Enhanced Photoelectrochemical Performance of 2D Bi2O3/TiO2 Heterostructure Film by Bi2S3 Surface Modification and Broadband Photodetector Application
Highlights
- Hierarchical ternary Bi2S3/Bi2O3/TiO2 composite film was successfully synthesized for the first time.
- A High-performance and dual functional PEC device based on Bi2S3/Bi2O3/TiO2 film was designed.
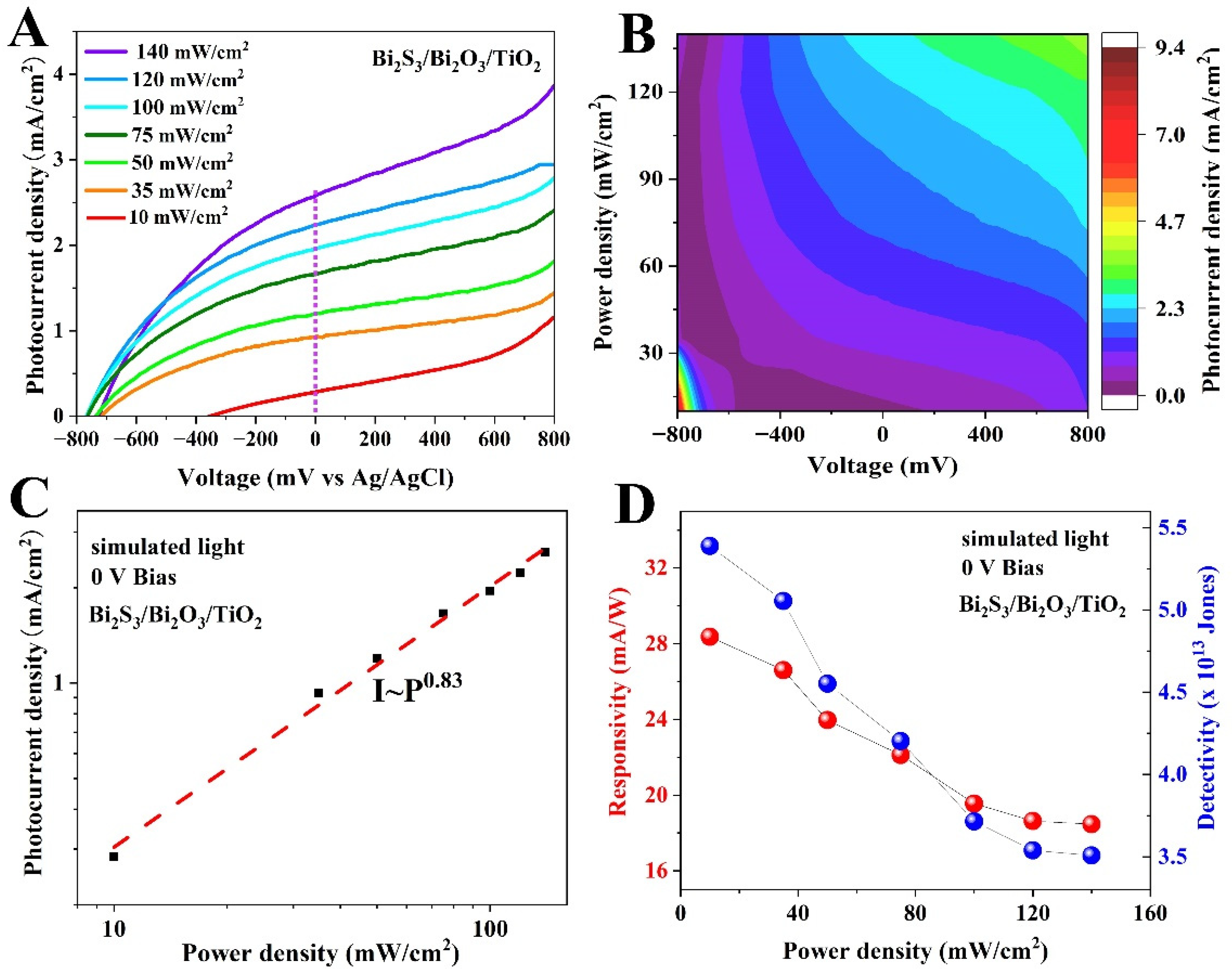
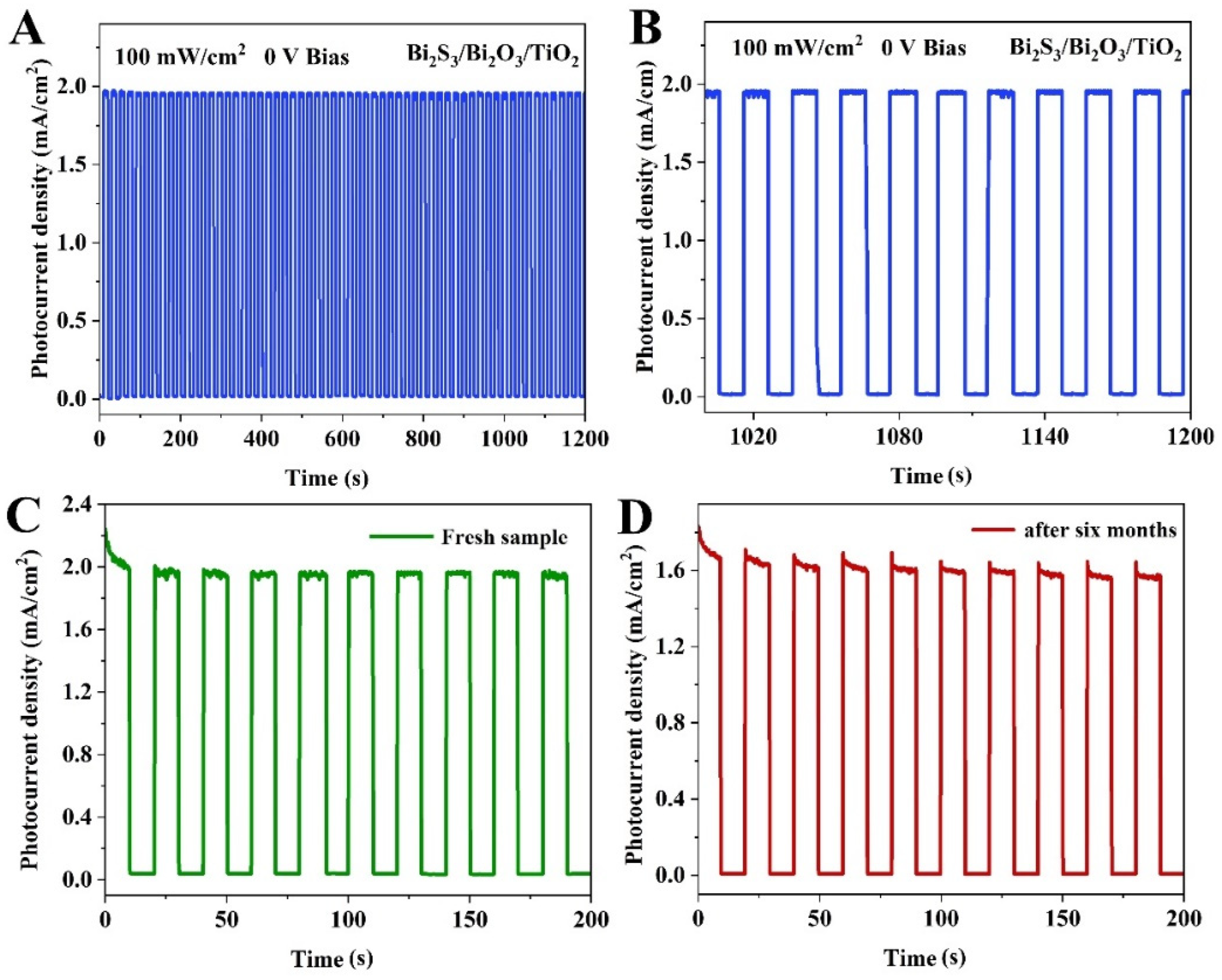
- The Bi2S3/Bi2O3/TiO2 PEC device exhibits a fast response speed of 63/95 ms and a high responsivity of 19.5 mA W−1 at a bias voltage of 0 V.
- The work mechanism of enhanced PEC performance for Bi2S3/Bi2O3/TiO2 photoelectrode was proposed.
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
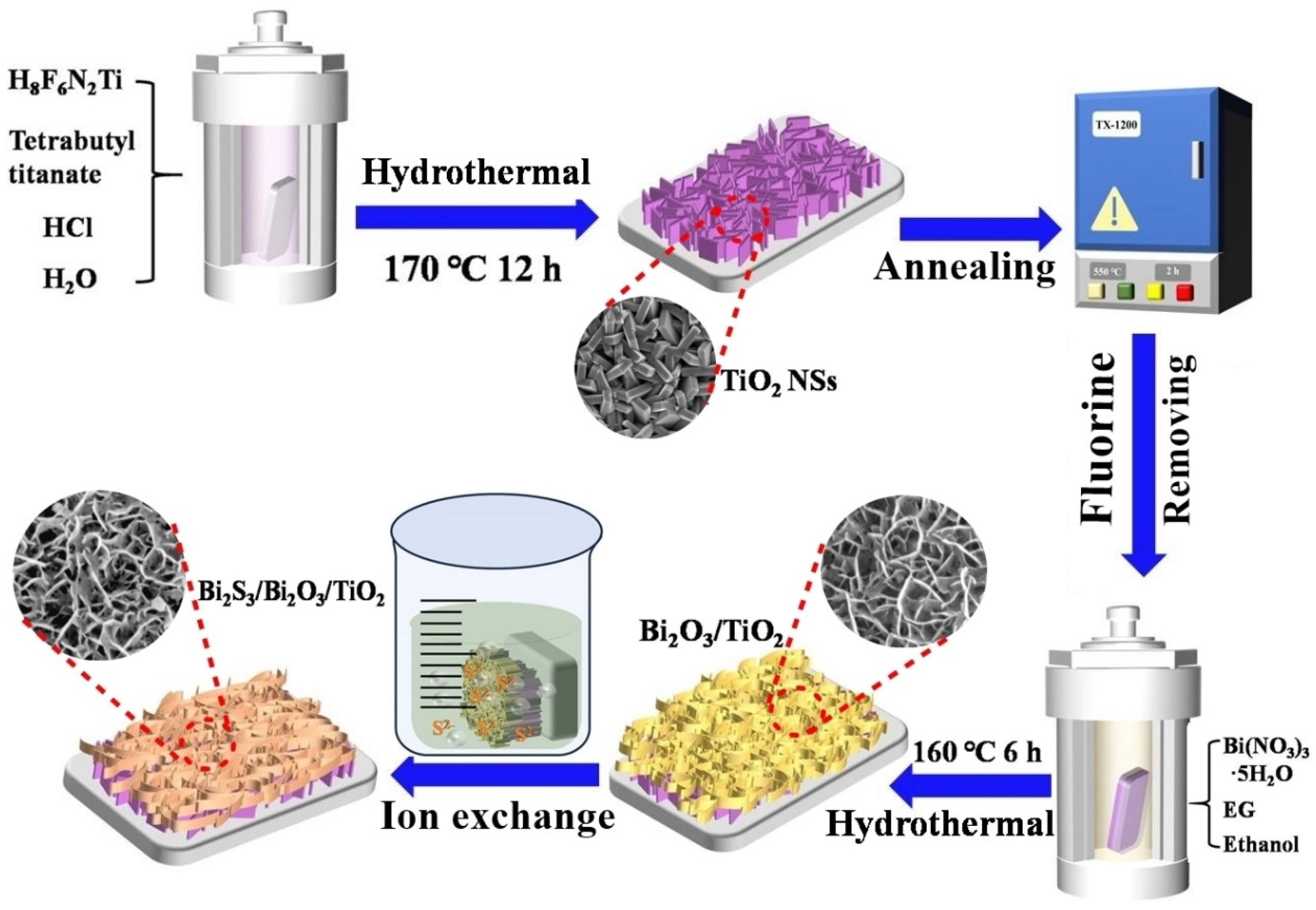
2.2. Synthesis of TiO2 NSs Array Film
2.3. Synthesis of Bi2S3/Bi2O3/TiO2 Dual Heterojunction Film
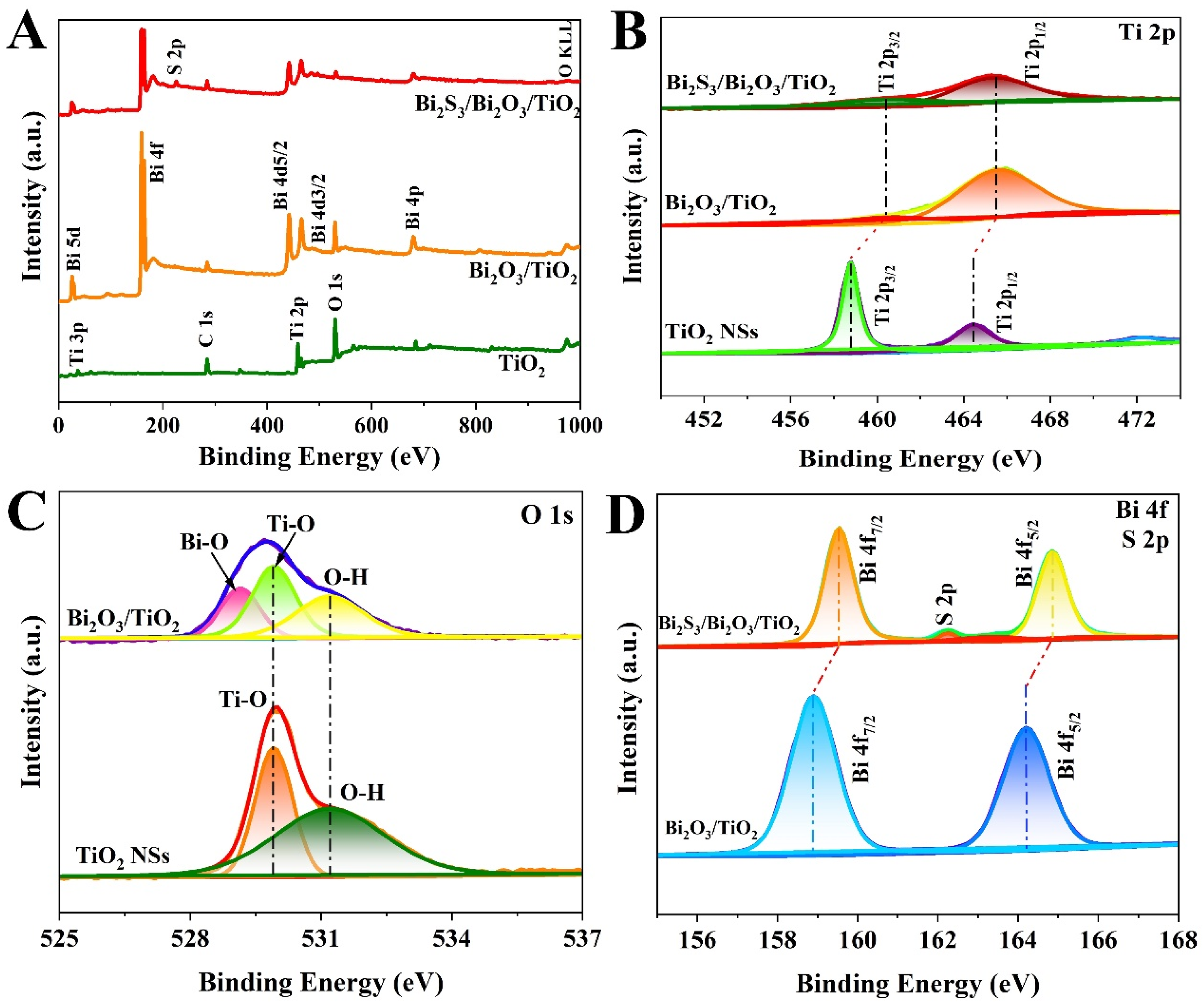
2.4. Characterization
2.5. Photodetection Performance Measurements
2.6. Photoelectrocatalytic Degradation of RhB
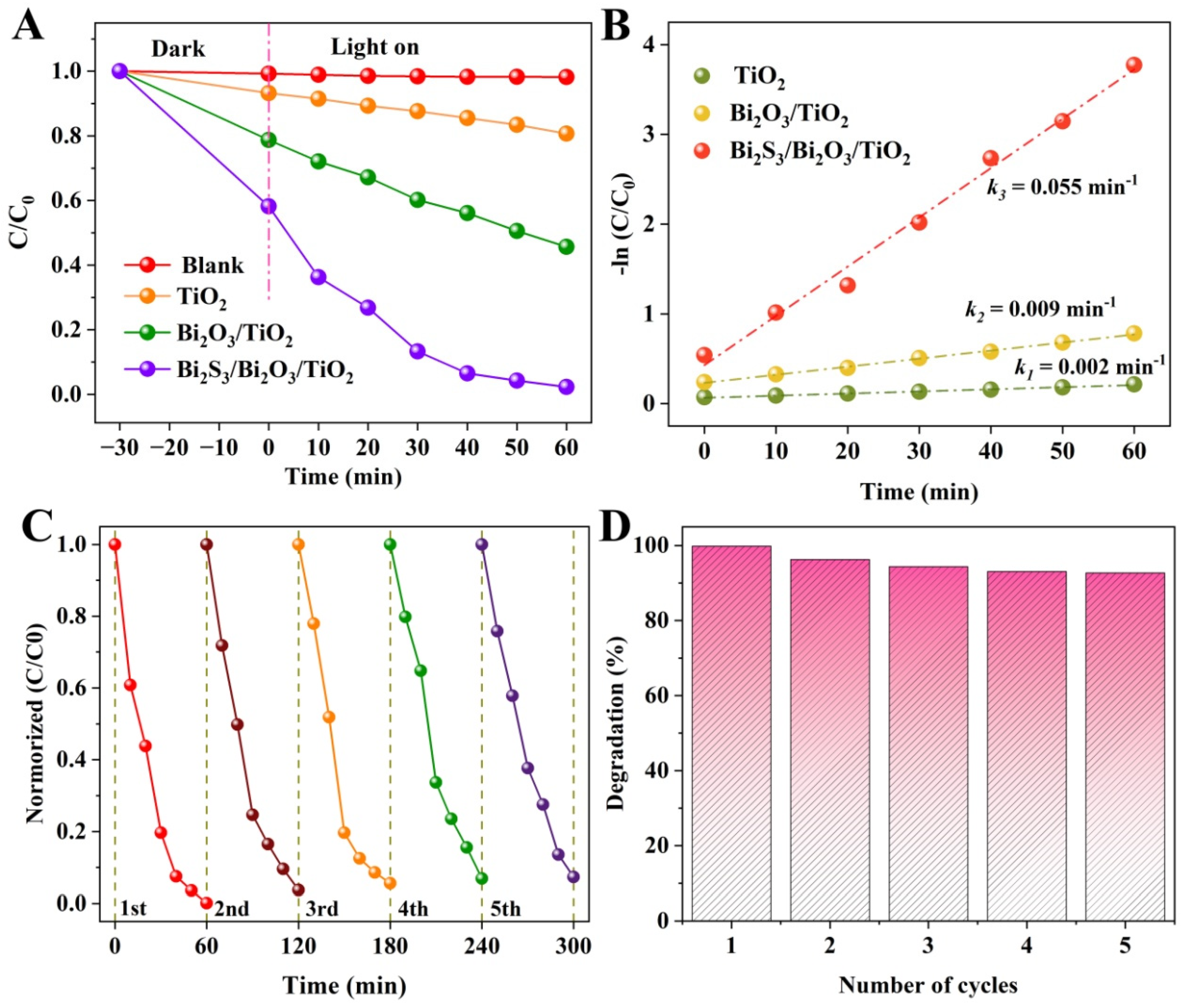
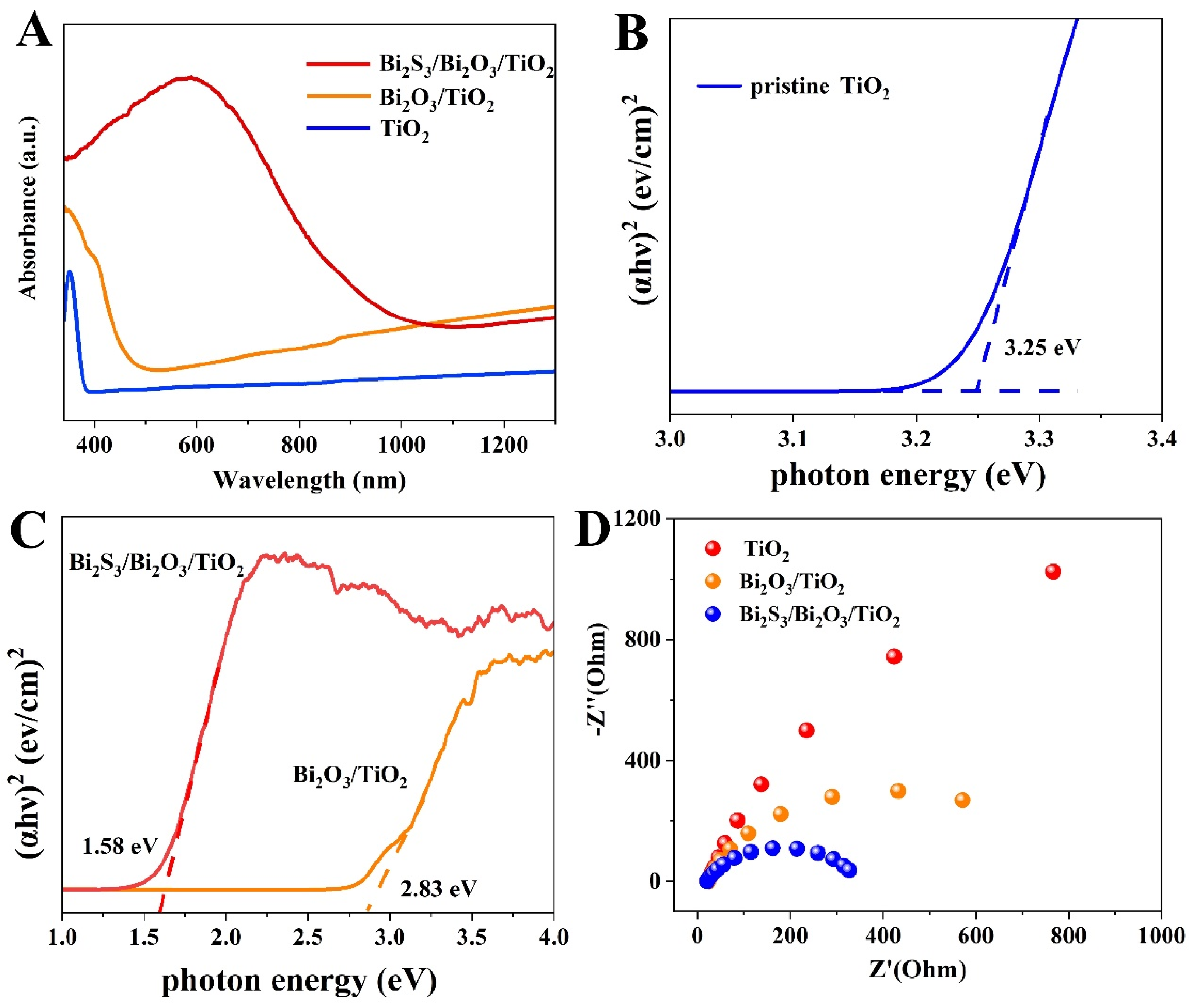
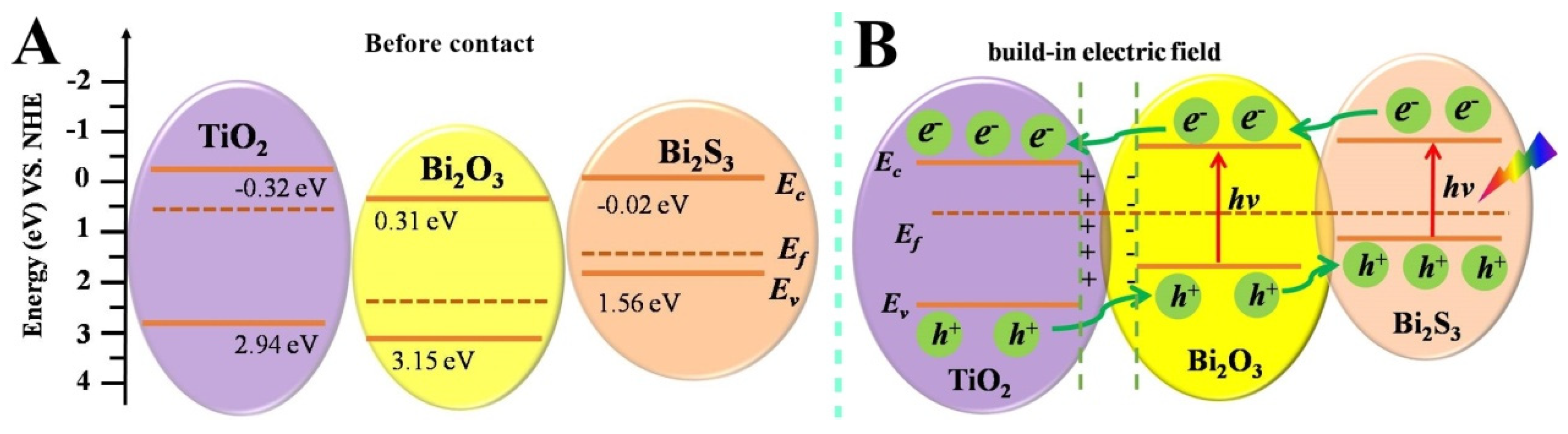
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, F.; Lei, S.; Wei, Y.; Zhao, D.; Gao, Y.; Ma, X.; Li, S.; Chang, S.; Wang, M.; et al. In situ grown two-dimensional TiO2/Ti3CN MXene heterojunction rich in Ti3+ species for highly efficient photoelectrocatalytic CO2 reduction. Chem. Eng. J. 2023, 452, 139392. [Google Scholar] [CrossRef]
- Sorokina, L.; Savitskiy, A.; Shtyka, O.; Maniecki, T.; Szynkowska-Jozwik, M.; Trifonov, A.; Pershina, E.; Mikhaylov, I.; Dubkov, S.; Gromov, D. Formation of Cu-Rh alloy nanoislands on TiO2 for photoreduction of carbon dioxide. J. Alloys Compd. 2022, 904, 164012. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Jing, J.; Mu, J.; Wang, R.; Du, C.; Su, Y. Photoelectrocatalytic peroxymonosulfate activation over CoFe2O4-BiVO4 photoanode for environmental purification: Unveiling of multi-active sites, interfacial engineering and degradation pathways. J. Colloid Interface Sci. 2023, 644, 519–532. [Google Scholar] [CrossRef]
- Xiong, Y.; Ma, S.; Hong, X.; Long, J.; Wang, G. Photoelectrocatalytic Processes of TiO2 Film: The Dominating Factors for the Degradation of Methyl Orange and the Understanding of Mechanism. Molecules 2023, 28, 7967. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, P.; Pineda-Arellano, C.A.; Millán-Ocampo, D.E.; Álvarez-Gallegos, A.; Sirés, I.; Silva-Martínez, S. Photoelectrocatalytic chemical oxygen demand analysis using a TiO2 nanotube array photoanode. Electrochim. Acta 2024, 476, 143710. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Shao, P.; Sun, H.; Fang, S.; Kang, Y.; Liang, K.; Jia, H.; Luo, Y.; Xue, J.; et al. Achieving record high external quantum efficiency >86.7% in solar-blind photoelectrochemical photodetection. Adv. Funct. Mater. 2022, 32, 2201604. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, C.; Han, J.; Mu, Y.; Gong, J.R.; Zhang, J. High-Performance self-powered PEC photodetectors based on 2D BiVO4/MXene Schottky Junction. Adv. Funct. Mater. 2025, 35, 2416922. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Y.; Huang, J.; Xu, Z.; Yang, Y.; Xie, C.; Zhang, B.; Ao, G.; Fu, Z.; Li, A.; et al. Optimizing Photoelectrochemical UV Imaging Photodetection: Construction of Anatase/Rutile Heterophase Homojunctions and Oxygen Vacancies Engineering in MOF-Derived TiO2. Molecules 2024, 29, 3096. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Leng, W.H.; Zhang, Z.; Zhang, J.Q.; Cao, C.N. Investigation of the kinetics of a TiO2 photoelectrocatalytic reaction involving charge transfer and recombination through surface states by electrochemical impedance spectroscopy. J. Phys. Chem. B 2005, 109, 15008–15023. [Google Scholar] [CrossRef]
- Sun, C.; Wu, L.; Hu, J.; Hussain, S.A.; Yang, J.; Jiao, F. A novel dual S-scheme heterojunction photocatalyst β-Bi2O3/NiAl-LDH/α-Bi2O3 induced by phase-transformed bismuth oxide for efficient degradation of antibiotics in full-spectrum: Degradation pathway, DFT calculation and mechanism insight. Chem. Eng. J. 2023, 474, 145616. [Google Scholar] [CrossRef]
- Shanmugapriya, V.; Arunpandiyan, S.; Hariharan, G.; Bharathi, S.; Selvakumar, B.; Arivarasan, A. Enhanced electrochemical performance of mixed metal oxide (Bi2O3/ZnO) loaded multiwalled carbon nanotube for high-performance asymmetric supercapacitors. J. Energy Storage 2022, 55, 105739. [Google Scholar] [CrossRef]
- Feng, X.; Zou, H.; Zheng, R.; Wei, W.; Wang, R.; Zou, W.; Lim, G.; Hong, J.; Duan, L.; Chen, H. Bi2O3/BiO2 nanoheterojunction for highly efficient electrocatalytic CO2 reduction to formate. Nano Lett. 2022, 22, 1656–1664. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Wang, Z.; Sun, J. Study on the Preparation and PEC-Type Photodetection Performance of β-Bi2O3 Thin Films. Materials 2024, 17, 3779. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.; Sun, Y.; Lin, H.; Chen, Z.; Chen, X.; Niu, L.; Zhang, Q.; Li, H. Fast and Long-Lasting Potassium-Ion Storage Enabled by Rationally Engineering Strain-Relaxation Bi/Bi2O3 Nanodots Embedded in Carbon Sheets. Adv. Funct. Mater. 2023, 33, 2307205. [Google Scholar] [CrossRef]
- Praveen, S.; Veeralingam, S.; Badhulika, S. A Flexible Self-Powered UV Photodetector and Optical UV Filter Based on β-Bi2O3/SnO2 Quantum Dots Schottky Heterojunction. Adv. Mater. Interfaces 2021, 8, 2100373. [Google Scholar] [CrossRef]
- Yang, S.; Jiao, S.; Nie, Y.; Lu, H.; Liu, S.; Zhao, Y.; Gao, S.; Wang, D.; Wang, J.; Li, Y. A self-powered high performance UV-Vis-NIR broadband photodetector based on β-Bi2O3 nanoparticles through defect engineering. J. Mater. Chem. C 2022, 10, 8364–8372. [Google Scholar] [CrossRef]
- Guo, P.; Yin, F.; Zhang, J.; Chen, B.; Ni, Z.; Shi, L.; Han, M.; Wu, Z.; Li, G. Crystal-Phase and Surface-Structure Engineering of Bi2O3 for Enhanced Electrochemical N2 Fixation to NH3. ACS Appl. Mater. Interfaces 2024, 16, 17540–17552. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, C.; Li, Y.; Deng, P.; Liu, Y.; Wu, R.; Liu, X.; Zheng, Y.; Zhang, W.; Wu, J.; et al. Enhanced supercapacitor performance of Bi2O3 by Mn doping. J. Alloys Compd. 2022, 914, 165258. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, X.; Tu, S.; Zuo, X.; Nan, J. Synthesis of flower-like heterostructured β-Bi2O3/Bi2O2CO3 microspheres using Bi2O2CO3 self-sacrifice precursor and its visible-light-induced photocatalytic degradation of o-phenylphenol. Appl. Catal. B Environ. 2015, 163, 510–519. [Google Scholar] [CrossRef]
- Khan, I.; Abdalla, A.; Qurashi, A. Synthesis of hierarchical WO3 and Bi2O3/WO3 nanocomposite for solar-driven water splitting applications. Int. J. Hydrogen Energy 2017, 42, 3431–3439. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; Haq, A.U.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.-Z.; Kang, Y.-R.; Sun, Z.-S.; Wang, X.-D.; Meng, Y.; Hong, M.-H.; Xie, W.-F. Cauliflower-shaped Bi2O3–ZnO heterojunction with superior sensing performance towards ethanol. J. Alloys Compd. 2021, 854, 157152. [Google Scholar] [CrossRef]
- Balachandran, S.; Swaminathan, M. Facile fabrication of heterostructured Bi2O3–ZnO photocatalyst and its enhanced Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 26306–26312. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, Y.; Wang, J.; Luo, D.; Fan, L.; Wu, J. Controllable fabrication of Bi2O3/TiO2 heterojunction with excellent visible-light responsive photocatalytic performance. Appl. Surf. Sci. 2017, 423, 119–130. [Google Scholar] [CrossRef]
- Sood, S.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Bi2O3/TiO2 heterostructures: Synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 2016, 290, 45–52. [Google Scholar] [CrossRef]
- Wang, C.; Tan, C.; Lv, W.; Zhu, G.; Wei, Z.; Zhang, K.H.L.; He, W. Coherent Bi2O3/TiO2 heterojunction material through oriented growth as an efficient photocatalyst for methyl orange degradation. Mater. Today Chem. 2018, 8, 36–41. [Google Scholar] [CrossRef]
- Taghinejad, H.; Taghinejad, M.; Abdollahramezani, S.; Li, Q.; Woods, E.V.; Tian, M.; Eftekhar, A.A.; Lyu, Y.; Zhang, X.; Ajayan, P.M.; et al. Ion-assisted nanoscale material engineering in atomic layers. Nano Lett. 2025, 25, 10123–10130. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Zhao, F.; Liu, Z.; Tong, Y.; Ji, H. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B: Environ. 2016, 185, 68–76. [Google Scholar] [CrossRef]
- Bhoi, Y.P.; Mishra, B.G. Single step combustion synthesis, characterization and photocatalytic application of α-Fe2O3-Bi2S3 heterojunctions for efficient and selective reduction of structurally diverse nitroarenes. Chem. Eng. J. 2017, 316, 70–81. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.; Zhang, H.; Duan, X.; Liang, P.; Li, X.; Tade, M.O.; Liu, S.; Wang, S. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Shinde, N.M.; Xia, Q.X.; Yun, J.M.; Shinde, P.V.; Shaikh, S.M.; Sahoo, R.K.; Mathur, S.; Mane, R.S.; Kim, K.H. Ultra-rapid chemical synthesis of mesoporous Bi2O3 micro-sponge-balls for supercapattery applications. Electrochim. Acta 2019, 296, 308–316. [Google Scholar] [CrossRef]
- Manjunatha, C.; Rastogi, C.K.; Rao, B.M.; Kumar, S.G.; Varun, S.; Raitani, K.; Maurya, G.; Karthik, B.; Swathi, C.; Sadrzadeh, M.; et al. Advances in hierarchical inorganic nanostructures for efficient solar energy harvesting systems. ChemSusChem 2024, 17, e202301755. [Google Scholar] [CrossRef]
- Mangolini, F.; McClimon, J.B.; Rose, F.; Carpick, R.W. Accounting for nanometer-thick adventitious carbon contamination in X-ray absorption spectra of carbon-based materials. Anal. Chem. 2014, 86, 12258–12265. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, S.; Wang, W.; Li, C.; Yang, Y.; Tian, Q.; Liu, Y. Plasmon-induced ultrafast charge transfer in single-particulate Cu1.94S–ZnS nanoheterostructures. Nanoscale Adv. 2021, 3, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Potlog, T.; Dumitriu, P.; Dobromir, M.; LuCa, D. XRD and XPS Analysis of TiO2 Thin Films Annealed in Different Environments. J. Mater. Sci. Eng. 2014, 4, 163–170. [Google Scholar]
- Zingg, D.S.; Hercules, D.M. Electron spectroscopy for chemical analysis studies of lead sulfide oxidation. J. Phys. Chem. 1978, 82, 1992–1995. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, J.; Wang, R.; Xing, C.; Li, Z.; Huang, W.; Jiang, X.; Guo, Z.; Luo, Z.; Li, Y.; et al. Ultrathin GeSe Nanosheets: From Systematic Synthesis to Studies of Carrier Dynamics and Applications for a High-Performance UV–Vis Photodetector. ACS Appl. Mater. Interfaces 2019, 11, 4278–4287. [Google Scholar] [CrossRef]
- Vashishtha, P.; Prajapat, P.; Sharma, A.; Singh, P.; Walia, S.; Gupta, G. Self-driven UVC–NIR broadband photodetector with high-temperature reliability based on a coco palm-like MoS2/GaN heterostructure. ACS Appl. Electron. Mater. 2023, 5, 1891–1902. [Google Scholar] [CrossRef]
- Vashishtha, P.; Abidi, H.I.; Giridhar, P.S.; Verma, K.A.; Prajapat, P.; Bhoriya, A.; Murdoch, B.J.; Tollerud, J.O.; Walia, S. CVD-grown monolayer MoS2 and GaN thin film heterostructure for a self-powered and bidirectional photodetector with an extended active spectrum. ACS Appl. Mater. Interfaces 2024, 16, 31294–31303. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kumar, M.D.; Kim, J.; Lim, D. Vertical growth of MoS2 layers by sputtering method for efficient photoelectric application. Sens. Actuators A Phys. 2018, 269, 355–362. [Google Scholar] [CrossRef]
- Vashishtha, P.; Prajapat, P.; Kumar, K.; Kumar, M.; Walia, S.; Gupta, G. Multiband spectral response inspired by ultra-high responsive thermally stable and self-powered Sb2Se3/GaN heterojunction based photodetector. Surf. Interfaces 2023, 42, 103376. [Google Scholar] [CrossRef]
- Vashishtha, P.; Tanwar, R.; Gautam, S.; Goswami, L.; Kushwaha, S.S.; Gupta, G. Wavelength-modulated polarity switch self-powered Bi2Se3/GaN heterostructure photodetector. Mater. Sci. Semicond. Process. 2024, 180, 108553. [Google Scholar] [CrossRef]
- Yang, X.; Qu, L.; Gao, F.; Hu, Y.; Yu, H.; Wang, Y.; Cui, M.; Zhang, Y.; Fu, Z.; Huang, Y.; et al. High-Performance broadband photoelectrochemical photodetectors Based on Ultrathin Bi2O2S Nanosheets. ACS Appl. Mater. Interfaces 2022, 14, 7175–7183. [Google Scholar] [CrossRef]
- Huang, W.; Xing, C.; Wang, Y.; Li, Z.; Wu, L.; Ma, D.; Dai, X.; Xiang, Y.; Li, J.; Fan, D.; et al. Facile fabrication and characterization of two-dimensional bismuth(iii) sulfide nanosheets for high-performance photodetector applications under ambient conditions. Nanoscale 2018, 10, 2404–2412. [Google Scholar] [CrossRef]
- Xing, C.; Huang, W.; Xie, Z.; Zhao, J.; Ma, D.; Fan, T.; Liang, W.; Ge, Y.; Dong, B.; Li, J.; et al. Ultrasmall Bismuth Quantum Dots: Facile liquid-Phase exfoliation, characterization, and application in high-performance UV–Vis photodetector. ACS Photonics 2018, 5, 621–629. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, X.; Cheng, H.; Jiang, X.; Wang, F. Ultrathin CuBi2O4 on a bipolar Bi2O3 nano-scaffold: A self-powered broadband photoelectrochemical photodetector with improved responsivity and response speed. Nanoscale 2023, 15, 6333–6342. [Google Scholar] [CrossRef]
- Ma, N.; Lu, C.; Liu, Y.; Han, T.; Dong, W.; Wu, D.; Xu, X. Direct Z-Scheme Heterostructure of Vertically Oriented SnS2 Nanosheet on BiVO4 Nanoflower for Self-Powered Photodetectors and Water Splitting. Small 2024, 20, 2304839. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Shen, J.; Ye, M. A controlled anion exchange strategy to synthesize Bi2S3 nanoparticles/plate-like Bi2WO6 heterostructures with enhanced visible light photocatalytic activities for Rhodamine B. Ceram. Int. 2016, 42 Pt B, 3154–3162. [Google Scholar] [CrossRef]
- Taghinejad, M.; Xia, C.; Hrton, M.; Lee, K.T.; Kim, A.S.; Li, Q.; Guzelturk, B.; Kalousek, R.; Xu, F.; Cai, W.; et al. Determining hot-carrier transport dynamics from terahertz emission. Science 2023, 382, 299–305. [Google Scholar] [CrossRef] [PubMed]



| Materials | Wavelength (nm) | Electrolyte | Response (ms) | R (mA W−1) | D* (1012 Jones) | Refs. |
|---|---|---|---|---|---|---|
| Bi2S3/Bi2O3/TiO2 | Sunlight | 0.5 M KOH | 63/95 | 19.5 | 37 | This work |
| BiVO4/MXene | Sunlight | 1 M Na2SO3 + 0.5 M phosphate | 8/14 | 40.95 | - | [8] |
| Bi2O2S | 365 | 1 M KOH | 10/45 | 13 | 2.34 × 10−2 | [45] |
| 2D Bi2S3 | 400 | 0.1 M KOH | 100/100 | 0.7 | 3.75 × 10−4 | [46] |
| Bi QDs | 350 | 1 M KOH | 100/200 | 0.295 | 9.09 × 10−4 | [47] |
| Bi2O3/CuBi2O4 | 380 | 0.3 M K2SO4 + 0.2 M phosphate | 0.18/0.19 | 75 | - | [48] |
| SnS2/BiVO4 | 500 | 1 M Na2SO3 + 0.5 M phosphate | 6/21 | 10.43 | - | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Yao, H. Enhanced Photoelectrochemical Performance of 2D Bi2O3/TiO2 Heterostructure Film by Bi2S3 Surface Modification and Broadband Photodetector Application. Materials 2025, 18, 3528. https://doi.org/10.3390/ma18153528
Liu L, Yao H. Enhanced Photoelectrochemical Performance of 2D Bi2O3/TiO2 Heterostructure Film by Bi2S3 Surface Modification and Broadband Photodetector Application. Materials. 2025; 18(15):3528. https://doi.org/10.3390/ma18153528
Chicago/Turabian StyleLiu, Lai, and Huizhen Yao. 2025. "Enhanced Photoelectrochemical Performance of 2D Bi2O3/TiO2 Heterostructure Film by Bi2S3 Surface Modification and Broadband Photodetector Application" Materials 18, no. 15: 3528. https://doi.org/10.3390/ma18153528
APA StyleLiu, L., & Yao, H. (2025). Enhanced Photoelectrochemical Performance of 2D Bi2O3/TiO2 Heterostructure Film by Bi2S3 Surface Modification and Broadband Photodetector Application. Materials, 18(15), 3528. https://doi.org/10.3390/ma18153528






