Amorphous MoTex Nanomaterials Promote Visible-Light Co-Catalytic Degradation of Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the MoTex Materials
2.3. Fabrication of MoTex@TiO2 Nanocomposites
2.4. Characterizations of Materials
3. Results and Discussion
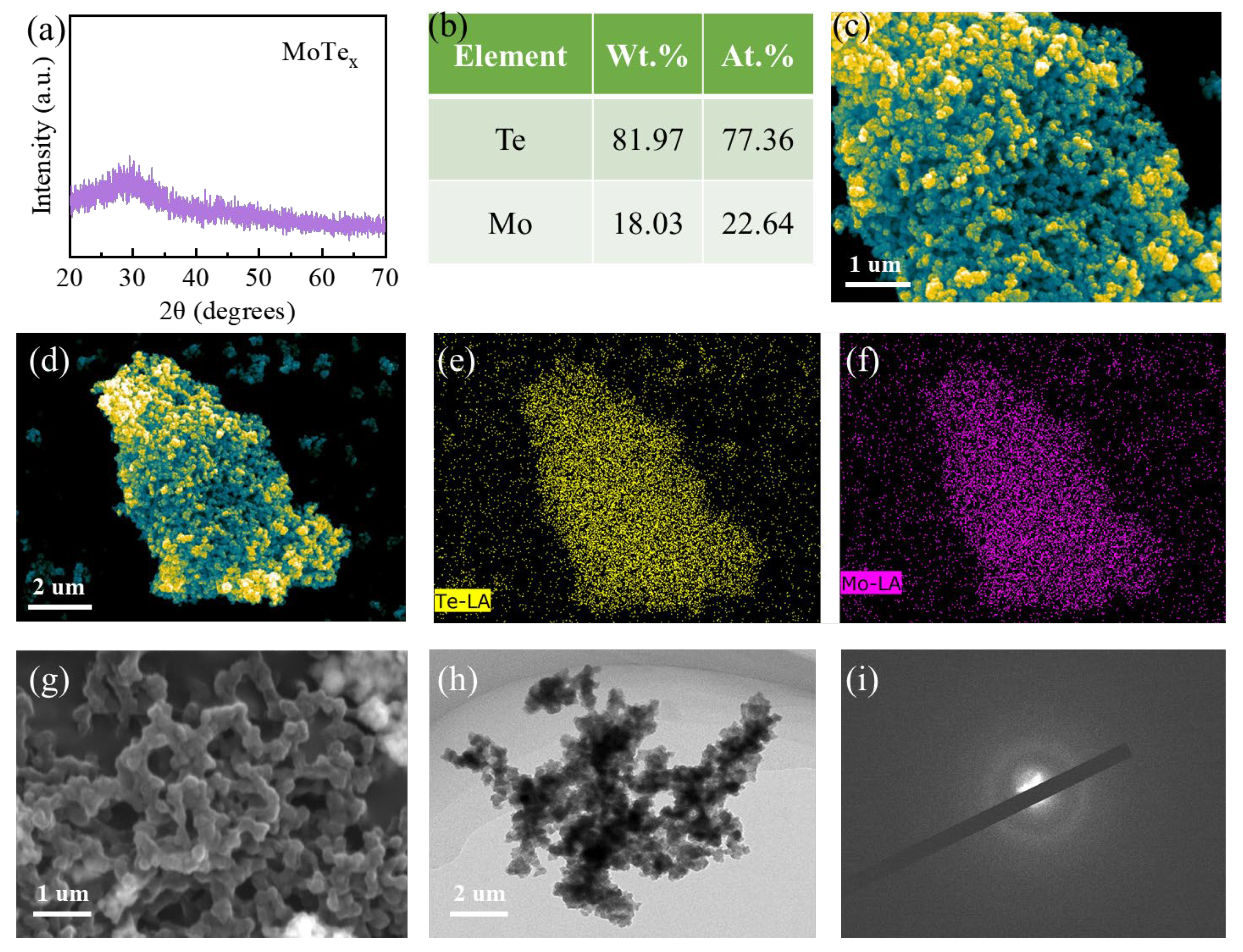
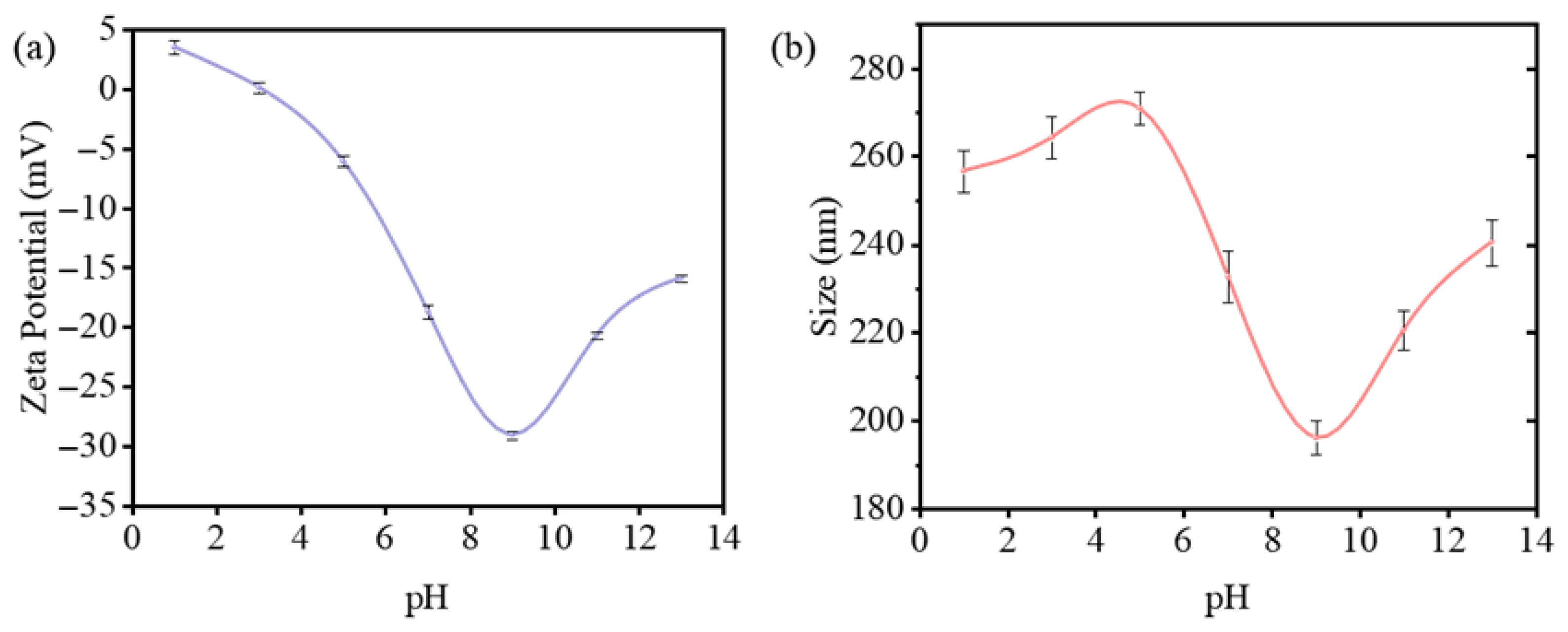
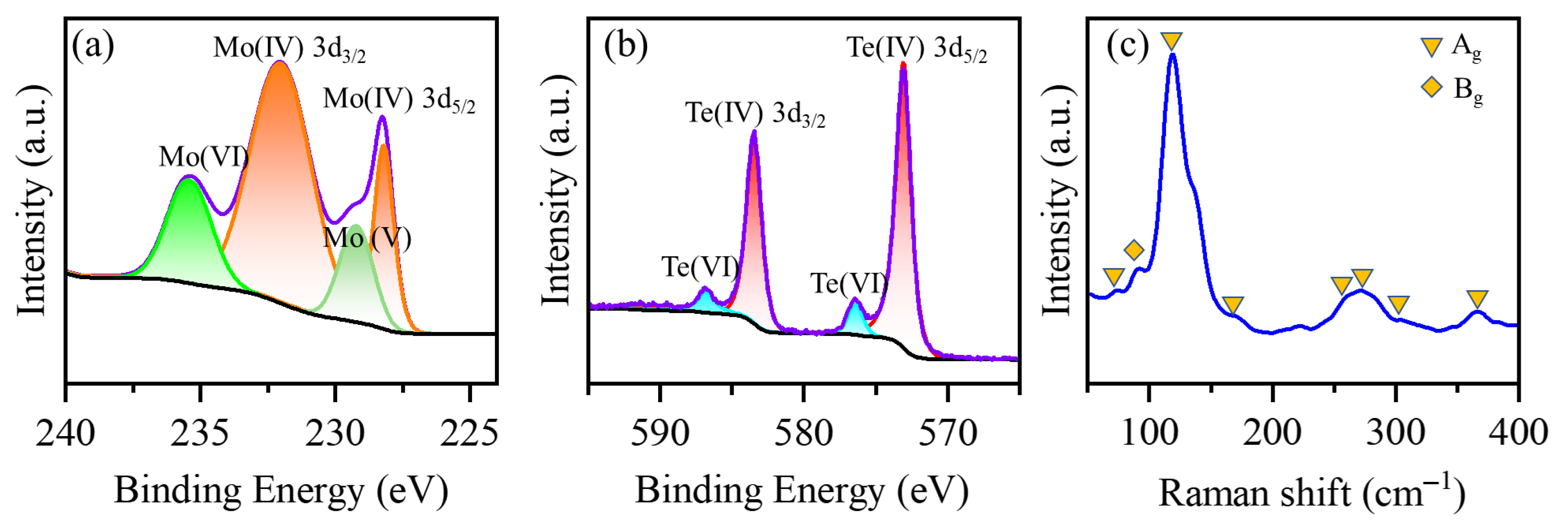
3.1. Morphology and Structure Characterization
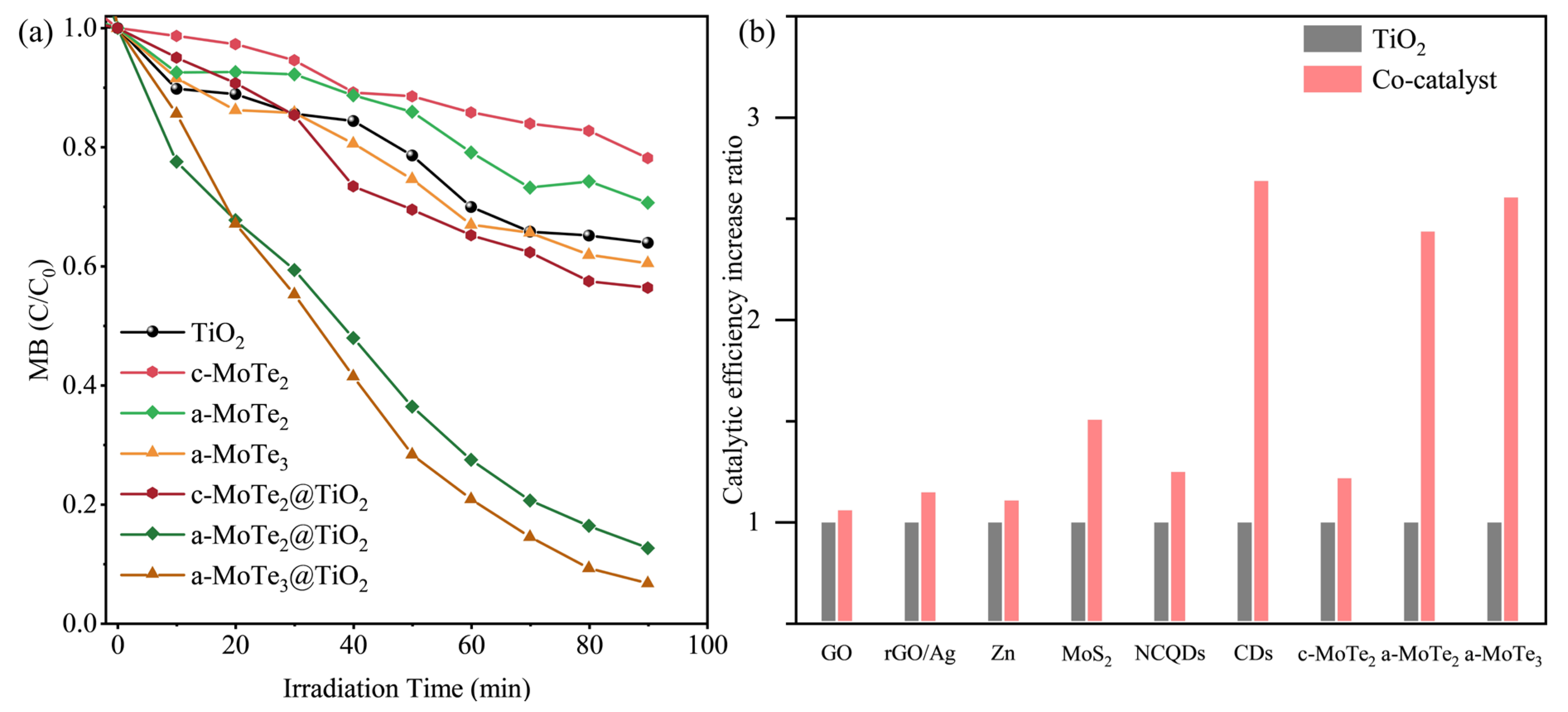
3.2. Photocatalyst Degradation of Methylene Blue
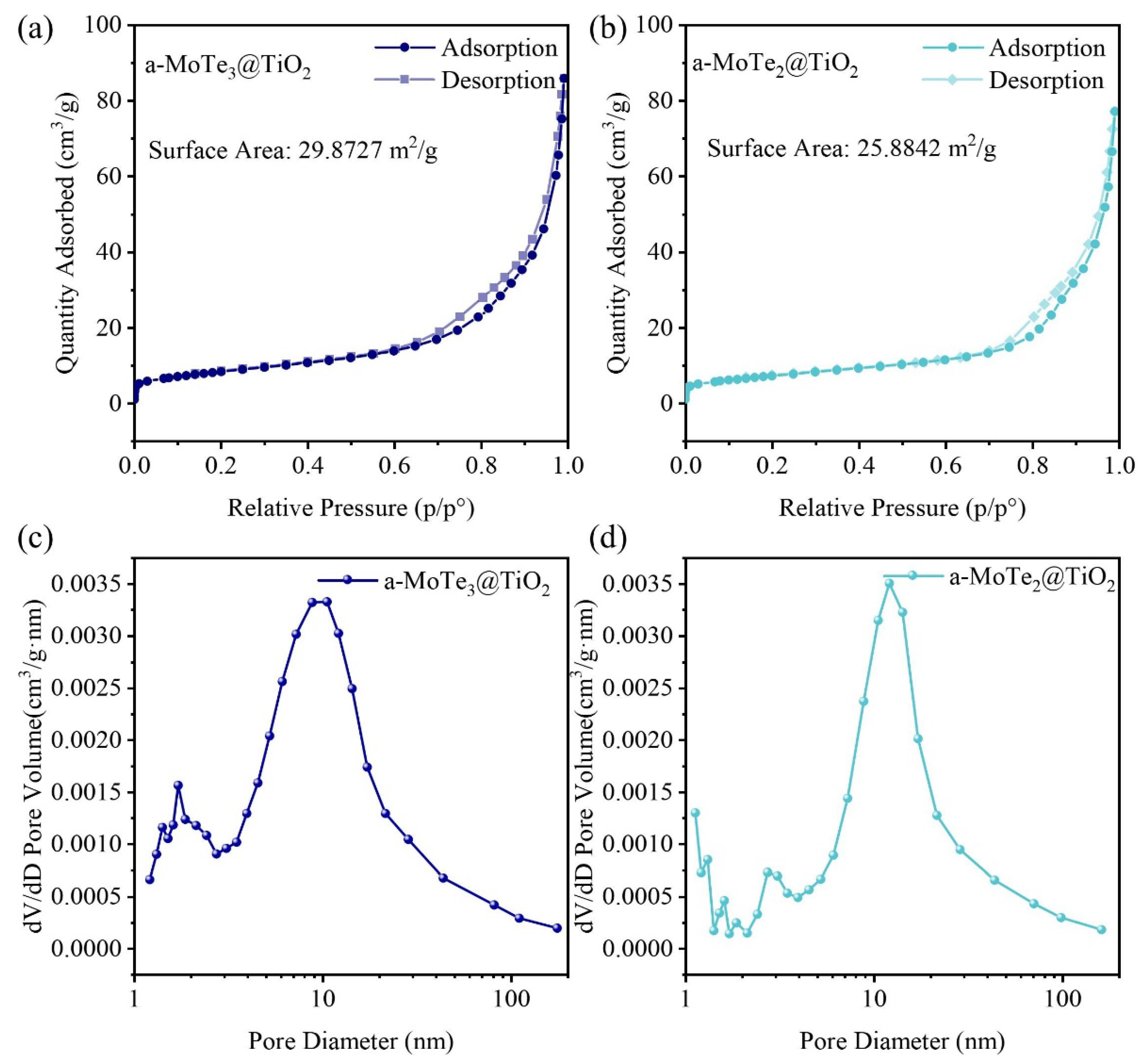
3.3. Specific Surface and Pore Size Analysis of MoTex@TiO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Qu, J.; Yin, G.; Zhang, T.; Zhao, H.-Y.; Jiao, F.-Z.; Liu, J.; Li, X.; Yu, Z.-Z. Omnidirectionally irradiated three-dimensional molybdenum disulfide decorated hydrothermal pinecone evaporator for solar-thermal evaporation and photocatalytic degradation of wastewaters. J. Colloid Interface Sci. 2023, 637, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Peng, Y.; Guo, S.; Hou, M.; Liang, Y.; Jiang, G.; Yang, L.; Ye, X.; Sun, H.; Chen, G. Fabrication of porous boron-doped diamond/Si electrodes for the electrocatalytic degradation of methylene blue. Appl. Surf. Sci. 2025, 684, 161944. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, X.; Zhao, S.; Du, Q.; Pi, X.; Jing, Z.; Jin, Y. Polydopamine coating for enhanced electrostatic adsorption of methylene blue by multiwalled carbon nanotubes in alkaline environments. J. Colloid Interface Sci. 2024, 675, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Cao, W.; Qiao, L.; Peng, X.; Yu, D.; Wang, S.; Li, C.; Wang, C. Degradation of methylene blue by ellipsoidal β-FeOOH@MnO2 core-shell catalyst: Performance and mechanism. Appl. Surf. Sci. 2023, 619, 156667. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, J.; Zhang, C.L.; Yue, Q.Y.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Yang, R.J.; Fan, Y.Y.; Zhang, Y.F.; Mei, L.; Zhu, R.S.; Qin, J.Q.; Hu, J.G.; Chen, Z.X.; Ng, Y.H.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202218016. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jana, S.; Singh, G.P.; Dey, R.K. Graphene-supported TiO2: Study of promotion of charge carrier in photocatalytic water splitting and methylene blue dye degradation. Adv. Compos. Hybrid Mater. 2020, 3, 127–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, X.; Lu, M.; Zhang, L.; Guo, B.; Liao, L.; Zhang, K.; Qin, A. CdSe/TiO2 nanowire arrays heterojunction enhanced self-driven photoelectrocatalytic degradation of mixed organic pollutants. Nano Energy 2025, 136, 110762. [Google Scholar] [CrossRef]
- Liu, S.; Lou, H.; Luo, J.; Albashir, D.; Shi, Y.; Chen, Q. A Novel In Situ Biosynthesized Bacterial Cellulose/MoS2/TiO2 Composite Film for Efficient Removal of Dyes and Pathogenic Bacteria from Industrial Wastewater under Sunlight Illumination. ACS Appl. Mater. Interfaces 2025, 17, 19543–19561. [Google Scholar] [CrossRef] [PubMed]
- Chawraba, K.; Medlej, H.; Toufaily, J.; Lalevee, J.; Hamieh, T. TiO2 Sensitized by Natural Dye Extracted from Cinnamon Bark for Photodegradation of Methylene Blue in Water Under LED Irradiation. Chem. Afr. 2024, 7, 2087–2101. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Experimental and computational study of metal oxide nanoparticles for the photocatalytic degradation of organic pollutants: A review. RSC Adv. 2023, 13, 18404–18442. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Mkhalid, I.A.; Baeissa, E.S.; Al-Rayyani, M.A. Photocatalytic Degradation of Methylene Blue by Fe/ZnO/SiO2 Nanoparticles under Visiblelight. J. Nanotechnol. 2012, 2012, 329082. [Google Scholar] [CrossRef]
- Liang, P.; Yang, W.; Peng, H.; Zhao, S. Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO. Molecules 2024, 29, 5584. [Google Scholar] [CrossRef] [PubMed]
- Paredes, P.; Rauwel, E.; Wragg, D.S.; Rapenne, L.; Estephan, E.; Volobujeva, O.; Rauwel, P. Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures. Nanomaterials 2023, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Parvees, A.; Priyadarshini, U.; Remya, N. Visible light induced photocatalytic degradation of methylene blue using carbon fibre cloth—Bismuth oxybromide—Water hyacinth derived silver nanocomposite. Environ. Res. 2024, 262, 119787. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, P.; Madasamy, P.D.; Pillay, K.; Brink, H. Sunlight-driven photocatalytic degradation of methylene blue using ZnO/biochar nanocomposite derived from banana peels. Biomass Convers. Biorefin. 2025, 15, 12347–12367. [Google Scholar] [CrossRef]
- Pan, G.; Xu, M.; Zhou, K.; Meng, Y.; Chen, H.; Guo, Y.; Wu, T. Photocatalytic Degradation of Methylene Blue Over Layered Double Hydroxides Using Various Divalent Metal Ions. Clays Clay Miner. 2019, 67, 340–347. [Google Scholar] [CrossRef]
- Lee, S.C.; Benck, J.D.; Tsai, C.; Park, J.; Koh, A.L.; Abild-Pedersen, F.; Jaramillo, T.F.; Sinclair, R. Chemical and phase evolution of amorphous molybdenum sulfide catalysts for electrochemical hydrogen production. ACS Nano 2016, 10, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: Theoretical and experimental studies. Adv. Mater. 2018, 30, 1800191. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Lee, J.T.; Chung, Y.J.; Srinivaas, M.; Wu, J.M. Ultrahigh efficient degradation activity of single- and few-layered MoSe2 nanoflowers in dark by piezo-catalyst effect. Nano Energy 2017, 40, 369–375. [Google Scholar] [CrossRef]
- Nirjhar, A.R.; Tan-Ema, S.J.; Sahriar, M.A.; Ahsan Dipon, M.N.; Hasan Abed, M.R.; Gainza, D.B.; Koneru, A.; Nishat, S.S.; Shorowordi, K.M.; Ahmed, S. Vacancy-induced spontaneous H2 evolution by overall water splitting on MoTe2/Ti2CO2: A two-dimensional direct Z scheme heterostructure. Int. J. Hydrogen Energy 2023, 48, 37273–37285. [Google Scholar] [CrossRef]
- Hellstern, T.R.; Kibsgaard, J.; Tsai, C.; Palm, D.W.; King, L.A.; Abild-Pedersen, F.; Jaramillo, T.F. Investigating catalyst–support interactions to improve the hydrogen evolution reaction activity of thiomolybdate [Mo3S13]2− nanoclusters. ACS Catal. 2017, 7, 7126–7130. [Google Scholar] [CrossRef]
- Sahu, A.; Steinmann, S.N.; Raybaud, P. Size-dependent structural, energetic, and spectroscopic properties of MoS3 polymorphs. Cryst. Growth Des. 2020, 20, 7750–7760. [Google Scholar] [CrossRef]
- Qu, D.; Liu, X.; Huang, M.; Lee, C.; Ahmed, F.; Kim, H.; Ruoff, R.S.; Hone, J.; Yoo, W.J. Carrier-type modulation and mobility improvement of thin MoTe2. Adv. Mater. 2017, 29, 1606433. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.H.; Han, S.K.; Park, J.; Lee, D.; Preston, D.J.; Kim, I.S.; Hersam, M.C.; Kwon, Y.; Shong, B.; et al. Strain-Enabled Local Phase Control in Layered MoTe2 for Enhanced Electrocatalytic Hydrogen Evolution. ACS Energy Lett. 2023, 8, 4716–4725. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, W.; Geng, D.; Fu, W.; Dan, J.; Xie, Y.; Kent, P.R.; Zhou, W.; Pennycook, S.J.; Loh, K.P. Edge segregated polymorphism in 2D molybdenum carbide. Adv. Mater. 2019, 31, 1808343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fu, D.; Ding, Z.; Zhang, Y.-Y.; Wan, D.; Tan, S.J.; Chen, Z.; Leng, K.; Dan, J.; Fu, W. Mo-terminated edge reconstructions in nanoporous molybdenum disulfide film. Nano Lett. 2018, 18, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Li, X.; Zhao, W.; Dong, J.; Rouleau, C.M.; Geohegan, D.B.; Ding, F.; Xiao, K.; Unocic, R.R. In situ edge engineering in two-dimensional transition metal dichalcogenides. Nat. Commun. 2018, 9, 2051. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Hu, Y.H. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016, 4, 285–304. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Z.; Kountoupi, E.; Tsoukalou, A.; Abdala, P.M.; Florian, P.; Fedorov, A.; Müller, C.R. Two-dimensional molybdenum carbide 2D-Mo2C as a superior catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 5510. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.-Y.; Ye, L.-H.; Zhang, X.-F.; Zhang, Y.-F.; Chen, W.-J. Exploring the reaction mechanism of H2S decomposition with MS3 (M = Mo, W) clusters. ACS Omega 2020, 5, 13324–13332. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: Fundamentals, progress, and beyond. Chem. Rev. 2021, 122, 1273–1348. [Google Scholar] [CrossRef] [PubMed]
- Er, D.; Ye, H.; Frey, N.C.; Kumar, H.; Lou, J.; Shenoy, V.B. Prediction of enhanced catalytic activity for hydrogen evolution reaction in Janus transition metal dichalcogenides. Nano Lett. 2018, 18, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Nguyen, P.D.; Nguyen, D.N.; Truong, Q.D.; Kim Chi, T.T.; Ung, T.T.D.; Honma, I.; Liem, N.Q.; Tran, P.D. Novel amorphous molybdenum selenide as an efficient catalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2018, 10, 8659–8665. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, P.; Wang, P.; Yu, J. Amorphous molybdenum sulfide as highly efficient electron-cocatalyst for enhanced photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 217–225. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J.; Lau, S.P. Synthesis, properties, and applications of 2D amorphous inorganic materials. J. Appl. Phys. 2020, 127, 220901. [Google Scholar] [CrossRef]
- Kang, J.; Yang, X.; Hu, Q.; Cai, Z.; Liu, L.-M.; Guo, L. Recent progress of amorphous nanomaterials. Chem. Rev. 2023, 123, 8859–8941. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Sun, Y.; Xiang, H.; Liang, X.; Yu, Y. 3D Amorphous Carbon with Controlled Porous and Disordered Structures as a High-Rate Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702434. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Li, D.-S.; Xu, J.; Tao, H.; Liu, B. Amorphous alloys for electrocatalysis: The significant role of the amorphous alloy structure. Nano Res. 2021, 16, 4277–4288. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Zhong, X.; Cheng, X.; Fan, Z.; Yu, Y. Amorphous Red Phosphorus Embedded in Sandwiched Porous Carbon Enabling Superior Sodium Storage Performances. Small 2018, 14, 1703472. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ma, Y.; Li, Z.; Cheng, M.; Ning, S.; Han, E.; Xu, M.; Zhang, P.-F.; Zhao, K.; Li, R. Disorder-tuned conductivity in amorphous monolayer carbon. Nature 2023, 615, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein-Zuta, G.; Arnon, Z.A.; Vijayakanth, T.; Messer, O.; Lusky, O.S.; Wagner, A.; Zilberman, G.; Aizen, R.; Michaeli, L.; Rencus-Lazar, S. A self-healing multispectral transparent adhesive peptide glass. Nature 2024, 630, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rosu-Finsen, A.; Davies, M.B.; Amon, A.; Wu, H.; Sella, A.; Michaelides, A.; Salzmann, C.G. Medium-density amorphous ice. Science 2023, 379, 474–478. [Google Scholar] [CrossRef]
- Liu, A.; Kim, Y.-S.; Kim, M.G.; Reo, Y.; Zou, T.; Choi, T.; Bai, S.; Zhu, H.; Noh, Y.-Y. Selenium alloyed tellurium oxide for amorphous p-channel transistors. Nature 2024, 629, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Escalera-López, D.; Lou, Z.; Rees, N.V. Benchmarking the activity, stability, and inherent electrochemistry of amorphous molybdenum sulfide for hydrogen production. Adv. Energy Mater. 2019, 9, 1802614. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, J.; Li, W.; Huang, R.; Wang, X.; Guo, J.; Choi, J.-H.; Lou, Y.; Wang, D.; Zou, G. Immobilized Precursor Particle Driven Growth of Centimeter-Sized MoTe2 Monolayer. J. Am. Chem. Soc. 2021, 143, 13314–13324. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Liang, Y.; Liu, F.; Zhang, M.; Feng, J.; Xi, B.; Xiong, S. Effect of Combination Model of MoTe2 and MXene Layers on Sodium Ion Storage. Adv. Mater. 2025, 2503252. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Luo, F.; Zhu, M.; Xu, X.; Ye, Y.; Li, B.; Wang, G.; Luo, W.; Zheng, X.; Wu, N.; et al. Correction and removal of expression of concern: Controllable 2H-to-1T′ phase transition in few-layer MoTe2. Nanoscale 2019, 11, 23498–23501. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.T.; Yin, L.-C.; Tran, P.D.; Saito, R. Simultaneous Anionic and Cationic Redox in the Mo3S11 Polymer Electrode of a Sodium-Ion Battery. J. Phys. Chem. C 2019, 123, 30856–30862. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Ji, D.; Guan, Q.; Thirumalraj, B.; Palmisano, G.; Mohamed, S.; Zheng, L. A highly efficient bifunctional Z-scheme photocatalyst with spatially-separated and synergistically-enhanced adsorption and photodegradation. Appl. Catal. B Environ. Energy 2025, 362, 124758. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical reduction of methylene blue in the presence of nanocatalysts: A critical review. Rev. Chem. Eng. 2020, 36, 749–770. [Google Scholar] [CrossRef]
- Zazpe, R.; Sopha, H.; Charvot, J.; Krumpolec, R.; Rodriguez-Pereira, J.; Michalička, J.; Mistrík, J.; Bača, D.; Motola, M.; Bureš, F.; et al. 2D MoTe2 nanosheets by atomic layer deposition: Excellent photo- electrocatalytic properties. Appl. Mater. Today 2021, 23, 101017. [Google Scholar] [CrossRef]
- Sabarinathan, M.; Bharathi, P.; Harish, S.; Hayakawa, Y. Two-dimensional layered MoS2@CuS nanostructures decorated over TiO2 spheres for the decomposition of organic pollutant under visible light. Inorg. Chem. Commun. 2023, 153, 110828. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Vasilaki, E.; Georgaki, I.; Vernardou, D.; Vamvakaki, M.; Katsarakis, N. Ag-loaded TiO2/reduced graphene oxide nanocomposites for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2015, 353, 865–872. [Google Scholar] [CrossRef]
- Yunarti, R.T.; Safitri, T.N.; Dimonti, L.C.C.; Aulia, G.; Khalil, M.; Ridwan, M. Facile synthesis of composite between titania nanoparticles with highly exposed (001) facet and coconut shell-derived graphene oxide for photodegradation of methylene blue. J. Phys. Chem. Solids 2022, 160, 110357. [Google Scholar] [CrossRef]
- Ibukun, O.; Evans, P.E.; Dowben, P.A.; Kyung Jeong, H. Titanium dioxide-molybdenum disulfide for photocatalytic degradation of methylene blue. Chem. Phys. 2019, 525, 110419. [Google Scholar] [CrossRef]
- Cong, S.; Cai, J.; Li, X.; You, J.; Wang, L.; Wang, X. Direct Z-Scheme Xylan-Based Carbon Dots@TiO2-x Nanocomposites for Visible Light Driven Photocatalytic of Dye Degradation and Antibacterial. Adv. Funct. Mater. 2024, 34, 2401540. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Hussain, T.; Karuppasamy, K.; Santhoshkumar, P.; Kim, K.-Y.; Manikandan, R.; Jung, J.; Kim, H.-S. Diverse chalcogen bonded molybdenum dichalcogenide alloy for the efficient photo- and electro-catalytic activity to eradicate the methylene blue and Congo red dyes. J. Clean. Prod. 2023, 426, 139127. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, W.; Wang, J.; Ren, F.; Chen, Z.; Sun, Z.; Ren, P.-G. Construction of biomass derived carbon quantum dots modified TiO2 photocatalysts with superior photocatalytic activity for methylene blue degradation. J. Alloys Compd. 2023, 932, 167627. [Google Scholar] [CrossRef]
- Karuppasamy, P.; Ramzan Nilofar Nisha, N.; Pugazhendhi, A.; Kandasamy, S.; Pitchaimuthu, S. An investigation of transition metal doped TiO2 photocatalysts for the enhanced photocatalytic decoloration of methylene blue dye under visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105254. [Google Scholar] [CrossRef]
- Chawraba, K.; Medlej, H.; Hamieh, M.; Lalevée, J.; Hamieh, T.; Toufaily, J. SBA-15 Loaded with Methylene Blue Dye for Efficient and Low-Cost Decontamination of Water under Visible Led Light. Preprints 2024. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, B.; Zhou, J.; Sun, Z. Amorphous MoTex Nanomaterials Promote Visible-Light Co-Catalytic Degradation of Methylene Blue. Materials 2025, 18, 3388. https://doi.org/10.3390/ma18143388
Zhang Z, Liu B, Zhou J, Sun Z. Amorphous MoTex Nanomaterials Promote Visible-Light Co-Catalytic Degradation of Methylene Blue. Materials. 2025; 18(14):3388. https://doi.org/10.3390/ma18143388
Chicago/Turabian StyleZhang, Zhen, Bin Liu, Jian Zhou, and Zhimei Sun. 2025. "Amorphous MoTex Nanomaterials Promote Visible-Light Co-Catalytic Degradation of Methylene Blue" Materials 18, no. 14: 3388. https://doi.org/10.3390/ma18143388
APA StyleZhang, Z., Liu, B., Zhou, J., & Sun, Z. (2025). Amorphous MoTex Nanomaterials Promote Visible-Light Co-Catalytic Degradation of Methylene Blue. Materials, 18(14), 3388. https://doi.org/10.3390/ma18143388









