
23]. The investigated sample served as the working electrode, while a saturated Ag/AgCl electrode and a graphite rod were employed as the reference and counter electrodes, respectively. Prior to testing, all samples were mechanically polished with silicon carbide (SiC) abrasive papers up to 4000 grit to achieve a consistent and smooth surface finish.
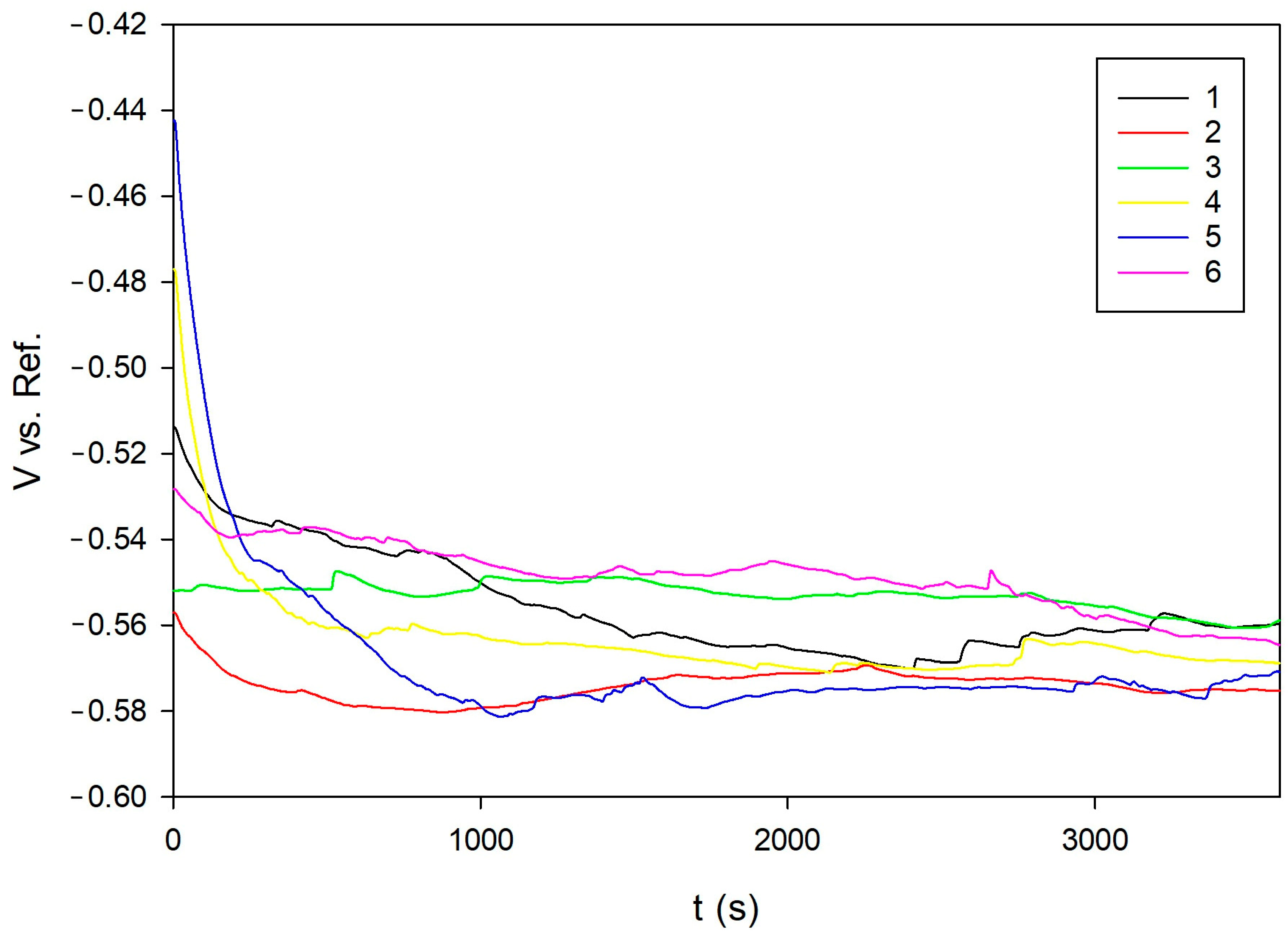
To monitor system stabilization, the open-circuit potential (OCP) was continuously recorded over a 60-min period [
24]. Following this stabilization phase, linear polarization resistance (LPR) measurements were conducted by applying a ±20 mV potential shift relative to the stabilized OCP. The polarization resistance (
Rp) was determined from the linear region of the current–potential response [
24,
25].
Electrochemical impedance spectroscopy (EIS) was utilized to assess corrosion mechanisms at the electrode–electrolyte interface. EIS spectra were collected at the OCP within the frequency range of 100 kHz to 0.01 Hz, applying a 10 mV sinusoidal signal [
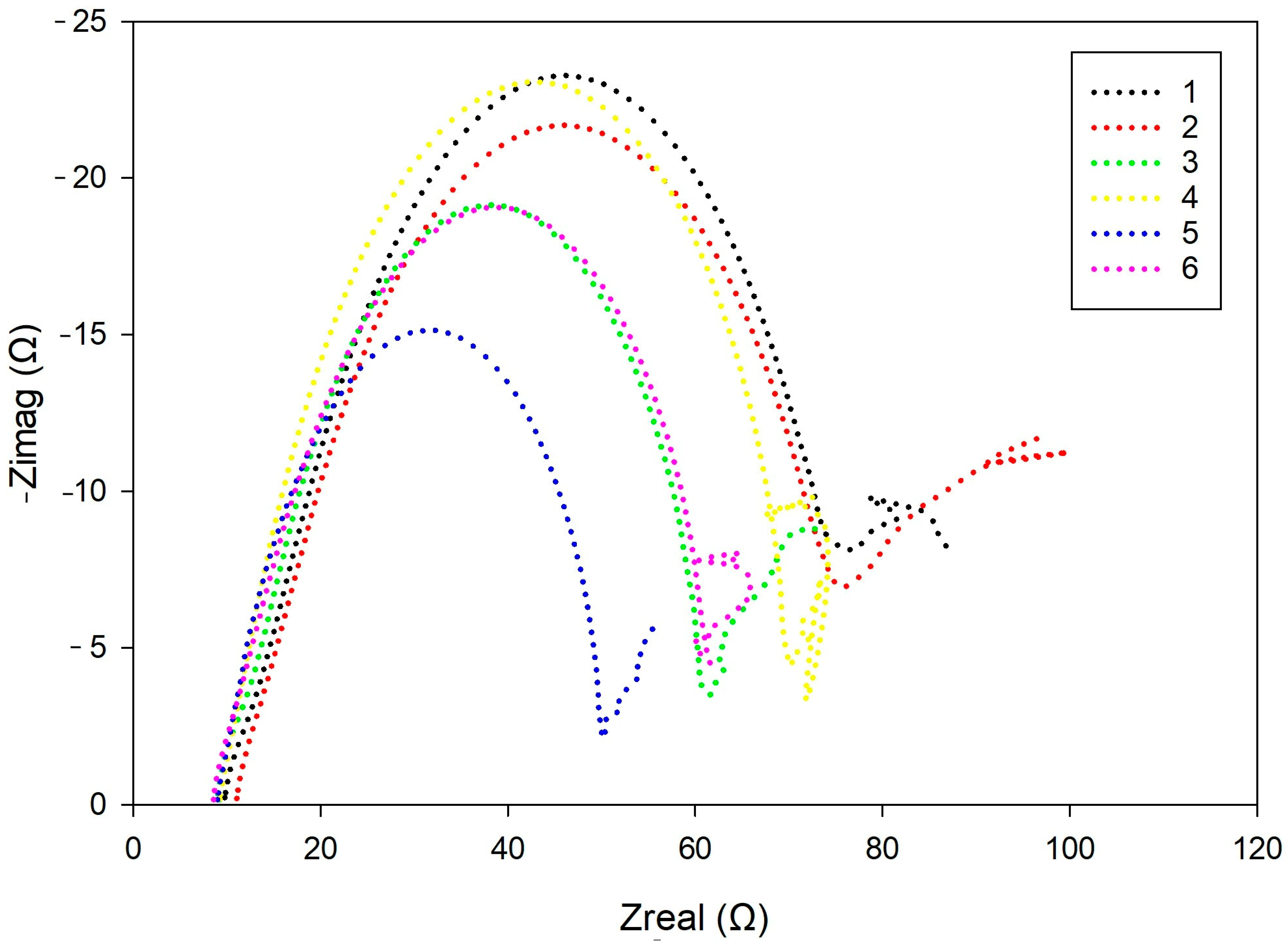
26]. The resulting data were interpreted by fitting to an equivalent electrical circuit to characterize the charge transfer and diffusion-related processes.
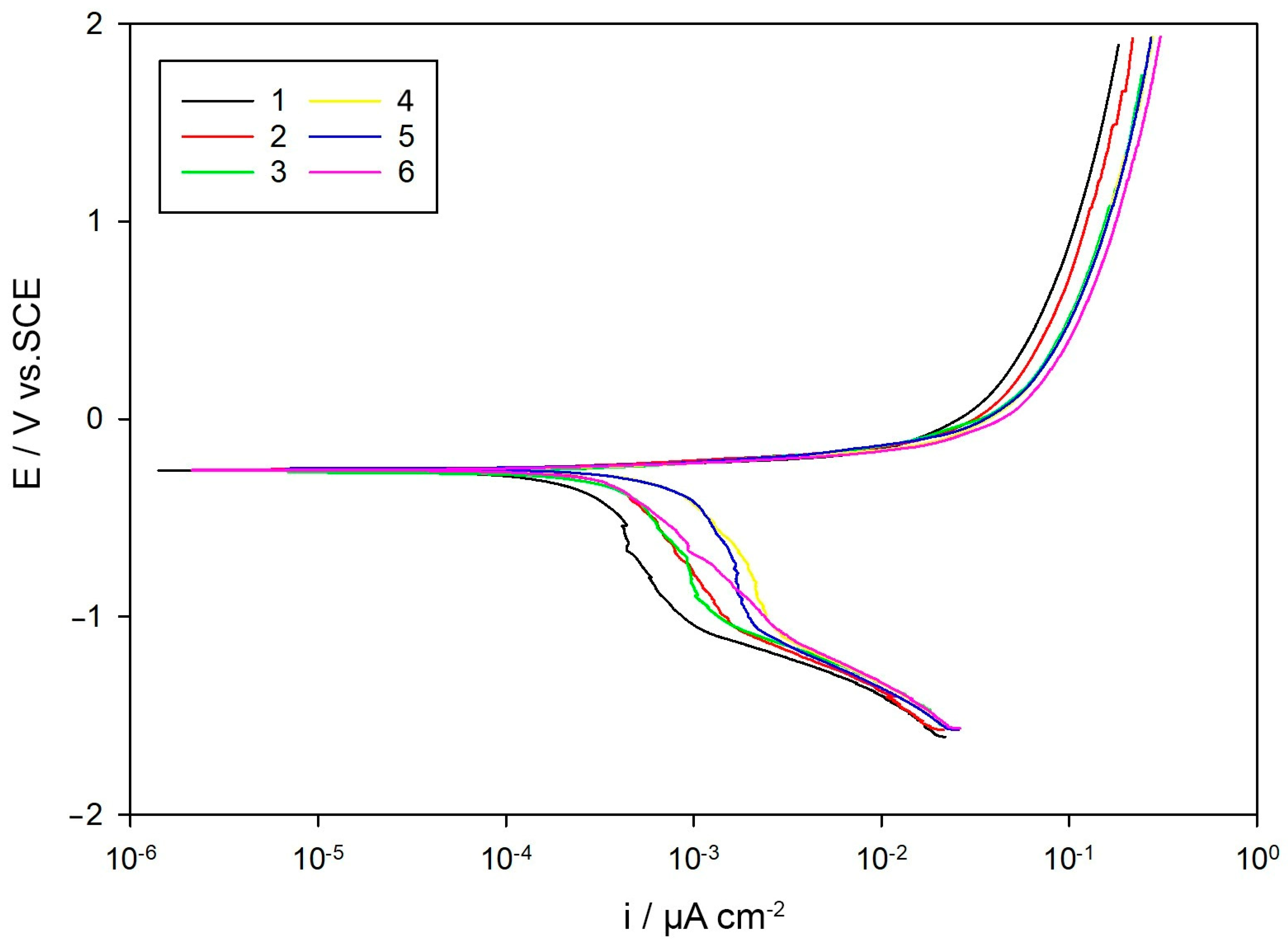
Additionally, potentiodynamic polarization tests were performed to study the corrosion kinetics in more detail. The potential scan ranged from –0.6 V to +2.5 V versus OCP, at a sweep rate of 2 mV/s. Key parameters including the corrosion potential (Ecorr), corrosion current density (icorr), and calculated corrosion rates were obtained using the Tafel extrapolation technique [
24,
27].
4. Discussion
To better understand the corrosion behavior of annealed 20MnCr5 steel, a comprehensive approach was adopted that integrated microstructural characterization with multi-method electrochemical analysis [
28]. While individual measurements such as open circuit potential (OCP), polarization resistance (LPR), impedance spectra (EIS), and potentiodynamic polarization (PDP) provide insights into specific aspects of corrosion mechanisms, a broader perspective was needed to identify interdependencies across the structural and electrochemical parameters.
Therefore, a correlation analysis was conducted to statistically link microstructural features (grain size, morphology, phase ratios), mechanical properties (hardness), and electrochemical responses (
Rp,
Icorr,
R1,
R2, corrosion rate, etc.) [
34,
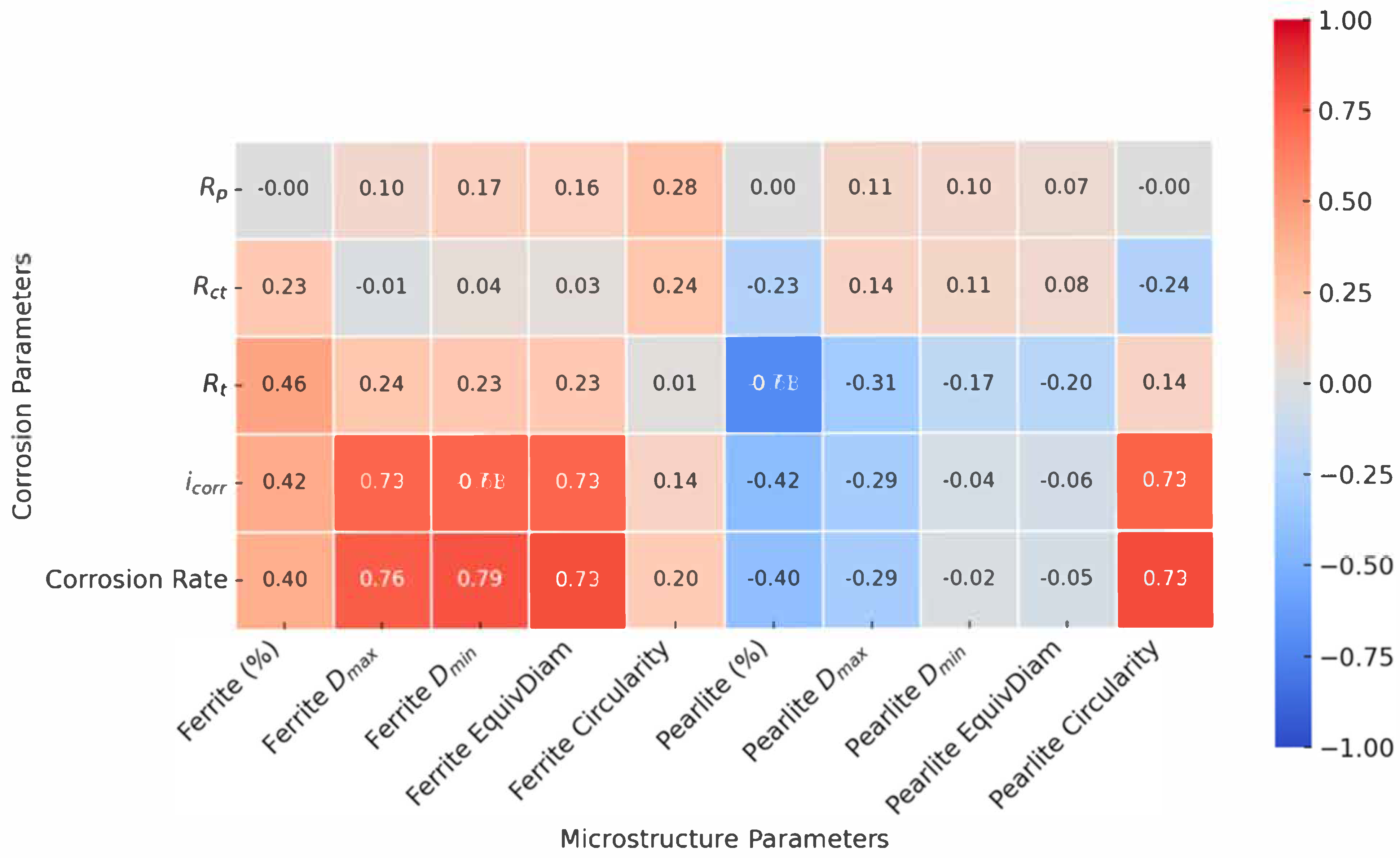
35]. The resulting correlations were visualized using a correlation diagram (
Figure 11), allowing for rapid identification of the strongest positive and negative relationships. This approach enabled an objective, data-driven interpretation of which microstructural characteristics most influenced the corrosion resistance, and provides a solid foundation for the discussion that follows.
In the revised analysis, the correlation diagram was adjusted to display only the relationships between the corrosion parameters and microstructural characteristics. Correlations within the same parameter groups (corrosion–corrosion or microstructure–microstructure) were excluded. The final diagram presents the corrosion properties on the y-axis and microstructural parameters on the x-axis, enabling a clearer interpretation of how microstructural features influence corrosion behavior (
Figure 11). Strong positive correlations (deep red) and strong negative correlations (deep blue) highlight statistically significant relationships. The correlation analysis was conducted using the Pearson correlation coefficient. Positive correlation values (r > 0) indicate that higher concentrations of a given element are associated with increased electrochemical parameter values, whereas negative values (r < 0) suggest an inverse relationship. Correlation strength was interpreted as follows: weak (|r| < 0.5), moderate (0.5 ≤ |r| < 0.8), and strong (|r| ≥ 0.8) [
35].
Based on the observed patterns, several key microstructural factors emerged as critical contributors to corrosion behavior, particularly the ferrite grain area, pearlite Dmax, and grain circularity.
The correlation map revealed a moderately strong positive correlation between the corrosion rate and pearlite content, especially when accompanied by a high pearlite D
max and low circularity. For instance, Sample 5 (AC), which had the highest pearlite content (71.53%) and large, irregular pearlitic grains, exhibited elevated corrosion current density and low substrate resistance. These results confirm that pearlite, although mechanically beneficial, can act as a cathodic domain within galvanic microcells [
36,
37]—particularly when its distribution is heterogeneous or its morphology irregular.
Sample 4 (FA), with the lowest pearlite content (40.51%), showed the highest overall corrosion rate. The correlation diagram clarified this apparent contradiction by highlighting the influence of grain coarsening. Specifically, the ferrite D
max and ferrite area were both strongly and positively correlated with the corrosion indicators (CR and
Icorr), suggesting that excessive grain growth undermines corrosion resistance by reducing the grain boundary density and disrupting uniform film formation [
37,
38,
39].
Sample 1 (N), with a moderate phase ratio and equiaxed grains, consistently aligned with favorable electrochemical indicators and mechanical properties. It demonstrated the highest Rp (167.3 Ω), the lowest Icorr (90.19 µA/cm2), and the most positive OCP (–0.5594 V), marking it as the condition with the best overall corrosion resistance.
The role of grain shape and size in governing corrosion behavior was further clarified through correlation with the EIS and PDP parameters. Grain circularity—both for ferrite and pearlite—showed a strong negative correlation with the corrosion rate and
Icorr, and a positive correlation with
R1 and
R2 [
34,
40]. This indicates that more equiaxed grains favor the development of more compact, continuous surface layers, which are less susceptible to localized degradation.
On the other hand, the grain area and maximum diameter were positively correlated with the corrosion indicators, indicating that coarsely grained microstructures are more vulnerable. Sample 6 (+FP), which had the most refined grains in terms of size, nonetheless exhibited low circularity and high morphological irregularity. These characteristics were strongly negatively correlated with
Rp and
R1, suggesting that extreme grain refinement, if not accompanied by geometric regularity, can result in poor corrosion performance [
37,
38,
39].
Additionally, the correlation analysis revealed that hardness (HRB) was negatively correlated with ferrite grain size (D
max, Equiv. Diameter, area) and positively correlated with ferrite circularity. These trends align with known metallurgical behavior, where finer and more uniform grains increase the dislocation density and hinder plastic deformation, thereby increasing hardness [
41,
42]. However, the relationship between hardness and corrosion resistance was not linear: Sample 6 exhibited the highest hardness but only moderate corrosion resistance due to poor grain morphology. This confirms that while grain refinement increases the mechanical strength, it must be balanced with morphological control to avoid compromising the corrosion performance [
41].
The electrochemical data were interpreted synergistically to confirm the trends revealed by the correlation analysis.
Open circuit potential (OCP) showed a weak correlation with the corrosion rate, confirming that thermodynamic indicators alone are insufficient for predicting actual degradation [
28].
Linear polarization resistance (LPR) values proved more predictive, with strong negative correlation with Icorr and CR and a positive correlation with R1. These trends were consistent with the microstructural findings; for example, Sample 1 exhibited both high Rp and a favorable grain structure.
Electrochemical impedance spectroscopy (EIS) reinforced these observations. Although
Rct and
Rf are commonly associated with the charge transfer resistance at the metal interface and the resistance of the porous surface layer, in this study, their low values, combined with high CPEs and dispersion, indicate that the surface layer was porous, actively degrading, and lacked protective barrier properties [
31,
32,
33]. Warburg impedance (
W), representing diffusional effects, was highest in samples with irregular surface morphology, supporting the link between grain geometry and electrolyte interaction [
40].
Potentiodynamic polarization (PDP) results offered kinetic validation. Both
Icorr and the corrosion rate correlated strongly with coarseness and irregularity in grain morphology. The correlation diagram clearly highlighted that ferrite and pearlite D
max, area, and perimeter were directly associated with higher corrosion activity, whereas equiaxed geometry and balanced phase distribution were consistently linked to reduced electrochemical reactivity [
34,
37,
38].
5. Conclusions
In this study, the influence of different annealing treatments on the microstructure and corrosion behavior of 20MnCr5 steel in a 3.5% NaCl solution was investigated. A combination of microstructural analysis, hardness testing, and electrochemical techniques, including open circuit potential (OCP), linear polarization resistance (LPR), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PDP), was used to characterize each condition. To improve data interpretability, a correlation analysis was performed and visualized through a correlation diagram, enabling statistical evaluation of the relationships between grain characteristics, phase distribution, mechanical properties, and corrosion indicators.
The results show that the corrosion resistance of 20MnCr5 steel is not determined by a single parameter but by the interaction of grain size, morphology, and phase equilibrium. Key findings from each characterization method include the following:
In terms of microstructure, the normalized sample (N) exhibited refined equiaxed grains and a balanced ferrite-pearlite distribution, which was beneficial for corrosion resistance. In contrast, the fully annealed (FA) and transformation-annealed (+FP) samples had coarser and irregular grains, which negatively impacted the corrosion performance. The spheroidizing annealed sample (AC) exhibited carbide spheroidization beneficial for mechanical properties, but the corrosion performance varied due to uneven carbide distribution.
Phase analysis confirmed that the cementite distribution varied with cooling rates and was particularly favorable for the normalized (N) and spheroidizing annealed (AC) samples due to improved carbide morphology and uniformity.
Hardness measurements indicated that the transformation-annealed sample (+FP) had the highest hardness due to grain refinement, although its corrosion resistance was moderate due to irregular grain morphology. The fully annealed (FA) and spheroidizing annealed (AC) samples exhibited lower hardness values and varying corrosion behaviors, highlighting the need for balancing mechanical and corrosion properties.
Electrochemical tests demonstrated that the normalized sample (N) exhibited the best corrosion resistance, characterized by the lowest corrosion current density, highest polarization resistance, and most positive open circuit potential (OCP). The spheroidizing annealed (AC) and fully annealed (FA) samples showed higher corrosion rates and lower resistance, indicating susceptibility to localized corrosion. The transformation-annealed (+FP) sample exhibited moderate corrosion resistance due to refined but morphologically irregular grains.
These results emphasize the importance of precisely controlling the annealing parameters to adjust microstructural features and achieve an optimal balance between mechanical strength and corrosion resistance in 20MnCr5 steel, especially for components used in aggressive chloride environments. This knowledge is particularly important for designing components exposed to chlorides in marine or industrial applications, where the microstructure significantly influences the long-term performance.