Durable Superhydrophobic Composite Coating Based on Hydrangea-like SiO2 Nanoparticles with Excellent Performance in Anticorrosion, Drag Reduction, and Antifouling
Abstract
1. Introduction
2. Experiment
2.1. Materials
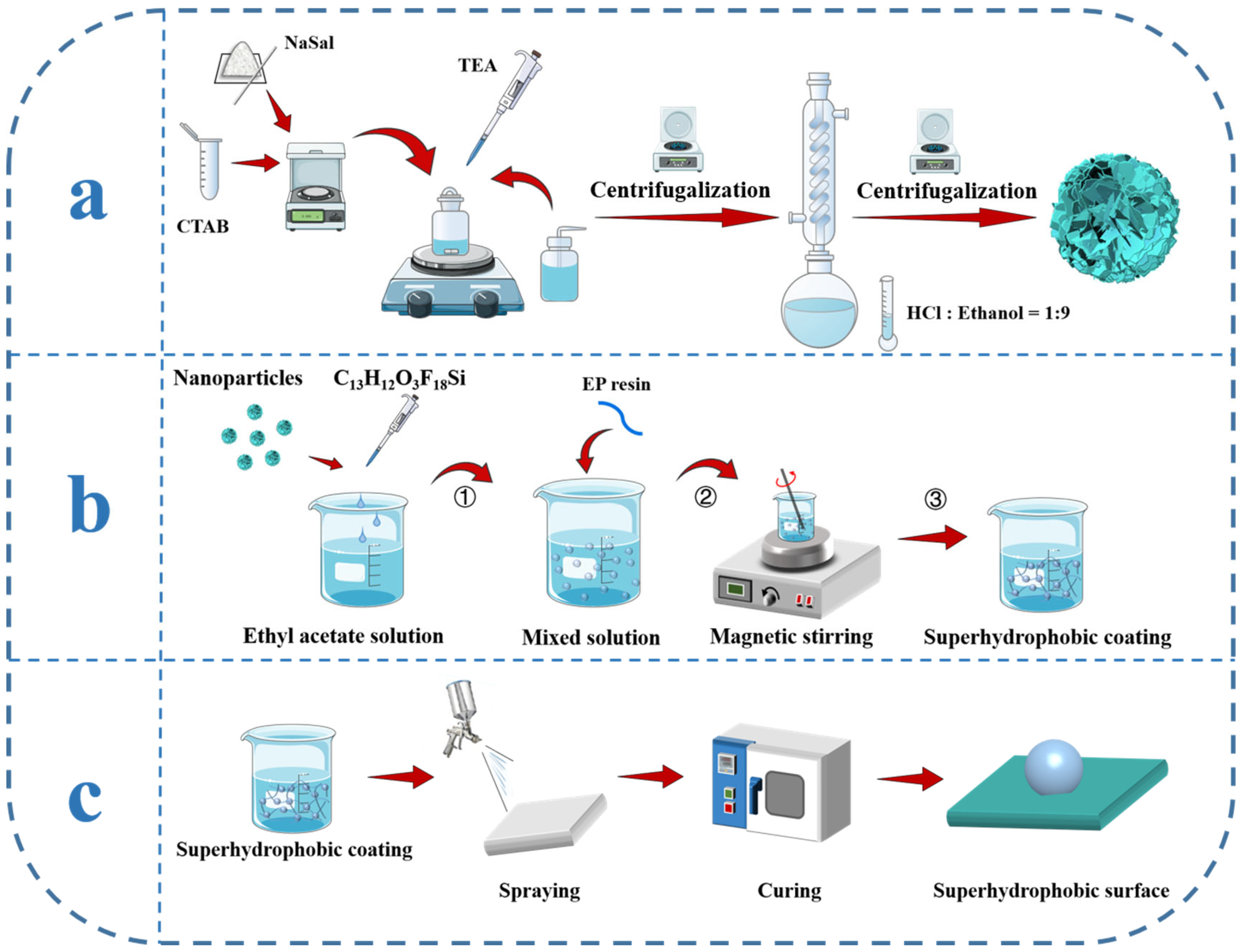
2.2. Synthesis of the Hydrangea-like Mesoporous SiO2 Nanoparticles
2.3. Preparation of h-SiO2@PFDT Superhydrophobic Nanoparticles
2.4. Preparation of h-SiO2@PFDT-EP Coating
2.5. Characterization and Morphology
2.6. Stability and Durability of Coatings
2.7. Corrosion Resistance of Coatings
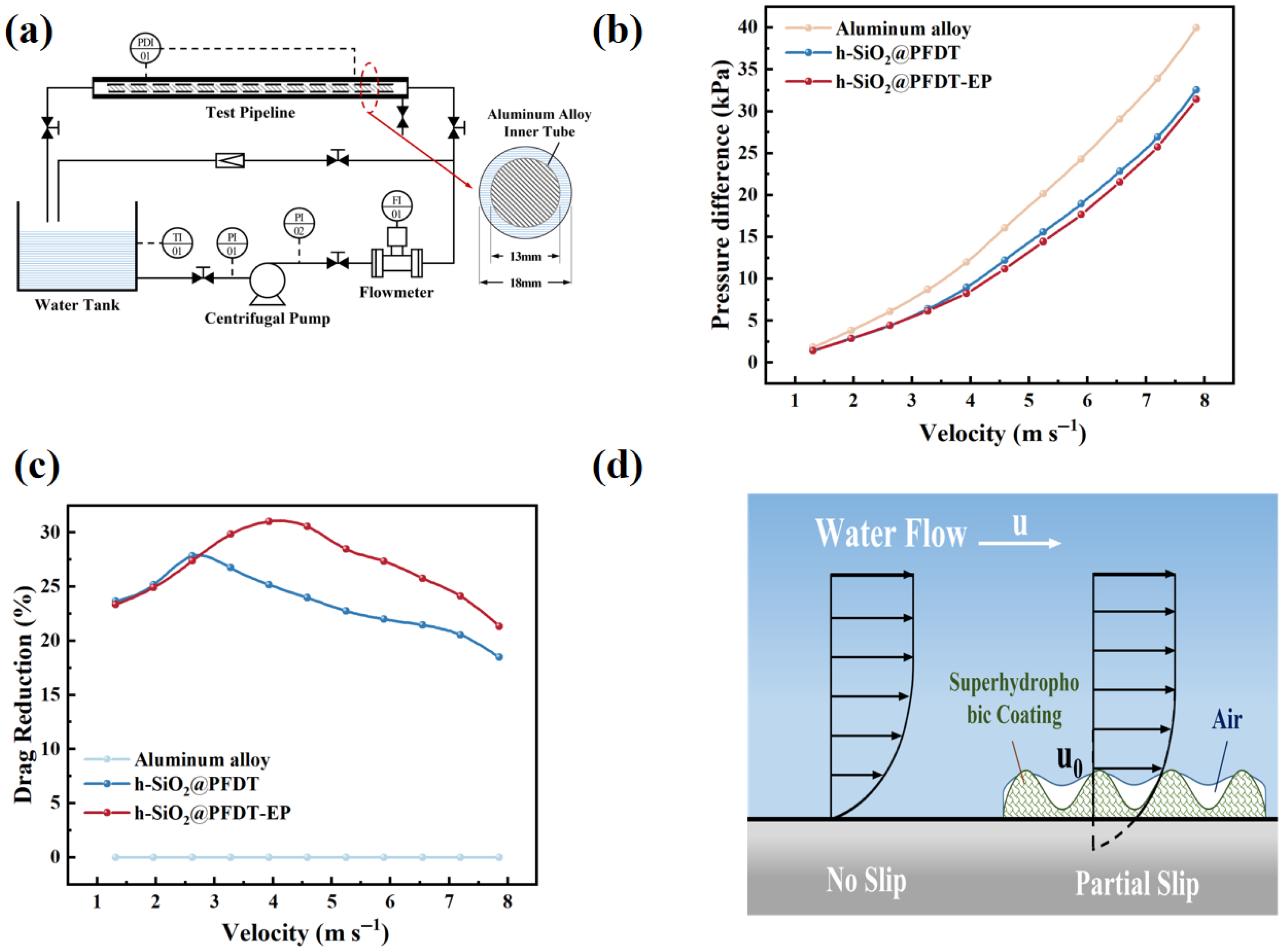
2.8. The Drag-Reduction Performance of Coatings
3. Results and Discussion
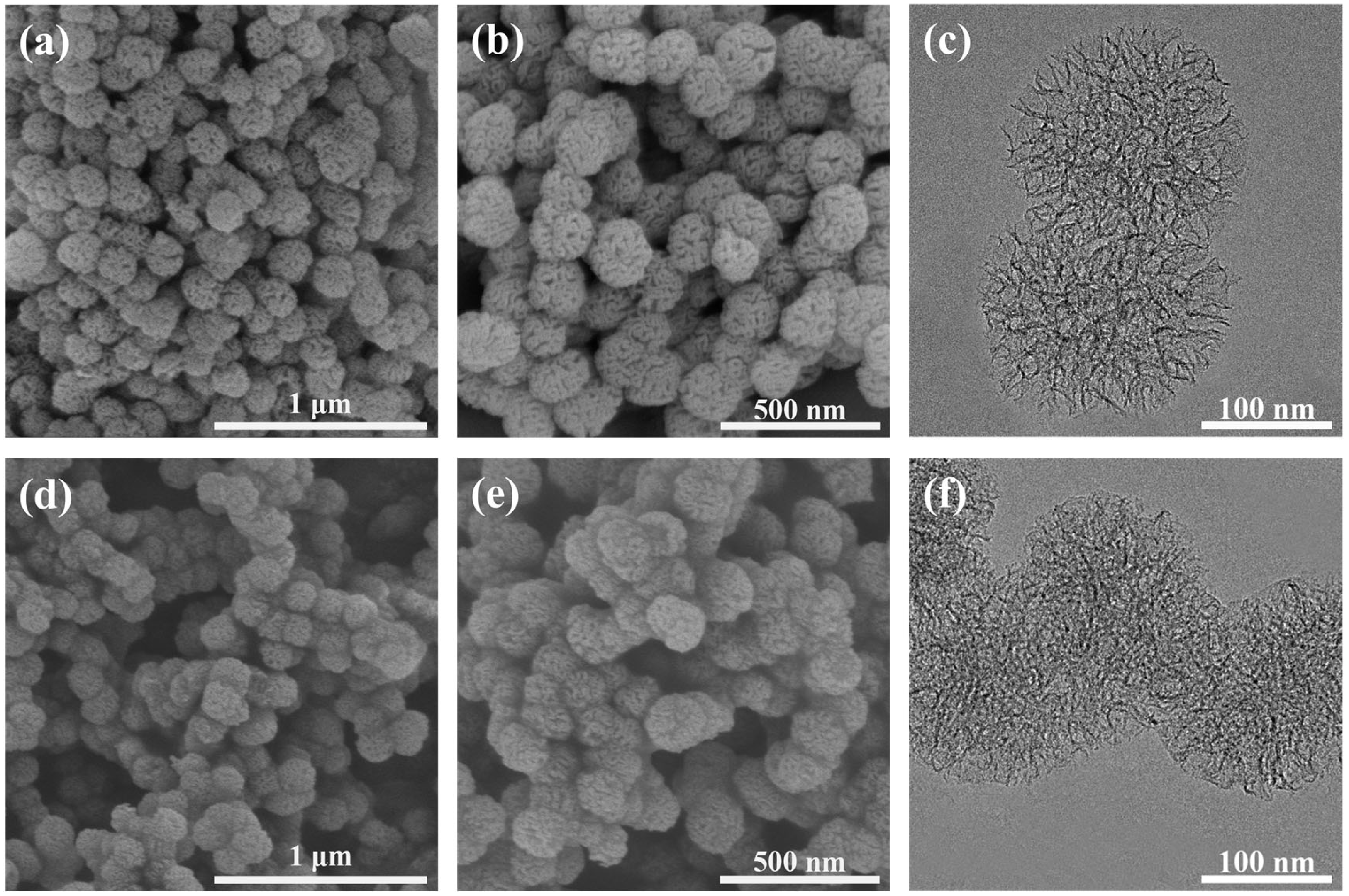
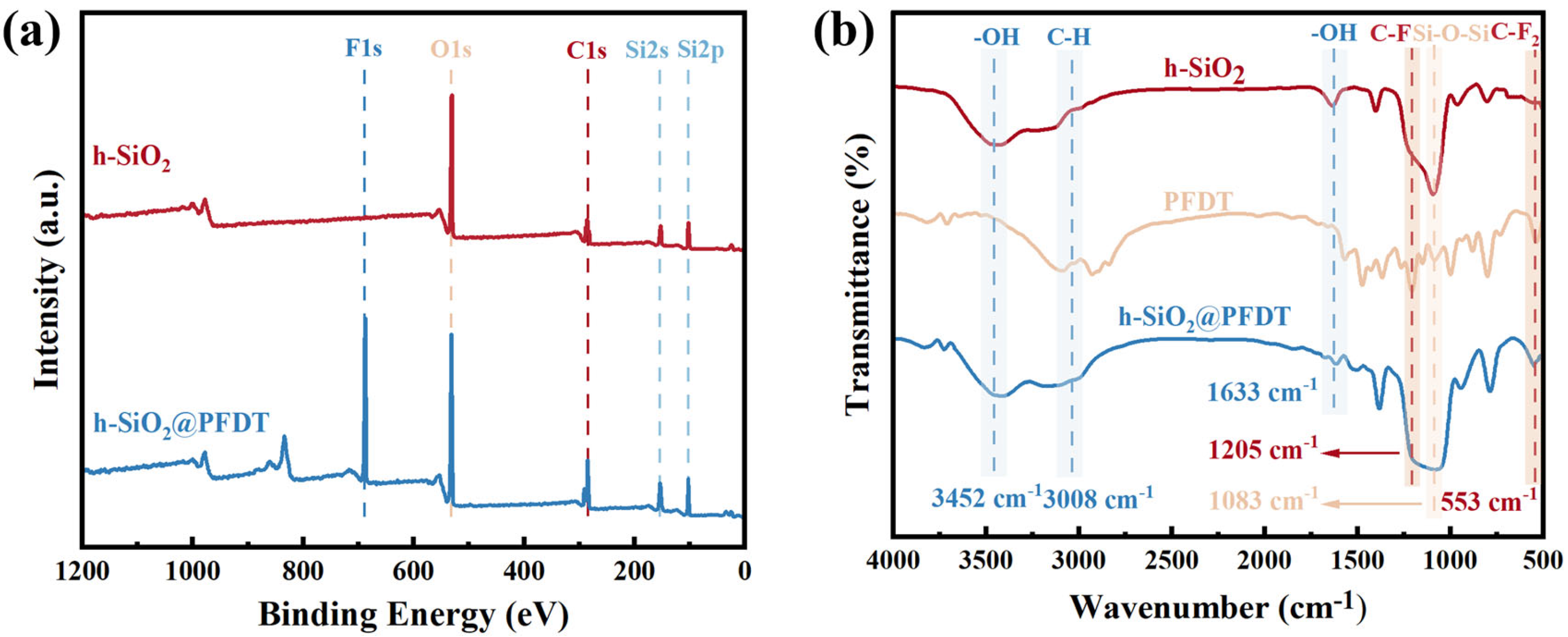
3.1. h-SiO2 Nanoparticles and h-SiO2@PFDT—Characterization of Nanocomposite Materials
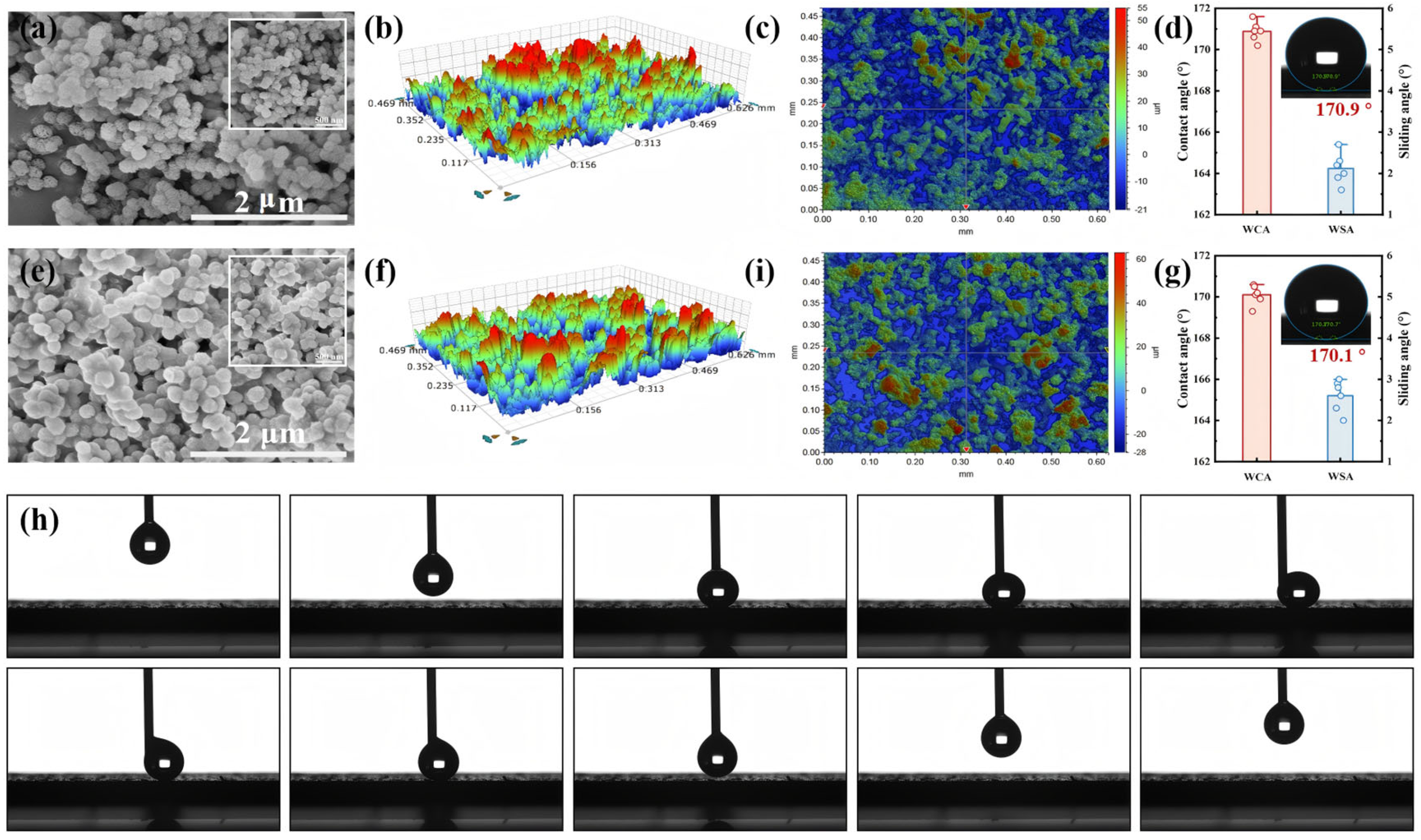
3.2. Morphology and Wettability of Coatings
3.3. Stability and Durability
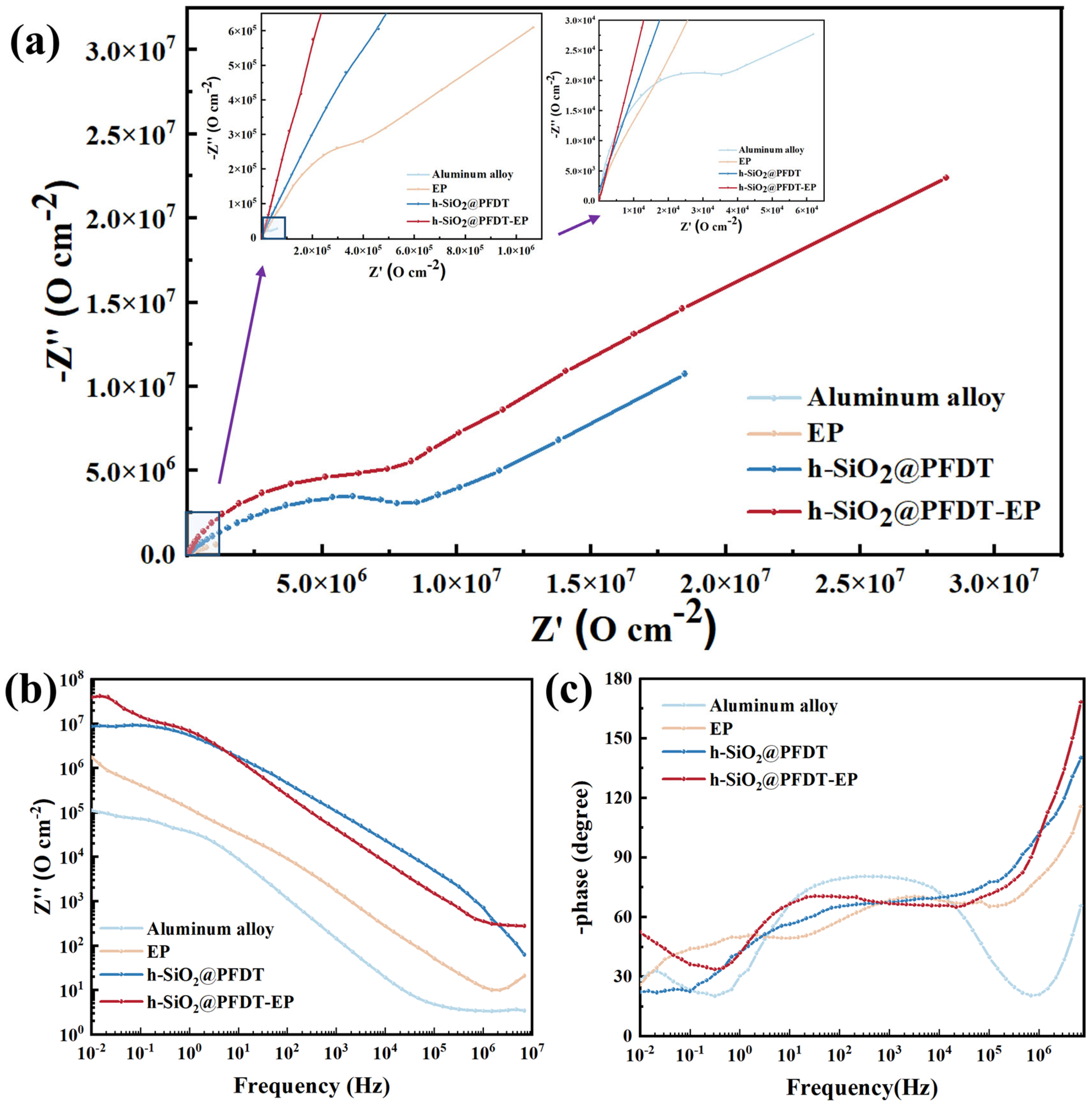
3.4. Corrosion Resistance
3.5. Drag-Reduction Performance
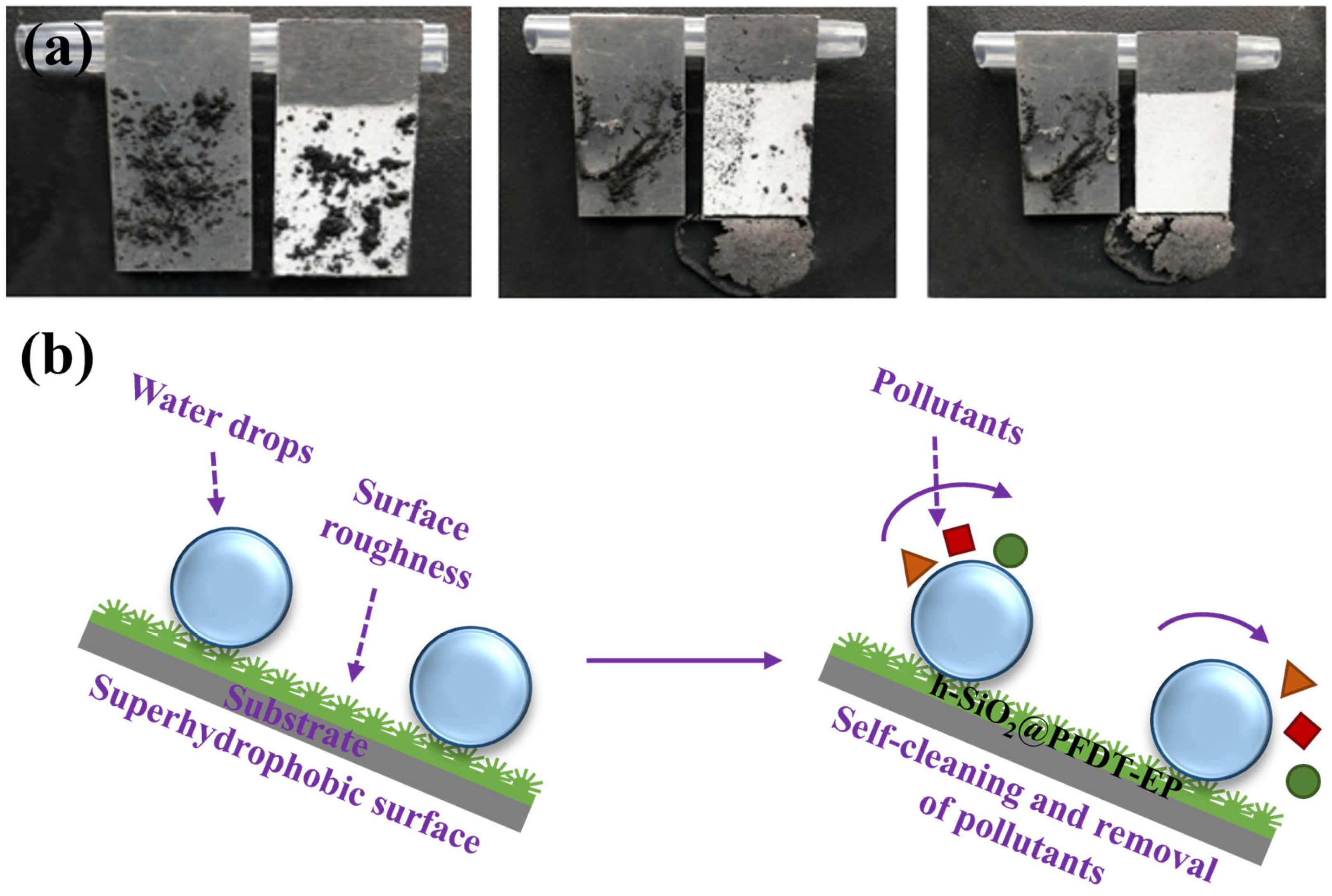
3.6. Antifouling Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.; Choi, C.H.; Kim, C.J. Superhydrophobic drag reduction in laminar flows: A critical review. Exp. Fluids 2016, 57, 176. [Google Scholar] [CrossRef]
- Park, H.; Choi, C.-H.; Kim, C.-J. Superhydrophobic drag reduction in turbulent flows: A critical review. Exp. Fluids 2021, 62, 229. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Yao, Z.; Hao, P.; Jiang, N. Mechanisms of drag reduction of superhydrophobic surfaces in a turbulent boundary layer flow. Exp. Fluids 2015, 56, 179. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Fan, B.; Zheng, X. Optimized anticorrosion of polypyrrole coating by inverted-electrode strategy: Experimental and molecular dynamics investigations. Polymers 2022, 14, 1356. [Google Scholar] [CrossRef]
- Radwan, A.B.; Mohamed, A.M.A.; Abdullah, A.M.; Al-Maadeed, M.A. Corrosion protection of electrospun PVDF-ZnO superhydrophobic coating. Surf. Coat. Technol. 2016, 289, 136–143. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Abbas, A.; Shenashen, M.A. Facile design of graphene oxide-ZnO nanorod-based ternary nanocomposite as a superhydrophobic and corrosion-barrier coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125793. [Google Scholar] [CrossRef]
- Radwan, A.B.; El-Hout, S.I.; Ibrahim, M.A.M.; Ismail, E.H.; Abdullah, A.M. Superior corrosion and UV-resistant highly porous poly(vinylidene fluoride-co-hexafluoropropylene)/alumina superhydrophobic coating. ACS Appl. Polym. Mater. 2022, 4, 1358–1367. [Google Scholar] [CrossRef]
- Fu, K.; Lu, C.; Liu, Y.; Zhang, H.; Zhang, B.; Zhang, H.; Zhou, F.; Zhang, Q.; Zhu, B. Mechanically robust, self-healing superhydrophobic anti-icing coatings based on a novel fluorinated polyurethane synthesized by a two-step thiol click reaction. Chem. Eng. J. 2021, 404, 127110. [Google Scholar] [CrossRef]
- Yap, S.W.; Johari, N.; Mazlan, S.A.; Hassan, N.A. Mechanochemical durability and self-cleaning performance of zinc oxide-epoxy superhydrophobic coating prepared via a facile one-step approach. Ceram. Int. 2021, 47, 16864–16872. [Google Scholar] [CrossRef]
- Lu, C.X.; Gao, Y.; Yu, S.J.; Zhou, H.; Wang, X.; Li, L.W. Non-fluorinated flexible superhydrophobic surface with excellent mechanical durability and self-cleaning performance. ACS Appl. Mater. Interfaces 2022, 14, 4750–4758. [Google Scholar] [CrossRef]
- Rahman, O.S.A.; Mukherjee, B.; Islam, A.; Keshri, A.K. Instant tuning of superhydrophilic to robust superhydrophobic and self-cleaning metallic coating: Simple, direct, one-step, and scalable technique. ACS Appl. Mater. Interfaces 2019, 11, 4616–4624. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Huo, M.D.; Huo, J.H.; Zhang, P.; Shao, X.; Zhang, X. Preparation of silica-epoxy superhydrophobic coating with mechanical stability and multifunctional performance via one-step approach. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129957. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhang, X.L.; Zhang, P.Y.; Gu, X.; Huo, M. A non-fluorinated superhydrophobic PDMS/STA/TiO2 coating with photocatalysis and environmental stability properties by one-step cold spraying. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132285. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhang, P.Y.; Gu, X.Q.; Zhang, X.; Huo, M. Preparation of PVDF-PDMS-SiO2 multi-stage rough superhydrophobic coating with excellent anti-corrosion and drag reduction performance via one-step cold spraying. Surf. Coat. Technol. 2023, 471, 129882. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, F.; Yin, Z.; Xue, M.; Chen, Y.; He, P.; Wu, J.; Ou, J.; Wang, F.; Luo, Y.; et al. Controllable fabrication of superhydrophobic alloys surface on 304 stainless steel substrate for anti-icing performance. Ceram. Int. 2023, 49, 25135. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/anti-icing properties of superhydrophobic surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Z.; Deng, Y.; Xue, M.; Chen, Y.; Ou, J.; Xie, Y.; Luo, Y.; Xie, C.; Hong, Z. Multifunctional CeO2-coated pulp/cellulose nanofibers (CNFs) membrane for wastewater treatment: Effective oil/water separation, organic contaminants photodegradation, and anti-bioadhesion activity. Ind. Crops Prod. 2023, 197, 116672. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Park, H. Effects of superhydrophobic surfaces on the flow around an NACA0012 hydrofoil at low Reynolds numbers. Exp. Fluids 2018, 59, 111. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.; Wu, Z.; Wang, J.; Shen, C.; Liu, Y.; Ren, L. Non-wet kingfisher flying in the rain: The water-repellent mechanism of elastic feathers. J. Colloid Interface Sci. 2019, 541, 56–64. [Google Scholar] [CrossRef]
- Zhao, B.; Jia, R. Preparation of super-hydrophobic films based on waterborne polyurethane and their hydrophobicity characteristics. Prog. Org. Coat. 2019, 135, 440–448. [Google Scholar] [CrossRef]
- Ni, Y.; Huang, J.; Li, S.; Wang, X.; Liu, L.; Wang, M.; Chen, Z.; Li, X.; Lai, Y. Underwater, multifunctional superhydrophobic sensor for human motion detection. ACS Appl. Mater. Interfaces 2021, 13, 4740–4749. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. Single-step prepared hybrid ZnO/CuO nanopowders for water repellent and corrosion resistant coatings. Ceram. Int. 2019, 45, 16864–16872. [Google Scholar] [CrossRef]
- Wei, D.-S.; Wang, J.-G.; Liu, Y.; Wang, D.; Li, S.; Wang, H. Controllable superhydrophobic surfaces with tunable adhesion on Mg alloys by a simple etching method and its corrosion inhibition performance. Chem. Eng. J. 2021, 404, 126444. [Google Scholar] [CrossRef]
- An, K.; Tong, W.; Wang, Y.-Q.; Qing, Y.; Sui, Y.; Xu, Y.; Ni, C. Eco-friendly superhydrophobic coupling conversion coating with corrosion resistance on magnesium alloy. Langmuir 2023, 39, 6355–6365. [Google Scholar] [CrossRef]
- Wang, T.-L.; Guo, Q.; Zhang, T.C.; Zhang, Y.-X.; Yuan, S. Large-scale prepared superhydrophobic HDTMS-modified diatomite/epoxy resin composite coatings for high-performance corrosion protection of magnesium alloys. Prog. Org. Coat. 2022, 170, 106999. [Google Scholar] [CrossRef]
- Li, B.Z.; Ouyang, Y.B.; Haider, Z.H.; Zhu, Y.; Qiu, R.; Hu, S.; Niu, H.; Zhang, Y.; Chen, M. One-step electrochemical deposition leading to superhydrophobic matrix for inhibiting abiotic and microbiologically influenced corrosion of Cu in seawater environment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126337. [Google Scholar] [CrossRef]
- Wang, D.H.; Sun, Q.Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-M.; Wang, Y.; Zhang, Y.; Liu, L.; Yuan, N.; Ding, J. Multifunctional coating on magnesium alloy: Superhydrophobic, self-healing, anti-corrosion and wear-resistant. Surf. Coat. Technol. 2023, 463, 129539. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Li, L.; Zhang, H.; Qiu, C. Drag-reductive and anti-corrosive superhydrophobic surface fabricated on aluminum with thin PDMS/SiO2 coating. Mater. Res. Express 2019, 6, 1065a8. [Google Scholar] [CrossRef]
- Li, D.-Y.; Cui, X.-F.; Wen, X.; Li, Y.; Liu, E.; Jin, G.; Zheng, W. Improved electrochemical behavior of Mg-Li alloys by superhydrophobic layered double hydroxides/Ni-based composite coatings. J. Alloys Compd. 2023, 947, 169666. [Google Scholar] [CrossRef]
- Zhang, L.P.; Xue, X.; Zhang, H.; Huang, Z.; Zhang, Z. Superhydrophobic surface with excellent mechanical robustness, water impact resistance and hydrostatic pressure resistance by two-step spray-coating technique. Compos. Part A Appl. Sci. Manuf. 2021, 146, 106405. [Google Scholar] [CrossRef]
- Wu, B.R.; Lu, J.J.; Peng, C.Y.; Jiang, D.; Yang, J.; Yang, J.; Xing, S.; Sheng, L. Inverse infusion processed hierarchical structure towards superhydrophobic coatings with ultrahigh mechanical robustness. Chem. Eng. J. 2020, 387, 124066. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Holkar, C.R.; Pinjari, D.V. Anticorrosive performance of superhydrophobic imidazole encapsulated hollow zinc phosphate nanoparticles on mild steel. Prog. Org. Coat. 2018, 114, 33–39. [Google Scholar] [CrossRef]
- Liu, X.; He, H.-Q.; Zhang, T.C.; Ouyang, L.; Zhang, Y.-X.; Yuan, S. Superhydrophobic and self-healing dual-function coatings based on mercaptobenzimidazole inhibitor-loaded magnesium silicate nanotubes for corrosion protection of AZ31B magnesium alloys. Chem. Eng. J. 2021, 404, 127106. [Google Scholar] [CrossRef]
- Shi, L.; Yan, H.; Zhao, S.; Zhang, L.; Fan, X. A durable superhydrophobic composite coating towards superior anticorrosion/wear properties. Appl. Surf. Sci. 2024, 655, 159662. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Ghannam, H.; Chahboun, A.; Turmine, M. Wettability of zinc oxide nanorod surfaces. RSC Adv. 2019, 9, 38289. [Google Scholar] [CrossRef]
- Emani, P.S.; Maddah, H.A.; Rangoonwala, A.; Che, S.W.; Prajapati, A.; Singh, M.R.; Gruen, D.M.; Berry, V.; Behura, S.K. Organophilicity of graphene oxide for enhanced wettability of ZnO nanorods. ACS Appl. Mater. Interfaces 2020, 12, 39772–39780. [Google Scholar] [CrossRef]


| Elements | Si | Cu | Mg | Fe | Mn | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|
| Mass fraction (wt.%) | 0.36 | 0.02 | 1.74 | 0.30 | 0.07 | 0.02 | 0.20 | 97.29 |
| Samples | Ecorr (V) | Icorr (A) | CR (mm·a−1) | η (%) |
|---|---|---|---|---|
| Aluminum alloy | −0.862 | 1.042 × 10−6 | 0.0334385 | 0 |
| EP coating | −0.775 | 1.182 × 10−7 | 0.0037931 | 88.66 |
| h-SiO2@PFDT coating | −0.612 | 1.169 × 10−8 | 0.0003751 | 98.38 |
| h-SiO2@PFDT-EP coating | −0.518 | 8.692 × 10−10 | 0.0000279 | 99.92 |
| Velocity of Flow (m s−1) | h-SiO2@PFDT (%) | h-SiO2@PFDT-EP (%) |
|---|---|---|
| 1.31 | 23.66 | 23.36 |
| 1.96 | 25.18 | 24.93 |
| 2.62 | 27.83 | 27.38 |
| 3.27 | 26.75 | 29.84 |
| 3.93 | 24.17 | 31.01 |
| 4.58 | 23.98 | 30.56 |
| 5.24 | 22.75 | 28.47 |
| 5.89 | 22.00 | 27.36 |
| 6.55 | 21.45 | 25.78 |
| 7.20 | 20.55 | 24.11 |
| 7.86 | 18.48 | 21.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhao, Y.; Gu, X.; Huo, M.; Yang, K.; Liu, M.; Fan, S.; Zhi, M. Durable Superhydrophobic Composite Coating Based on Hydrangea-like SiO2 Nanoparticles with Excellent Performance in Anticorrosion, Drag Reduction, and Antifouling. Materials 2025, 18, 3443. https://doi.org/10.3390/ma18153443
Xue Y, Zhao Y, Gu X, Huo M, Yang K, Liu M, Fan S, Zhi M. Durable Superhydrophobic Composite Coating Based on Hydrangea-like SiO2 Nanoparticles with Excellent Performance in Anticorrosion, Drag Reduction, and Antifouling. Materials. 2025; 18(15):3443. https://doi.org/10.3390/ma18153443
Chicago/Turabian StyleXue, Yuhao, Yamei Zhao, Xiaoqi Gu, Mengdan Huo, Kunde Yang, Mingyu Liu, Sixian Fan, and Maoyong Zhi. 2025. "Durable Superhydrophobic Composite Coating Based on Hydrangea-like SiO2 Nanoparticles with Excellent Performance in Anticorrosion, Drag Reduction, and Antifouling" Materials 18, no. 15: 3443. https://doi.org/10.3390/ma18153443
APA StyleXue, Y., Zhao, Y., Gu, X., Huo, M., Yang, K., Liu, M., Fan, S., & Zhi, M. (2025). Durable Superhydrophobic Composite Coating Based on Hydrangea-like SiO2 Nanoparticles with Excellent Performance in Anticorrosion, Drag Reduction, and Antifouling. Materials, 18(15), 3443. https://doi.org/10.3390/ma18153443




