The Use of a Composite of Modified Construction Aggregate and Activated Carbon for the Treatment of Groundwater Contaminated with Heavy Metals and Chlorides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Cadmium chloride dihydrate (CdCl2·2.5H2O);
- Copper(II) chloride dihydrate (CuCl2·2H2O);
- Nickel(II) sulphate heptahydrate (NiSO4·7H2O);
- Lead(II) nitrate (Pb(NO3)2);
- Zinc chloride (ZnCl2);
- Sodium chloride (NaCl).
2.2. Materials Modifications
2.3. Preliminary Flow Tests
2.4. Batch Test
- PN-EN ISO 11885:2009: Heavy metals in solution were measured using ICP-ASA (atomic absorption spectrometry) and ICP-AES (inductively coupled plasma atomic emission spectrometry) from Thermo Scientific, Waltham, MA, USA [30];
- PN-ISO 9297:1994: Chloride ions were analyzed through titration (Mohr method) [31].
3. Results
3.1. Preliminary Flow Test Results
3.2. Modification Results
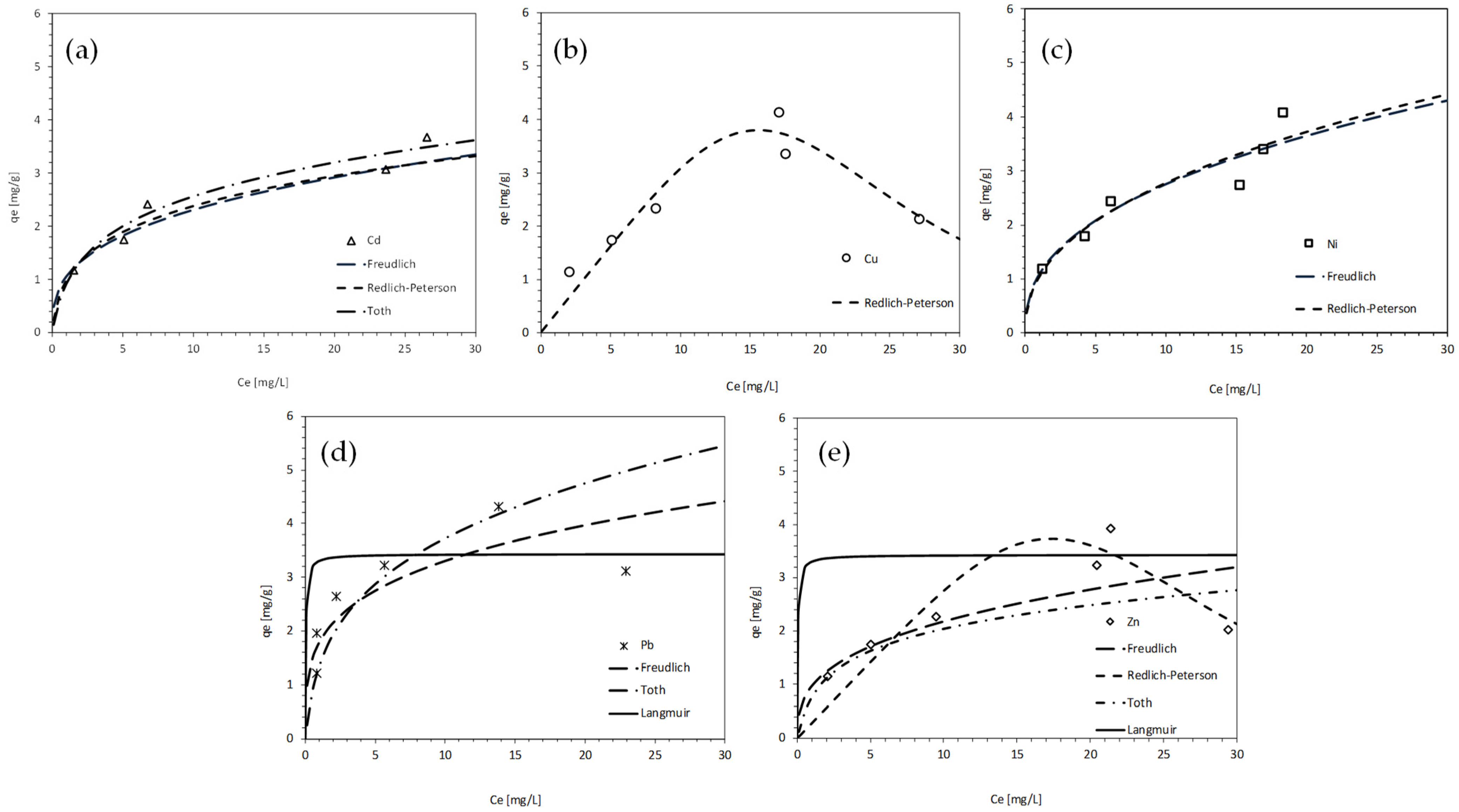
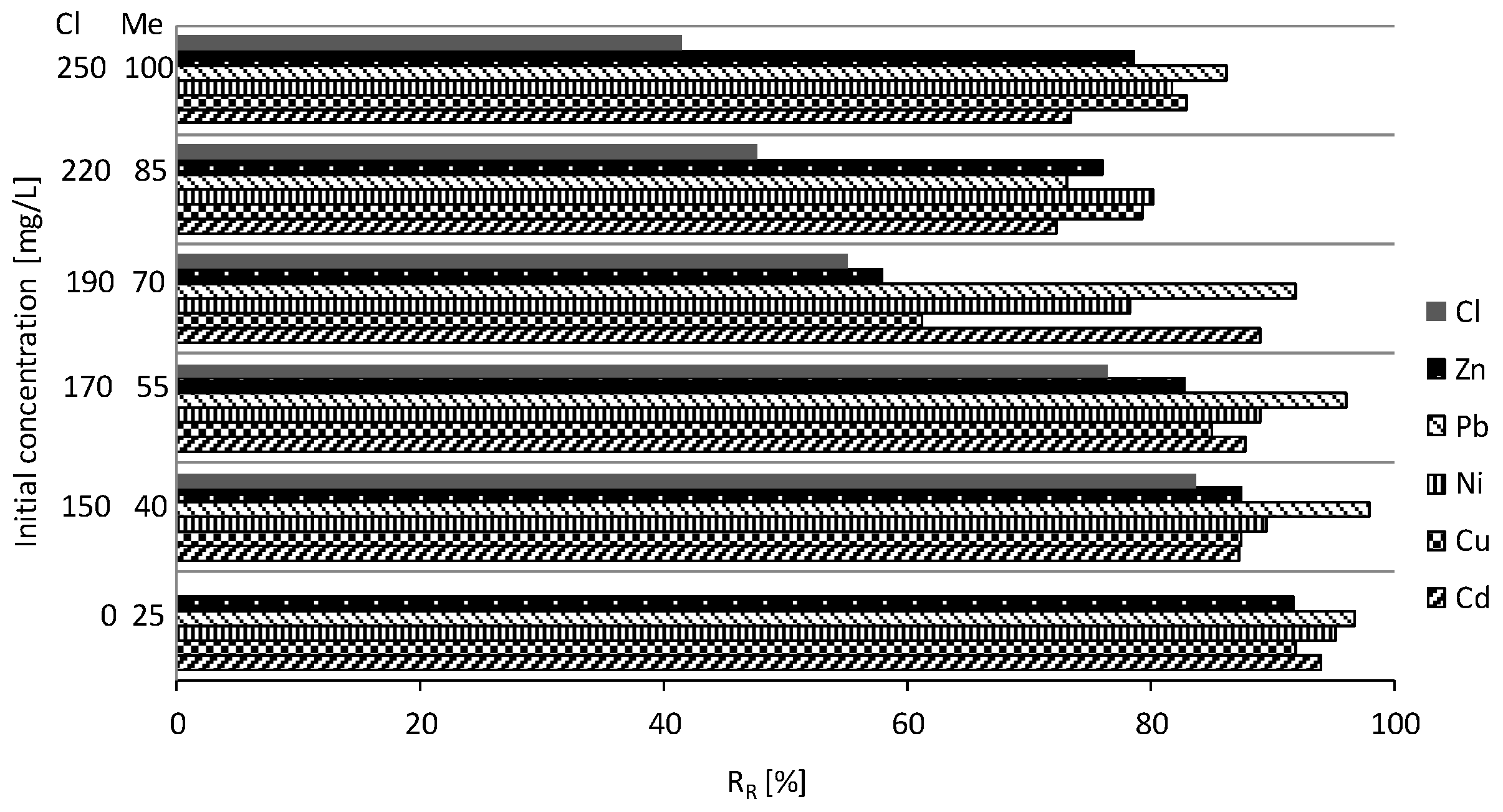
3.3. Batch Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 on substances particularly harmful to the aquatic environment and the conditions to be met when discharging sewage into water or soil, as well as when discharging rainwater or meltwater into water or water facilities. Journal of Laws 2019, Item 1311.
- Lewandowská, Š.; Lhotský, O.; Vaňková, Z.; Cajthaml, T.; Pacáková, B.; Yarmalinskaya, Y.; Komárek, M. Permeable Reactive Barrier with Zerovalent Iron Grit for the Remediation of Metal Contaminated Groundwater with High Mineralization: A Comprehensive Field Assessment. ACS EST Water 2024, 5, 425–433. [Google Scholar] [CrossRef]
- Pourkhorshidi, S.; Sangiorgi, C.; Torreggiani, D.; Tassinari, P. Using recycled aggregates from construction and demolition waste in unbound layers of pavements. Sustainability 2020, 12, 9386. [Google Scholar] [CrossRef]
- Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; De Brito, J. Magnesia (MgO) production and characterization, and its influence on the performance of cementitious materials: A review. Materials 2020, 13, 4752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Dastgheib, S.A.; Karanfil, T. Adsorption of dissolved natural organic matter by modified activated carbons. Water Res. 2005, 39, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, Y.; Liu, H.; Wu, S.; Lu, C. CO2 Capture Performance Using Limestone Modified with Propionate Acid During Calcium Looping Cycle. In Cleaner Combustion and Sustainable World; Qi, H., Zhao, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1163–1169. [Google Scholar] [CrossRef]
- Stavropoulos, G.G.; Samaras, P.; Sakellaropoulos, G.P. Effect of activated carbons modification on porosity, surface structure and phenol adsorption. J. Hazard. Mater. 2008, 151, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, P.; Guo, X.; Han, D.; Chao, Y. High–temperature CO2 capture cycles of hydrated limestone prepared with aluminum (hydr) oxides derived from kaolin. Energy Convers. Manag. 2014, 86, 1147–1153. [Google Scholar] [CrossRef]
- Arora, M.; Eddy, N.K.; Mumford, K.A.; Babab, Y.; Perera, J.M.; Stevens, G.W. Surface modification of natural zeolite by chitosan and its use for nitrate removal in cold regions. Cold Reg. Sci. Technol. 2010, 62, 92–97. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Li, C.M.; Xu, Z.X.; Zhang, C.M.; Wang, J.L.; Li, K.X. Surface modification of activated carbon for CO2 adsorption. Carbon 2014, 76, 471. [Google Scholar] [CrossRef]
- Marzaioli, V.; Aguilar–Pimentel, J.A.; Weichenmeier, I.; Luxenhofer, G.; Wiemann, M.; Landsiedel, R.; Wohlleben, W.; Eiden, S.; Mempel, M.; Behrendt, H.; et al. Surface modifications of silica nanoparticles are crucial for their inert versus proinflammatory and immunomodulatory properties. Int. J. Nanomed. 2014, 9, 2815–2832. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Zhao, X.; Wang, Y.; Jin, X.; Ji, J.; Cheng, Z.; Yand, G.; Li, H.; Chen, G. Research progress of biochar modification technology and its application in environmental remediation. Biomass Bioenergy 2024, 184, 107178. [Google Scholar] [CrossRef]
- Lin, R.S.; Huang, G.; Ma, F.Y.; Pan, T.H.; Wang, X.Y.; Han, Y.; Liao, Y.P. Investigation of phosphogypsum-based cementitious materials: The effect of lime modification. Dev. Built Environ. 2024, 18, 100477. [Google Scholar] [CrossRef]
- Moussavi, G.; Aghapour, A.A.; Yaghmaeian, K. The degradation and mineralization of catechol using ozonation catalyzed with MgO/GAC composite in a fluidized bed reactor. Chem. Eng. J. 2014, 249, 302–310. [Google Scholar] [CrossRef]
- Ramachandran, D.K.; Clemens, F.; Glasscock, A.J.; Søgaarda, M.; Kaisera, A. Tailoring the micro-structure of porous MgO supports for asymmetric oxygen separation membranes: Optimization of thermoplastic feedstock systems. Ceram. Int. 2014, 40, 10465–10473. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of magnesium oxide. Metall. Mater. Trans. 1999, 30, 1009–1015. [Google Scholar] [CrossRef]
- Szymoniak, L.; Claveau-Mallet, D.; Haddad, M.; Barbeau, B. Application of magnesium oxide media for remineralization and removal of divalent metals in drinking water treatment: A review. Water 2022, 14, 633. [Google Scholar] [CrossRef]
- Teringo, J. III. Magnesium hydroxide reduces sludge/improves filtering. Pollut. Eng. 1987, 19, 78–83. [Google Scholar]
- Koo-Amornpattana, W.; Phadungbut, P.; Kunthakudee, N.; Jonglertjunya, W.; Ratchahat, S.; Hunsom, M. Innovative metal oxides (CaO, SrO, MgO) impregnated waste-derived activated carbon for biohydrogen purification. Sci. Rep. 2023, 13, 4705. [Google Scholar] [CrossRef] [PubMed]
- Ghoniem, M.G.; Ben Aissa, M.A.; Ali, F.A.M.; Khairy, M. Efficient and rapid removal of Pb (II) and Cu (II) heavy metals from aqueous solutions by MgO nanorods. Inorganics 2022, 10, 256. [Google Scholar] [CrossRef]
- Kawamura, M.; Kasai, Y. Determination of saturated surface-dry condition of clay–sand mixed soils for soil–cement concrete construction. Mater. Struct. 2010, 43, 571–582. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Asouhidou, D.D. Kinetics of soptive removal of chromium (VI) from aqueous solution by calcined Mg–AlC03 hydrotaleite. Water Res. 2003, 37, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Lv, L.; Pan, B.C.; Zhang, Q.J.; Zhang, W.M.; Zhang, Q.X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Ho, Y. Second–order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non–linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo–second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Tang, Z.; Kim, S.J.; Reddy, G.K.; Dong, J.; Smirniotis, P. Modified zeolite membrane reactor for high temperature water gas shift reaction. J. Membr. Sci. 2010, 354, 114–122. [Google Scholar] [CrossRef]
- Ho, Y.S.; Wang, C.C. Pseudo–isotherms for the sorption of cadmium ion onto tree fern. Process Biochem. 2004, 39, 759–763. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al–Muhtaseb, A.H.; Al–Laqtah, N.A.; Walker, G.; Allen, S.; Ahmad, M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Kiari, M.; Konan, A.; Mamane, O.; Ouattara, L.; Grema, M.; Boukari, M.; Yao, K. Adsorption kinetics, thermodynamics, modeling and optimization of bisphenol A on activated carbon based on Hyphaene Thebaica shells. Case Stud. Chem. Environ. Eng. 2024, 10, 100903. [Google Scholar] [CrossRef]
- PN-EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2009.
- PN-ISO 9297:1994; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). ISO: Geneva, Switzerland, 1994.
- Yan, Y.; Chen, X.; Zhang, B.; Zhao, X. Heterogeneous adsorption of chloride ions on ce-ment-based materials: Mechanism and modeling. Constr. Build. Mater. 2011, 25, 159–164. [Google Scholar] [CrossRef]
- Navarro, C.R.; Cardell, C.; Martín, V. Influence of pH on the removal of heavy metals from groundwater by MgO. Appl. Geochem. 2006, 21, 55–65. [Google Scholar] [CrossRef]
- Okoye, A.I.; Ejikeme, P.M.; Onukwuli, O.D. Lead removal from wastewater using fluted pumpkin seed shell activated carbon: Adsorption modeling and kinetics. Int. J. Environ. Sci. Technol. 2010, 7, 793–800. [Google Scholar] [CrossRef]
- Jeon, C.; Kim, D.; Kim, S.; Kim, Y. Adsorption characteristics of heavy metals by granular activated carbon coated with magnesium oxide. Desalin. Water Treat. 2012, 40, 17–24. [Google Scholar] [CrossRef]
- He, J.; Lin, H.; Kong, L. Removal of heavy metals from aqueous solution by MgO nanoparticles. J. Hazard. Mater. 2010, 175, 308–313. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Yang, X. Removal of chloride ions from aqueous solution using modified activated carbon. Water Sci. Technol. 2013, 67, 2731–2738. [Google Scholar] [CrossRef]

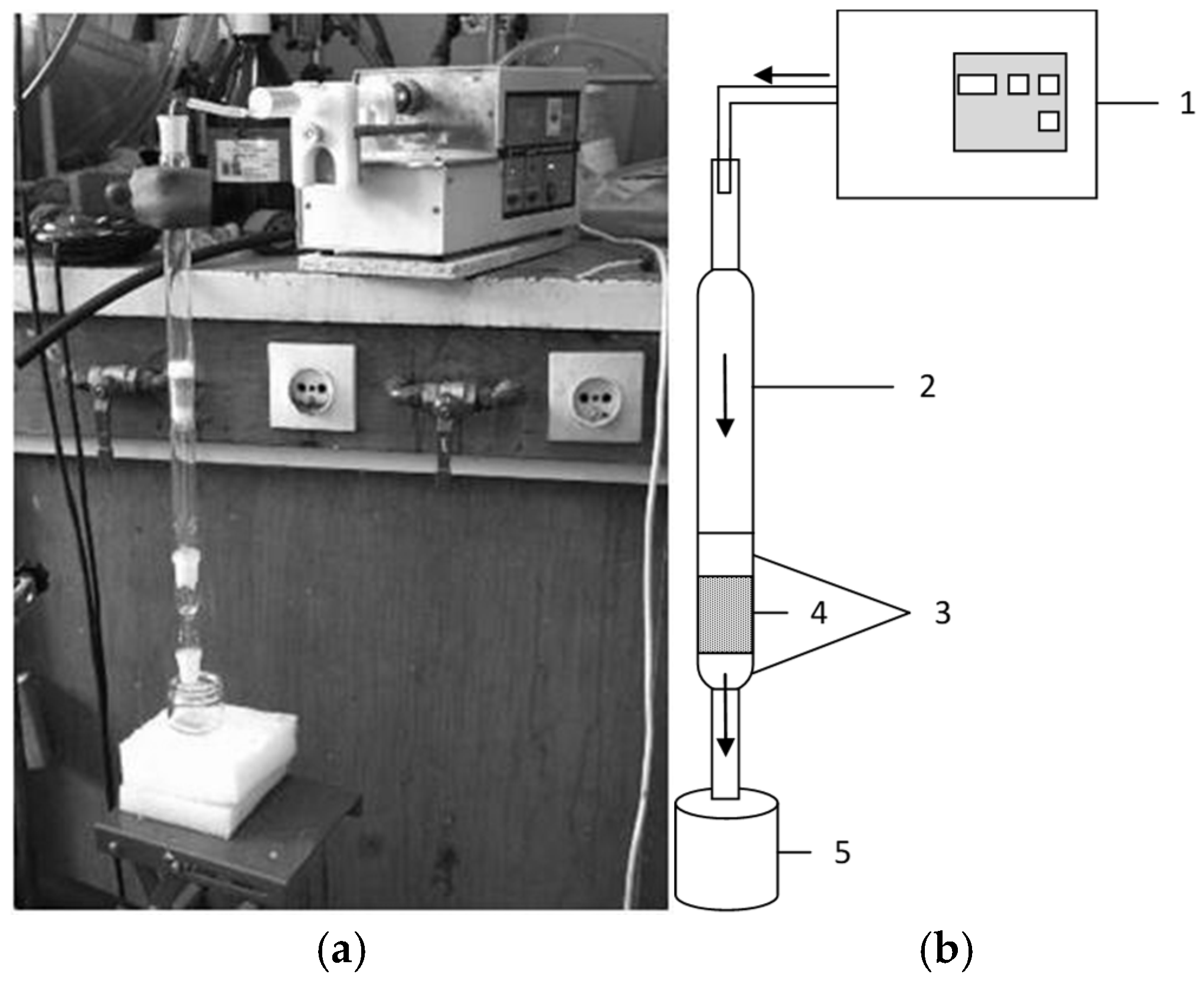
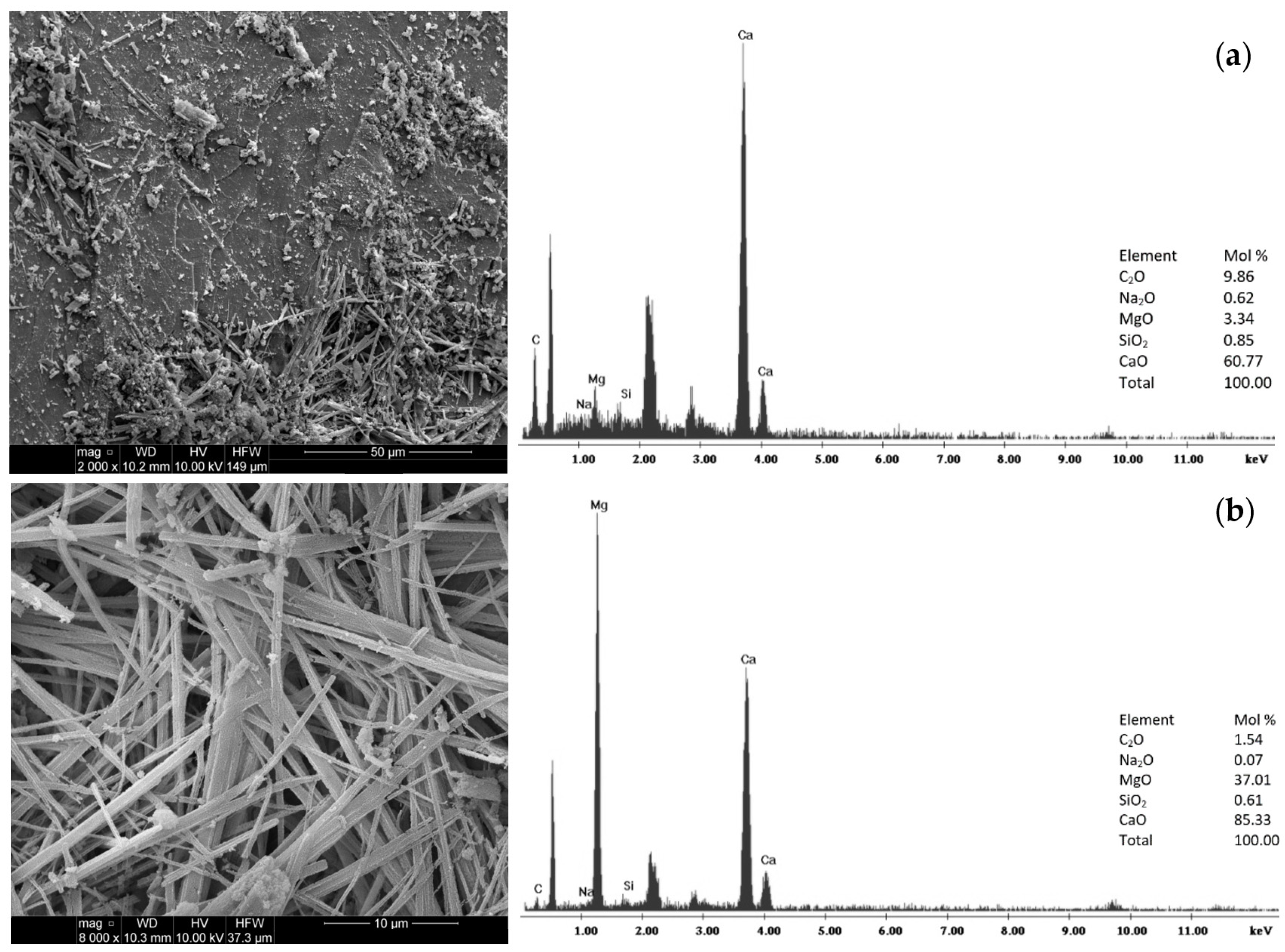
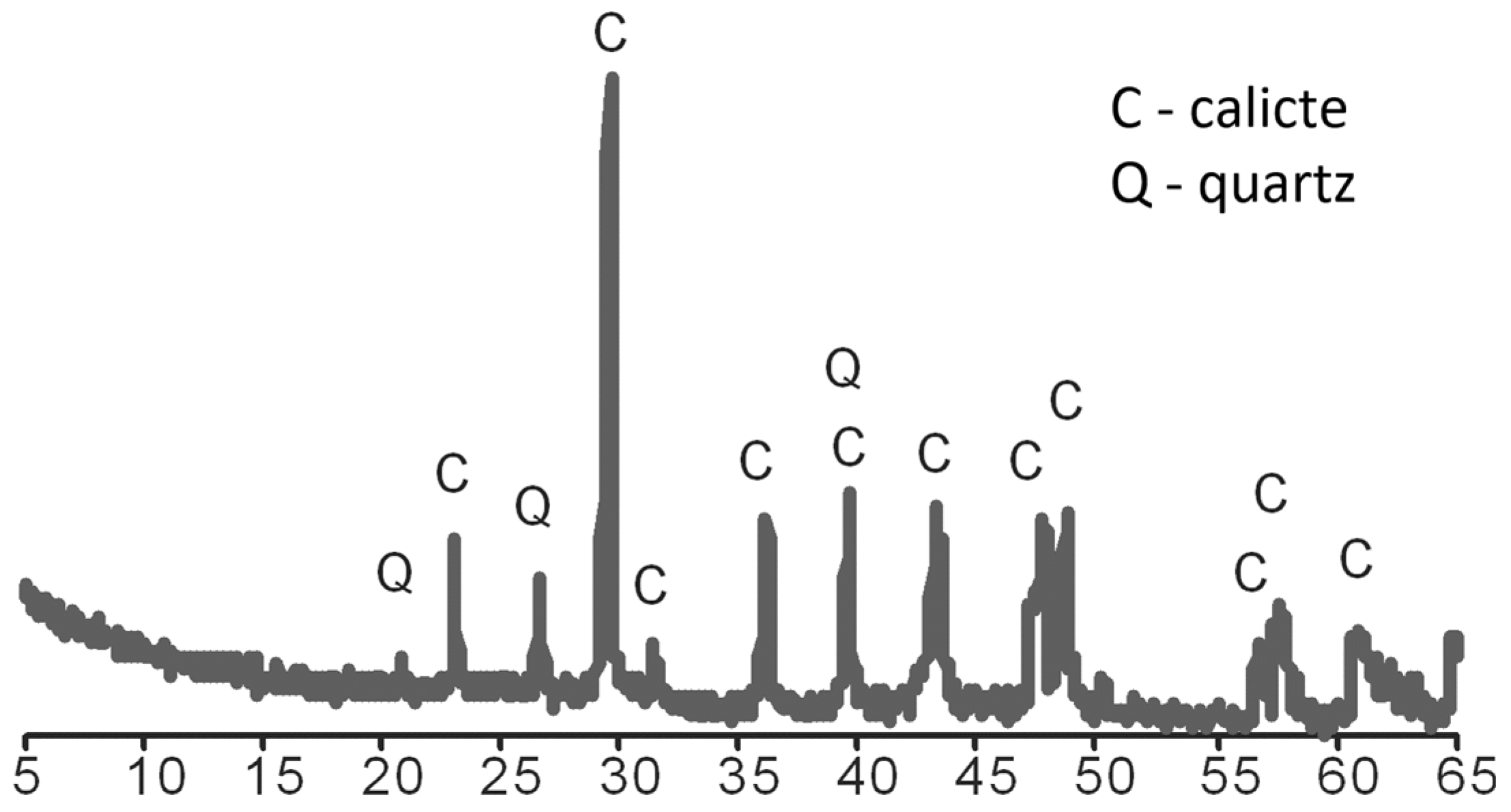


| Materials | CA | MCA | AC | MAC | ||||
|---|---|---|---|---|---|---|---|---|
| Conditions | Before | After | Before | Before | After | After | Before | After |
| volume of solution in syringe [mL] | 20.0 | 9.5 | 20.0 | 9.5 | 20.0 | 9.5 | 20.0 | 9.5 |
| mass of material [g] | 6.7000 | 6.7042 | 5.0935 | 5.5171 | 1.6936 | 1.7179 | 1.9689 | 9.0299 |
| mass of quartz wool [g] | 0.2080 | 0.5198 | 0.208 | 0.5033 | 0.2080 | 0.6185 | 0.208 | 0.4613 |
| mass of reactor [g] | 66.9382 | 70.7000 | 65.28 | 68.7893 | 65.9535 | 69.781 | 62.1948 | 66.6346 |
| mass of vessels [g] | 55.0165 | 55.9210 | 39.4487 | 40.8032 | 50.3204 | 51.1857 | 55.5052 | 56.115 |
| volume of r-liquid in receiver [mL] | 5.5 | 5.5 | 5.5 | 5.5 | ||||
| velocity of flow through bed [m/s] | 1.56 × 10−5 | 1.54 × 10−5 | 1.39 × 10−5 | 9.50 × 10−6 | ||||
| t [s] | 2491.8 | 2593.2 | 2902.2 | 4206.0 | ||||
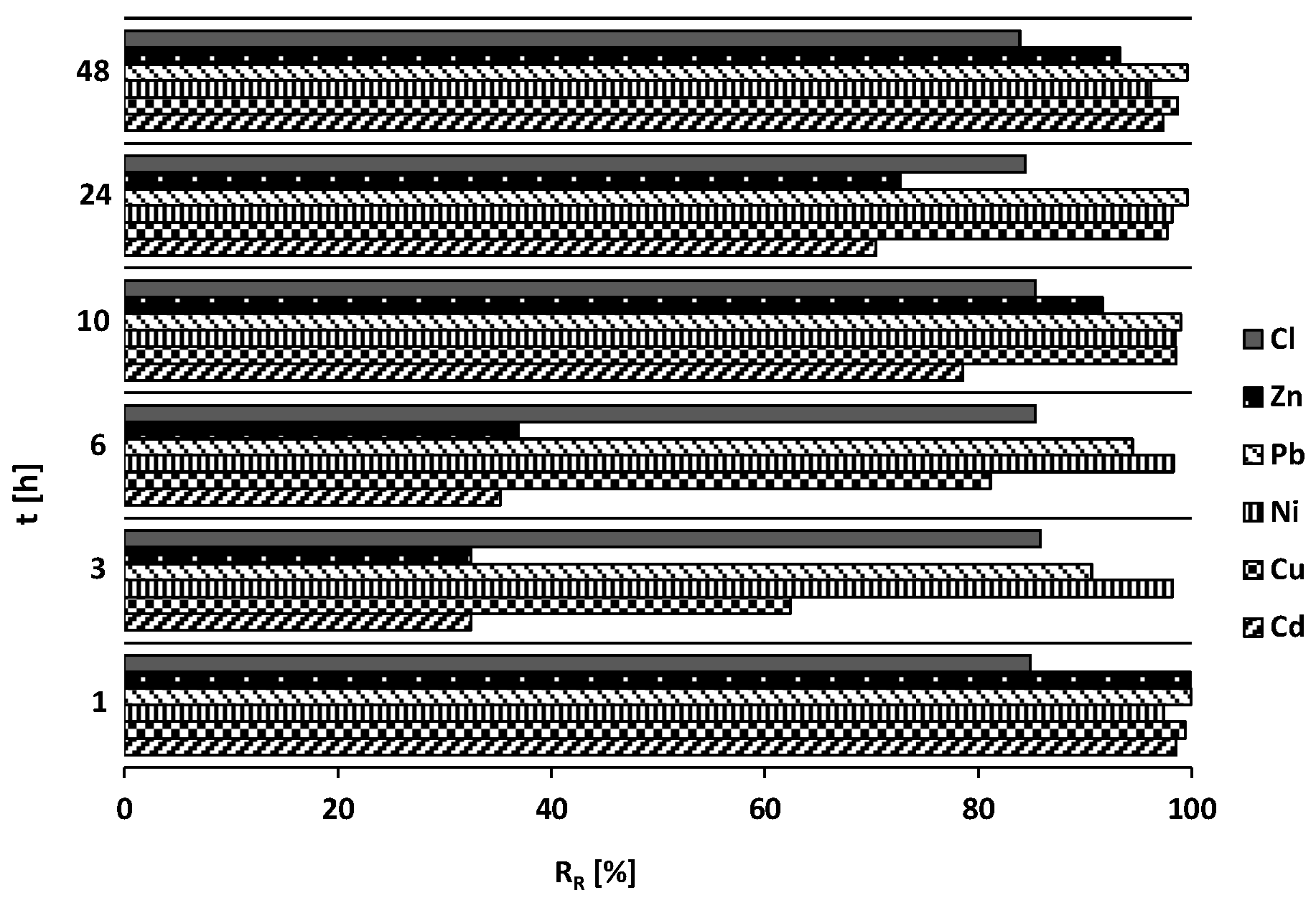
| RR [%] | 0.34 | 20.43 | 0.57 | 60.07 | ||||
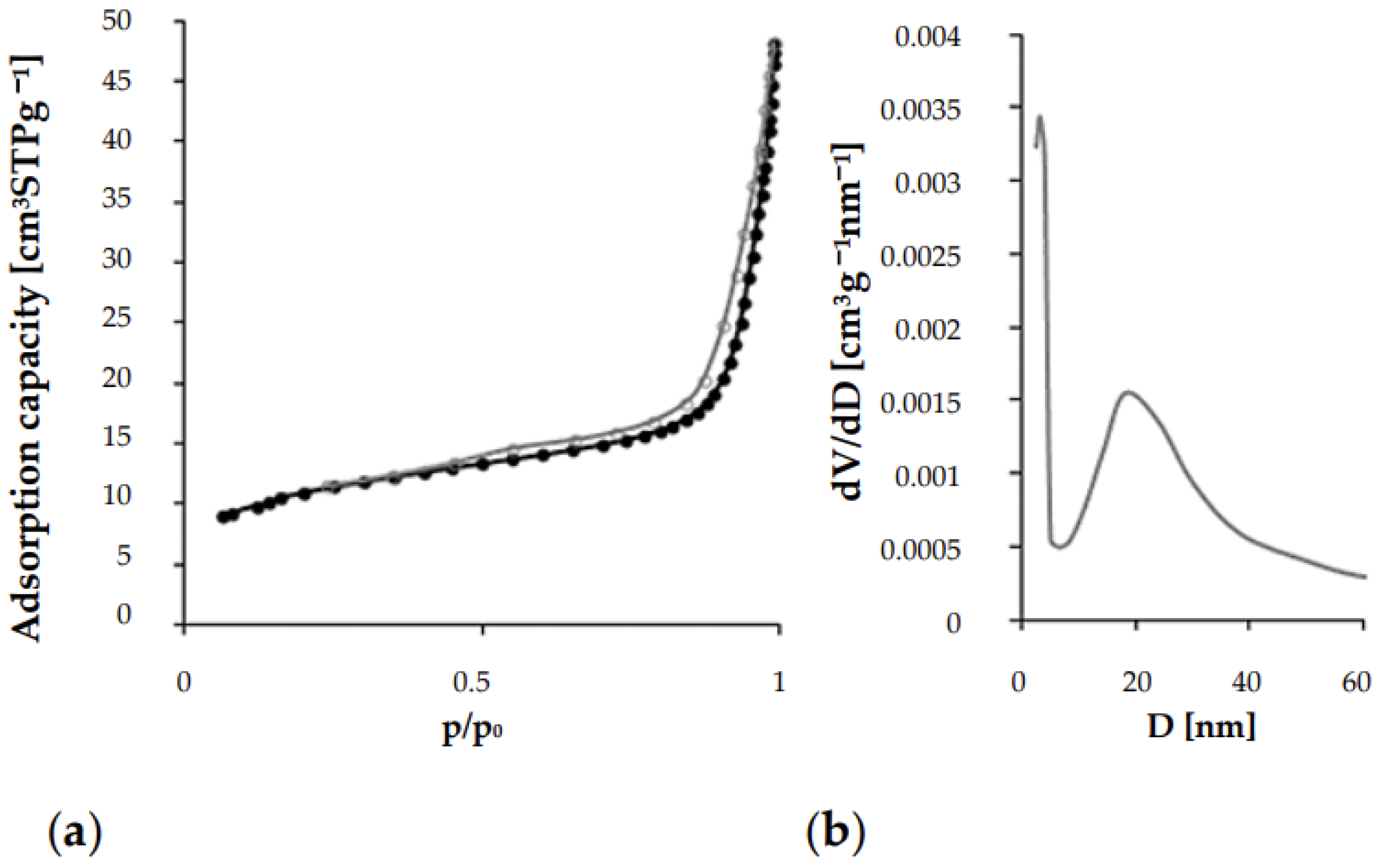
| Material | SBET [m2/g] | Vmic [cm3/g] | Smic [m2/g] | Vmes [cm3/g] | Smes [m2/g] | D [nm] | DBJH/DBJH [nm] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Ads. | Des. | Ads. | Des. | Ads. | Des | |||||
| AC | 856.05 | 0.1600 | 366.41 | 0.51 | 0.50 | 177.10 | 92.31 | 3.65 | 4.85 | 0.40 |
| CA | 2.82 | 0.0002 | 0.28 | 0.00 | 0.00 | 0.95 | 1.35 | 12.12 | 11.65 | 14.13 |
| MIX | 38.73 | 0.0700 | - | 0.07 | 0.07 | 22.74 | 17.99 | 12.02 | 13.61 | 6.91 |
| Contamination | Cl | Cd | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|
| Model | |||||||
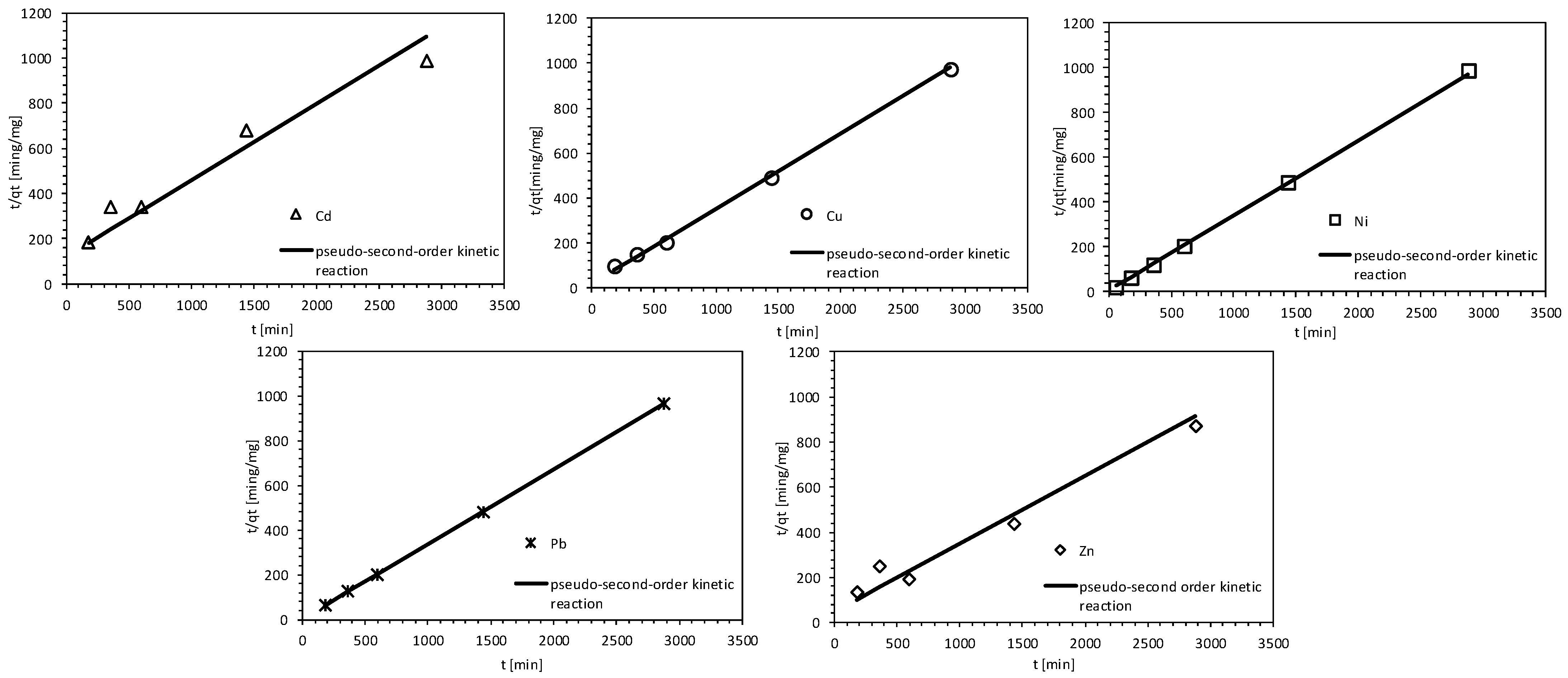
| Second- order kinetic | Cs [mg/L] | - | 1.6 | 0.78 | - | 0.23 | 0.95 |
| k2 [min−1] | - | 0.0027 | 0.0121 | - | 0.0559 | 0.004 | |
| R2 [-] | - | 0.88 | 0.83 | - | 0.92 | 0.75 | |
| F [-] | - | 29.31 | 32.10 | - | 54.17 | 15.19 | |
| p-value [-] | - | 0.012 | 0.011 | - | 0.005 | 0.029 | |
| Pseudo- second-order kinetic | Cs [mg/L] | 13.72 | 1.6 | 0.78 | 1.76 | 0.23 | 0.95 |
| kp2 [min−1] | 0.098 | 0.001 | 0.007 | 0.017 | 0.037 | 0.002 | |
| R2 [-] | 0.99 | 0.93 | 0.99 | 0.99 | 0.99 | 0.96 | |
| F [-] | 95,679.11 | 93.91 | 2900.70 | 9768.36 | 91,246.94 | 121.73 | |
| p-value [-] | 7.45 × 10−8 | 0.002 | 7.45 × 10−8 | 6.29 × 10−10 | 8.00 × 10−8 | 0.002 | |
| Intraparticle diffusion | Cs [mg/L] | - | 1.6 | - | - | - | - |
| kint [min−1] | - | 0.0574 | - | - | - | - | |
| R2 [-] | - | 0.92 | - | - | - | - | |
| F [-] | - | 72.35 | - | - | - | - | |
| p-value [-] | - | 0.003 | - | - | - | - | |
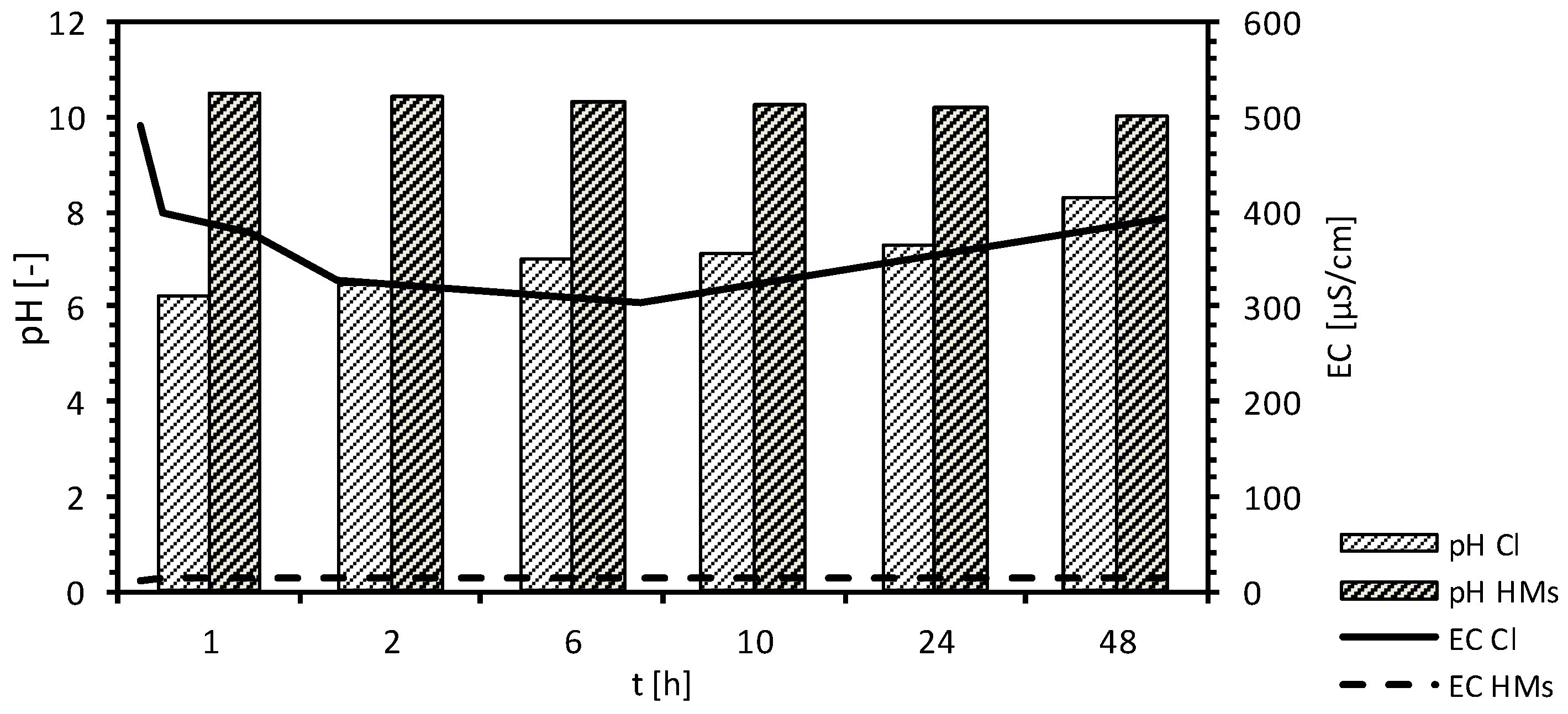
| pH [-] | 10.00–10.41 | 6.17–8.30 | |||||
| EC [µS/cm] | 176.00–491.00 | 17.70–15.08 | |||||
| Contamination | Cl | Cd | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|
| Model | |||||||
| Langmuir | qmax [-] | - | - | 2.69 | 4.38 | 3.43 | 2.66 |
| KL [L/mg] | - | - | 10.94 | 3.92 | 25.97 | 7.44 | |
| RL [L/mg] | - | - | 0.002 | 0.005 | 0.001 | 0.003 | |
| R2 [-] | - | - | 0.80 | 0.88 | 0.96 | 0.79 | |
| F [-] | - | - | 16.12 | 53.68 | 101.62 | 14.68 | |
| p-value [-] | - | - | 0.02 | 0.005 | 0.001 | 0.018 | |
| Freundlich | KF [mg/L] | - | 1.02 | 0.98 | 1.06 | 1.79 | 0.98 |
| NF [-] | - | 0.38 | 0.38 | 0.44 | 0.26 | 0.38 | |
| R2 [-] | - | 0.95 | 0.62 | 0.97 | 0.71 | 0.65 | |
| F [-] | - | 65.96 | 6.67 | 113.94 | 9.61 | 7.44 | |
| p-value [-] | - | 0.004 | 0.061 | 0.002 | 0.036 | 0.053 | |
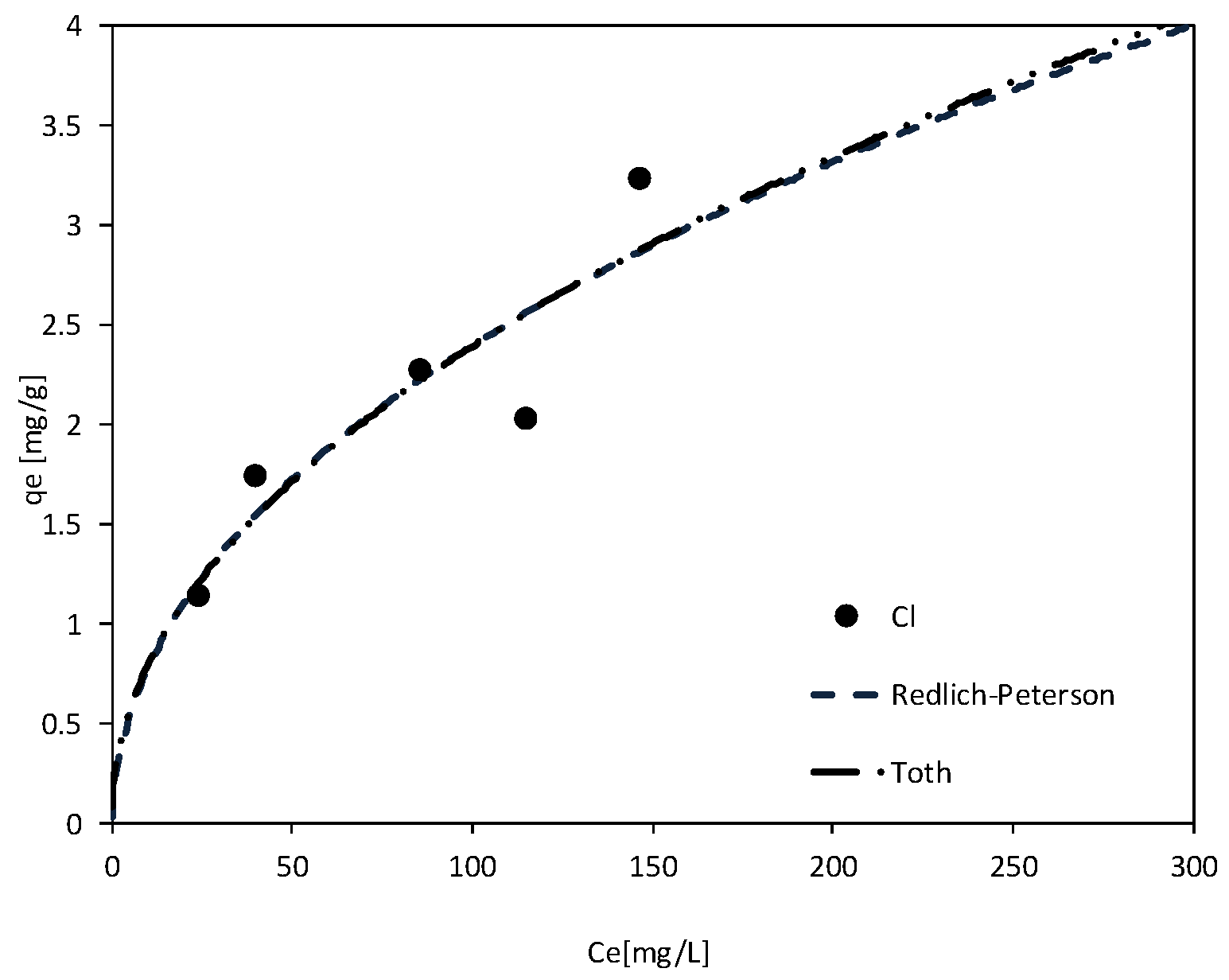
| Redlich–Peterson | KR [L/mg] | 0.62 | 2.43 | 0.32 | 89.56 | - | 0.29 |
| BR [-] | 1.97 | 1.64 | 0.00 | 85.09 | - | 0.00 | |
| β [-] | 0.55 | 0.73 | 3.99 | 0.56 | - | 4.05 | |
| R2 [-] | 0.79 | 0.96 | 0.96 | 0.96 | - | 0.91 | |
| F [-] | 12.21 | 52.69 | 47.81 | 78.23 | - | 27.64 | |
| p-value [-] | 0.040 | 0.005 | 0.002 | 0.003 | - | 0.006 | |
| Toth | KT [-] | 0.26 | 1.43 | - | - | 1.82 | 1.27 |
| bT [-] | 0.001 | 0.831 | - | - | 0.54 | 0.911 | |
| β [-] | 1.93 | 1.39 | - | - | 1.48 | 1.31 | |
| R2 [-] | 0.80 | 0.75 | - | - | 0.66 | 0.65 | |
| F [-] | 12.30 | 33.57 | - | - | 19.05 | 8.11 | |
| p-value [-] | 0.039 | 0.010 | - | - | 0.012 | 0.046 | |
| pH [-] | 10.00–10.30 | 6.28–11.79 | |||||
| EC [µS/cm] | 190.00–1331.00 | 7.68–25.50 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawluk, K.; Lendo-Siwicka, M.; Wrzesiński, G.; Szymanek, S.; Osawaru, O.Y. The Use of a Composite of Modified Construction Aggregate and Activated Carbon for the Treatment of Groundwater Contaminated with Heavy Metals and Chlorides. Materials 2025, 18, 3437. https://doi.org/10.3390/ma18153437
Pawluk K, Lendo-Siwicka M, Wrzesiński G, Szymanek S, Osawaru OY. The Use of a Composite of Modified Construction Aggregate and Activated Carbon for the Treatment of Groundwater Contaminated with Heavy Metals and Chlorides. Materials. 2025; 18(15):3437. https://doi.org/10.3390/ma18153437
Chicago/Turabian StylePawluk, Katarzyna, Marzena Lendo-Siwicka, Grzegorz Wrzesiński, Sylwia Szymanek, and Osazuwa Young Osawaru. 2025. "The Use of a Composite of Modified Construction Aggregate and Activated Carbon for the Treatment of Groundwater Contaminated with Heavy Metals and Chlorides" Materials 18, no. 15: 3437. https://doi.org/10.3390/ma18153437
APA StylePawluk, K., Lendo-Siwicka, M., Wrzesiński, G., Szymanek, S., & Osawaru, O. Y. (2025). The Use of a Composite of Modified Construction Aggregate and Activated Carbon for the Treatment of Groundwater Contaminated with Heavy Metals and Chlorides. Materials, 18(15), 3437. https://doi.org/10.3390/ma18153437








