Extraction of Rubidium and Cesium from a Variety of Resources: A Review
Highlights
- Extraction technology of rubidium cesium from different resources
- The current situation and uses of rubidium and cesium resources
- The occurrence states and mineral structures of rubidium and cesium in different resources
- Conservation and green recycling of rare metal resources
Abstract
1. Introduction
2. Minerals of the Mica Group
2.1. Lepidolite
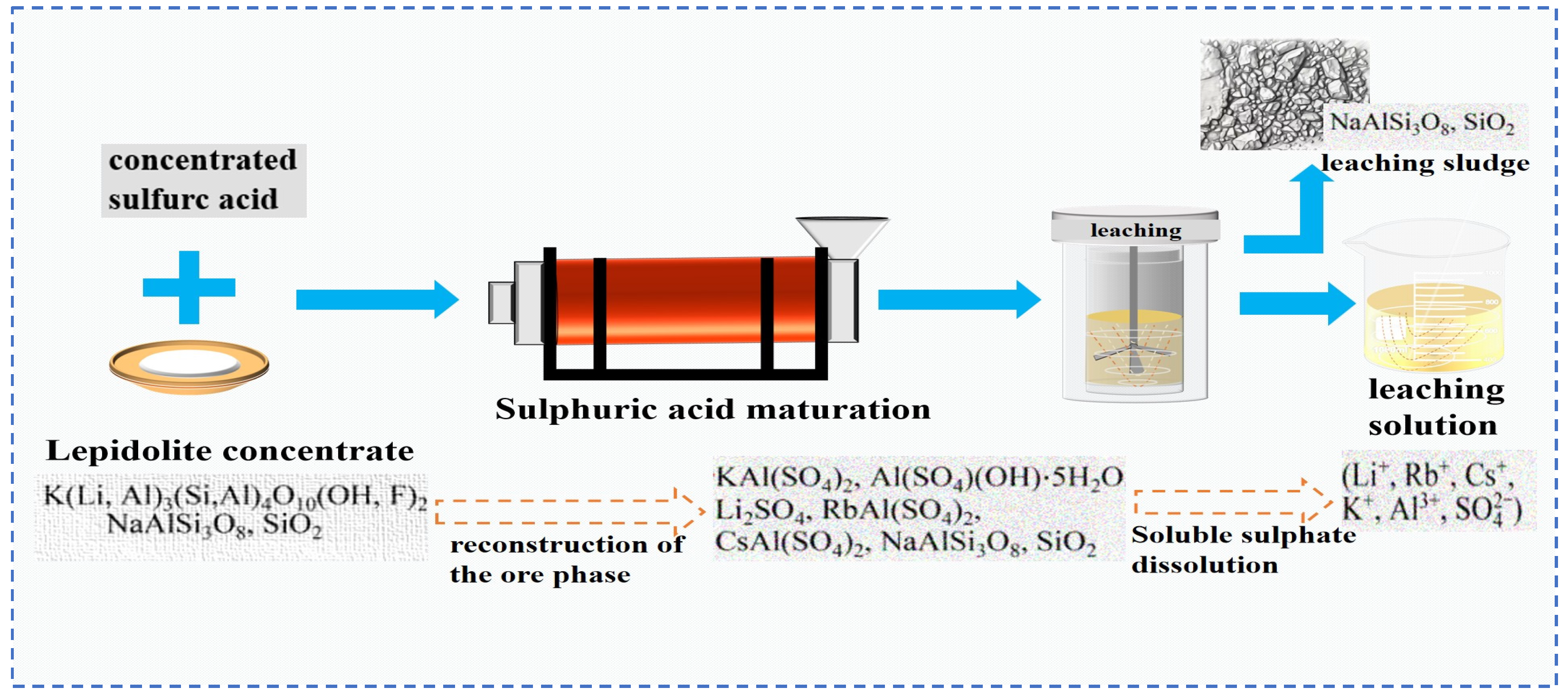
2.1.1. Acid Leaching
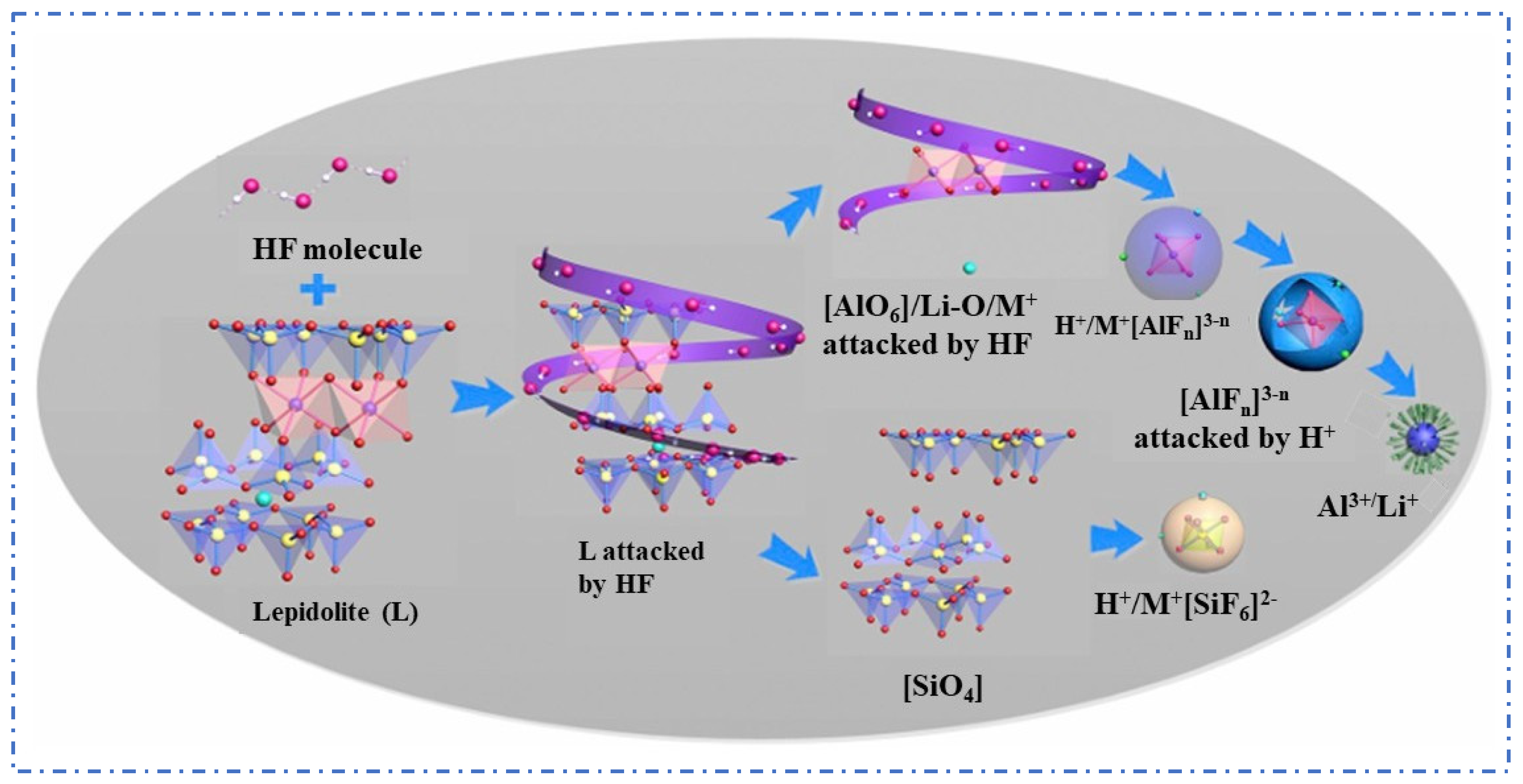
2.1.2. Fluoride Chemical Method
2.1.3. Alkaline Leaching Method
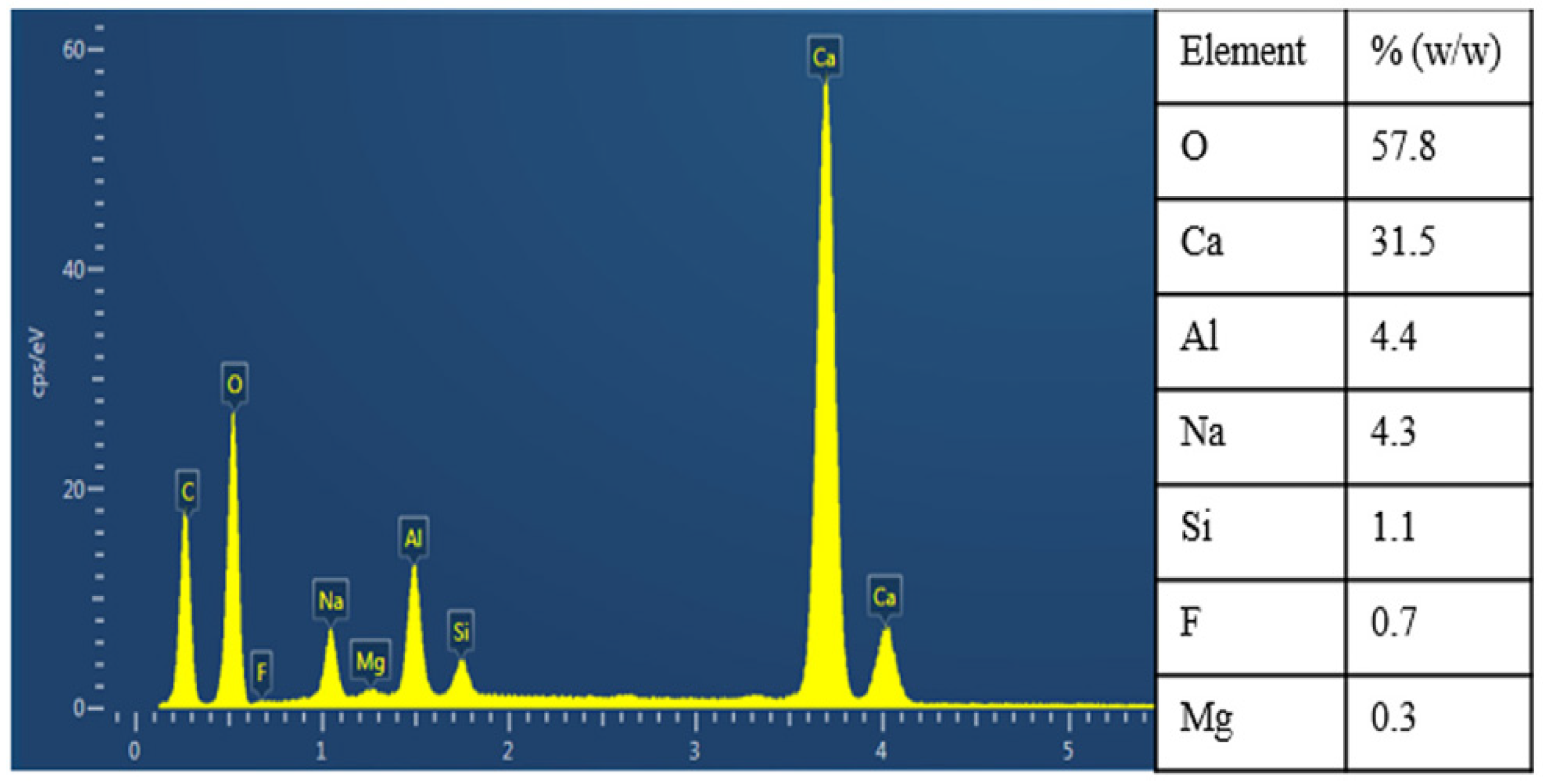
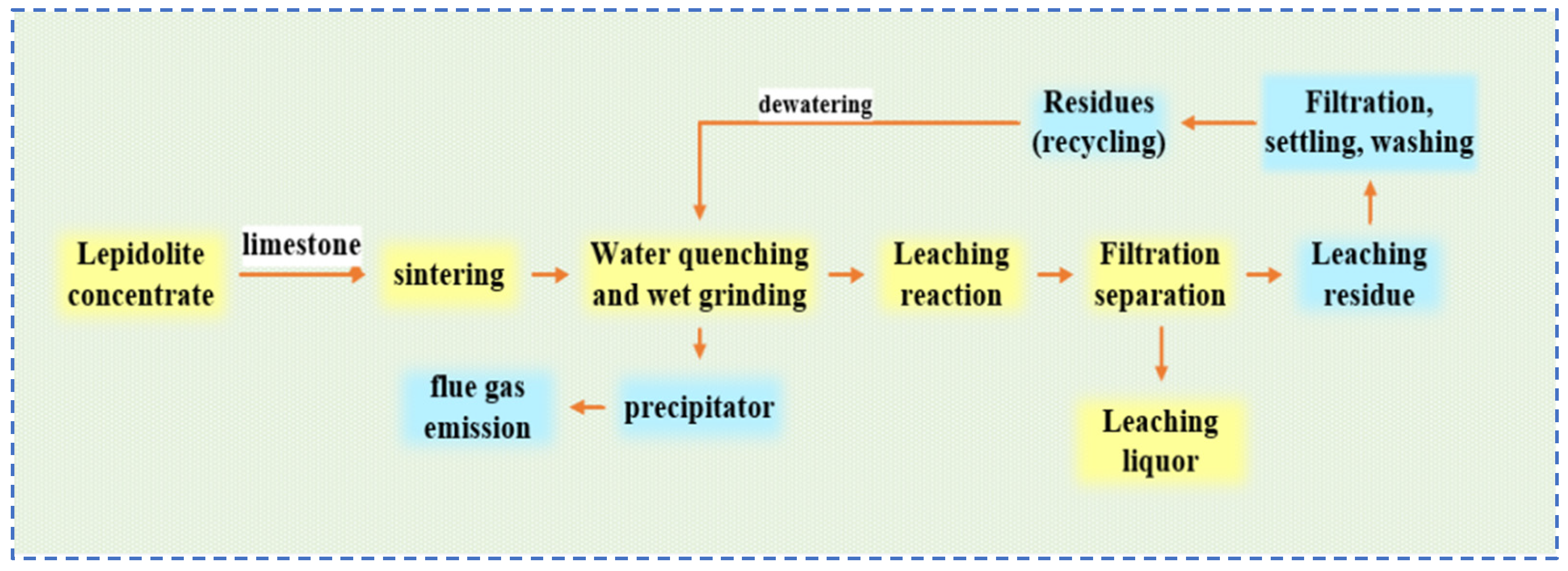
2.1.4. Salt Roasting-Leaching Method
2.2. Zinnwaldite
3. Salt Lake Brine and Lithium Ore Leachate
3.1. Precipitation Method
3.2. Solvent Extraction Method
3.3. Adsorption
4. Silicate Minerals
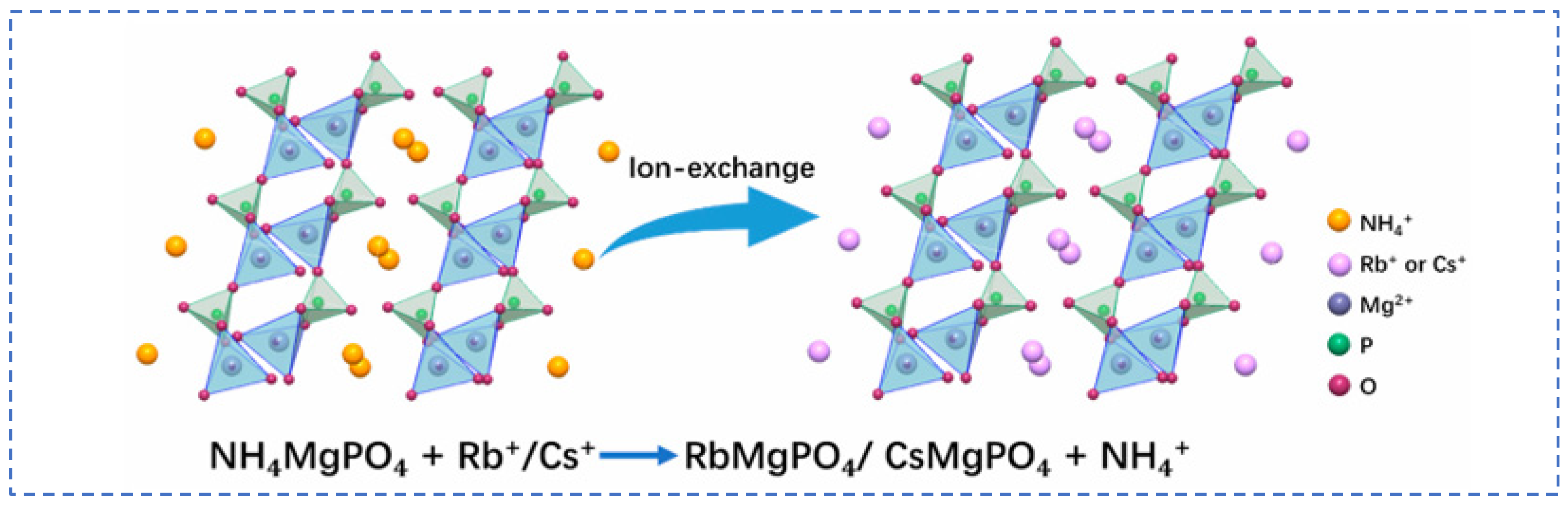
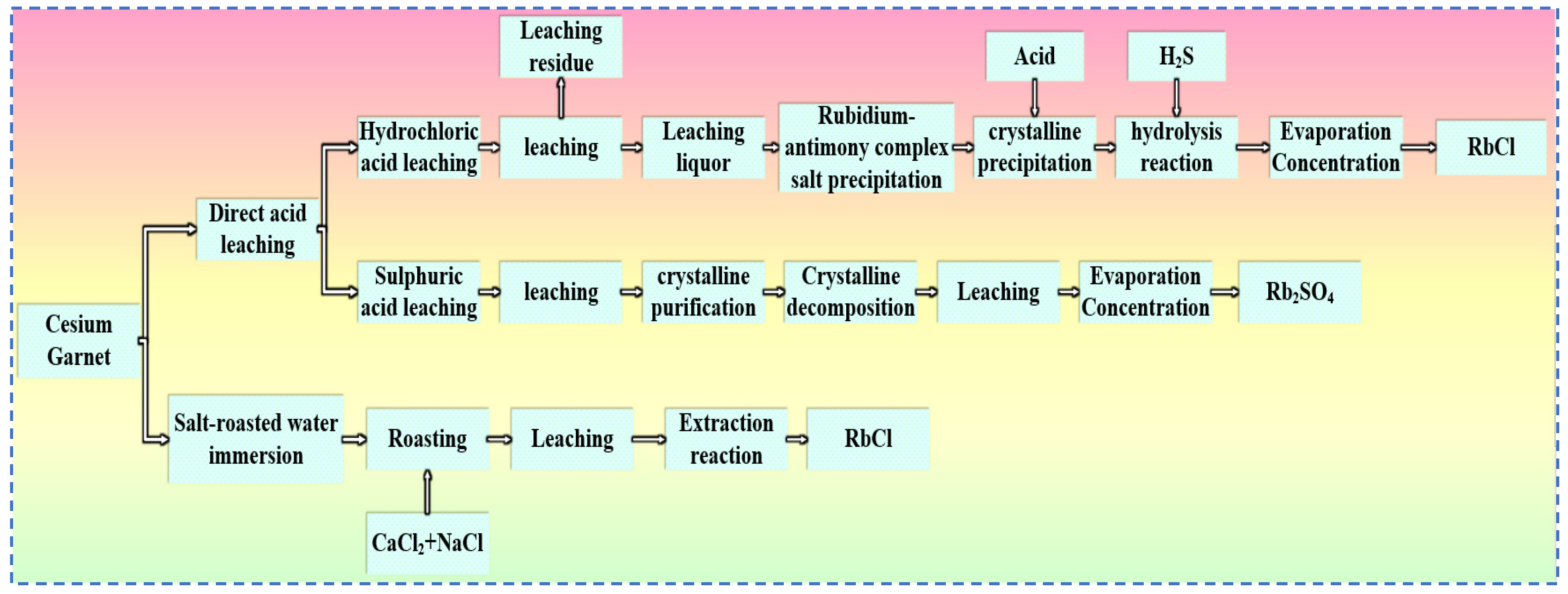
4.1. Rubidium Extraction from Pollucite
4.1.1. Hydrochloric Acid Leaching Method
4.1.2. Sulfuric Acid Leaching Method
4.1.3. Salt Roasting-Water Leaching Method
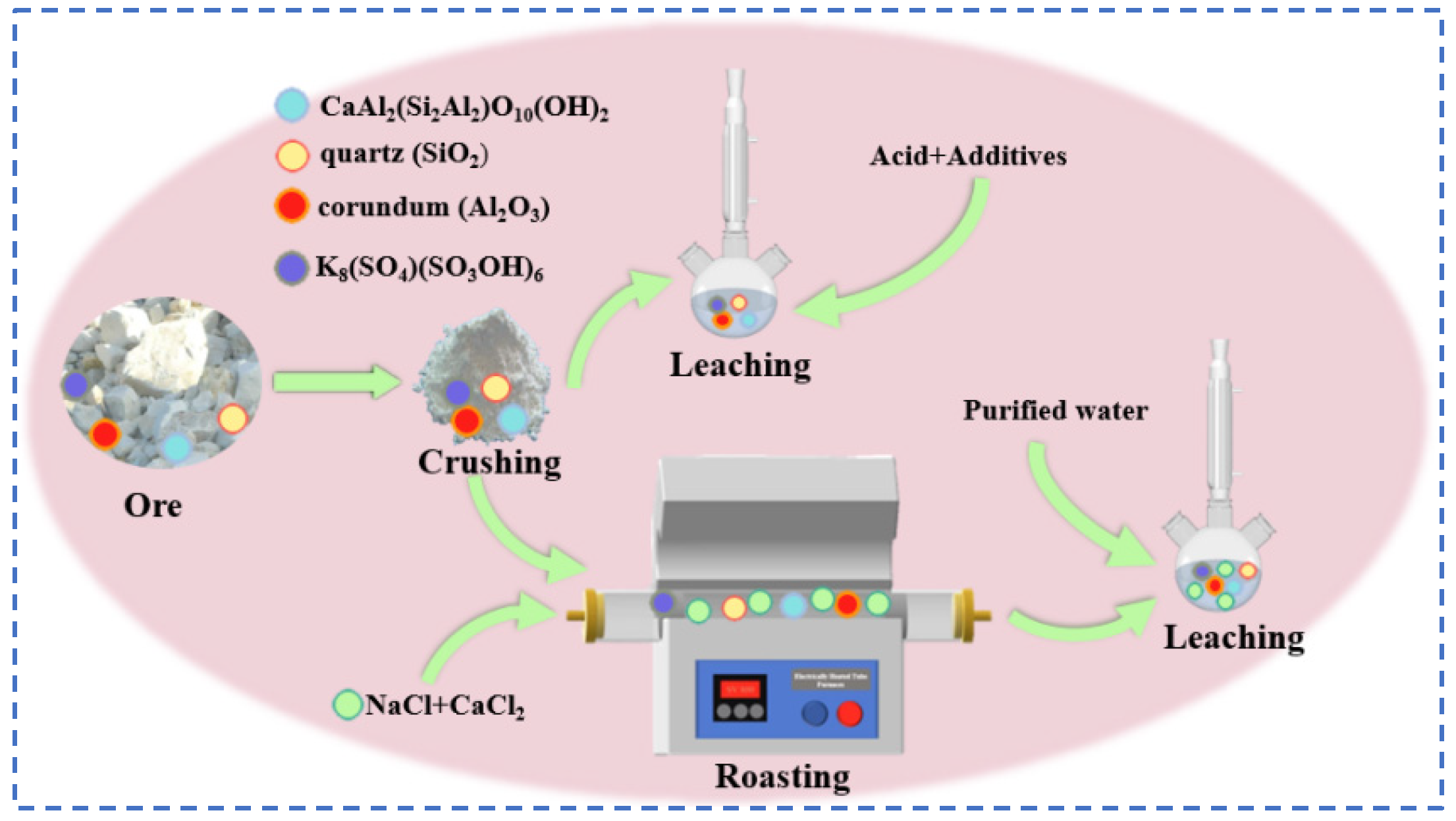
4.2. Rubidium Extraction from Potash Feldspar
4.2.1. Acid Leaching Method
4.2.2. Salt Roasting-Water Leaching Method
5. Potential Resource
5.1. Flotation Tailings
5.2. Kaolin
5.3. Geothermal Water
5.4. Radioactive Wastewater
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thi, T.H.-D.; Van, T.L.; Giere, R.; Tam, T. Extraction of lithium from lepidolite via iron sulphide roasting and water leaching. Hydrometallurgy 2015, 153, 154–159. [Google Scholar] [CrossRef]
- Arnold, W.D.; Crouse, D.J.; Brown, K.B. Solvent Extraction of Cesium (and Rubidium) from Ore Liquors with Substituted Phenols. Ind. Eng. Chem. Proc. Des. Dev. 1965, 4, 249–254. [Google Scholar] [CrossRef]
- Wu, J. Applications of Rare Metals Rubidium and Cesium and Their Compounds in China. Xinjiang Nonferr. Met. 2023, 46, 55–56. [Google Scholar]
- Li, J.; Xu, S. Metals with Eyes: Caesium and Rubidium. Chem. World 2005, 117, 85–108. [Google Scholar]
- Cao, Y.; Gao, Z.; Wang, S.; Wang, W.; Liu, H. Extraction of rubidium from a difficult-to-process rubidium ore. Met. Mines 2015, 44, 83–87. [Google Scholar]
- Zhong, J. Experimental study on the extraction of rubidium from rubidium-bearing minerals using chlorination roasting. Hunan Nonferr. Met. 2016, 32, 43–45. [Google Scholar]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Jandova, J.; Dvorak, P.; Formanek, J.; Vu, H.N. Recovery of rubidium and potassium alums from lithium-bearing minerals. Hydrometallurgy 2012, 119, 73–76. [Google Scholar] [CrossRef]
- Sitando, O.; Crouse, P.L. Processing of a Zimbabwean petalite to obtain lithium carbonate. Int. J. Miner. Process. 2012, 102, 45–50. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Yan, S.; Tang, M.; Deng, T.; Huang, Z. Separation and extraction of rubidium and caesium from brine. Miner. Rocks 1993, 13, 113–119. [Google Scholar]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Wang, R.; Xie, L.; Zhu, Z.; Hu, H. Mica: An Important Indicator Mineral for Rare Metal Mineralisation in Granite-Pegmatite Deposits. Acta Petrol. Sin. 2019, 35, 69–75. [Google Scholar]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of lepidolite and recovery of lithium hydroxide from purified alkaline pressure leach liquor by phosphate precipitation and lime addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Guo, H.; Lv, M.; Kuang, G.; Cao, Y.; Wang, H. Stepwise heat treatment for fluorine removal on selective leachability of Li from lepidolite using HF/H2SO4 as lixiviant. Sep. Purif. Technol. 2021, 259, 118194. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, Y.; Zhang, L.; Tan, X.; Li, C.; Wang, W. Study on Sulphuric Acid Maturation of Lithium Mica Concentrate. Miner. Prot. Util. 2018, 59–62. [Google Scholar] [CrossRef]
- Tian, J.; Li, T.; Wang, M.; Zhao, H.; Shi, J. Research Progress on Lithium Extraction Technology from Typical Lithium Ore. J. Hubei Univ. Nat. Sci. Ed. 2020, 42, 56–60. [Google Scholar]
- Yu, Y.; Cui, L.; Wang, Y.; Zhang, L. Research progress on lithium extraction technology from lithium mica. J. Nonferr. Met. Soc. China 2023, 33, 1972–1993. [Google Scholar]
- Li, G.; Yang, J.; Yang, J. Review of the progress in the decomposition of lithium mica and the process of lithium leaching. Bull. Silic. Res. 2017, 36, 1599–1604. [Google Scholar]
- Fu, X.; Wang, L. Review of extraction processes for rubidium from silicate ore resources. Compr. Util. Miner. Resour. 2020, 171–179. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.; Wang, H. Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Li, C.; Yi, Y.; Liu, W.; Zhang, L. Energy-efficient and simultaneous extraction of lithium, rubidium and cesium from lepidolite concentrate via sulfuric acid baking and water leaching. Hydrometallurgy 2019, 185, 244–249. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Selective recovery and efficient separation of lithium, rubidium, and cesium from lepidolite ores. Sep. Purif. Technol. 2022, 288, 120667. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Zhang, L.; Wang, W.; Liu, H.; Cao, Y. Extraction of lithium from lithium mica concentrate using a sulphurisation roasting-water leaching process. Hydrometallurgy 2021, 40, 6–9. [Google Scholar]
- Zhang, L.; Zhang, Y.; Zhang, X.; Tan, X. Extraction of lithium, rubidium, and caesium from lithium mica using a sulphuric acid leaching-water immersion process. Nonferr. Met. Smelt. Sect. 2019, 39–42. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, Y.; Tan, X.; Zhang, L.; Wang, W.; Liu, W. Mechanism and kinetic characteristics of lithium, rubidium, and caesium extraction from lithium mica using sulphuric acid leaching. J. Cent. South Univ. Nat. Sci. Ed. 2021, 52, 3093–3102. [Google Scholar]
- Liu, J.; Yin, Z.; Li, X.; Hu, Q.; Liu, W. A novel process for the selective precipitation of valuable metals from lepidolite. Miner. Eng. 2019, 135, 29–36. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Z.; Liu, W.; Li, X.; Hu, Q. Treatment of aluminum and fluoride during hydrochloric acid leaching of lepidolite. Hydrometallurgy 2020, 191, 105222. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Li, H.; Pei, W.; Wang, H. Enhanced lithium leaching from lepidolite in continuous tubular reactor using H2SO4+H2SiF6 as lixiviant. Trans. Nonferr. Met. Soc. China 2021, 31, 2165–2173. [Google Scholar] [CrossRef]
- Wang, D.; Chen, S.; Liu, X.; Cheng, B.; Ji, Q. Research on a New Process for Lithium Extraction from Lithium Mica Using Alkali Solution. Inorg. Salt Ind. 2014, 46, 26–28. [Google Scholar]
- Huang, Z.; Feng, W.; Lou, J.; Yang, L.; Xie, C. Experimental study on the mixed alkali decomposition process of lithium mica. Fine Chem. Intermed. 2017, 47, 55–60. [Google Scholar]
- Sun, Y. Ways to improve the Li2O recovery rate of lithium mica-limestone sintering. Rare Met. Hard Alloys 2000, 23–27. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Liu, Z. Research on a New Process for the Pressure Leaching of Lithium Mica in Yichun. Nonferr. Met. Smelt. Sect. 2001, 5, 19–21. [Google Scholar]
- Zhang, X.; Chen, Z.; Rohani, S.; He, M.; Tan, X.; Liu, W. Simultaneous extraction of lithium, rubidium, cesium and potassium from lepidolite via roasting with iron(II) sulfate followed by water leaching. Hydrometallurgy 2022, 208, 105820. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Wen, X.; Pu, J.; Wang, Y.; Yuan, D. Study on Lithium Extraction from Lithium Mica Using Sulphate Method. Inorg. Salt Ind. 2014, 46, 41–44. [Google Scholar]
- Guo, C.; Zhou, J.; Wen, X.; Pu, J.; Wang, Y.; Yuan, D. Study on the extraction of lithium, rubidium, and caesium from lithium mica using the sulphate method. Nonferr. Met. Smelt. Sect. 2015, 31–33. [Google Scholar] [CrossRef]
- Yan, Q.X.; Li, X.H.; Wang, Z.X.; Wang, J.X.; Guo, H.J.; Hu, Q.Y.; Peng, W.J.; Wu, X.F. Extraction of lithium from lepidolite using chlorination roasting–water leaching process. Trans. Nonferr. Met. Soc. China 2012, 22, 1753–1759. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110–111, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Wu, K. Extraction of lithium, rubidium and cesium from lithium porcelain stone. Hydrometallurgy 2020, 191, 105233. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, L.; Ouyang, D.; Hu, Y.; Zhong, H.; He, Z. Process optimization on the extraction of rubidium from rubidium-bearing biotite. Miner. Eng. 2019, 137, 87–93. [Google Scholar] [CrossRef]
- Lin, G.; Li, J.; Zeng, B.; Wang, W.; Li, C.; Zhang, L. Leaching of rubidium from biotite ore by chlorination roasting and ultrasonic enhancement. Arab. J. Chem. 2022, 15, 104275. [Google Scholar] [CrossRef]
- Dai, W.; Fang, Y.; Yu, L.; Zhao, G.; Yan, X. Rubidium ion capture with composite adsorbent PMA@HKUST-1. J. Taiwan Inst. Chem. Eng. 2018, 84, 222–228. [Google Scholar] [CrossRef]
- Sharma, S.K.; Truong, D.Q.; Guo, J.; An, A.K.; Naidu, G.; Deka, B.J. Recovery of rubidium from brine sources utilizing diverse separation technologies. Desalination 2023, 556, 116578. [Google Scholar] [CrossRef]
- Lv, Y.; Xing, P.; Ma, B.; Liu, Y.; Wang, C.; Zhang, W.; Chen, Y. Efficient Extraction of Lithium and Rubidium from Polylithionite via Alkaline Leaching Combined with Solvent Extraction and Precipitation. ACS Sustain. Chem. Eng. 2020, 8, 14462–14470. [Google Scholar] [CrossRef]
- Li, Z.; Pranolo, Y.; Zhu, Z.; Cheng, C.Y. Solvent extraction of cesium and rubidium from brine solutions using 4-tert-butyl-2-(α-methylbenzyl)-phenol. Hydrometallurgy 2017, 171, 1–7. [Google Scholar] [CrossRef]
- Liu, S.M.; Liu, H.H.; Huang, Y.J.; Yang, W.J. Solvent extraction of rubidium and cesium from salt lake brine with t-BAMBP-kerosene solution. Trans. Nonferr. Met. Soc. China 2015, 25, 329–334. [Google Scholar] [CrossRef]
- Chen, W.-S.; Lee, C.-H.; Chung, Y.-F.; Tien, K.-W.; Chen, Y.-J.; Chen, Y.-A. Recovery of Rubidium and Cesium Resources from Brine of Desalination through t-BAMBP Extraction. Metals 2020, 10, 607. [Google Scholar] [CrossRef]
- Pang, D.; Fu, Z.; Zhang, Z.; Zhang, Y.; Ma, Y.; Wang, J.; Zhao, D. Extraction of Cesium and Rubidium with 4-tert-butyl-2-(α-methylbenzyl) phenol from Salt Lake Brine. Salt Lake Res. 2019, 27, 111–119. [Google Scholar]
- Lv, Y.; Ma, B.; Liu, Y.; Wang, C.; Zhang, W.; Chen, Y. Selective extraction of cesium from high concentration rubidium chloride leach liquor of lepidolite. Desalination 2022, 530, 115673. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, M.; Xu, Y.; Yu, J.; Sun, H.; Kang, M. Separation of manganese from waste liquid of mica extraction and the mechanism of t-BAMBP extraction. Nonferr. Met. Eng. 2022, 12, 83–90. [Google Scholar]
- Zhang, X.; Qin, Z.; Aldahri, T.; Rohani, S.; Ren, S.; Liu, W. Separation and recovery of cesium sulfate from the leach solution obtained in the sulfuric acid baking process of lepidolite concentrate. Hydrometallurgy 2021, 199, 105537. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, D.; Zhang, Y.; Peng, X.; Xie, S.; Li, L.; Niu, Y.; Wang, Y.; Song, F. Cesium extraction from an alkali-free solution using a 4-tert-butyl-2-(α-methylbenzyl) phenol–di-(2-ethylhexyl) phosphoric acid synergistic system: A conceptual process flowsheet for separating cesium salt from salt-lake brine. Hydrometallurgy 2024, 228, 106353. [Google Scholar] [CrossRef]
- Pang, D.; Zhang, Z.; Zhou, Y.; Fu, Z.; Li, Q.; Zhang, Y.; Wang, G.; Jing, Z. The process and mechanism for cesium and rubidium extraction with saponified 4-tert-butyl-2-(a-methylbenzyl) phenol. Chin. J. Chem. Eng. 2022, 46, 31–39. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Q.; Shen, X. Complexation and extraction investigation of rubidium ion by calixcrown-C2mimNTf2 system. Sep. Purif. Technol. 2019, 227, 115704. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Wang, Z.; Liu, X.; Zhang, M.; Fan, J.; Zhou, Z.; Ren, Z. Extraction of Rb(I) Ions from Aqueous Solution Using Novel Imprinting Materials. Ind. Eng. Chem. Res. 2019, 58, 5269–5279. [Google Scholar] [CrossRef]
- Hashemi, B.; Shamsipur, M. Synthesis of novel ion-imprinted polymeric nanoparticles based on dibenzo-21-crown-7 for the selective pre-concentration and recognition of rubidium ions. J. Sep. Sci. 2015, 38, 4248–4254. [Google Scholar] [CrossRef]
- Xu, J.; Pu, Z.; Xu, X.; Wang, Y.; Yang, D.; Zhang, T.; Qiu, F. Simultaneous adsorption of Li(I) and Rb(I) by dual crown ethers modified magnetic ion imprinting polymers. Appl. Organomet. Chem. 2019, 33, e4778. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, S.; Ma, N.; Dai, W. Well-designed a novel phosphomolybdic-acid@PCN-224 composite with efficient simultaneously capture towards rubidium and cesium ions. Polyhedron 2021, 207, 115402. [Google Scholar] [CrossRef]
- Qian, Y.; Ding, D.; Li, K.; Fang, D.; Wang, Y.; Hu, Y.; Ye, X.; Liu, H.; Wu, Z.; Li, J. Extraction of rubidium and cesium from a leach solution of lepidolite with biomass carbon adsorbents. Hydrometallurgy 2022, 208, 105802. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, B.; Liu, Y.; Wang, C.; Chen, Y. A novel adsorbent potassium magnesium ferrocyanide for selective separation and extraction of rubidium and cesium from ultra-high salt solutions. J. Water Process Eng. 2023, 55, 104225. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhang, Q.; Han, W.; Zhang, H.; Ye, X. High-Efficiency Selective Adsorption of Rubidium and Cesium from Simulated Brine Using a Magnesium Ammonium Phosphate Adsorbent. Separations 2024, 11, 277. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Li, K.; Wang, C.; Fang, D.; Han, W.; Lu, M.; Ye, X.; Zhang, H.; Liu, H.; et al. Efficient Selective Adsorption of Rubidium and Cesium from Practical Brine Using a Metal-Organic Framework-Based Magnetic Adsorbent. Langmuir 2024, 40, 9688–9701. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, T.; He, L.; Zhao, Z.; Sun, F.; Xu, W.; Liu, D. Electrochemical extraction of rubidium from salt lake by using cupric ferrocyanide based on potassium shuttle. Desalination 2023, 549, 116331. [Google Scholar] [CrossRef]
- Nie, W.; Wen, S.; Xian, Y.; Li, Y.; Han, G.; Jiang, Y. Leaching rubidium from a low-grade rubidium-bearing aluminosilicate ore. J. Mater. Res. Technol-JMRT 2021, 13, 1546–1554. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Song, Y.; Li, W.; Liu, S.; Liu, Y. Mineralogical Characteristics of Pegmatite Tailings and Beneficiation Assessment of Pollucite in Recovering Cesium. Minerals 2022, 12, 541. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Y.; Gao, Z.; Liu, H. Research Progress on Rubidium and Cesium Separation and Extraction Technology. Miner. Prot. Util. 2013, 54–58. [Google Scholar] [CrossRef]
- Liu, C.; Guo, L.; Tong, X. Hydrothermal decomposition of K-feldspar with Cs enrichment into pollucite in Cs-included alkaline solution. Adv. Powder Technol. 2022, 33, 103591. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, B.; Wang, C.; Chen, Y.; Zhang, W. Comprehensive utilization of complex rubidium ore resources: Mineral dissociation and selective leaching of rubidium and potassium. Int. J. Miner. Metall. Mater. 2023, 30, 857–867. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Ma, Y.; Li, Y.; Li, R. Study on the extraction of rubidium and caesium from rubidium-caesium feldspar. China Sci. Technol. J. Database Ind. A 2022, 4, 81–84. [Google Scholar]
- Xing, P.; Wang, C.; Chen, Y.; Ma, B. Rubidium extraction from mineral and brine resources: A review. Hydrometallurgy 2021, 203, 105644. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Zhao, L.; Cui, H.; Wei, X.; Shang, Y. Extraction of valuable metals rubidium, lithium, and potassium from tungsten tailings using a chlorination roasting-water leaching process. Hydrometallurgy 2019, 38, 267–270. [Google Scholar]
- Gao, X. Research on the comprehensive recovery of rubidium and tin from fluorite flotation tailings. Min. Metall. Eng. 2021, 41, 71–75. [Google Scholar]
- Liu, H.; Tong, L.; Su, M.; Chen, D.; Song, G.; Zhou, Y. The latest research trends in the removal of cesium from radioactive wastewater: A review based on data-driven and visual analysis. Sci. Total Environ. 2023, 869, 161664. [Google Scholar] [CrossRef]
- Luo, M.; Liu, C.; Jiang, Y.; Xue, J.; Li, P.; Yu, J. Green recovery of potassium and aluminum elements from alunite tailings using gradient leaching process. J. Clean. Prod. 2017, 168, 1080–1090. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Liu, H.; Cao, Y.; Zhang, B.; Wang, H.; Zhao, H. Research on the extraction of rubidium from tungsten tailings. Nonferr. Met. Smelt. Sect. 2018, 17–20. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, X.; Zhang, X.; Wu, Y. Experimental study on leaching of rubidium and potassium from rubidium-potassium tailings. Hydrometallurgy 2019, 38, 347–351. [Google Scholar]
- Zhou, L.; Yuan, T.; Li, R.; Zhong, Y.; Lei, X. Extraction of rubidium from kaolin clay waste: Process study. Hydrometallurgy 2015, 158, 61–67. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, C.; He, Y.; Li, M.; Liu, G.; Guo, Y.; Ji, D.; Deng, T. Novel layered iron antimony thiostannate adsorbent of K1.61Fe0.04Sb0.03Sn3.1S7 for cesium green recovery from geothermal water. J. Clean. Prod. 2022, 347, 131332. [Google Scholar] [CrossRef]
- Mercado, S.; Hurtado, R. Potash extraction from cerro prieto geothermal brine. Geothermics 1992, 21, 759–764. [Google Scholar] [CrossRef]
- Sugita, H.; Matsunaga, I.; Yamaguchi, T.; Kato, K.; Ueda, A. Silica removal performance of seed from geothermal fluids. Geothermics 2003, 32, 171–185. [Google Scholar] [CrossRef]
- Yang, W.J.; Liu, S.M.; Li, Y.J.; Huang, Y.J.; Luo, X.S. Process Analysis of Rb+ and Cs+ Adsorption from Salt Lake Brine by Ammonium Molybdophosphate Composite Material. Adv. Mater. Res. 2013, 785–786, 812–816. [Google Scholar] [CrossRef]
- Vitolo, S.; Cialdella, M.L. Silica separation from reinjection brines at monte amiata geothermal plants, Italy. Geothermics 1994, 23, 257–266. [Google Scholar] [CrossRef]
- Dozol, J.F.; Asfari, Z.; Arnaud-Neu, F.; Vicens, J.; Thuéry, P. Extraction of rubidium and caesium from strongly alkaline media. Radiochim. Acta 2004, 92, 175–182. [Google Scholar] [CrossRef]
- Lehto, J.; Koivula, R.; Leinonen, H.; Tusa, E.; Harjula, R. Removal of Radionuclides from Fukushima Daiichi Waste Effluents. Sep. Purif. Rev. 2019, 48, 122–142. [Google Scholar] [CrossRef]
- Du, Z.; Jia, M.; Men, J. Removal of cesium from aqueous solution using PAN-based ferrocyanide composite spheres: Adsorption on a fixed-bed column. Appl. Mech. Mater. 2014, 496–500, 259–263. [Google Scholar] [CrossRef]
- Sheha, R.R. Synthesis and characterization of magnetic hexacyanoferrate (II) polymeric nanocomposite for separation of cesium from radioactive waste solutions. J. Colloid Interface Sci. 2012, 388, 21–30. [Google Scholar] [CrossRef]
- Naidu, G.; Nur, T.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Selective sorption of rubidium by potassium cobalt hexacyanoferrate. Sep. Purif. Technol. 2016, 163, 238–246. [Google Scholar] [CrossRef]
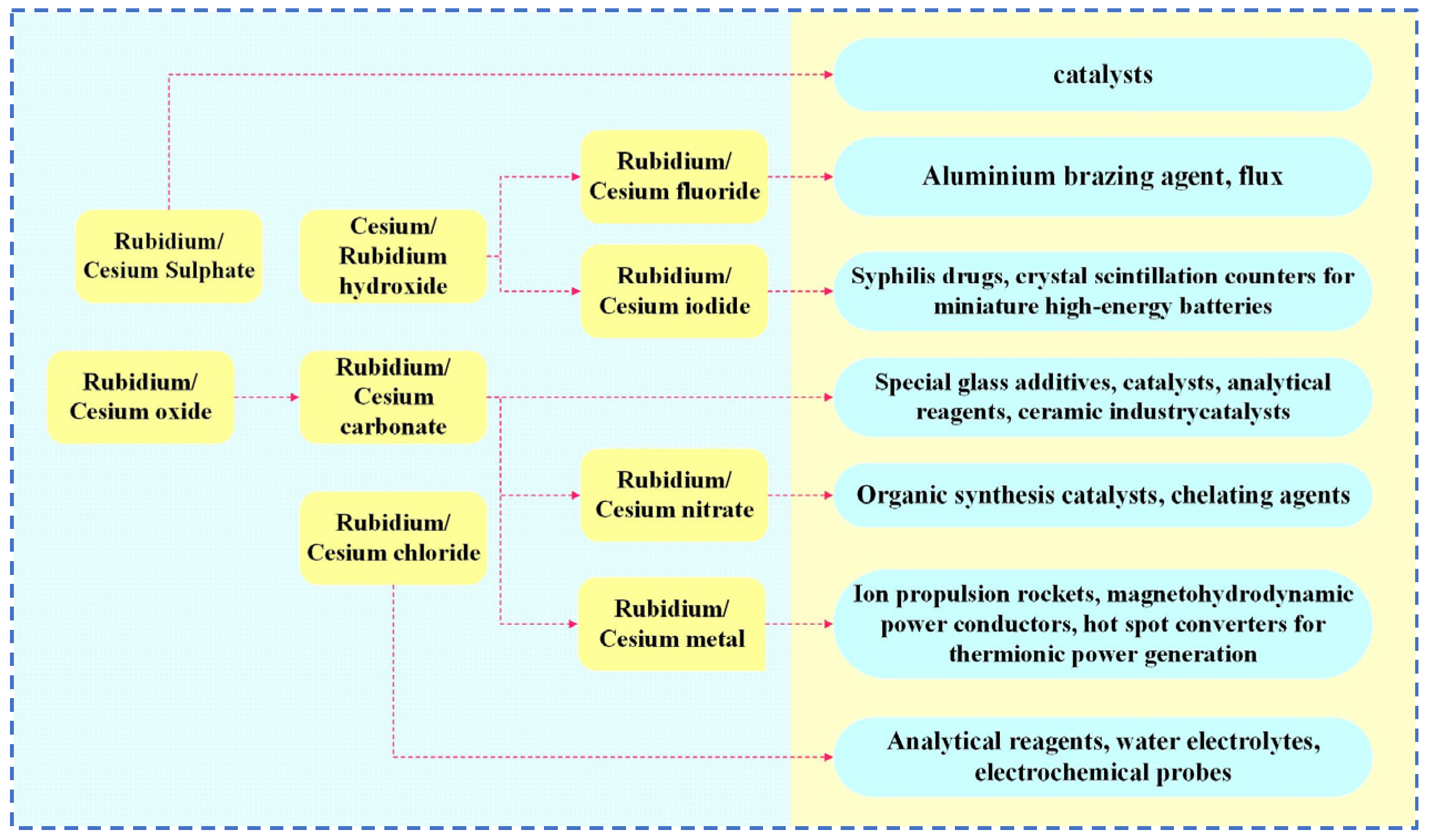
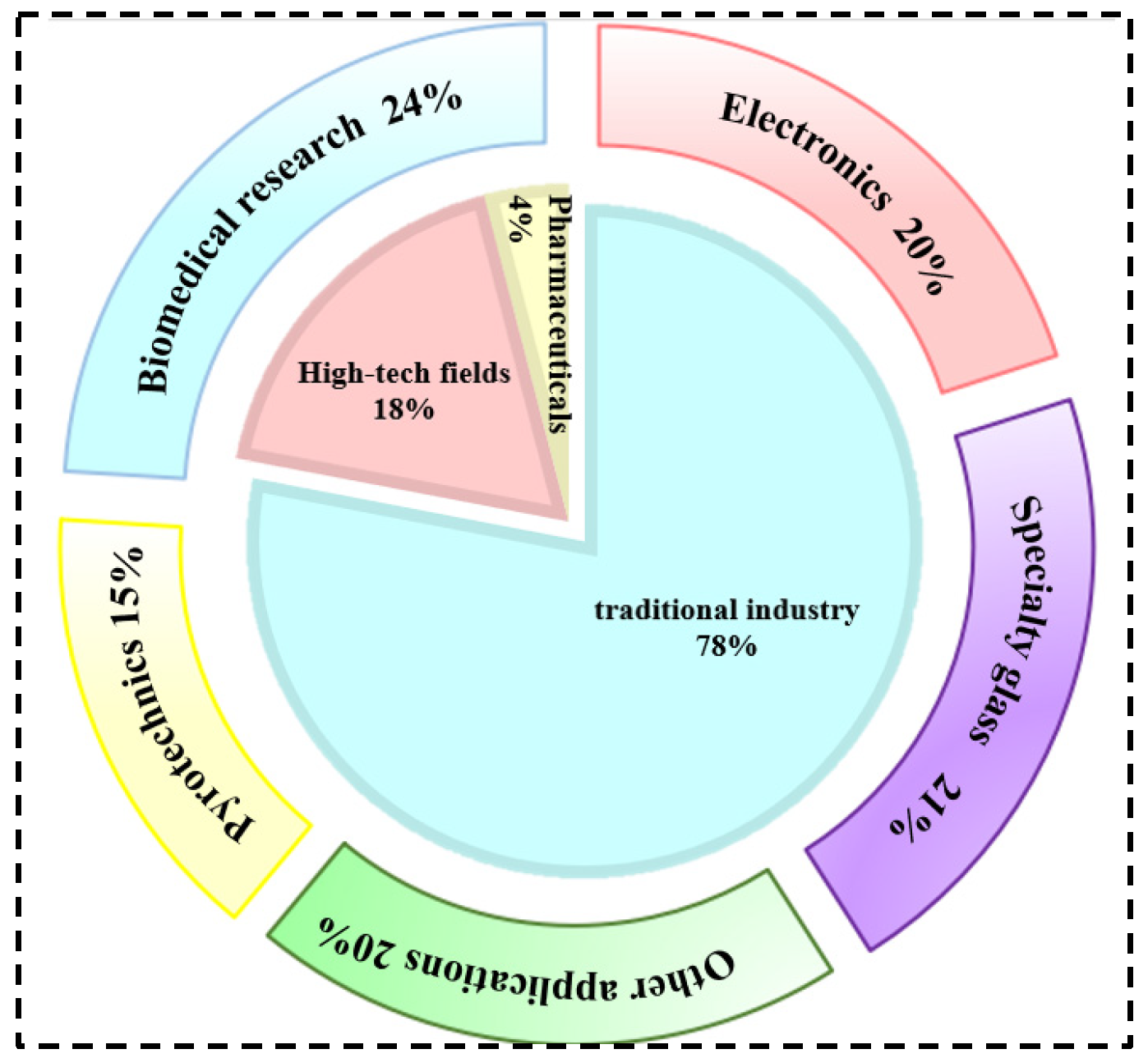
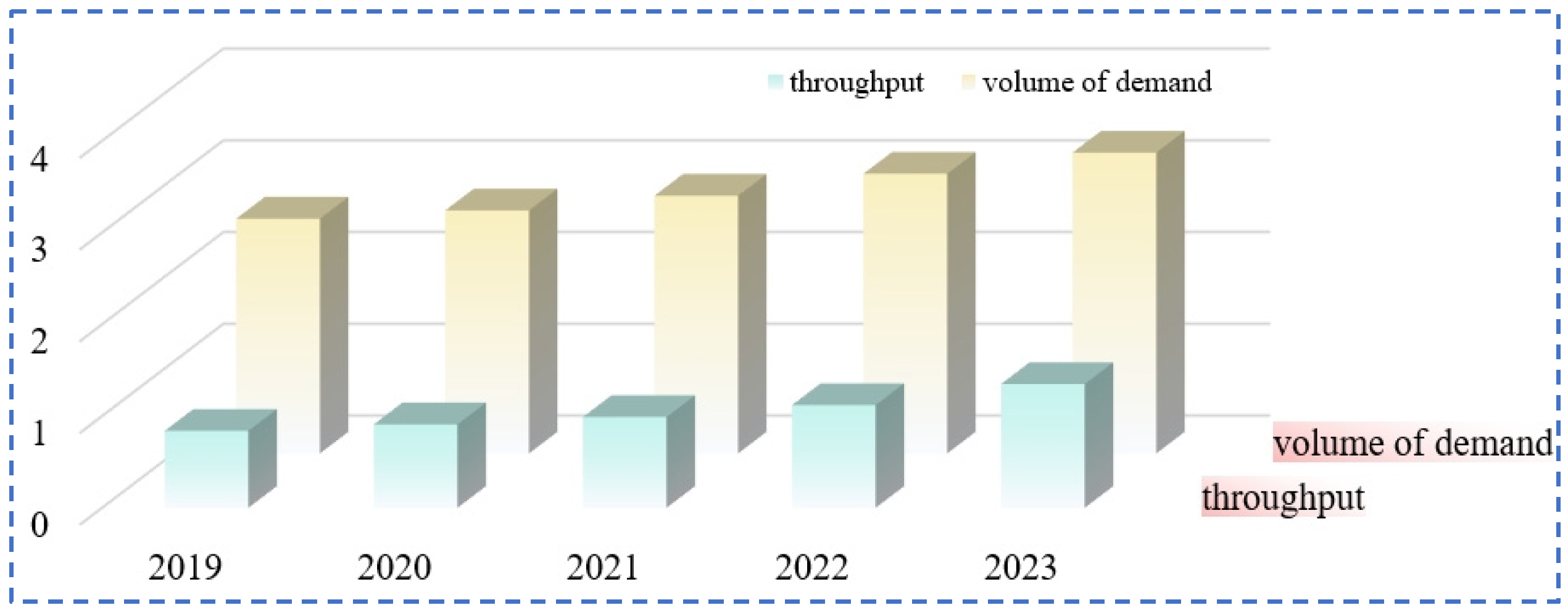

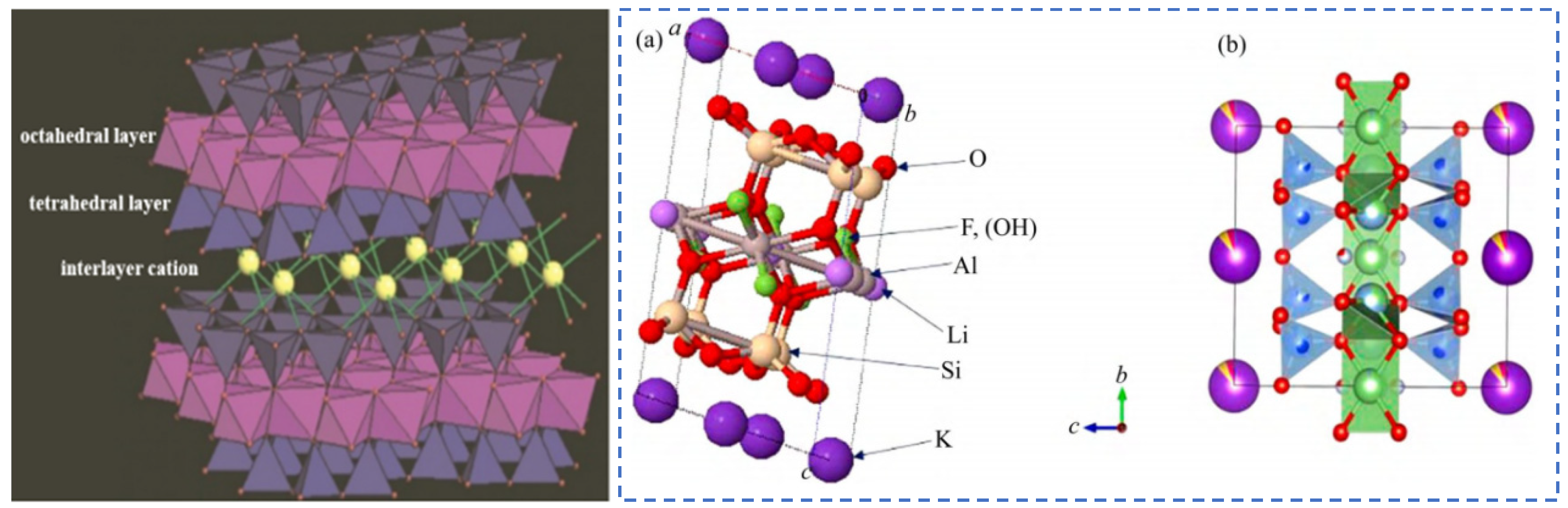


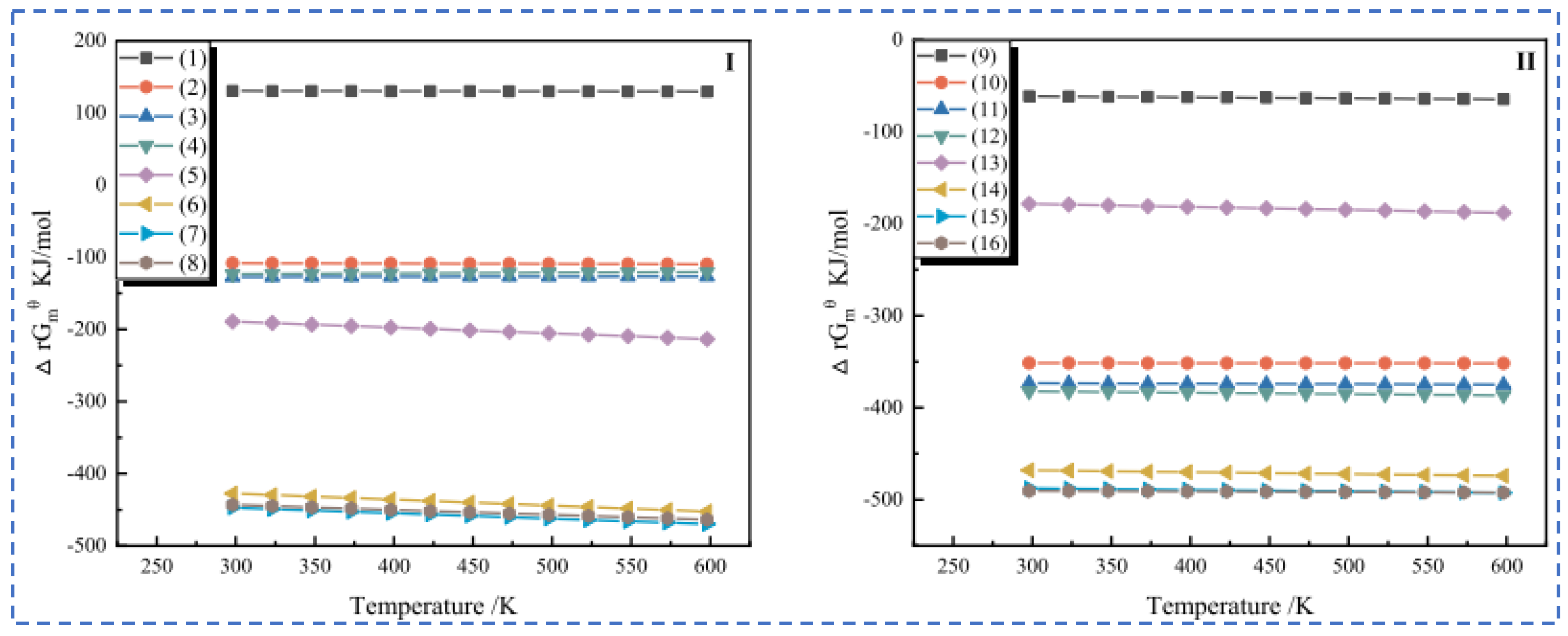
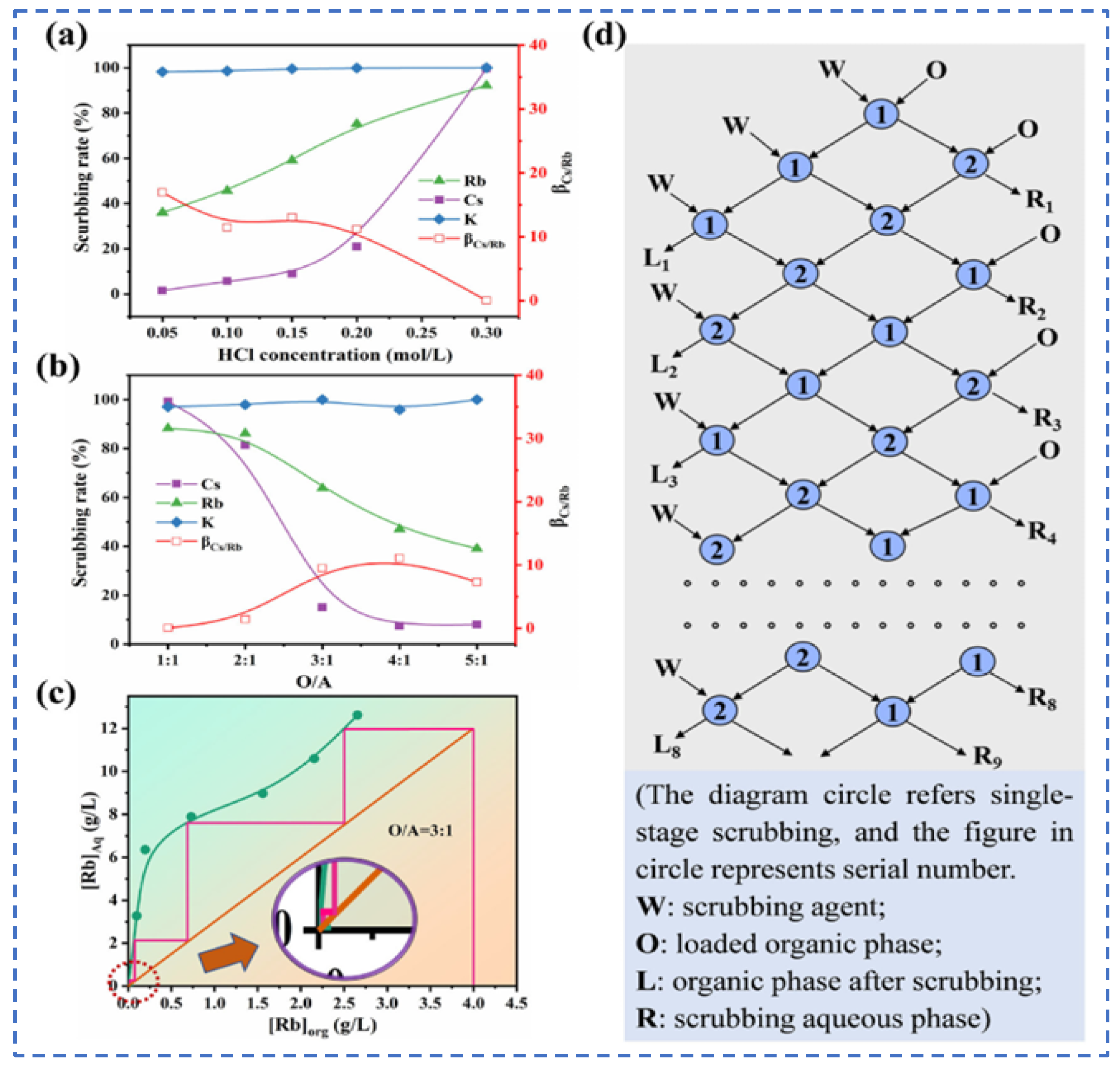

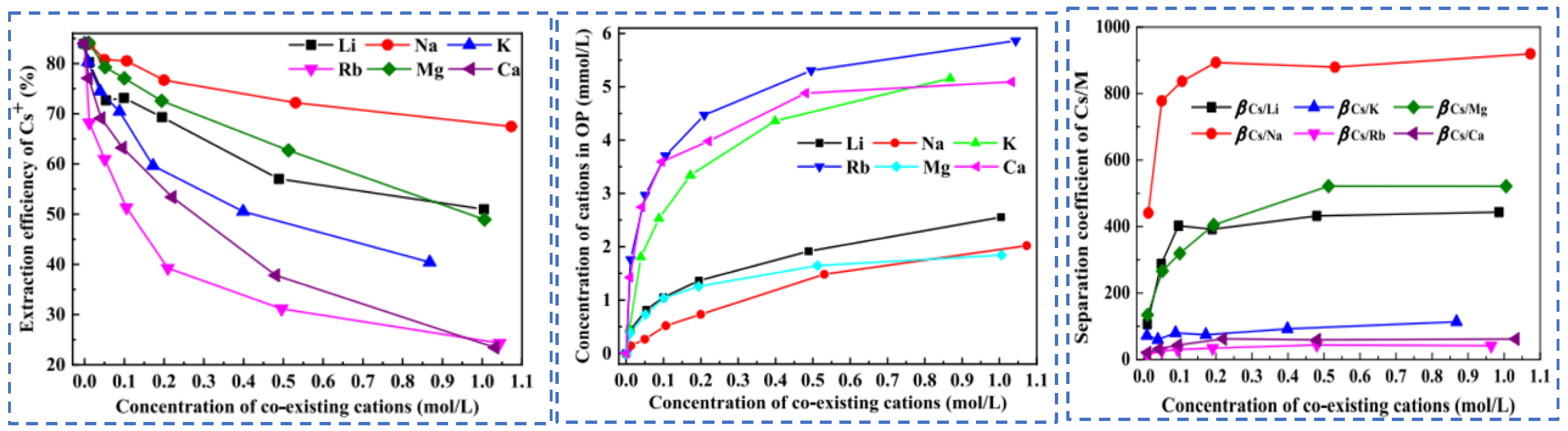

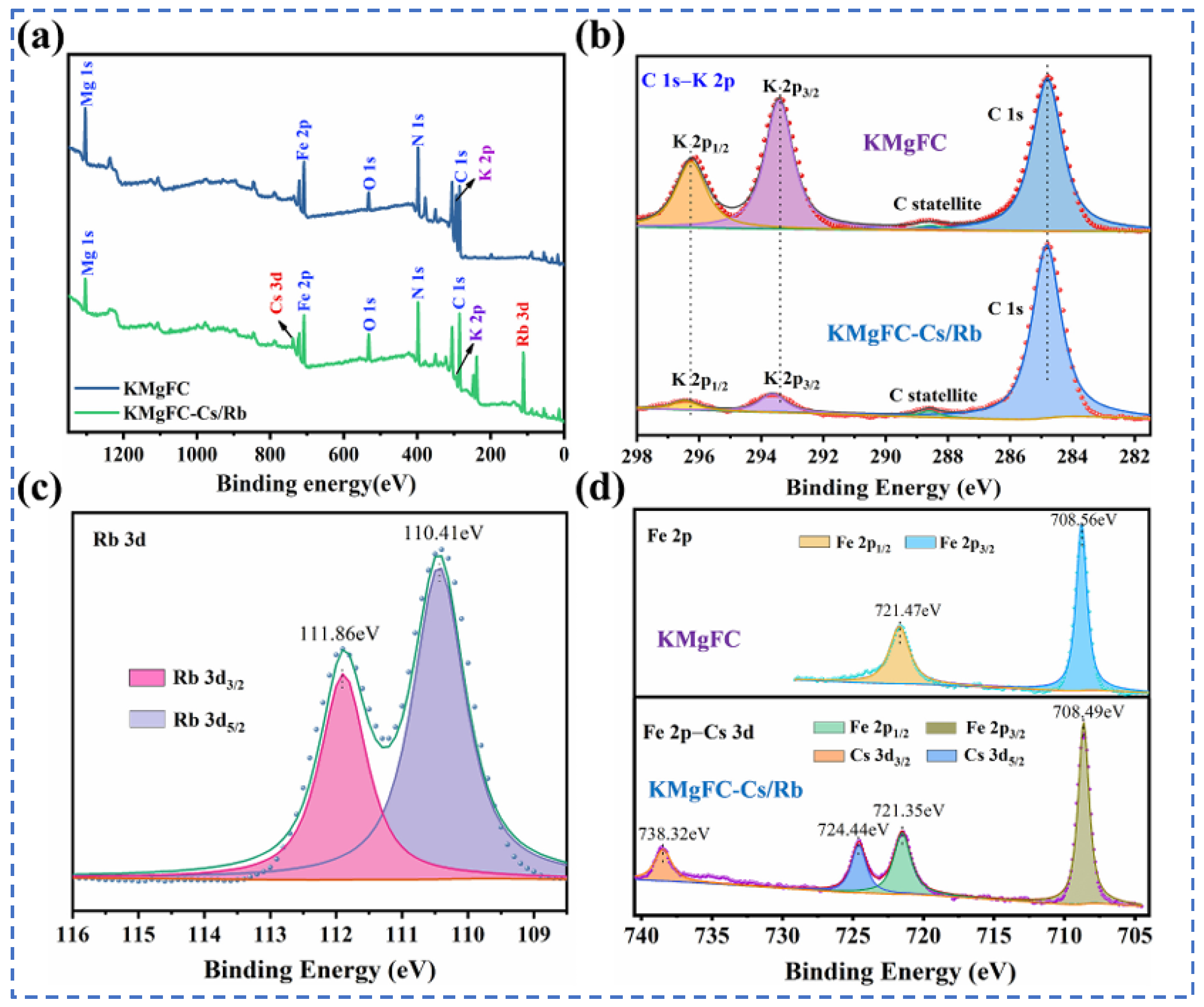

| Characterization | Purpose |
|---|---|
| Ultrafine jump frequencies of electrons in the outer layers of atoms | Atomic clocks, frequency standards, satellite navigation, aerospace measurement and control |
| Photosensitivity | Night vision equipment, optoelectronic equipment |
| Easily ionized | Magnetic fluid power generation, ion rockets, fuel cells |
| Biochemical | Sedatives and hypnotics for the treatment of epilepsy |
| Absorption | Suction agent for vacuum tubes |
| Radioactivity | Cs137 can be used as a radioactive source to treat cancer |
| Quantum effect | Quantum computing |
| Deposit Type | Main Minerals | Genesis | Typical Deposits |
|---|---|---|---|
| Pegmatite | Cesium garnet, lithium mica, potassium feldspar, cesium beryl, borofluoropotassium, cesium boron clonorite, cesium manganese stellar feldspar | Crystallization of residual magma of the aerogenic-pegmatitic phase | Tanco deposit in Canada, Bikita deposit in Zimbabwe, Sinclair deposit in Australia. |
| Granite type | Potassium feldspar, skarn, (iron) lithium mica, black mica, white mica and so on | Residual magma crystallization, hydrothermal crystallization or accounting | Inner Mongolia Lime Kiln, Zhaojinggou, Hunan Zhengchong, Guangdong Tiantang Mountain, Gansu Guobao Mountain (China) |
| Salt Lake | Halite, potash, carnallite. | Evaporation deposition of concentrated brines | Salton Sea Salt Lake Brine, Qaidam Basin, U.S.A. |
| Brine and hot spring type | Silica waffle, granular opal, colloidal opal, hydromica, chalcedony square quartz. | Concentration or deposition | Tibet Hitching Post, Tibet Gulu (China) |
| Mineral Name | Chemical Formula | Content of Rb2O (%) | Content of Cs2O (%) |
|---|---|---|---|
| Cesium garnet | Cs(A1Si2O6) nH2O | 0.3–1.4 | 23.5–36.5 |
| potassium feldspar | K2O·A12O3·6SiO2 | 3 | - |
| Lithium Mica | K(Li,A1)3(Si,A1)O10(OH,F)2 | 3.75 | 0.4–1 |
| Precipitant | Precipitation Form | Characteristics |
|---|---|---|
| Silicomolybdenum (tungsten) acid | Rb4H4[Si(Mo2O7)6], Cs8[Si(Mo2O7)6] | High recovery of Rb, Cs but precipitated compounds are unstable and prone to decomposition |
| Potassium iodobismuthate | M3B2I9 | Recovery of Rb, Cs product purity of nearly 100 per cent, the process is complex and requires secondary purification |
| Chloroplatinic acid | M2(PCl6) | Rb, Cs recovery is high and expensive |
| Stannum tetrachloride | M2SnCl6 | Simple process, but time-consuming and costly |
| Antimony trichloride | 3MC1·2SbCl3 | The process is easy to operate but the cost of chemicals is high |
| Iodine chloride | MICl2 | Recovered Rb, Cs products are nearly 100 per cent pure, but the crystallization process needs to be repeated |
| Aluminum sulfate | MAI(SO4)2·12H2O | No pollution to the environment, but the process is complex and difficult to operate |
| Geothermal Brine | Ion/Metal Concentration (mg/L) | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Rb | Cs | Li | Na | Mg | K | ||
| Salton Sea (USA) | 170 | 20 | 194 | 53,000 | 33 | 16,700 | [79] |
| Brawley (USA) | 67 | 19 | 219 | 47,600 | 114 | 126,000 | [80] |
| Wairakei (New Zealand) | 2.90 | 2.50 | 13.2 | 1250 | 0.04 | 210 | [81] |
| Salak (Indonesia) | 5 | 4.50 | 17 | 5000 | 0.10 | 990 | [82] |
| Cerro Prieto (Mexico) | 11 | 39 | 27 | 8300 | 0.50 | 2210 | [83] |
| Hvergerdi (Iceland) | 0.04 | <0.02 | 0.30 | 212 | - | 27 | [84] |
| Mote Amiata (Italy) | 2.10 | 0.70 | 21.90 | 1977 | <0.50 | 558 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, H.; Yu, M.; Mubula, Y.; Zeng, L.; Xu, K.; Zhu, Z.; He, G. Extraction of Rubidium and Cesium from a Variety of Resources: A Review. Materials 2025, 18, 3378. https://doi.org/10.3390/ma18143378
Niu H, Yu M, Mubula Y, Zeng L, Xu K, Zhu Z, He G. Extraction of Rubidium and Cesium from a Variety of Resources: A Review. Materials. 2025; 18(14):3378. https://doi.org/10.3390/ma18143378
Chicago/Turabian StyleNiu, Heyue, Mingming Yu, Yusufujiang Mubula, Ling Zeng, Kun Xu, Zhehan Zhu, and Guichun He. 2025. "Extraction of Rubidium and Cesium from a Variety of Resources: A Review" Materials 18, no. 14: 3378. https://doi.org/10.3390/ma18143378
APA StyleNiu, H., Yu, M., Mubula, Y., Zeng, L., Xu, K., Zhu, Z., & He, G. (2025). Extraction of Rubidium and Cesium from a Variety of Resources: A Review. Materials, 18(14), 3378. https://doi.org/10.3390/ma18143378







