Fatigue Resistance of Customized Implant-Supported Restorations
Abstract
1. Introduction
2. Method
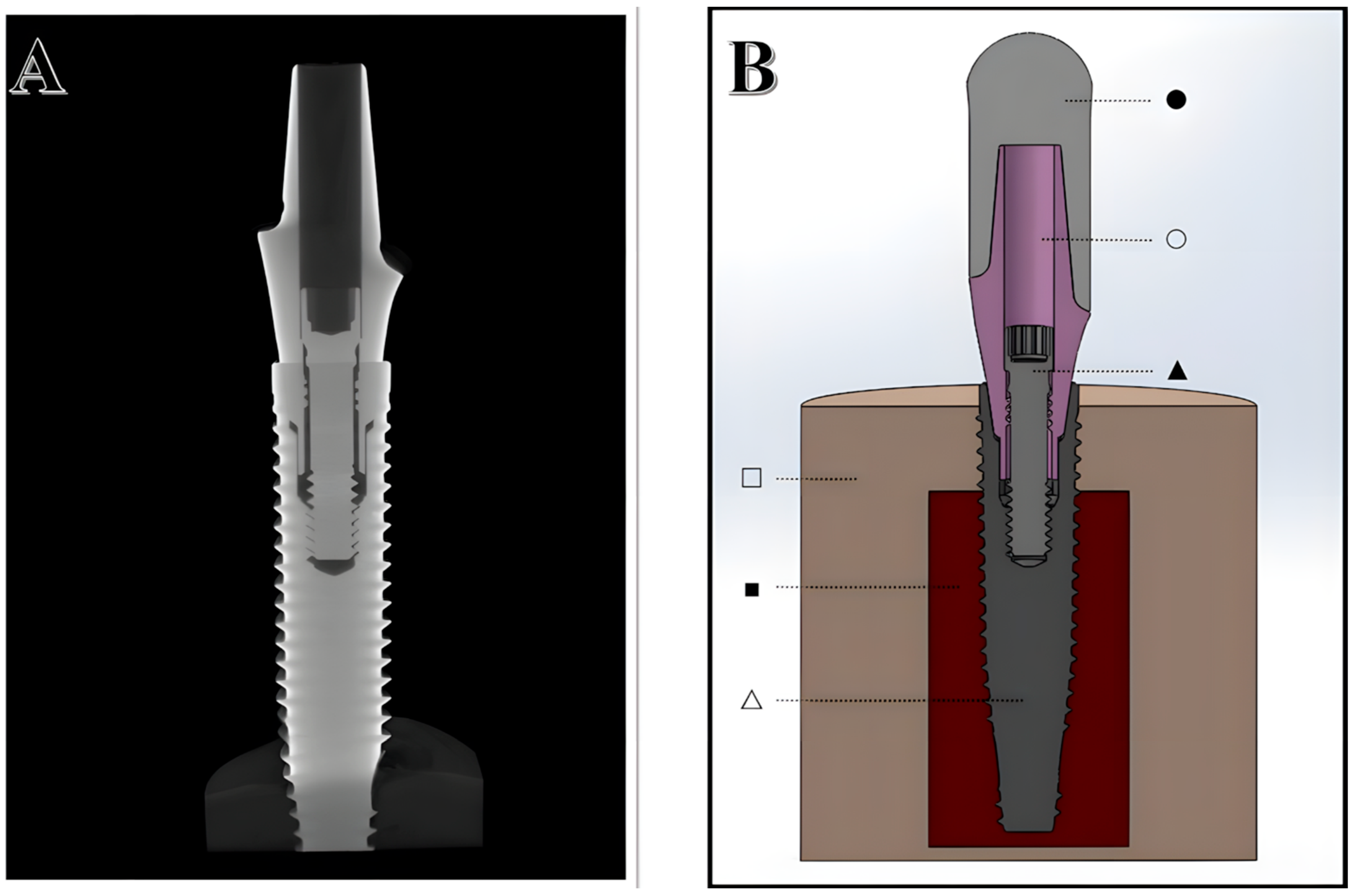
2.1. Digitalization of Structures
2.2. Design of Experiments
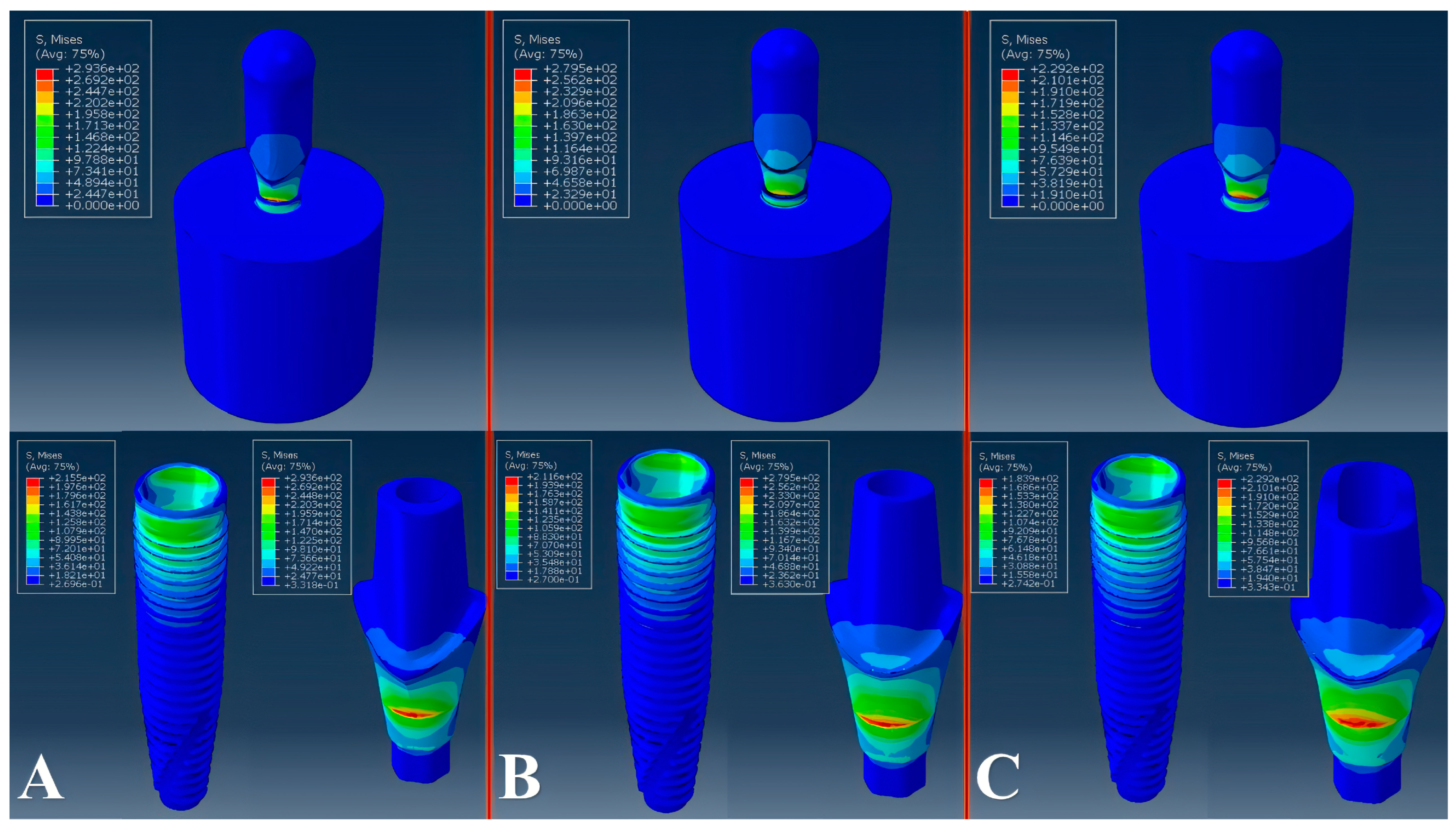
2.3. Virtual Mechanical Testing
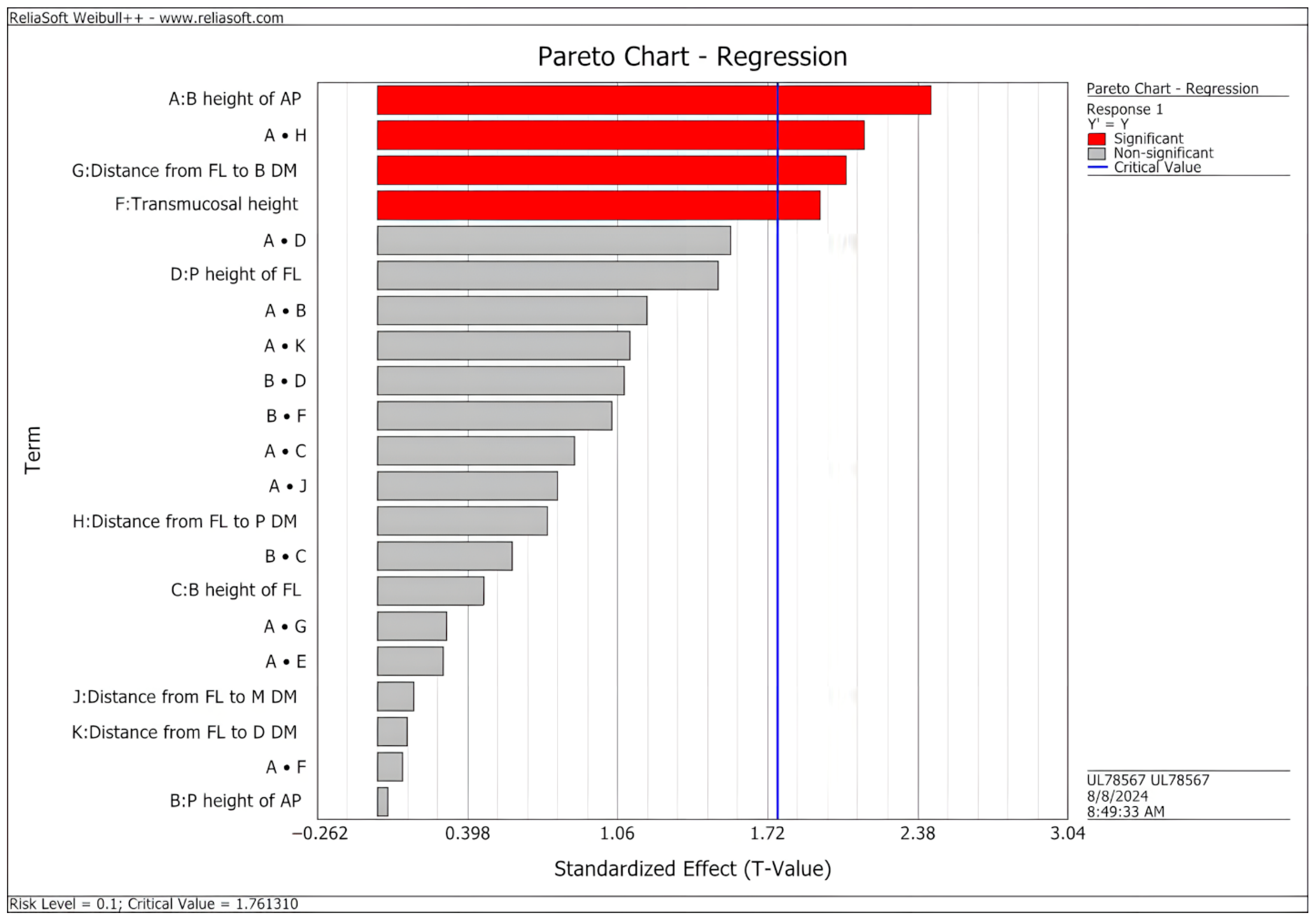
3. Results
4. Discussion
5. Conclusions
- The analysis of customized abutments identified design factors that have significant effects on the fatigue behavior of implant-abutment systems, as predicted by virtual modeling;
- Lower abutment preparation and transmucosal heights increased the predicted fatigue limit of the implant-abutment system;
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- French, D.; Ofec, R.; Levin, L. Long Term Clinical Performance of 10 871 Dental Implants with up to 22 Years of Follow-up: A cohort study in 4247 patients. Clin. Implant Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, R.; Dopico, J.; Kalemaj, Z.; Buti, J.; Pardo Zamora, G.; Mardas, N. Comparison of clinical outcomes of immediate versus delayed placement of dental implants: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2022, 33, 231–277. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Scutellà, F.; Wang, H.; Zucchelli, G. Implant placement in the esthetic area: Criteria for positioning single and multiple implants. Periodontol. 2000 2018, 77, 176–196. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Meda, R.; Esquivel, J.; Blatz, M.B. The esthetic biological contour concept for implant restoration emergence profile design. J. Esthet. Restor. Dent. 2021, 33, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Gonzalez-Martin, O.; Weisgold, A.; Lee, E. Considerations of implant abutment and crown contour: Critical contour and subcritical contour. Int. J. Periodontics Restor. Dent. 2010, 30, 335–343. [Google Scholar]
- González-Martín, O.; Lee, E.; Weisgold, A.; Veltri, M.; Su, H. Contour Management of Implant Restorations for Optimal Emergence Profiles: Guidelines for Immediate and Delayed Provisional Restorations. Int. J. Periodontics Restor. Dent. 2020, 40, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Meda, R.; Blatz, M. The Impact of 3D Implant Position on Emergence Profile Design. Int. J. Periodontics Restor. Dent. 2021, 41, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lops, D.; Bressan, E.; Parpaiola, A.; Sbricoli, L.; Cecchinato, D.; Romeo, E. Soft tissues stability of cad-cam and stock abutments in anterior regions: 2-year prospective multicentric cohort study. Clin. Oral Implant. Res. 2015, 26, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Barwacz, C.; Shah, K.; Bittner, N.; Parker, W.; Neumeier, T.; Thalji, G.; De Kok, I. A Retrospective, Multicenter, Cross-Sectional Case Series Study Evaluating Outcomes of CAD/CAM Abutments on Implants from Four Manufacturers: 4-Year Mean Follow-up. Int. J. Oral Maxillofac. Implant. 2021, 36, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Gurbanov, S.; Hess, P.; Nentwig, G.-H.; Schwarz, F.; Sader, R. Crestal bone loss and implant failure of prefabricated versus customized abutments: A 10-year retrospective radiological study. Clin. Oral Investig. 2022, 26, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Erhan Çömlekoğlu, M.; Nizam, N.; Çömlekoğlu, M.D. Immediate definitive individualized abutments reduce peri-implant bone loss: A randomized controlled split-mouth study on 16 patients. Clin. Oral Investig. 2018, 22, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Gamborena, I.; Sasaki, Y.; Blatz, M.B. Predictable immediate implant placement and restoration in the esthetic zone. J. Esthet. Restor. Dent. 2021, 33, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Zarauz, C.; Pitta, J.; Pradies, G.; Sailer, I. Clinical Recommendations for Implant Abutment Selection for Single-Implant Reconstructions: Customized vs Standardized Ceramic and Metallic Solutions. Int. J. Periodontics Restor. Dent. 2020, 40, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, X.; Zou, L.; He, J.; Zhao, B. Mechanical properties and marginal fit of prefabricated versus customized dental implant abutments: A comparative study. Clin. Implant Dent. Relat. Res. 2022, 24, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, F.A.; Bonfante, E.A.; Vollmer, F.; Gierthmuehlen, P.C. Failure Load of Monolithic Lithium Disilicate Implant-Supported Single Crowns Bonded to Ti-base Abutments versus to Customized Ceramic Abutments after Fatigue. J. Prosthodont. 2022, 31, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Rheu, G.; Kim, Y.; Jeong, C.; Lee, J.; Shin, S. Influence of Implant transmucosal design on early peri-implant tissue responses in beagle dogs. Clin. Oral Implant. Res. 2014, 25, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Leal, E.; Orsi, I.A.; Salamanca, C.; Dias, F.J.; Weber, B. Influence of transmucosal height in abutments of single and multiple implant-supported prostheses: A non-linear three-dimensional finite element analysis. Comput. Methods Biomech. Biomed. Engin. 2018, 21, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kul, E.; Korkmaz, İ.H. Effect of different design of abutment and implant on stress distribution in 2 implants and peripheral bone: A finite element analysis study. J. Prosthet. Dent. 2021, 126, 664.e1–664.e9. [Google Scholar] [CrossRef] [PubMed]
- Pellizzer, E.P.; Lemos, C.A.A.; Almeida, D.A.F.; de Souza Batista, V.E.; Santiago Júnior, J.F.; Verri, F.R. Biomechanical analysis of different implant-abutments interfaces in different bone types: An in silico analysis. Mater. Sci. Eng. C 2018, 90, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.I.M.; Gomes, R.S.; Ruggiero, M.M.; Bergamo, E.T.P.; Bonfante, E.A.; Marcello-Machado, R.M.; Del Bel Cury, A.A. Probability of survival and stress distribution of narrow diameter implants with different implant-abutment taper angles. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Lillo, R.; Parra, C.; Fuentes, R.; Borie, E.; Engelke, W.; Beltrán, V. Compressive Resistance of Abutments with Different Diameters and Transmucosal Heights in Morse-Taper Implants. Braz. Dent. J. 2015, 26, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Lenz, U.; Santos, R.B.; Griggs, J.A.; Estrela, C.; Bueno, M.d.R.; Porto, O.C.L.; Della Bona, A. Longevity of different abutments placed on narrow diameter implants: Assessment of structural damage and loosening. Dent. Mater. 2024, 40, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.B.D.; Lenz, U.; Griggs, J.A.; Estrela, C.; Bueno, M.D.; Porto, O.C.L.; Della Bona, A. Structural and torque changes in implant components of different diameters subjected to mechanical fatigue. Dent. Mater. 2024, 40, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, M.; Duan, Y.; Betts, L.; Priddy, M.; Griggs, J.A. Effect of Bone Remodeling on Dental Implant Fatigue Limit Predicted Using 3D Finite Element Analysis. J. Dent. Oral Epidemiol. 2022, 2. [Google Scholar] [CrossRef]
- Oberoi, G.; Kornfellner, E.; Aigner, D.A.; Unger, E.; Schwentenwein, M.; Bomze, D.; Staudigl, C.; Pahr, D.; Moscato, F. Design and optimization of a novel patient-specific subperiosteal implant additively manufactured in yttria-stabilized zirconia. Dent. Mater. 2024, 40, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sarangi, S.K. An Optimized Dental Implant Model Using Finite Element Analysis and Design of Experiment. Int. J. Oral Maxillofac. Implant. 2023, 38, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Poovarodom, P.; Rungsiyakull, C.; Suriyawanakul, J.; Li, Q.; Sasaki, K.; Yoda, N.; Rungsiyakull, P. Multi-objective optimization of custom implant abutment design for enhanced bone remodeling in single-crown implants using 3D finite element analysis. Sci. Rep. 2024, 14, 15867. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, M.; Loeb, M.; Jose, R.M.; Sinclair, M.J.; Duan, Y.; Salazar Marocho, S.M.; Roach, M.D.; Griggs, J.A. Screening dental implant design parameters for effect on the fatigue limit of reduced-diameter implants. Dent. Mater. 2025, 41, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, M.; Jose, R.M.; Duan, Y.; Griggs, J.A. Effects of abutment screw preload and preload simulation techniques on dental implant lifetime. JADA Found. Sci. 2022, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Griggs, J.A. Effect of loading frequency on cyclic fatigue lifetime of a standard-diameter implant with an internal abutment connection. Dent. Mater. 2018, 34, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Experiment Design and Analysis Reference; Reliasoft Publishing: Tucson, AZ, USA, 2008.
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Döring, H.; Nonhoff, J. Performance of conical abutment (Morse Taper) connection implants: A systematic review. J. Biomed. Mater. Res. Part A 2014, 102, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Belli, R.; Wendler, M.; de Ligny, D.; Cicconi, M.R.; Petschelt, A.; Peterlik, H.; Lohbauer, U. Chairside CAD/CAM materials. Part 1: Measurement of elastic constants and microstructural characterization. Dent. Mater. 2017, 33, 84–98. [Google Scholar] [CrossRef] [PubMed]
- TIMET TIMETAL. Titanium Alloy (Ti-6Al-4V ELI; ASTM Grade 23) (Annealed; 2.50–4.00 in Rod or Thickness; Per ASTM F136). MatWeb 2025. Available online: https://www.matweb.com/search/datasheet.aspx?MatGUID=cdc9162a3dda4b428b0dea2ce3b105da&ckck=1 (accessed on 20 July 2024).
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. Biomed Res. Int. 2016, 2016, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef] [PubMed]
- ISO 14801; Dentistry–Implants–Dynamic Loading test for Endosseous Dental Implants. International Organization for Standardization: Geneva, Switzerland, 2016.
- Duan, Y.; Gonzalez, J.A.; Kulkarni, P.A.; Nagy, W.W.; Griggs, J.A. Fatigue lifetime prediction of a reduced-diameter dental implant system: Numerical and experimental study. Dent. Mater. 2018, 34, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Guijarro, M.; Pérez-Pevida, E.; Chávarri-Prado, D.; Estrada-Martínez, A.; Diéguez-Pereira, M.; Sánchez-Lasheras, F.; Brizuela-Velasco, A. Biomechanical Effects of Ti-Base Abutment Height on the Dental Implant System: A Finite Element Analysis. J. Funct. Biomater. 2024, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Mirzaee, S.; Falahchai, M. The effect of different implant-abutment types and heights on screw loosening in cases with increased crown height space. Clin. Exp. Dent. Res. 2024, 10, e894. [Google Scholar] [CrossRef] [PubMed]
- Bordin, D.; Cury, A.A.D.B.; Faot, F. Influence of Abutment Collar Height and Implant Length on Stress Distribution in Single Crowns. Braz. Dent. J. 2019, 30, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Quispe-López, N.; Guadilla, Y.; Gómez-Polo, C.; López-Valverde, N.; Flores-Fraile, J.; Montero, J. The influence of implant depth, abutment height and mucosal phenotype on peri-implant bone levels: A 2-year clinical trial. J. Dent. 2024, 148, 105264. [Google Scholar] [CrossRef] [PubMed]
- Mattheos, N.; Janda, M.; Acharya, A.; Pekarski, S.; Larsson, C. Impact of design elements of the implant supracrestal complex (ISC) on the risk of peri-implant mucositis and peri-implantitis: A critical review. Clin. Oral Implant. Res. 2021, 32, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Lee, Y.; Hong, S.-J.; Song, S.J.; Choi, Y.; Cho, E.; Paek, J. Risk factors associated with screw loosening in CAD-CAM custom abutments: A 6-year retrospective study. J. Prosthet. Dent. 2023, 133, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Pol, C.W.P.; Cune, M.S.; Raghoebar, G.M.; Naves, L.Z.; Meijer, H.J.A. Mechanical strength of stock and custom abutments as original and aftermarket components after thermomechanical aging. Clin. Exp. Dent. Res. 2024, 10, e892. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.; Woo, Y.H.; Kim, H.S.; Pae, A.; Noh, K.; Lee, H.; Kwon, K.R. Comparative Analysis of Screw Loosening with Prefabricated Abutments and Customized CAD/CAM Abutments. Implant Dent. 2016, 25, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Klongbunjit, D.; Aunmeungtong, W.; Khongkhunthian, P. Implant-abutment screw removal torque values between customized titanium abutment, straight titanium abutment, and hybrid zirconia abutment after a million cyclic loading: An in vitro comparative study. Int. J. Implant Dent. 2021, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pevida, E.; Chávarri-Prado, D.; Diéguez-Pereira, M.; Estrada-Martínez, A.; Montalbán-Vadillo, O.; Jiménez-Garrudo, A. Consequences of Peri-Implant Bone Loss in the Occlusal Load Transfer to the Supporting Bone in terms of Magnitude of Stress, Strain, and Stress Distribution: A Finite Element Analysis. Biomed Res. Int. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Mito, T.; Yoda, N.; Sato, E.; Shigemitsu, R.; Han, J.; Sasaki, K. Effect of peri-implant bone resorption on mechanical stress in the implant body: In vivo measured load-based finite element analysis. J. Oral Rehabil. 2020, 47, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
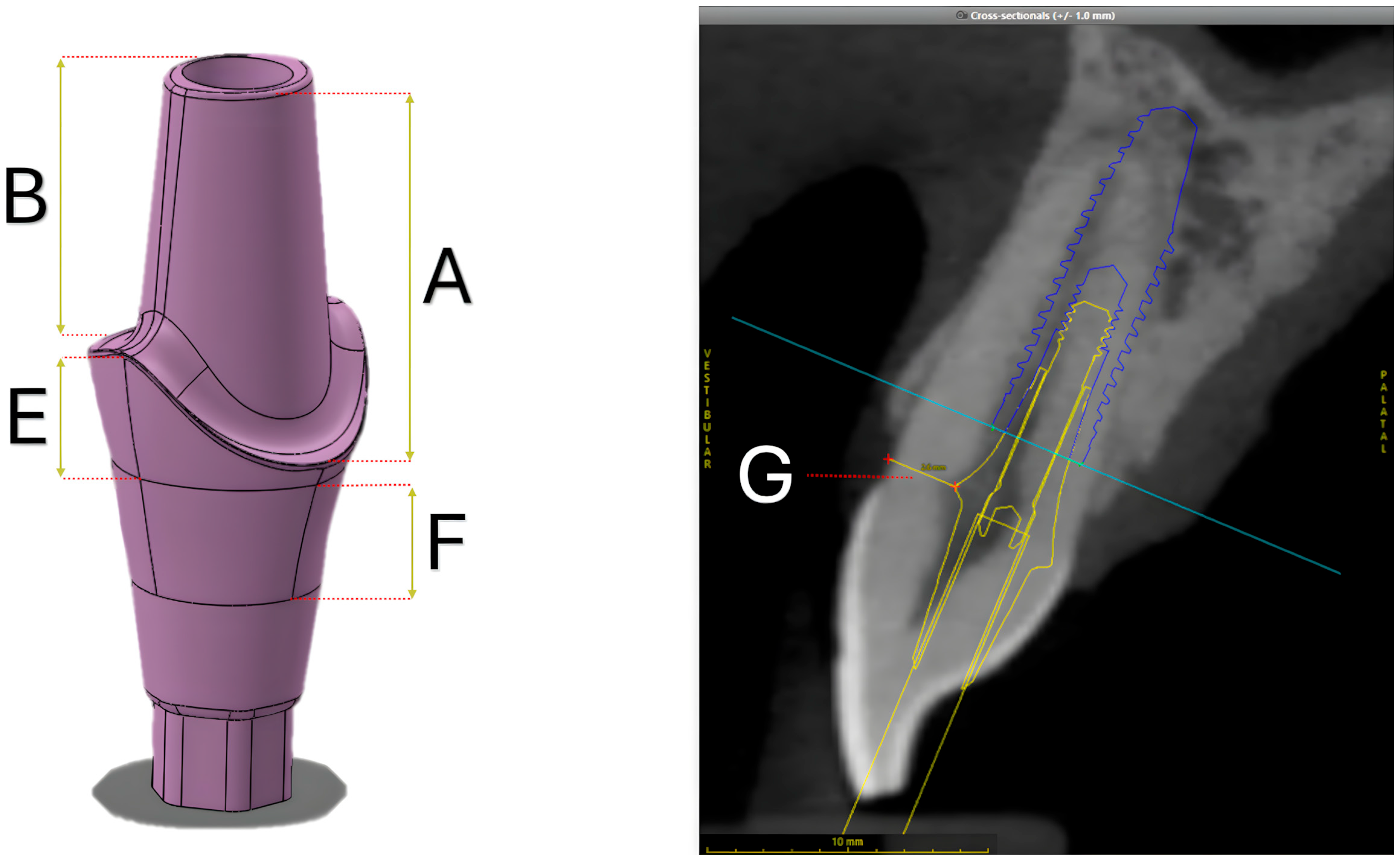
| Design Parameters | 20% Lower | Reference | 20% Higher | |
|---|---|---|---|---|
| A | Buccal height of the abutment preparation | 4.25 | 5.32 | 6.38 |
| B | Palatal height of the abutment preparation | 3.41 | 4.26 | 5.11 |
| C | Buccal height of the finishing line | 0.16 | 0.2 | 0.24 |
| D | Palatal height of the finishing line | 1.28 | 1.6 | 1.92 |
| E | Interproximal height of finishing line | 1.64 | 2.05 | 2.46 |
| F | Transmucosal height | 1.6 | 2 | 2.4 |
| G | Distance from the prosthetic finishing line to buccal dentin margin | 2.06 | 2.57 | N/A |
| H | Distance from the prosthetic finishing line to palatal dentin margin | 0.6 | 0.77 | N/A |
| I | Distance from the prosthetic finishing line to mesial dentin margin | 0.54 | 0.67 | N/A |
| J | Distance from the prosthetic finishing line to distal dentin margin | 0.5 | 0.62 | N/A |
| Component | Material | Young’s Modulus (GPa) | Poisson’s Ratio | Ultimate Tensile Strength (MPa) |
|---|---|---|---|---|
| Cap | Zirconia [33] | 205 | 0.30 | - |
| Abutment | Ti 6Al-4V ELI [34] | 113.8 | 0.31 | 825 |
| Connector screw | Ti 6Al-4V ELI [34] | 113.8 | 0.31 | 825 |
| Implant body | CP Ti Grade 4 [35] | 103 | 0.34 | 660 |
| Cortical bone | Cortical bone [36] | 20 | 0.30 | - |
| Cancellous bone | Cancellous bone [36] | 14 | 0.30 | - |
| Evaluated Abutment | Parameter A | Parameter B | Parameter C | Parameter D | Parameter E | Parameter F | Parameter G | Parameter H | Parameter I | Parameter J | Peak Von Mises Stress | Fatigue Limit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RA | 6.25 | 6.25 | 0.2 | 1.6 | 2.05 | 2 | 2.57 | 0.77 | 0.67 | 0.62 | 279 | 140 |
| CA 1 | 5 | 5 | 0.2 | 1.6 | 1.64 | 2 | 2.06 | 0.6 | 0.67 | 0.62 | 260 | 165 |
| CA 2 | 6.25 | 5 | 0.16 | 1.6 | 2.05 | 1.6 | 2.57 | 0.6 | 0.54 | 0.62 | 242 | 137 |
| CA 3 | 5 | 6.25 | 0.2 | 1.28 | 1.64 | 2 | 2.06 | 0.6 | 0.54 | 0.5 | 302 | 142 |
| CA 4 | 7.5 | 6.25 | 0.2 | 1.28 | 2.46 | 2 | 2.06 | 0.6 | 0.54 | 0.5 | 288 | 149 |
| CA 5 | 7.5 | 5 | 0.16 | 1.6 | 2.46 | 1.6 | 2.57 | 0.6 | 0.54 | 0.62 | 252 | 144 |
| CA 6 | 6.25 | 6.25 | 0.2 | 1.28 | 2.05 | 2 | 2.06 | 0.6 | 0.54 | 0.5 | 293 | 133 |
| CA 7 | 7.5 | 5 | 0.16 | 1.28 | 2.46 | 1.6 | 2.06 | 0.6 | 0.54 | 0.5 | 242 | 163 |
| CA 8 | 7.5 | 5 | 0.2 | 1.6 | 2.46 | 2 | 2.06 | 0.6 | 0.67 | 0.62 | 282 | 146 |
| CA 9 | 5 | 6.25 | 0.16 | 1.6 | 1.64 | 2 | 2.06 | 0.77 | 0.54 | 0.62 | 252 | 168 |
| CA 10 | 5 | 6.25 | 0.16 | 1.28 | 1.64 | 1.6 | 2.06 | 0.77 | 0.67 | 0.62 | 254 | 170 |
| CA 11 | 5 | 6.25 | 0.16 | 1.6 | 1.64 | 2 | 2.57 | 0.6 | 0.67 | 0.5 | 263 | 147 |
| CA 12 | 7.5 | 6.25 | 0.16 | 1.28 | 2.46 | 1.6 | 2.06 | 0.77 | 0.67 | 0.62 | 283 | 147.5 |
| CA 13 | 7.5 | 6.25 | 0.16 | 1.6 | 2.46 | 2 | 2.57 | 0.6 | 0.67 | 0.5 | 263 | 145 |
| CA 14 | 7.5 | 6.25 | 0.2 | 1.6 | 2.46 | 1.6 | 2.57 | 0.77 | 0.54 | 0.5 | 255 | 142 |
| CA 15 | 6.25 | 5 | 0.16 | 1.28 | 2.05 | 2 | 2.57 | 0.77 | 0.67 | 0.5 | 275 | 147 |
| CA 16 | 7.5 | 6.25 | 0.16 | 1.6 | 2.46 | 2 | 2.06 | 0.77 | 0.54 | 0.62 | 288 | 155 |
| CA 17 | 5 | 5 | 0.2 | 1.28 | 1.64 | 2 | 2.57 | 0.77 | 0.54 | 0.62 | 254 | 156 |
| CA 18 | 5 | 5 | 0.2 | 1.6 | 1.64 | 1.6 | 2.06 | 0.77 | 0.67 | 0.5 | 263 | 152 |
| CA 19 | 7.5 | 5 | 0.2 | 1.6 | 2.46 | 1.6 | 2.06 | 0.77 | 0.67 | 0.5 | 273 | 146 |
| CA 20 | 6.25 | 5 | 0.16 | 1.28 | 2.05 | 1.6 | 2.06 | 0.6 | 0.54 | 0.5 | 271 | 149 |
| CA 21 | 6.25 | 5 | 0.2 | 1.28 | 2.05 | 2 | 2.57 | 0.77 | 0.54 | 0.62 | 284 | 141 |
| CA 22 | 6.25 | 5 | 0.2 | 1.6 | 2.05 | 2 | 2.06 | 0.6 | 0.67 | 0.62 | 280 | 141 |
| CA 23 | 5 | 5 | 0.16 | 1.28 | 1.64 | 1.6 | 2.06 | 0.6 | 0.54 | 0.5 | 262 | 143 |
| CA 24 | 6.25 | 6.25 | 0.16 | 1.6 | 2.05 | 2 | 2.06 | 0.77 | 0.54 | 0.62 | 282 | 155 |
| CA 25 | 6.25 | 5 | 0.2 | 1.6 | 2.05 | 1.6 | 2.06 | 0.77 | 0.67 | 0.5 | 247 | 164 |
| CA 26 | 7.5 | 6.25 | 0.2 | 1.28 | 2.46 | 1.6 | 2.57 | 0.6 | 0.67 | 0.62 | 271 | 141.5 |
| CA 27 | 7.5 | 5 | 0.2 | 1.28 | 2.46 | 2 | 2.57 | 0.77 | 0.54 | 0.62 | 244 | 150 |
| CA 28 | 5 | 5 | 0.16 | 1.28 | 1.64 | 2 | 2.57 | 0.77 | 0.67 | 0.5 | 257 | 145.5 |
| CA 29 | 5 | 6.25 | 0.2 | 1.28 | 1.64 | 1.6 | 2.57 | 0.6 | 0.67 | 0.62 | 258 | 143.5 |
| CA 30 | 7.5 | 5 | 0.16 | 1.28 | 2.46 | 2 | 2.57 | 0.77 | 0.67 | 0.5 | 265 | 141 |
| CA 31 | 6.25 | 6.25 | 0.2 | 1.6 | 2.05 | 1.6 | 2.57 | 0.77 | 0.54 | 0.5 | 238 | 163.5 |
| CA 32 | 5 | 5 | 0.16 | 1.6 | 1.64 | 1.6 | 2.57 | 0.6 | 0.54 | 0.62 | 250 | 144 |
| CA 33 | 6.25 | 6.25 | 0.16 | 1.28 | 2.05 | 1.6 | 2.06 | 0.77 | 0.67 | 0.62 | 264 | 167 |
| CA 34 | 5 | 6.25 | 0.2 | 1.6 | 1.64 | 1.6 | 2.57 | 0.77 | 0.54 | 0.5 | 229 | 173.5 |
| CA 35 | 6.25 | 6.25 | 0.16 | 1.6 | 2.05 | 2 | 2.57 | 0.6 | 0.67 | 0.5 | 260 | 148 |
| CA 36 | 6.25 | 6.25 | 0.2 | 1.28 | 2.05 | 1.6 | 2.57 | 0.6 | 0.67 | 0.62 | 238 | 154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, U.; dos Santos, R.B.; Satpathy, M.; Griggs, J.A.; Della Bona, A. Fatigue Resistance of Customized Implant-Supported Restorations. Materials 2025, 18, 3420. https://doi.org/10.3390/ma18143420
Lenz U, dos Santos RB, Satpathy M, Griggs JA, Della Bona A. Fatigue Resistance of Customized Implant-Supported Restorations. Materials. 2025; 18(14):3420. https://doi.org/10.3390/ma18143420
Chicago/Turabian StyleLenz, Ulysses, Renan Brandenburg dos Santos, Megha Satpathy, Jason A. Griggs, and Alvaro Della Bona. 2025. "Fatigue Resistance of Customized Implant-Supported Restorations" Materials 18, no. 14: 3420. https://doi.org/10.3390/ma18143420
APA StyleLenz, U., dos Santos, R. B., Satpathy, M., Griggs, J. A., & Della Bona, A. (2025). Fatigue Resistance of Customized Implant-Supported Restorations. Materials, 18(14), 3420. https://doi.org/10.3390/ma18143420







