Surface Coatings on Biomedical Magnesium Alloys
Abstract
1. Introduction
- (1)
- Material Alloying [14]: By designing appropriate alloying strategies involving elements such as Fe [15], Al [16], Ca [17], Zn [18], Zr [19], and Y [20], the mechanisms of grain boundary strengthening and solid solution hardening can be achieved, which effectively reduces the corrosion rate of magnesium to some extent. Gu et al. [21] fabricated Sr-Mg binary alloys with varying Sr contents through alloying and hot-rolling processes, demonstrating that the addition of 0–2 wt% Sr reduced micro-shrinkage porosity and refined grain size, consequently enhancing both corrosion resistance and corrosion uniformity. However, excessive Sr addition resulted in deteriorated mechanical properties and accelerated corrosion rates in the as-rolled Mg-Sr alloys. Although alloying remains widely employed, significant challenges persist in corrosion protection, as these alloys lack the capacity to form protective oxide layers on their surfaces, leading to uncontrolled corrosion rates [10]. Moreover, extensive alloying may induce galvanic coupling reactions in Mg alloys, thereby reducing the corrosion resistance of the Mg matrix. The majority of alloying elements tend to form detrimental impurities or secondary phases that adversely affect the microstructure [22].
- (2)
- Amorphous Phase Engineering [23]: Metallic glasses exhibit unique structural characteristics including single-phase homogeneous solid solutions and multi-component compositions. This chemically homogeneous structure eliminates intergranular corrosion but increases susceptibility to localized pitting and selective corrosion in amorphous alloys. The inevitable stress exposure during implantation makes amorphous alloys particularly vulnerable to corrosion fatigue and stress corrosion cracking, which significantly accelerates degradation and reduces service lifespan [24]. Zhou et al. [25] synthesized Mg68−xZn28Ca4Ndx (x = 0, 0.5, 1, 1.5) alloys with 2 mm diameters to investigate the effects of Nd content on glass-forming ability and corrosion resistance. The Mg67.5Zn28Ca4Nd0.5 amorphous specimen demonstrated optimal corrosion resistance among the tested compositions. Li et al. [26] investigated the corrosion fatigue behavior of Mg66Zn30Ca3Sr1 amorphous alloy in phosphate-buffered saline under cyclic loading conditions. Their findings revealed that cyclic loading accelerates corrosion rates through repeated elastic deformation that disrupts passive films, inducing galvanic corrosion and crevice corrosion, ultimately leading to localized exfoliation and catastrophic brittle fractures in amorphous alloys. The study concluded that fatigue corrosion in amorphous alloys exhibits high sensitivity to cyclic stress levels.
- (3)
- Processing Optimization: Techniques such as extrusion and rolling can refine the grain structure of Mg alloys, thereby enhancing their corrosion resistance. Cai et al. [27] melted Mg–Zn alloy materials in an electric resistance furnace at 750–800 °C, followed by casting into permanent steel molds preheated to 200 °C, ultimately fabricating Mg–5Zn alloy specimens with a measured tensile strength of 194.59 MPa and self-corrosion potential of −1.477 V. Jana et al. [28] prepared pure magnesium and Mg–Gd–Nd–Zr–Zn alloys through powder compaction sintering with subsequent heat treatment, demonstrating that post-treatment materials exhibited superior compressive strength compared to as-sintered counterparts. The heat-treated magnesium alloy displayed a self-corrosion potential of −1.49 V, whereas the untreated material registered −1.51 V, indicating improved electrochemical stability through thermal processing. Although processing optimization can enhance both corrosion resistance and mechanical properties, certain manufacturing techniques still fail to meet clinically required mechanical performance standards and biocompatibility thresholds.
2. Degradation Mechanism of Magnesium Alloy
3. Coatings Classification
3.1. Inorganic Coatings
3.1.1. Micro-Arc Oxidation Coatings
3.1.2. Phosphate Coatings
3.1.3. Fluorinated Coatings
3.1.4. Hydrothermal Coatings
3.1.5. Bioceramic Coatings
3.2. Organic Coatings
3.2.1. Synthetic Polymer Coatings
3.2.2. Natural Polymer Coatings
3.3. Composite Coatings
3.3.1. Sol-Gel Coatings
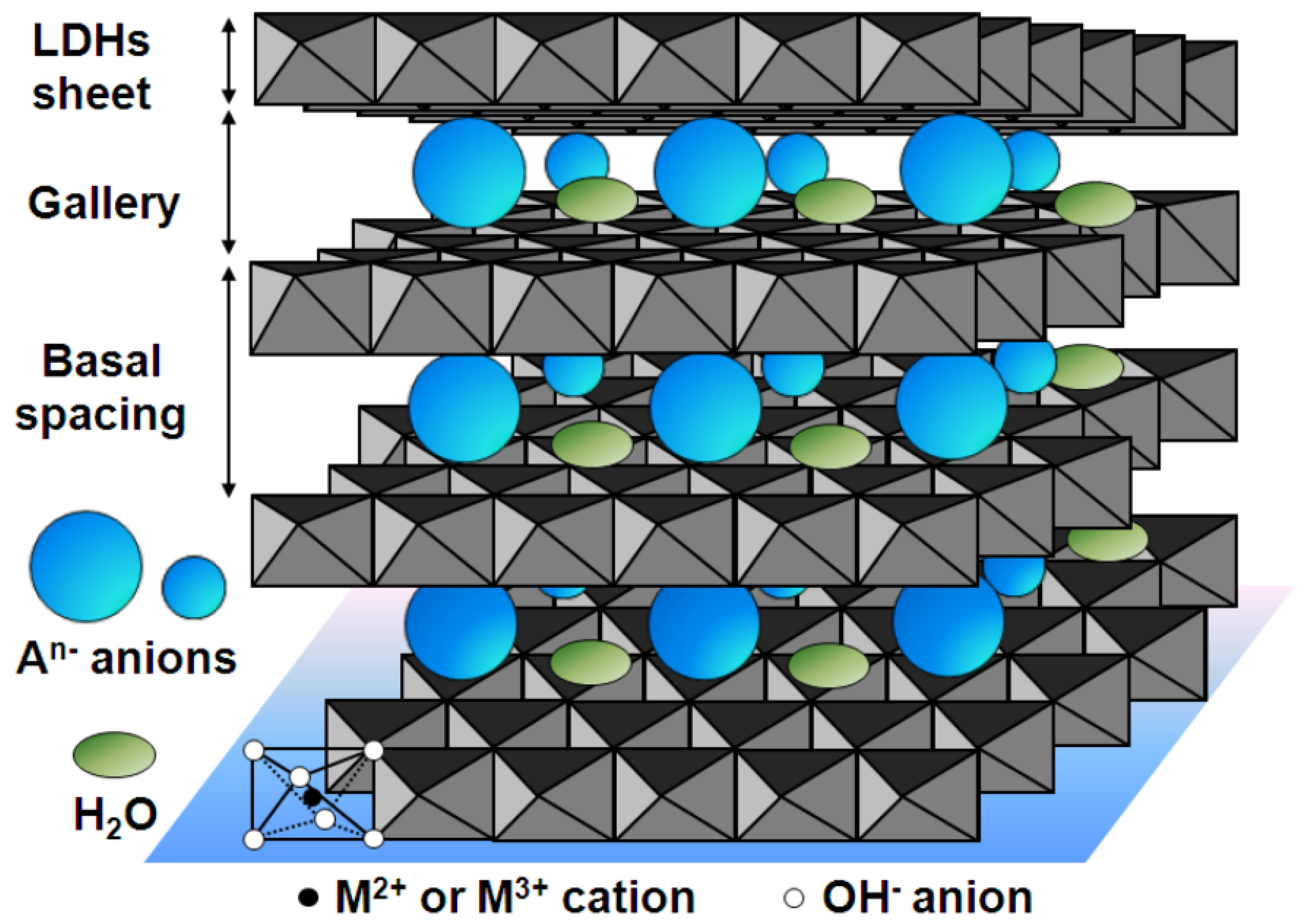
3.3.2. Layered Coatings
3.3.3. Ionic Liquid Conversion Coatings
3.3.4. Hybrid Coatings of Bioactive Molecules
3.4. Metal-Based Coatings
3.4.1. Metal Hydroxide Coatings
3.4.2. Metal Oxide Coatings
4. Potential Challenges of Surface Modification of Medical Magnesium Alloys
5. Summary
- The interaction mechanism between the magnesium alloy matrix and the modified layer and the modified layer and the biological interface should be deeply studied and the interface characteristics of the coating fully considered, such as the influence of adhesion force, interphase diffusion, and mechanical properties, to provide a theoretical basis for the preparation of the surface coating.
- Develop multifunctional composite coatings according to the requirements of high corrosion resistance, self-degradation, drug resistance, and biosafety of clinical biomedical implant materials. Prepare the bottom layer with a good bond to the substrate, introduce the polymer coating onto the prepared coating by chemical combination, and then conjugate the corrosion inhibitor molecules to the hybrid coating. It can effectively avoid the shortcomings of limited interface bonding strength between different coatings in the composite coating and prepare a multifunctional and integrated coating with controlled release, self-healing, and good biocompatibility.
- Nanoscale characterization at the interface is still lacking, especially in situ deposition/growth, and cannot yet provide strong evidence for the interaction between degradation products and surrounding tissues. New characterization systems can be established by combining advanced sensing technology and big data analysis.
- Optimizing material design to regulate alloy phases and nanostructures, thereby constructing functionally graded magnesium alloy coating systems through multi-scale collaborative design, will alleviate the constraints imposed by hydrogen evolution at elevated resorption rates in large-sized magnesium alloy implants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, C.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Li, W. Electrochemical behavior of anodized Mg alloy AZ91D in chloride containing aqueous solution. Corros. Sci. 2005, 47, 2816–2831. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Bian, H.; Zhou, Y.; Gao, C.; Shuai, C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Wei, L.; Liu, J.; Liu, X.; Zhen, Z.; Zheng, Y. Research Progress in Bioresorbable Magnesium Scaffolds. Acta Metall. Sin. 2017, 53, 1153–1167. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K. Research Progress on Corrosion Behavior and Mechanism of Magnesium Alloys. Corros. Sci. Prot. Technol. 2015, 27, 78–84. [Google Scholar]
- Zeng, R.C.; Chen, J.; Zhang, J. Research and Progress on Galvanic Corrosion of Magnesium Alloys. Mater. Rev. 2008, 2008, 107–109+117. [Google Scholar] [CrossRef]
- Zeng, R.C.; Cui, L.Y.; Wei, K. Biomedical magnesium alloys: Composition, microstructure and corrosion. Acta Metal. Sin. 2018, 54, 1215–1235. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, J.; Xue, F.; Zeng, R.; Wang, G.; Chu, P.K.; Chu, C. Smart self-healing coatings on biomedical magnesium alloys: A review. Smart Mater. Manuf. 2023, 1, 100022. [Google Scholar] [CrossRef]
- Liu, X.; Peng, F. Progress in Surface Modification of Biodegradable Magnesium Alloys for Biomedical Application. J. Chin. Ceram. Soc. 2017, 45, 1421–1431. [Google Scholar] [CrossRef]
- Gholap, S.S.; Kale, K.B. Advances in magnesium alloys and coatings for biomedical applications: A comprehensive review. AIP Conf. Proc. 2024, 3122, 050012. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe–Mn alloys for stents. J. Biomed. Mater. Res. Part A 2010, 93A, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Siddiqui, A.R.; Balani, K. An environment-friendly phosphate chemical conversion coating on novel Mg-9Li-7Al-1Sn and Mg-9Li-5Al-3Sn-1Zn alloys with remarkable corrosion protection. Appl. Surf. Sci. 2018, 443, 429–440. [Google Scholar] [CrossRef]
- Jin, Y.; Blawert, C.; Yang, H.; Wiese, B.; Feyerabend, F.; Bohlen, J.; Mei, D.; Deng, M.; Campos, M.S.; Scharnagl, N.; et al. Microstructure-corrosion behaviour relationship of micro-alloyed Mg-0.5Zn alloy with the addition of Ca, Sr, Ag, In and Cu. Mater. Des. 2020, 195, 108980. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Pei, J.; Tian, Y.; Zhang, J.; Jiang, C.; Huang, J.; Pang, Z.; Cao, Y.; Wang, X.; et al. Degradation and osteogenic induction of a SrHPO4-coated Mg–Nd–Zn–Zr alloy intramedullary nail in a rat femoral shaft fracture model. Biomaterials 2020, 247, 119962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hou, R.; Yang, J.; Sheng, Y.; Li, Z.; Chen, L.; Li, W.; Wang, X. Influence of Zirconium (Zr) on the microstructure, mechanical properties and corrosion behavior of biodegradable zinc-magnesium alloys. J. Alloys Compd. 2020, 840, 155792. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Z.; Guan, S.; Zhu, S.; Duan, T.; Zheng, Q.; Liu, S. Degradation of Mg-Zn-Y-Nd alloy intestinal stent and its effect on the growth of intestinal endothelial tissue in rabbit model. J. Magnes. Alloys 2022, 10, 2208–2219. [Google Scholar] [CrossRef]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y.; Qin, L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloys 2022, 10, 627–669. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, H. Research Progress in Application and Surface Modification of Medical Degradable Magnesium Alloys. Surf. Technol. 2024, 53, 15–30. [Google Scholar] [CrossRef]
- Wang, D.; Ding, H.; Ma, Y.; Gong, P.; Wang, Y. Research progress on corrosion resistance of metallic glasses. J. Chin. Soc. Corros. Prot. 2021, 41, 277–288. [Google Scholar]
- Zhou, J.; Li, K.; Wang, B.; Ai, F. The influence of adding Nd on the amorphous formation ability and corrosion resistance of Mg-Zn-Ca alloy. Mater. Rev. 2019, 33, 73–77. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Pang, S.; Liaw, P.K.; Zhang, T. Corrosion fatigue behavior of a Mg-based bulk metallic glass in a simulated physiological environment. Intermetallics 2016, 73, 31–39. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. Effect of heat treatment on microstructure, mechanical, corrosion and biocompatibility of Mg-Zn-Zr-Gd-Nd alloy. J. Alloys Compd. 2020, 821, 153462. [Google Scholar] [CrossRef]
- Heimann, R.B. Magnesium alloys for biomedical application: Advanced corrosion control through surface coating. Surf. Coat. Technol. 2021, 405, 126521. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent progress on coatings of biomedical magnesium alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Li, D.D.; Dai, D.; Xiong, G.; Lan, S.; Zhang, C. Composite Nanocoatings of Biomedical Magnesium Alloy Implants: Advantages, Mechanisms, and Design Strategies. Adv. Sci. 2023, 10, e2300658. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, J.; Liu, Q. Biodegradable behavior of the micro-arc oxidation coating with micro-nano structure formed by femtosecond laser on magnesium alloy AZ31B. Ceram. Int. 2025, 51, 9864–9876. [Google Scholar] [CrossRef]
- Golshirazi, A.; Kharaziha, M.; Golozar, M. Polyethylenimine/kappa carrageenan: Micro-arc oxidation coating for passivation of magnesium alloy. Carbohydr. Polym. 2017, 167, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ning, C.; Zhang, G.; Shang, L.; Zhong, L.; Zhang, Y. Properties of polydimethylsiloxane hydrophobic modified duplex microarc oxidation/diamond-like carbon coatings on AZ31B Mg alloy. J. Magnes. Alloys 2021, 9, 1285–1296. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Liu, L.; Lei, Q.; Dong, J.; Zhang, T. Preparation of Conductive and Corrosion Resistant Phosphate Conversion Coating on AZ91D Magnesium Alloy. Coatings 2023, 13, 1706. [Google Scholar] [CrossRef]
- Dziková, J.; Fintová, S.; Kajánek, D.; Florková, Z.; Wasserbauer, J.; Doležal, P. Characterization and Corrosion Properties of Fluoride Conversion Coating Prepared on AZ31 Magnesium Alloy. Coatings 2021, 11, 675. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Z.; Lei, Y.; Zeng, L.; Dai, Y.; Luo, W.; Duan, Z.; Sun, Q.; Yang, H.; Lin, X. Robust superhydrophobic mg alloys integrating hydrothermal processing with TA-APTES/PDMS coatings for synergistically enhanced corrosion protection. Prog. Org. Coat. 2025, 200, 109055. [Google Scholar] [CrossRef]
- Hernández, L.; González, J.E.; Barranco, V.; Veranes-Pantoja, Y.; Galván, J.; Gattorno, G.R. Biomimetic hydroxyapatite (HAp) coatings on pure Mg and their physiological corrosion behavior. Ceram. Int. 2022, 48, 1208–1222. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Q.-K.; Liu, Y.-Z.; Xiao, Z.; Xia, B.-H.; Li, S.-Q.; Zhang, F.; Cui, L.-Y.; Zeng, R.-C. Polyphosphate assisted hydrothermal synthesis of hydroxyapatite coating on Mg alloys: Enhanced mechanical properties and corrosion resistance. Surf. Coat. Technol. 2022, 432, 128033. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.; Wen, C. Realization and characterization of double-layer Ca-P coating on WE43 Mg alloy for biomedical applications. Surf. Coat. Technol. 2020, 398, 126091. [Google Scholar] [CrossRef]
- Zaludin, M.A.F.; Jamal, Z.A.Z.; Derman, M.N.; Kasmuin, M.Z. Fabrication of calcium phosphate coating on pure magnesium substrate via simple chemical conversion coating: Surface properties and corrosion performance evaluations. J. Mater. Res. Technol. 2019, 8, 981–987. [Google Scholar] [CrossRef]
- Bugdayci, M.; Baslayici, S.; Açma, M.E. Corrosion behaviour of hydroxyapatite coatings on AZ31 and AZ91 magnesium alloys by plasma spray. J. Ceram. Process. Res. 2021, 22, 98–105. [Google Scholar]
- Bansal, P.; Singh, G.; Sidhu, H.S. Plasma-Sprayed Hydroxyapatite-Strontium Coating for Improved Corrosion Resistance and Surface Properties of Biodegradable AZ31 Mg Alloy for Biomedical Applications. J. Mater. Eng. Perform. 2021, 30, 1768–1779. [Google Scholar] [CrossRef]
- Zhang, C.; Lan, C.; Jiajia, L.; Dongwei, S.; Jun, Z.; Huinan, L. In vitro evaluation of degradation, cytocompatibility and antibacterial property of polycaprolactone/hydroxyapatite composite coating on bioresorbable magnesium alloy. J. Magnes. Alloys 2022, 10, 2252–2265. [Google Scholar] [CrossRef]
- Grewal, N.S.; Batra, U.; Kumar, K.; Mahapatro, A. Novel PA encapsulated PCL hybrid coating for corrosion inhibition of biodegradable Mg alloys: A triple triggered self-healing response for synergistic multiple protection. J. Magnes. Alloys 2023, 11, 1440–1460. [Google Scholar] [CrossRef]
- Surmeneva, M.; Vladescu, A.; Cotrut, C.; Tyurin, A.; Pirozhkova, T.; Shuvarin, I.; Elkin, B.; Oehr, C.; Surmenev, R. Effect of parylene C coating on the antibiocorrosive and mechanical properties of different magnesium alloys. Appl. Surf. Sci. 2018, 427, 617–627. [Google Scholar] [CrossRef]
- de Sousa Santos, F.; Binder, L.; Scharnagl, N.; da Conceição, T.F. Sustainable smart coatings of chitosan and LDH loaded with natural inhibitors for corrosion protection of Mg AZ31 alloy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133639. [Google Scholar] [CrossRef]
- Agarwal, S.; Riffault, M.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Biomimetic hyaluronic acid-lysozyme composite coating on AZ31 Mg Alloy with combined antibacterial and osteoinductive activities. ACS Biomater. Sci. Eng. 2017, 3, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Merino, E.; Durán, A.; Ceré, S.; Castro, Y. Hybrid Epoxy-Alkyl Sol–Gel Coatings Reinforced with SiO2 Nanoparticles for Corrosion Protection of Anodized AZ31B Mg Alloy. Gels 2022, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wang, S.; Wu, Z. Corrosion Behavior Study of AZ31B magnesium Alloy by Sol-Gel Silica-based Hybrid Coating. Int. J. Electrochem. Sci. 2017, 12, 8269–8279. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Yuwono, J.A.; Meng, G.; Feng, Z. Developing levodopa-modified sol-gel coating with enhanced anticorrosion performance on Mg alloy AZ31. Anti-Corros. Methods Mater. 2023, 70, 129–138. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Zeng, Y.; Chen, C.; Guo, H.; Lei, B.; Zhang, P.; Feng, Z.; Meng, G. A novel sol-gel coating via catechol/lysine polymerization for long-lasting corrosion protection of Mg alloy AZ31. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130361. [Google Scholar] [CrossRef]
- Durán, K.; Hernández, N.; Rueda, L.; Hernández-Barrios, C.; Coy, A.; Viejo, F. Design of multilayer hybrid sol-gel coatings with bifunctional barrier-bioactive response on the Elektron 21 magnesium alloy for biomedical applications. J. Magnes. Alloys 2021, 9, 2097–2112. [Google Scholar] [CrossRef]
- Benzarti, Z.; Itani, S.; Castro, J.D.; Carvalho, S.; Ramos, A.S. Design multifunctional Mg–Zr coatings regulating Mg alloy bioabsorption. J. Magnes. Alloys 2024, 12, 1461–1478. [Google Scholar] [CrossRef]
- Li, X.; Ke, R.; Lin, E.; Liu, J.; Chen, D.; Kure-Chu, S.-Z.; Xiao, X. Multifunctional MOF-based composite coating on Mg alloy for biodegradable orthopedic implants. Appl. Surf. Sci. 2025, 692, 162727. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Li, Z.; Yuan, W.; Zheng, Y.; Cui, Z.; Yang, X.; Yeung, K.W.; Wu, S. A combined coating strategy based on atomic layer deposition for enhancement of corrosion resistance of AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 1101–1111. [Google Scholar] [CrossRef]
- Nadaraia, K.; Suchkov, S.; Imshinetskiy, I.; Mashtalyar, D.; Kosianov, D.; Belov, E.; Sinebryukhov, S.; Gnedenkov, S. New superhydrophobic composite coatings on Mg-Mn-Ce magnesium alloy. J. Magnes. Alloys 2023, 11, 1721–1739. [Google Scholar] [CrossRef]
- Howlett, P.; Gramet, S.; Lin, J.; Efthimiadis, J. Conversion coatings of Mg-alloy AZ91D using trihexyl(tetradecyl) phosphonium bis(trifluoromethanesulfonyl)amide ionic liquid. Sci. China Chem. Engl. Ed. 2012, 5, 1598–1607. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Kim, K.-S.; Lee, K.-B.; Lee, M.-H. Enhancement of bone formation on LBL-coated Mg alloy depending on the different concentration of BMP-2. Colloids Surf. B Biointerfaces 2019, 173, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-H.; Xu, D.; Shu, Y.; Yong, Q.; Wu, L.; Yu, G. Environmentally friendly and facile Mg(OH)2 film for electroless nickel plating on magnesium alloy for enhanced galvanic corrosion inhibition. Surf. Coat. Technol. 2024, 478, 130371. [Google Scholar] [CrossRef]
- Wang, J.-M.; Sun, X.; Song, L.; Kannan, M.B.; Zhang, F.; Cui, L.-Y.; Zou, Y.-H.; Li, S.-Q.; Zeng, R.-C. Corrosion resistance of Mg-Al-LDH steam coating on AZ80 Mg alloy: Effects of citric acid pretreatment and intermetallic compounds. J. Magnes. Alloys 2023, 11, 2967–2979. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; John, D.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Min, S.; Liu, Y.; Liu, B.; Hu, Y.; Wang, Z.; Mao, F.; Wang, C.; Ma, X.; et al. The effect of pore size on the mechanical properties, biodegradation and osteogenic effects of additively manufactured magnesium scaffolds after high temperature oxidation: An in vitro and in vivo study. Bioact. Mater. 2023, 28, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Sugahara, K.; Yamamoto, A.; Tsubakino, H. Corrosion rate of magnesium and its alloys in buffered chloride solutions. Corros. Sci. 2002, 44, 603–610. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, B.; Zhao, R.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhu, X.; Iulian, A.V.; Zhang, X. Evaluation on the corrosion resistance, antibacterial property and osteogenic activity of biodegradable Mg-Ca and Mg-Ca-Zn-Ag alloys. J. Magnes. Alloys 2022, 10, 3380–3396. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B.; He, Y.; Sandham, A. Effect of surface roughness on the in vitro degradation behaviour of a biodegradable magnesium-based alloy. Appl. Surf. Sci. 2013, 279, 343–348. [Google Scholar] [CrossRef]
- Kirkland, N.; Lespagnol, J.; Birbilis, N.; Staiger, M. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. A mechanistic in vitro study of the microgalvanic degradation of secondary phase particles in magnesium alloys. J. Biomed. Mater. Res. Part A 2015, 103, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Schilling, M.; Eifler, R.; Hering, B.; Reifenrath, J.; Besdo, S.; Windhagen, H.; Willbold, E.; Weizbauer, A. Corrosion behavior, biocompatibility and biomechanical stability of a prototype magnesium-based biodegradable intramedullary nailing system. Mater. Sci. Eng. C 2016, 59, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Bobbert, F.; Lietaert, K.; Dong, J.-H.; Leeflang, M.; Zhou, J.; Zadpoor, A. Corrosion fatigue behavior of additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 106, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Lin, X.; Tan, L.; Yang, K. Effect of surface coating on antibacterial behavior of magnesium based metals. Mater. Lett. 2011, 65, 3509–3511. [Google Scholar] [CrossRef]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Liu, Y.; He, X.; Liu, D.; Chen, M. In vitro and in vivo biocompatibility of Mg–Zn–Ca alloy operative clip. Bioact. Mater. 2019, 4, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, X.; Tang, H.; Sun, H.; Qin, L.; Zheng, Y.; Gu, X.; Fan, Y. In vitro and in vivo degradation behavior of Mg–2Sr–Ca and Mg–2Sr–Zn alloys. Bioact. Mater. 2020, 5, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, S.; Wang, Y.; Liu, H.; Wang, H.; Wang, L.; Ren, C.; Zhu, S.; Chen, K. In vivo degradation behavior of Ca-deficient hydroxyapatite coated Mg–Zn–Ca alloy for bone implant application. Colloids Surf. B Biointerfaces 2011, 88, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Yerokhin, A.; Snizhko, L.; Gurevina, N.; Leyland, A.; Pilkington, A.; Matthews, A. Spatial characteristics of discharge phenomena in plasma electrolytic oxidation of aluminium alloy. Surf. Coat. Technol. 2004, 177–178, 779–783. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, D.; Yu, J.; Di, S. Effects of Al2O3 Nano-additive on Performance of Micro-arc Oxidation Coatings Formed on AZ91D Mg Alloy. J. Mater. Sci. Technol. 2014, 30, 984–990. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Y.; Xu, W.; Su, J.; Zhuo, X.; Wang, D.; Virtanen, S.; Wang, Y. Fabrication and degradation behavior of MAO-HA coating on Zn-Mg-Ag alloy. Surf. Coat. Technol. 2025, 503, 131959. [Google Scholar] [CrossRef]
- Qian, L.; Sun, M.; Huang, N.; Yang, P.; Jing, F.; Zhao, A.; Akhavan, B. Biodegradable PTMC-MAO composite coatings on AZ31 Mg-alloys for enhanced corrosion-resistance. J. Alloys Compd. 2024, 998, 175017. [Google Scholar] [CrossRef]
- Ning, C.-M.; Cui, X.-J.; Shang, L.-L.; Zhang, Y.-J.; Zhang, G.-A. Structure and properties of different elements doped diamond-like carbon on micro-arc oxidation coated AZ31B Mg alloy. Diam. Relat. Mater. 2020, 106, 107832. [Google Scholar] [CrossRef]
- Pommiers, S.; Frayret, J.; Castetbon, A.; Potin-Gautier, M. Alternative conversion coatings to chromate for the protection of magnesium alloys. Corros. Sci. 2014, 84, 135–146. [Google Scholar] [CrossRef]
- Zhen, Y.; Lin, C.; Hu, Y.; Ren, Z.; Zhou, P.; Dou, B.; Xiao, J.; Sun, J.; Wang, X.; Yuan, Y. Electrically conductive and corrosion resistant coating on Mg Li alloys, Part I: Chemical conversion coating. Corros. Commun. 2024, 15, 24–35. [Google Scholar] [CrossRef]
- Jayaraj, J.; Kumar, S.A.; Srinivasan, A.; Raghu, K.; Arunchandran, C.; Rajinikanth, V. Corrosion and in vitro characteristics of cerium phosphate based chemical conversion coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2024, 644, 158797. [Google Scholar] [CrossRef]
- Xia, X.; Nie, J.; Weibo, C.; Jingli, S.; Yong, Y.; Lisong, Z.; Xiaoxue, W.; Zehua, D. Preparation and Corrosion Resistance of Superhydrophobic Phosphate Chemical Conversion Coatings for Magnesium Alloys. Surf. Technol. 2024, 53, 116–128. Available online: https://qikan.cqvip.com/Qikan/Article/Detail?id=7112794976 (accessed on 4 June 2025).
- Chiu, K.; Wong, M.; Cheng, F.; Man, H. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Barajas, J.; Joya, J.; Durán, K.; Hernández-Barrios, C.; Coy, A.; Viejo, F. Relationship between microstructure and formation-biodegradation mechanism of fluoride conversion coatings synthesised on the AZ31 magnesium alloy. Surf. Coat. Technol. 2019, 374, 424–436. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, H.; Dou, L. Layered Double Hydroxide-Based Nanocarriers for Drug Delivery. Pharmaceutics 2014, 6, 298–332. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Zerankeshi, M.; Zohrevand, M.; Alizadeh, R. Hydrothermal Coating of the Biodegradable Mg-2Ag Alloy. Metals 2023, 13, 1260. [Google Scholar] [CrossRef]
- Gerashi, E.; Jamalpour, M.; Alizadeh, R.; Labbaf, S.; Mahmudi, R. Effects of hydrothermal coating on the degradation behavior and biocompatibility of an Mg–4Zn–0.3Sr alloy. Mater. Lett. 2023, 330, 133224. [Google Scholar] [CrossRef]
- Zhang, A.-M.; Lenin, P.; Zeng, R.-C.; Kannan, M.B. Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys. J. Magnes. Alloys 2022, 10, 1154–1170. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Xuan, S.; Zhang, W.; Li, G.; Li, H. Modulating the corrosion performance of magnesium alloys through hydroxyapatite coating. Chem. Eng. J. 2024, 495, 16. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S. Phytic acid and its interactions: Contributions to protein functionality, food processing, and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2081–2105. [Google Scholar] [CrossRef] [PubMed]
- Tiyyagura, H.R.; Rudolf, R.; Gorgieva, S.; Fuchs-Godec, R.; Boyapati, V.R.; Mantravadi, K.M.; Kokol, V. The chitosan coating and processing effect on the physiological corrosion behaviour of porous magnesium monoliths. Prog. Org. Coat. 2016, 99, 147–156. [Google Scholar] [CrossRef]
- Adhikari, U.; Rijal, N.P.; Khanal, S.; Pai, D.; Sankar, J.; Bhattarai, N. Magnesium incorporated chitosan based scaffolds for tissue engineering applications. Bioact. Mater. 2016, 1, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Manzur, J.; Aizaz, A.; Nawaz, M.H.; Zaman, B.; Ahmad, K.; Rehman, M.A.U. Electrophoretic deposition of moringa loaded chitosan coatings on Mg: A study on antibacterial properties and degradation kinetics. Mater. Lett. 2024, 354, 135430. [Google Scholar] [CrossRef]
- Agarwal, S.; Labour, M.-N.; Hoey, D.; Duffy, B.; Curtin, J.; Jaiswal, S. Enhanced corrosion resistance and cytocompatibility of biomimetic hyaluronic acid functionalised silane coating on AZ31 Mg alloy for orthopaedic applications. J. Mater. Sci. Mater. Med. 2018, 29, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Jang, Y.-S.; Kim, S.-Y.; Lee, M.-H. Corrigendum to “Functions achieved by the hyaluronic acid derivatives coating and hydroxide film on bio-absorbed Mg” [Appl. Surf. Sci. 473 (2019) 31–39]. Appl. Surf. Sci. 2020, 512, 145087. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Reetz, M.T. Entrapment of biocatalysts in hydrophobic sol-gel materials for use in organic chemistry. Adv. Mater. 1997, 9, 943–954. [Google Scholar] [CrossRef]
- Wright, J.D.; Sommerdijk, N.A. Sol-Gel Materials: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Yao, W.; Tan, Y.; Lu, Q.; Yi, H.; Cheng, C.; Wu, L.; Saji, V.S.; Pan, F. Recent advances in protective coatings and surface modifications for corrosion protection of Mg alloys. J. Mater. Res. Technol. 2024, 31, 3238–3254. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Zarkov, A. Sol–Gel Technology Applied to Materials Science: Synthesis, Characterization and Applications. Materials 2024, 17, 462. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Guglielmi, M. Sol-gel coatings on metals. J. Sol-Gel Sci. Technol. 1997, 8, 443–449. [Google Scholar] [CrossRef]
- Talha, M.; Wang, Q.; Ma, Y.; Lin, Y. Self-assembled hybrid silane/ZnO coatings for corrosion protection of resorbable magnesium alloy. Int. J. Adhes. Adhes. 2023, 120, 103281. [Google Scholar] [CrossRef]
- Li, D.; Dai, D.; Wang, J.; Ai, Z.; Zhang, C. Fish scale-inspired biomimetic nanocoatings on magnesium implants for vascularized bone regeneration in infected bone defects. J. Magnes. Alloys 2025, 13, 311–329. [Google Scholar] [CrossRef]
- Dai, L.; Cui, H.; Chen, X.; Xu, R.; Zhang, Y.; Li, L. A multi-level biomimetic LDH coatings with super hydrophobicity, corrosion resistance, anti-icing and anti-fouling properties on magnesium alloy. J. Magnes. Alloys, 2025; in press. [Google Scholar] [CrossRef]
- Li, W.; Kumara, C.; Luo, H.; Meyer, H.M.; He, X.; Ngo, D.; Kim, S.H.; Qu, J. Ultralow Boundary Lubrication Friction by Three-Way Synergistic Interactions among Ionic Liquid, Friction Modifier, and Dispersant. ACS Appl. Mater. Interfaces 2020, 12, 17077–17090. [Google Scholar] [CrossRef] [PubMed]
- Elsentriecy, H.H.; Luo, H.; Meyer, H.M.; Grado, L.L.; Qu, J. Effects of pretreatment and process temperature of a conversion coating produced by an aprotic ammonium-phosphate ionic liquid on magnesium corrosion protection. Electrochim. Acta 2014, 123, 58–65. [Google Scholar] [CrossRef]
- Elsentriecy, H.H.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M. Improving corrosion resistance of AZ31B magnesium alloy via a conversion coating produced by a protic ammonium-phosphate ionic liquid. Thin Solid Film. 2014, 568, 44–51. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gu, C.; Feng, J.; Guo, Y.; Jin, Y.; Tu, J. Hydrophobic epoxy resin coating with ionic liquid conversion pretreatment on magnesium alloy for promoting corrosion resistance. J. Mater. Sci. Technol. 2020, 37, 9–18. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Yan, W.; Tu, J.; Ding, X. Fabrication and corrosion property of conversion films on magnesium alloy from deep eutectic solvent. Surf. Coat. Technol. 2018, 344, 702–709. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, Y.-L.; Tian, Y.-Q.; Chen, L.-S. Research progress on surface protective coatings of biomedical degradable magnesium alloys. J. Alloys Compd. 2021, 885, 161001. [Google Scholar] [CrossRef]
- Amerstorfer, F.; Schober, M.; Valentin, T.; Klim, S.; Leithner, A.; Fischerauer, S.; Glehr, M. Risk of reinfection after two- or multiple-stage knee revision surgery using superficial vancomycin coating and conventional spacers. J. Orthop. Res. 2021, 39, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Nadaraia, K.; Mashtalyar, D.; Piatkova, M.; Pleshkova, A.; Imshinetskiy, I.; Gerasimenko, M.; Belov, E.; Kumeiko, V.; Kozyrev, D.; Fomenko, K.; et al. Antibacterial HA-coatings on bioresorbable Mg alloy. J. Magnes. Alloys 2024, 12, 1965–1985. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, G.; Zhang, Y.-H.; Zhao, Q. Growth and characterization of Mg(OH)2 film on magnesium alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Wang, L. Effect of Mg(OH)2 layer on the wear and corrosion properties of AZ91 magnesium alloy. Phys. Scr. 2025, 100, 065912. [Google Scholar] [CrossRef]
- Wang, C.; Tong, Y.; Wang, Q.; Li, X.; Song, Y.; Pan, D.; Sun, R.; Tang, Y. In-situ growth of Mg(OH)2 coating on AZ31B magnesium alloy with the assistance of Ti3C2Tx MXene. Mater. Lett. 2022, 327, 133013. [Google Scholar] [CrossRef]
- Duan, Y.; Zhu, J.; Wu, J. In-situ hydrothermal Mg(OH)2-SiO2-Al(OH)3 composite coatings on AZ91D magnesium alloy with enhanced corrosion properties. Vacuum 2023, 217, 112515. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Tan, J.; Liu, X. In-situ growth of layered double hydroxide films on biomedical magnesium alloy by transforming metal oxyhydroxide. Appl. Surf. Sci. 2019, 496, 143690. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Qian, K.; Zhang, T.; Wang, F. Incorporation of LDH nanocontainers into plasma electrolytic oxidation coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 1236–1246. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Zhang, P. Excellent plasma electrolytic oxidation coating on AZ61 magnesium alloy under ordinal discharge mode. J. Magnes. Alloys 2022, 10, 2460–2474. [Google Scholar] [CrossRef]
- Dong, X.; Xia, M.; Wang, F.; Yang, H.; Ji, G.; Nyberg, E.; Ji, S. A super wear-resistant coating for Mg alloys achieved by plasma electrolytic oxidation and discontinuous deposition. J. Magnes. Alloys 2023, 11, 2939–2952. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, P.; Zhang, W.; Sun, X.; Du, Y. AlO2—Effects on Plasma Electrolytic Oxidation Coating of AZ61 Mg Alloy in KF-KOH Electrolyte. JOM 2025, 77, 4246–4259. [Google Scholar] [CrossRef]
- Hou, S. Solvothermal fabrication of TiO2 nanosheet films on degradable Mg–Zn alloys. Surf. Eng. 2016, 32, 745–749. [Google Scholar] [CrossRef]
- Mashtalyar, D.; Imshinetskiy, I.; Nadaraia, K.; Gnedenkov, A.; Suchkov, S.; Opra, D.; Pustovalov, E.; Ustinov, A.Y.; Sinebryukhov, S.; Gnedenkov, S. Effect of TiO2 nanoparticles on the photocatalytic properties of PEO coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 735–752. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Ma, J.; Ma, X. Corrosion Resistance and In Vitro Biological Properties of TiO2 on MAO-Coated AZ31 Magnesium Alloy via ALD. Coatings 2024, 14, 1198. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Yao, W.; Wu, Y.; Gao, Y.; Yang, Y.; Wu, L.; Serdechnova, M.; Blawert, C.; Pan, F. Recent developments in coatings on biodegradable Mg alloys: A review. J. Magnes. Alloys 2025, 13, 1405–1427. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Noeske, P.M.; Grunwald, I.; Haag, R. A Universal Approach to Crosslinked Hierarchical Polymer Multilayers as Stable and Highly Effective Antifouling Coatings. Adv. Mater. 2014, 26, 2688–2693. [Google Scholar] [CrossRef] [PubMed]


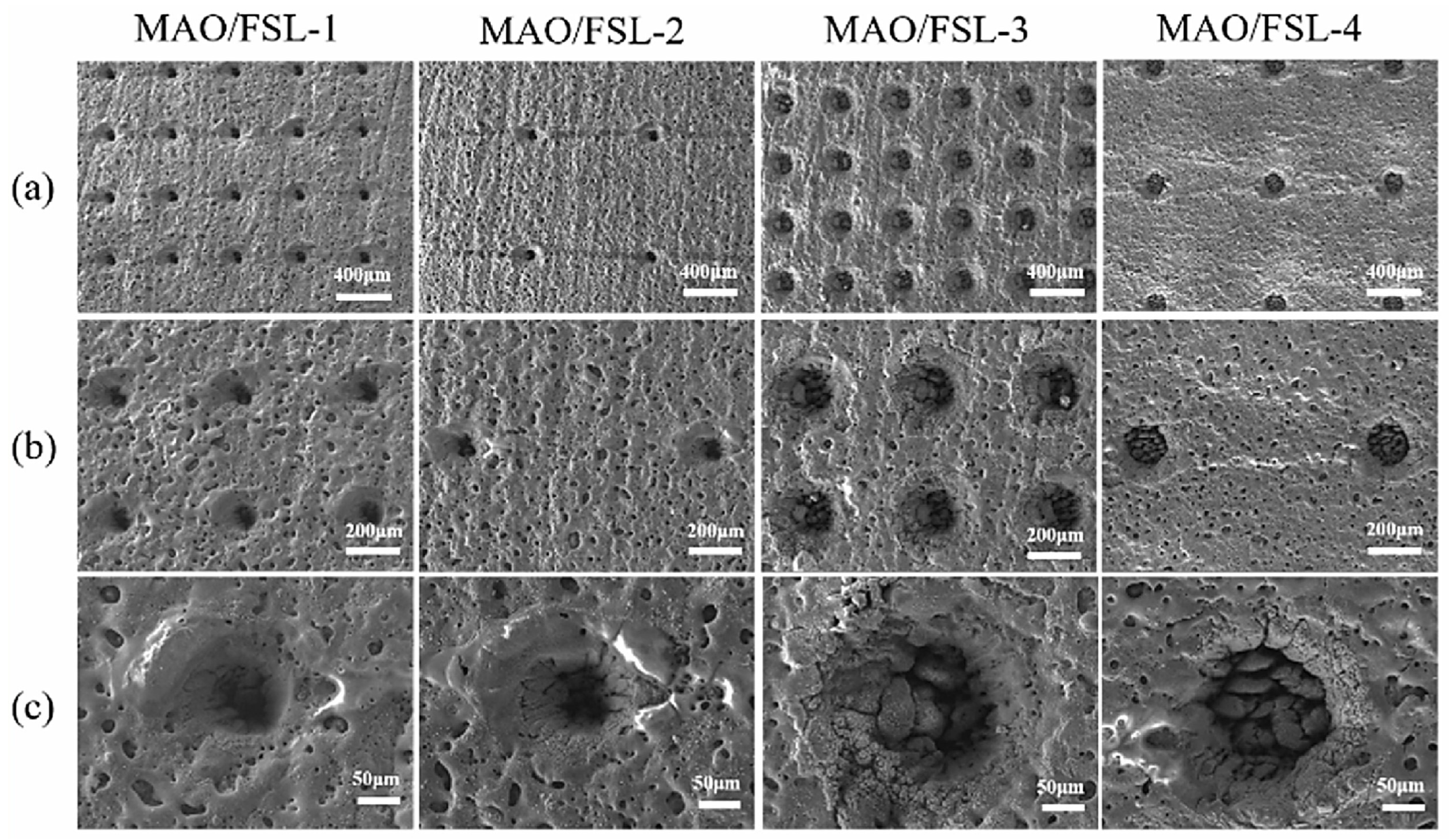
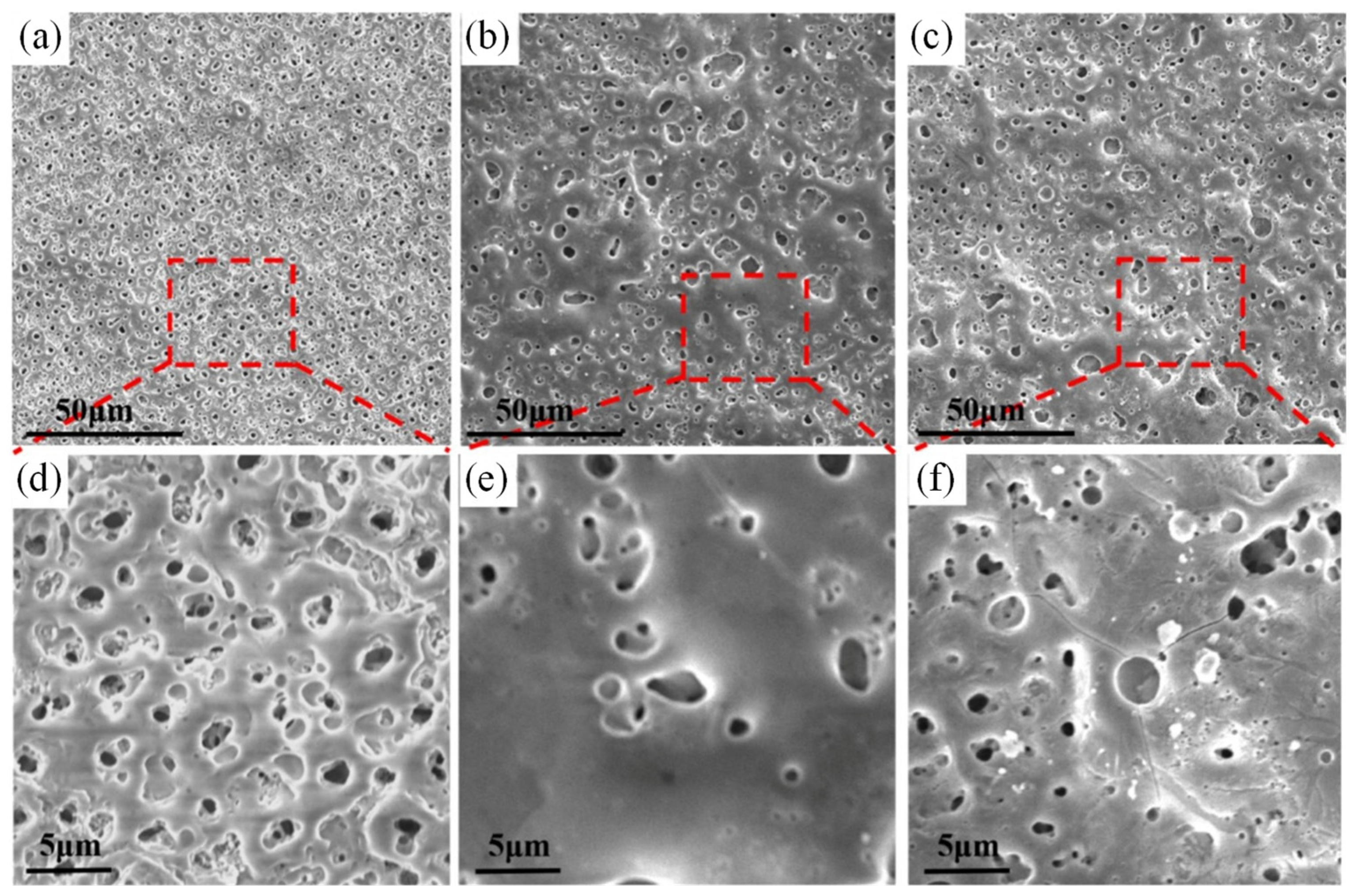

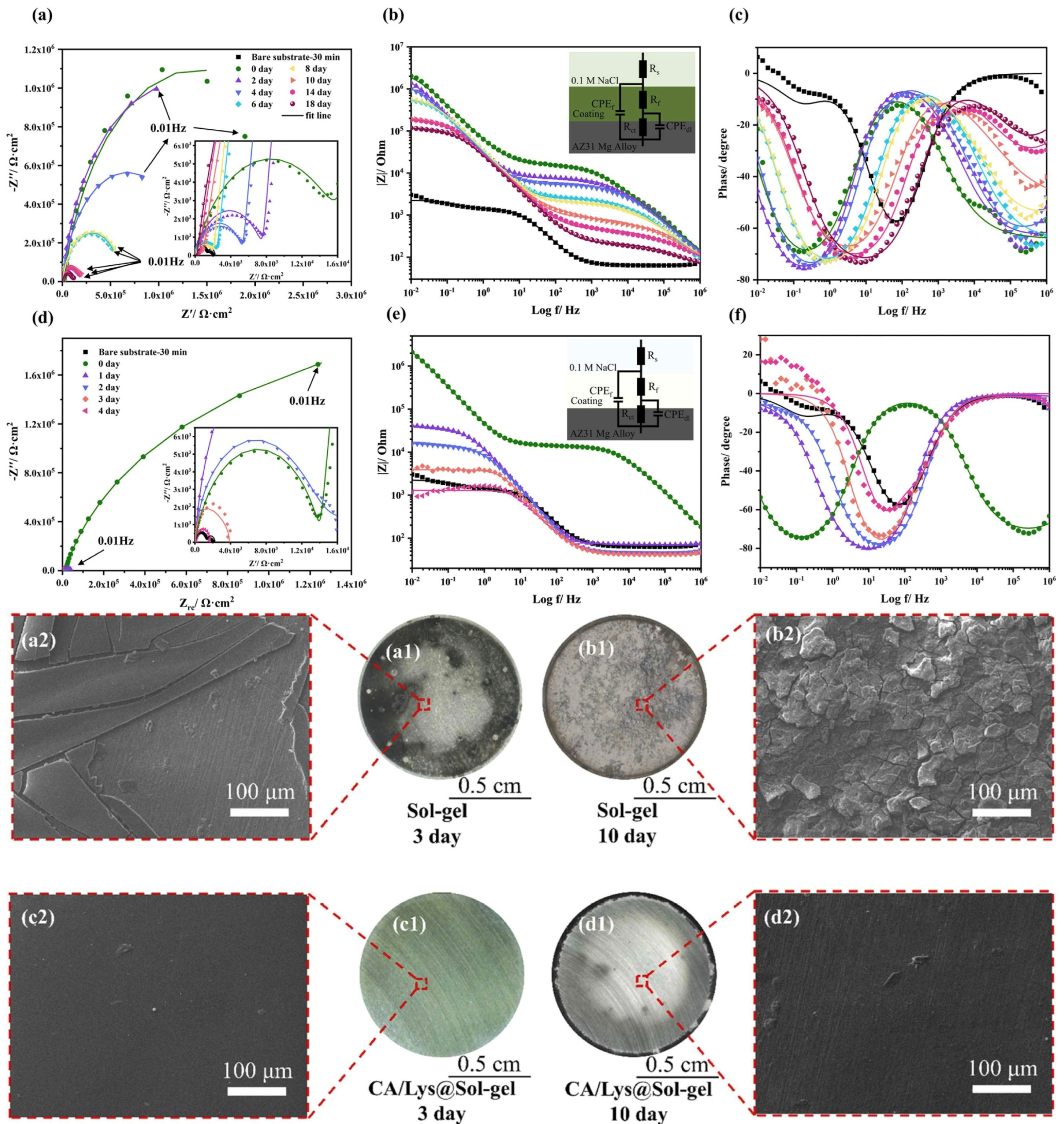
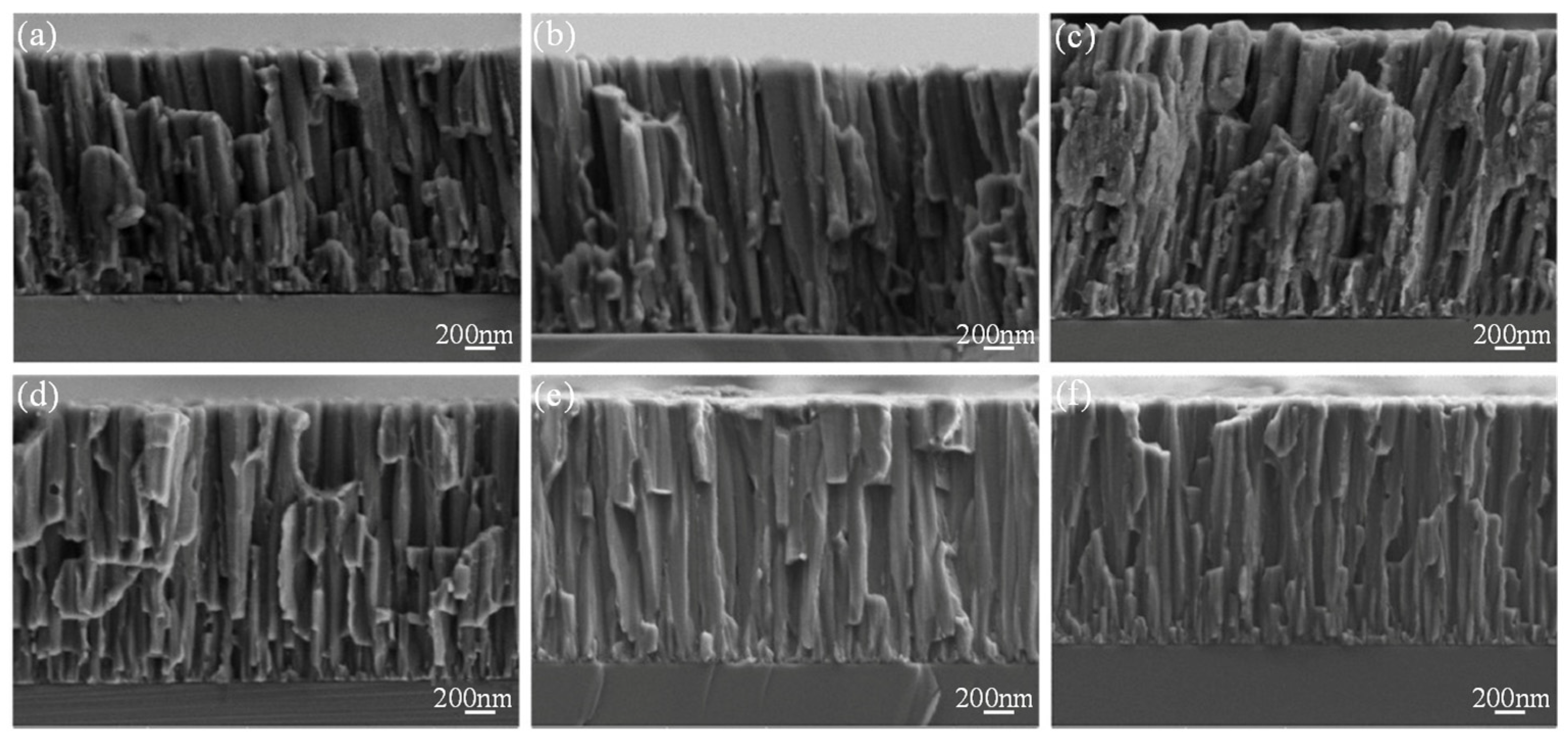


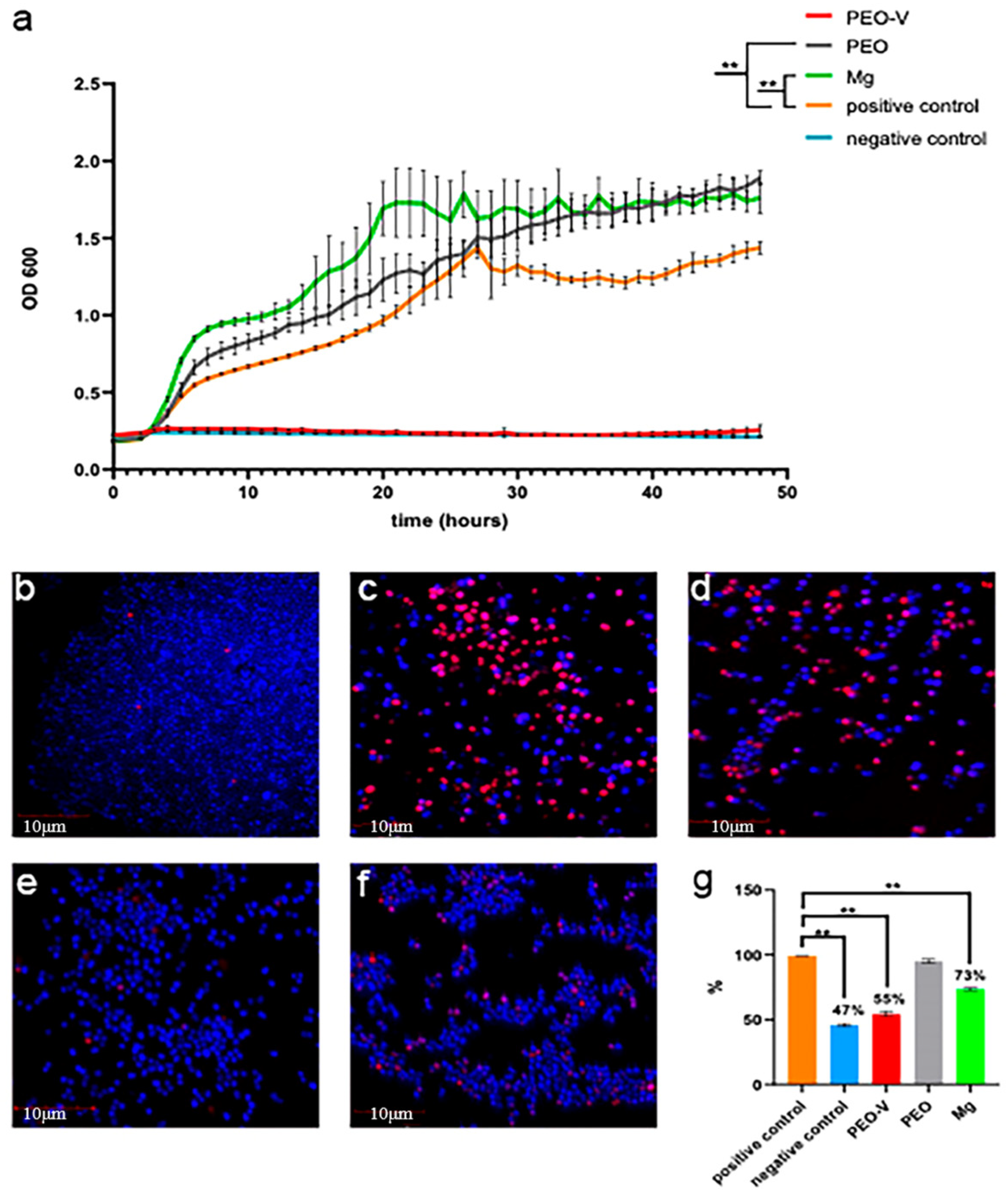
| Substrate | Materials | Coating | Results | Ref. |
|---|---|---|---|---|
| AZ31B | MAO/FSL | HAp | It has the most reasonable degradation rate and exhibits excellent biocompatibility and antibacterial performance. | [32] |
| AZ91 | MAO | MAO-LBL | The mechanical interlocking and adhesion of the coating have been enhanced, further preventing the penetration of corrosive media. | [33] |
| AZ31B | MAO | MAO/DLC | After PDMS penetrates the pores of the coating, it can enhance the density, adhesion, wear resistance, and corrosion resistance of the coating. | [34] |
| AZ91D | Chemical conversion | Phosphate | The presence of the strong oxidant KMnO4 forms an extremely thin passivation film on the protruding β phase, which inhibits the formation of phosphate crystals and prevents the shielding of conductive points. | [35] |
| AZ31 | Soaking | MgF2 | The coating significantly reduces the corrosion rate of AZ31 magnesium alloy, but the defect still leads to local corrosion. | [36] |
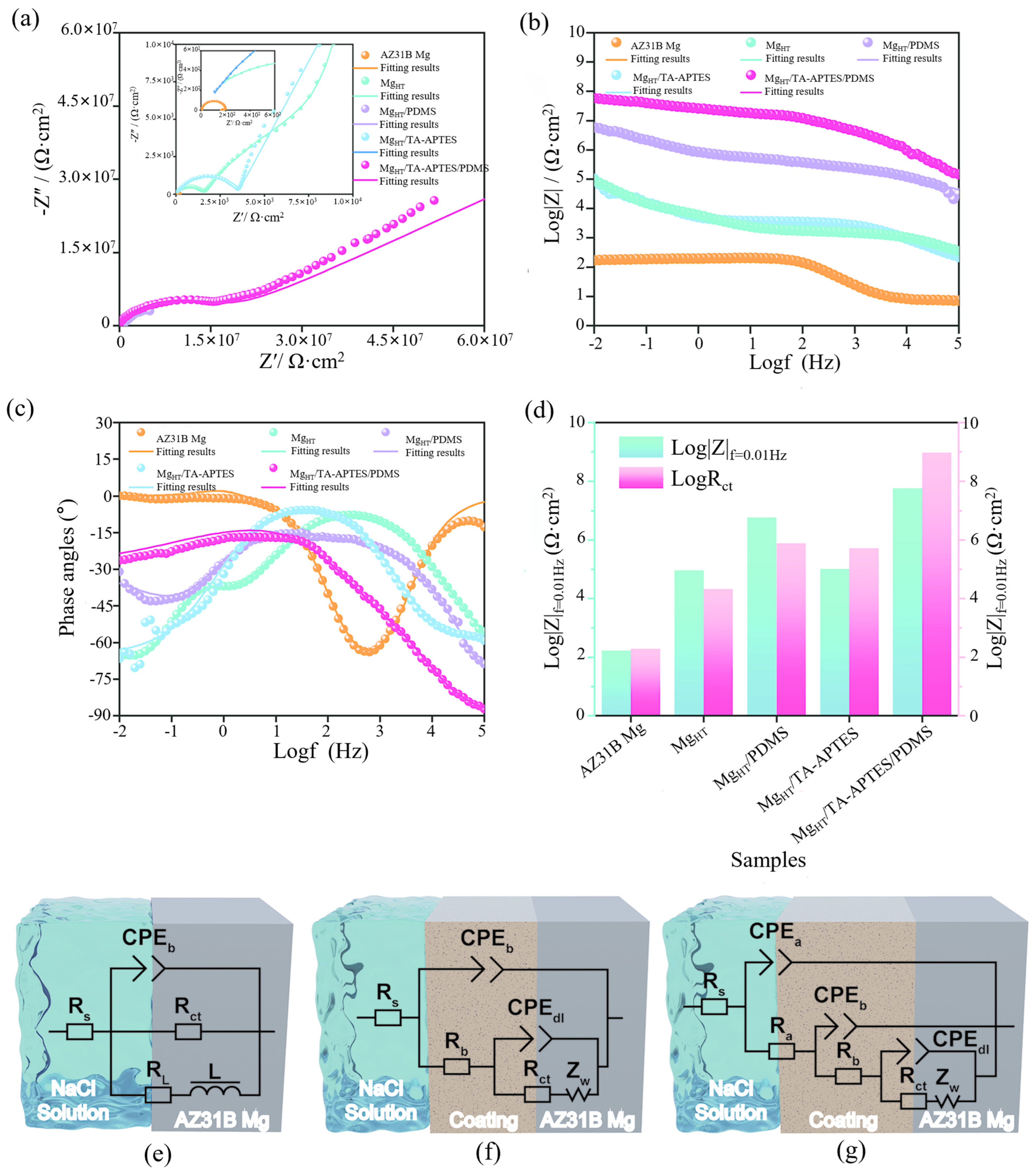
| AZ31B | Hydrothermal | MgHT/TA-APTES/PDMS | The corrosion rate of magnesium alloys is significantly reduced, and they possess excellent chemical stability and mechanical durability. | [37] |
| Pure Mg | Biomimetic deposition method | HAp | The corrosion behavior of the coating is influenced by surface pretreatment and deposition time. The coating deposited 6 h after heat treatment has higher corrosion resistance. | [38] |
| Mg alloy | Hydrothermal | PPA-HAp | Compared with traditional HA coatings, PPA-HA coatings have higher adhesion and better hardness and elastic moduli and have demonstrated excellent long-term corrosion resistance in in vitro experiments. | [39] |
| WE43 | Anodic oxidation | Ca-P | The HA coating is converted from DCPD through alkaline treatment and exhibits excellent microstructure, adhesion strength, and corrosion resistance. | [40] |
| Pure Mg | Soaking | CaP | Both coatings significantly enhance the corrosion resistance of magnesium substrates. Among them, the DCPD coating demonstrates superior corrosion resistance due to its low porosity, thickness, and good coverage. | [41] |
| AZ31, AZ91 | Plasma spraying | HAp | The experimental results show that the HAp coating significantly reduces the corrosion rate of the alloy in the simulated fluid, from 1.2 mm/year without coating to 0.4 mm/year. | [42] |
| AZ31 | Plasma spraying | HAp/Sr | Electrochemical tests show that the corrosion current density of the HA + 12%Sr coating is the lowest, demonstrating the best corrosion resistance. | [43] |
| AZ31 | Hydrothermal and dipping methods | PCL/HAp | PCL fills the pores of HA crystals to form a dense structure, which enhances the bonding strength of the coating and reduces the electrochemical corrosion rate to 6.9 mm/year. | [44] |
| ZM21 | Combining double emulsification and phase separation techniques | PA-PCL | The increase in pH value and the increase in Mg2 concentration respectively increase the PA release by 2.5 times and 3.1 times, further enhancing the self-repairing ability of the coating. | [45] |
| AZ31, WE43, AZ91 | Chemical vapor deposition | Parylene C | The results show that the organic coating significantly enhances the corrosion resistance of the alloy; Parylene C can effectively delay the degradation of magnesium alloys while maintaining mechanical adaptability. | [46] |
| AZ31 | Coprecipitation method | Chitosan/LDH | This intelligent coating can trigger the release of corrosion inhibitors at different pH values, and the coating containing gallic acid ions significantly improves the corrosion resistance of magnesium alloys. | [47] |
| AZ31 | PEO | HA/CMC | It was found through experiments that the HA coating significantly improves the initial corrosion resistance and has a self-healing ability, which could quickly self-repair the damage in the scratch test. | [48] |
| AZ31B | Anodic oxidation | Sol-Gel | The results show that the coating can significantly improve the corrosion resistance of magnesium alloy at both 110 °C and 160 °C curing temperatures, and the protection efficiency of the 110 °C curing coating reaches 98.6% after 72 h immersion. | [49] |
| AZ31B | Sol-Gel | GPTMS/TEOS | The coating is uniform and dense and bonds well with the substrate. Moreover, this coating can reduce the corrosion current density by three orders of magnitude, demonstrating excellent anti-corrosion performance. | [50] |
| AZ31 | Sol-Gel | DOPA-modified | The experimental results show that the DOPA-modified coating can provide long-term corrosion protection for more than 14 days in 0.1 M NaCl solution, while the unmodified coating could only maintain 2–3 days. | [51] |
| AZ31 | CA/Ly polymerization | Sol-Gel | Characterization confirms that CA/Lys is environmentally friendly by bonding to silane networks via hydrogen and chemical bonding (CA toxicity is lower than chromate). | [52] |
| Elektron 21 | Sol-Gel | TEOS–GPTMS | The study confirms that the TEOS–GPTMS hybrid system is an effective method to construct dual-function coatings, but the salt content and aging time need to be optimized to balance corrosion protection and biological activity. | [53] |
| Mg | DCMS | Mg-Zr | With the increase of Zr content, the corrosion resistance and hardness of the coating also increase, while the surface morphology and free energy also change. | [54] |
| Mg-1Zn-1Gd | LBL | PEO-ZnO@MOF | According to the in vitro biocompatibility assays, the PEO-ZnO@MOF sample shows uniquely outstanding properties of cell proliferation, cell adhesion, and cell viability, compared with those of the PEO, PEO–ZnO, and bare ZG11 samples. | [55] |
| AZ31 | ALD | ZrO2/PLGA | PLGA further fills the nanogap and delays electrolyte penetration. However, once the ZrO2 membrane is damaged, the local acid and galvanic corrosion caused by PLGA hydrolysis will accelerate the substrate corrosion. | [56] |
| Mg-Mn-Ce | PEO | MCC | The coating has irregular delamination results, low-surface free energy, and a contact Angle of 171 ± 2°, showing superhydrophobic properties. | [57] |
| AZ91D | ILC | [P6,6,6,14][NTf2] | The experimental results show that the ionic liquid conversion coating provides effective corrosion protection for magnesium alloy AZ91D, especially when combined with A + C pretreatment, which provides a new way for chromate replacement coating. | [58] |
| AZ31B | LBL | BMP-2 | The BMP-2 concentration group of 50 ng/mL has the best effect in promoting bone formation and stabilizing bone growth. | [59] |
| AZ31 | Electroless | Ni-P | The experimental results show that this coating exhibits excellent resistance to galvanic corrosion in neutral salt spray tests and NaCl solution immersion. | [60] |
| AZ80 | Steam method | LDH | The addition of LDH nano-containers and inhibitors significantly enhances the corrosion resistance of the coating, enabling it to exhibit more stable and excellent corrosion protection capabilities in NaCl solution. | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Zhao, Z.; Li, H.; Wang, D.; Shuai, C.; Yang, Y. Surface Coatings on Biomedical Magnesium Alloys. Materials 2025, 18, 3411. https://doi.org/10.3390/ma18143411
Ren J, Zhao Z, Li H, Wang D, Shuai C, Yang Y. Surface Coatings on Biomedical Magnesium Alloys. Materials. 2025; 18(14):3411. https://doi.org/10.3390/ma18143411
Chicago/Turabian StyleRen, Jiapeng, Zhenyu Zhao, Hua Li, Dongsheng Wang, Cijun Shuai, and Youwen Yang. 2025. "Surface Coatings on Biomedical Magnesium Alloys" Materials 18, no. 14: 3411. https://doi.org/10.3390/ma18143411
APA StyleRen, J., Zhao, Z., Li, H., Wang, D., Shuai, C., & Yang, Y. (2025). Surface Coatings on Biomedical Magnesium Alloys. Materials, 18(14), 3411. https://doi.org/10.3390/ma18143411







