Multiphase NiCoFe-Based LDH for Electrocatalytic Sulfion Oxidation Reaction Assisting Efficient Hydrogen Production
Abstract
1. Introduction
2. Experimental Section
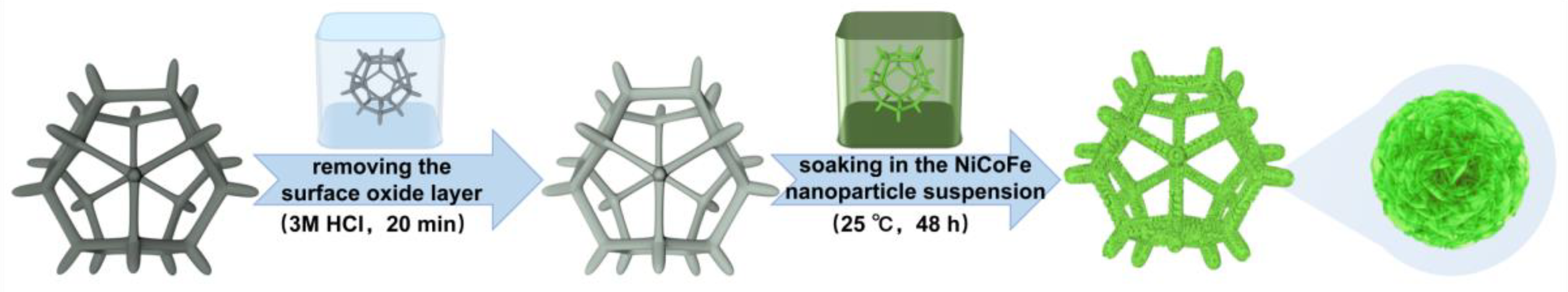
2.1. Materials Preparation
2.2. Physicochemical Characterization
2.3. Electrochemical Measurements
2.4. Theoretical Calculation Method
3. Results and Discussion
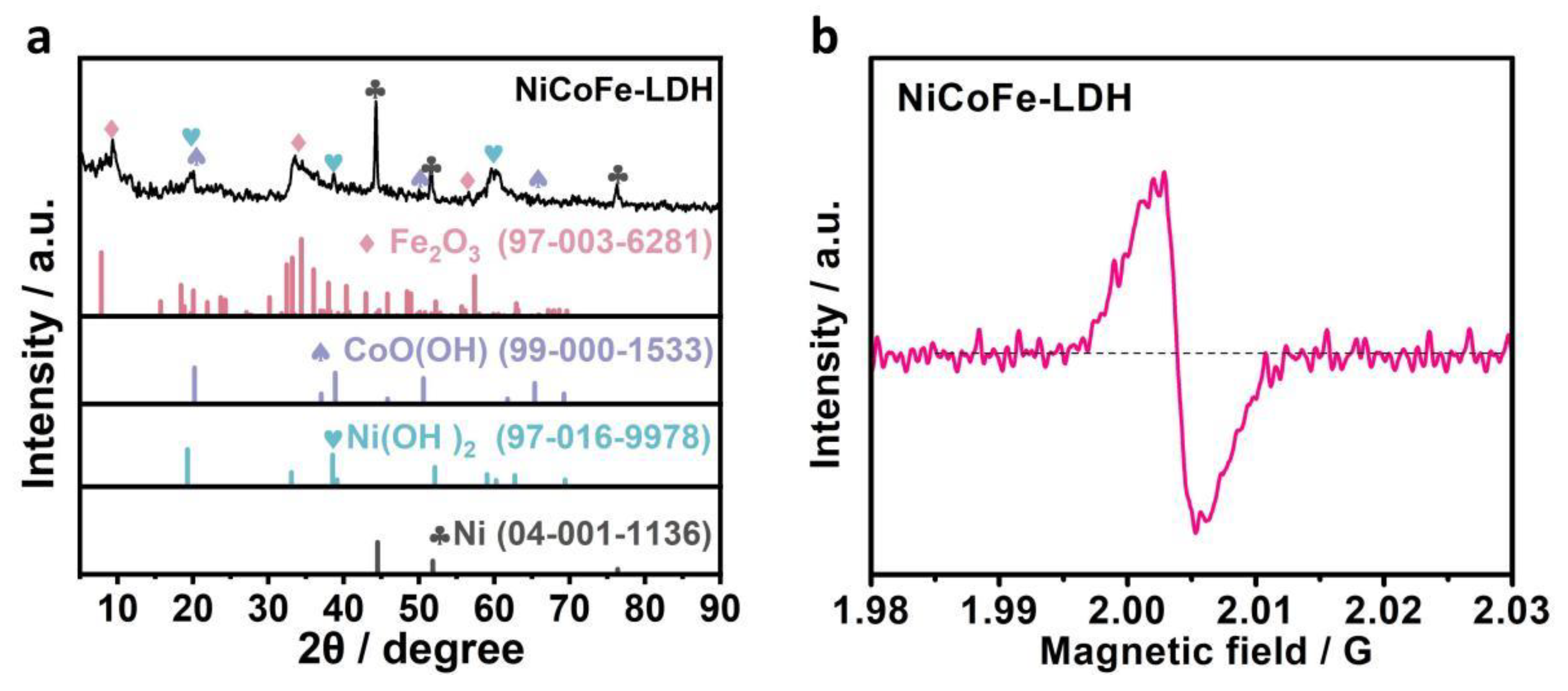
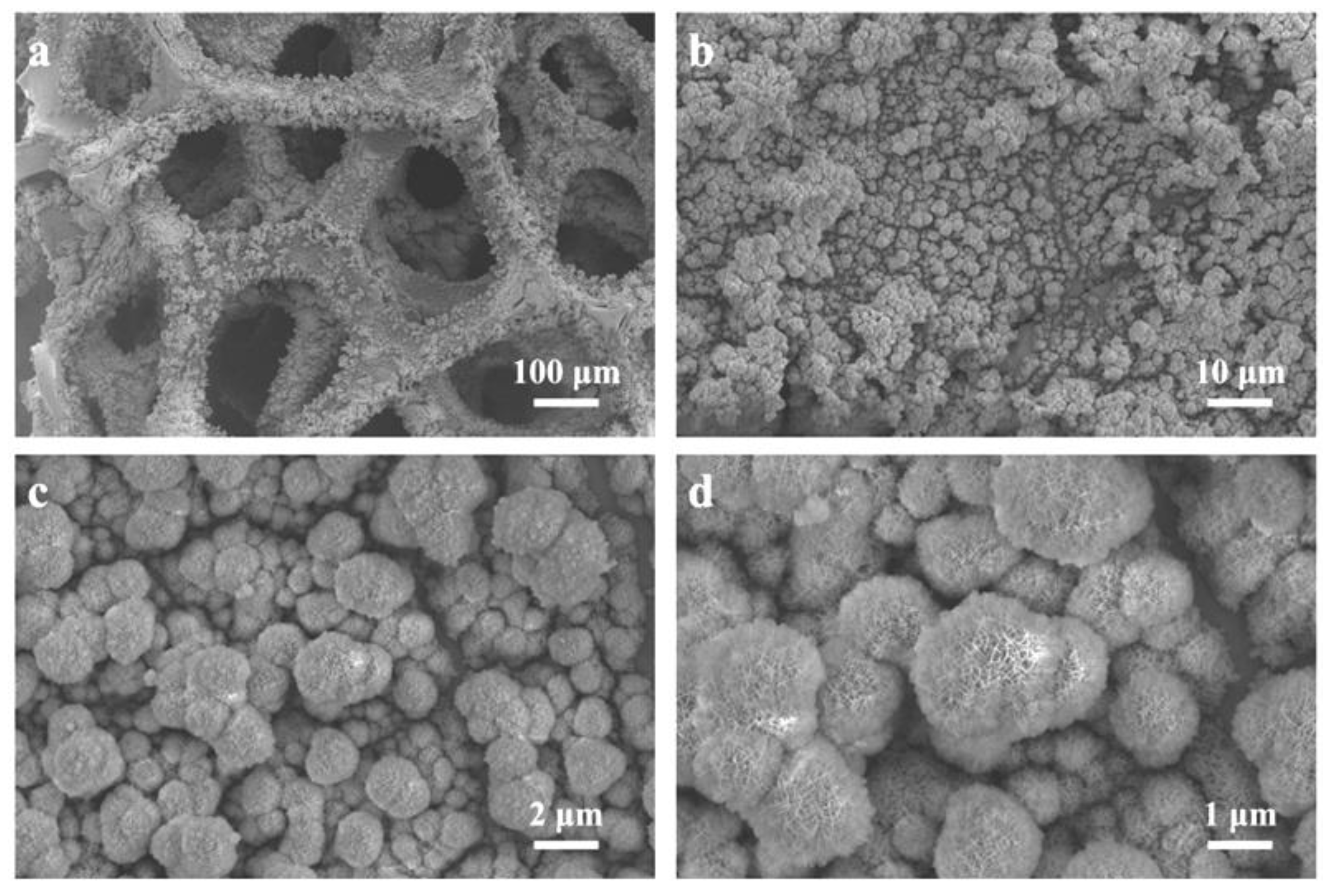
3.1. Microstructure Analysis
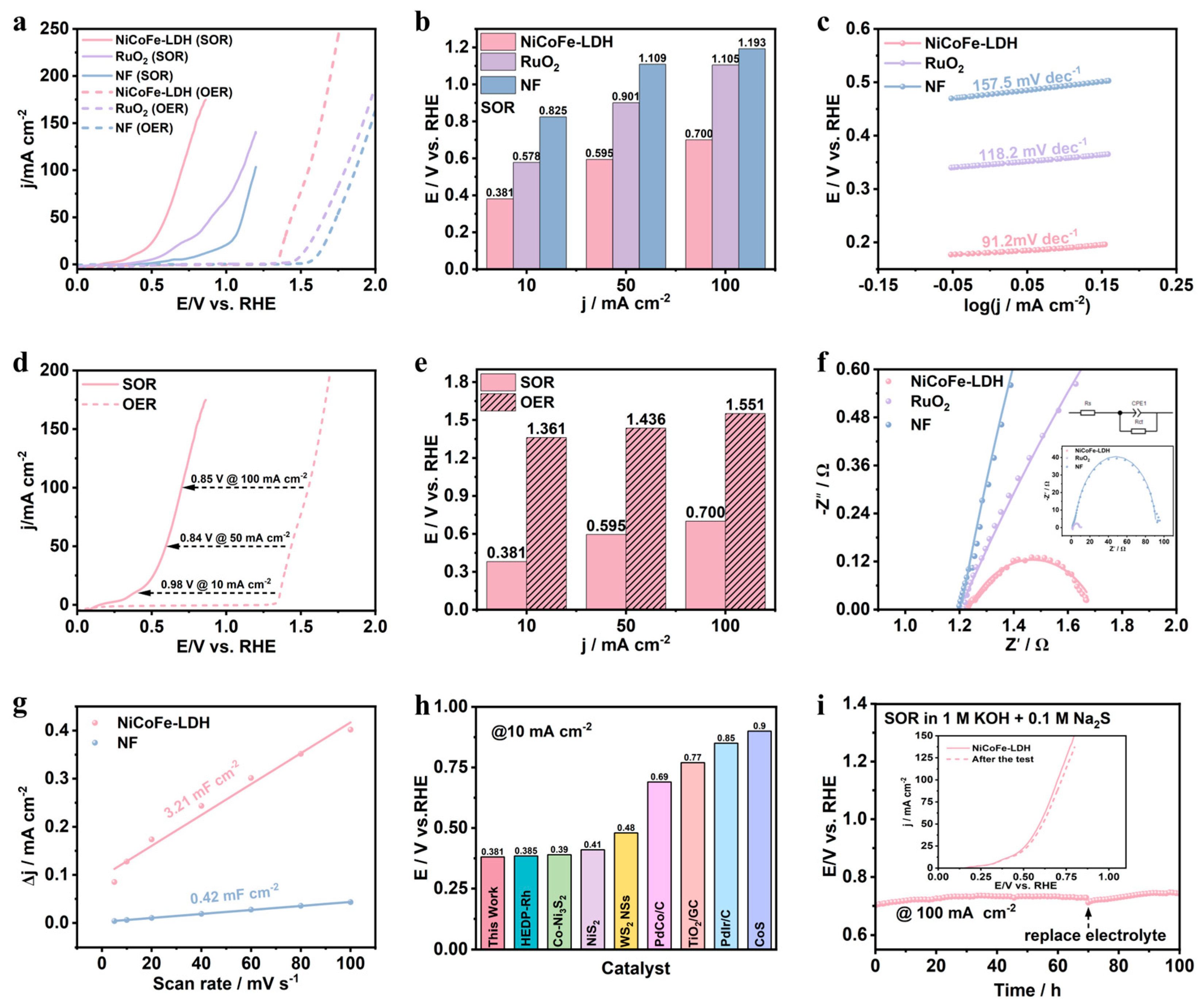
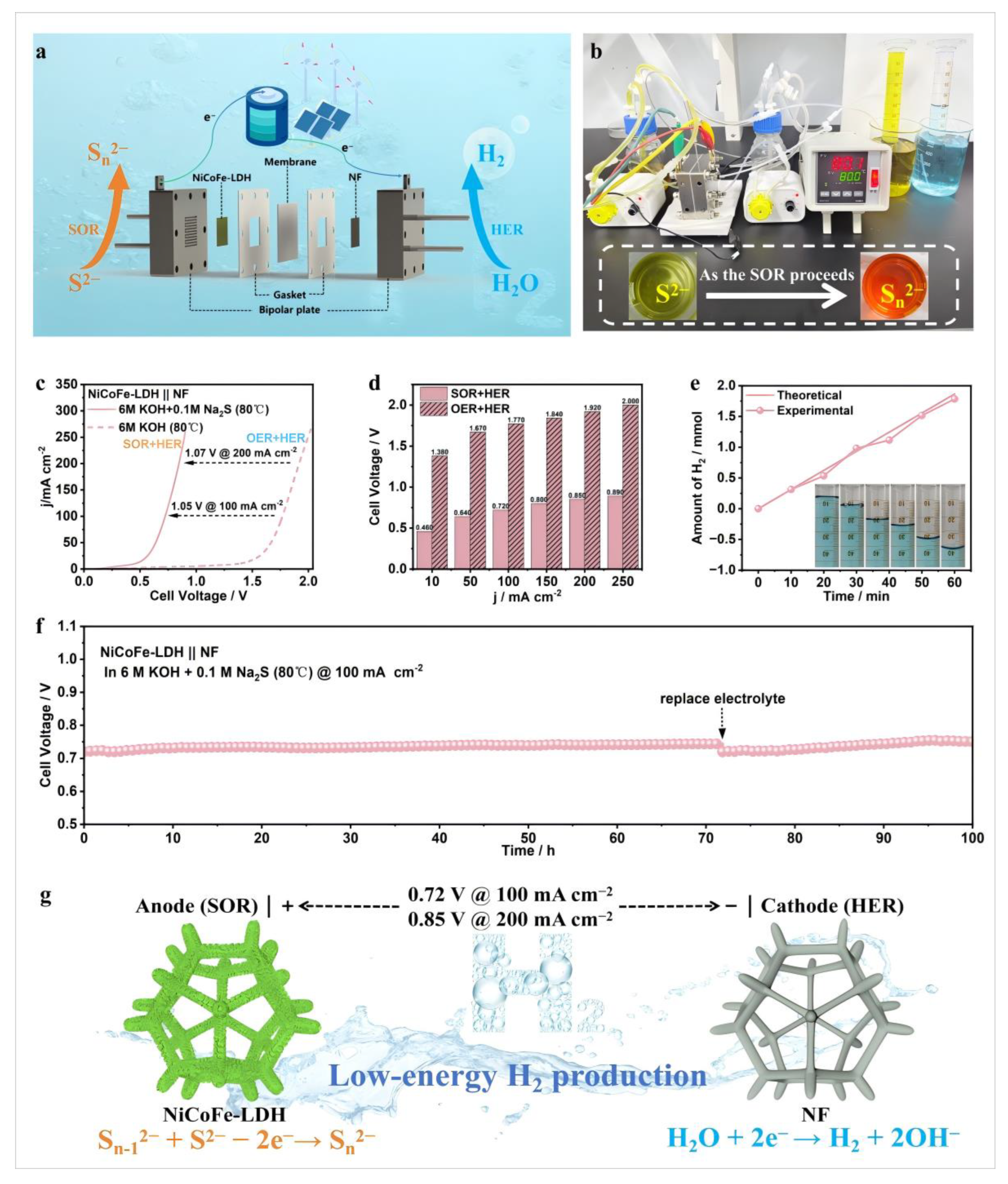
3.2. Electrocatalytic Activity
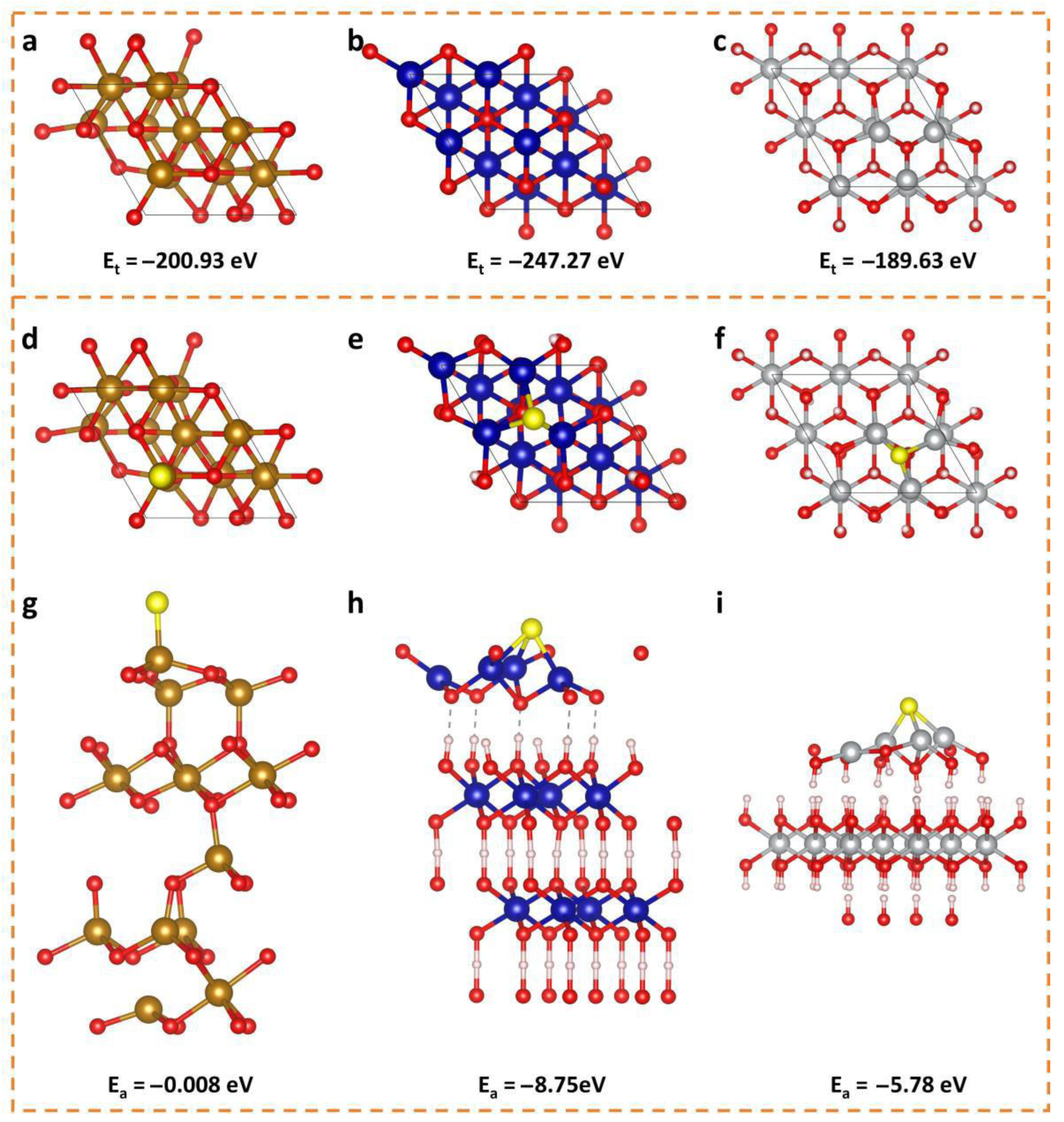
3.3. Theoretical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, Y.; Peng, M.; Lan, J.; Jiang, K.; Chen, D.; Tan, Y. Active-site engineering in dealloyed nanoporous catalysts for electrocatalytic water splitting. J. Mater. Chem. A 2022, 11, 495–511. [Google Scholar] [CrossRef]
- Zhou, Q.; Hao, Q.; Li, Y.; Yu, J.; Xu, C.; Liu, H.; Yan, S. Free-standing trimodal porous NiZn intermetallic and Ni heterojunction as highly efficient hydrogen evolution electrocatalyst in the alkaline electrolyte. Nano Energy 2021, 89, 106402. [Google Scholar] [CrossRef]
- Bu, X.; Liang, X.; Bu, Y.; Quan, Q.; Meng, Y.; Lai, Z.; Wang, W.; Liu, C.; Lu, J.; Wu, C.-M.L.; et al. NiMo@C3N5 heterostructures with multiple electronic transmission channels for highly efficient hydrogen evolution from alkaline electrolytes and seawater. Chem. Eng. J. 2022, 438, 135379. [Google Scholar] [CrossRef]
- Karthikeyan, S.C.; Ramakrishnan, S.; Prabhakaran, S.; Subramaniam, M.R.; Mamlouk, M.; Kim, D.H.; Yoo, D.J. Low-Cost Self-Reconstructed High Entropy Oxide as an Ultra-Durable OER Electrocatalyst for Anion Exchange Membrane Water Electrolyzer. Small 2024, 20, 2402241. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, H.; Ji, L.; Zhang, J.; Wang, C.; Guan, R. Durable Hierarchical Porous PtCo Nanoparticle Films for Hydrogen Evolution Reaction in Simulated Seawater. ACS Appl. Nano Mater. 2025, 8, 8176–8186. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Cheng, H.; Ma, J. Alkaline Hydrogen Evolution Reaction Electrocatalysts for Anion Exchange Membrane Water Electrolyzers: Progress and Perspective. JACS Au 2024, 4, 4639–4654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rong, J.; Fan, Q.; Sun, M.; Deng, Q.; Ni, Z.; Li, X.; Hu, T. Facile engineering of CoS/rGO heterostructures on carbon cloth for efficient all-pH hydrogen evolution reaction and alkaline water electrolysis. J. Mater. Chem. A 2024, 13, 486–498. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, D.; Wei, Y.; Sun, F.; Xie, Y.; Wang, L.; Fu, H. Modulating the Electronic Structure of Cobalt-Vanadium Bimetal Catalysts for High-Stable Anion Exchange Membrane Water Electrolyzer. Adv. Mater. 2024, 36, 2408634. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, M.; Fu, R.; Ge, J.; Yang, W.; Hong, X.; Sun, C.; Liao, X.; Zhao, Y.; Wang, Z. Iron Molybdenum Sulfide-Supported Ultrafine Ru Nanoclusters for Robust Sulfion Degradation-Assisted Hydrogen Production. Adv. Funct. Mater. 2024, 34, 2315326. [Google Scholar] [CrossRef]
- Lyu, C.; Li, Y.; Cheng, J.; Yang, Y.; Wu, K.; Wu, J.; Wang, H.; Lau, W.-M.; Tian, Z.; Wang, N.; et al. Dual Atoms (Fe, F) Co-Doping Inducing Electronic Structure Modulation of NiO Hollow Flower-Spheres for Enhanced Oxygen Evolution/Sulfion Oxidation Reaction Performance. Small 2023, 19, 2302055. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Xu, H.-M.; Shuai, T.-Y.; Zhan, Q.-N.; Zhang, Z.-J.; Li, G.-R. Modulation Strategies for the Preparation of High-Performance Catalysts for Urea Oxidation Reaction and Their Applications. Small 2023, 19, 2301130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Miao, L.; Zheng, S.; Qin, H.; Cao, X.; Yang, L.; Jiao, L. Interfacial Engineering of Ni3N/Mo2N Heterojunctions for Urea-Assisted Hydrogen Evolution Reaction. ACS Catal. 2023, 13, 4091–4100. [Google Scholar] [CrossRef]
- Liu, W.-J.; Xu, Z.; Zhao, D.; Pan, X.-Q.; Li, H.-C.; Hu, X.; Fan, Z.-Y.; Wang, W.-K.; Zhao, G.-H.; Jin, S.; et al. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, T.Y.; Lin, Z.J.; Liu, S.X.; Liu, X.; Zhou, R.X.; Cai, Z.; Huang, Y.H.; Li, Q. Spontaneous Hydrogen Production Coupled with Glucose Valorization through Modulating Au-Pt Coordination on Ultrathin Au3Pt Twin Nanowires. Angew. Chem. Int. Ed. 2025, 64, e202424476. [Google Scholar] [CrossRef]
- Pei, A.; Xie, R.K.; Zhu, L.H.; Wu, F.S.; Huang, Z.A.; Pang, Y.Y.; Chang, Y.C.; Chai, G.L.; Pao, C.W.; Gao, Q.S.; et al. Methanol-Enhanced Low-Cell-Voltage Hydrogen Generation at Industrial-Grade Current Density by Triadic Active Sites of Pt1-Pdn-(Ni,Co)(OH)x. J. Am. Chem. Soc. 2025, 147, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liang, L.; Li, T.; Bao, M.; Yu, Z.; Wang, J.; Thalluri, S.M.; Lin, F.; Liu, Q.; Cui, Z.; et al. Pt1.8Pd0.2CuGa Intermetallic Nanocatalysts with Enhanced Methanol Oxidation Performance for Efficient Hybrid Seawater Electrolysis. Adv. Mater. 2024, 36, 2403792. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Lv, M.; Shao, J.; Wu, H.; Zhou, W.; Qi, S.; Deng, C.; Chai, X.; Yang, H.; Hu, Q.; et al. Lattice Strain Engineering of Ni2P Enables Efficient Catalytic Hydrazine Oxidation-Assisted Hydrogen Production. Adv. Mater. 2023, 35, 2305598. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-L.; Zhang, H.; Suo, T.-Y.; Cunha, J.; Yu, Z.-P.; Xu, W.-Y.; Chen, L.; Hou, Z.-H.; Yin, H. Ru nanoclusters immobilized in N-doped porous carbon for efficient hydrazine-assisted hydrogen production and Zn–hydrazine battery. Rare Met. 2025, 44, 2502–2512. [Google Scholar] [CrossRef]
- Yu, Z.; Boukhvalov, D.W.; Tan, H.; Xiong, D.; Feng, C.; Wang, J.; Wang, W.; Zhao, Y.; Xu, K.; Su, W.; et al. Sulfur and phosphorus co-doped FeCoNiCrMn high-entropy alloys as efficient sulfion oxidation reaction catalysts enabling self-powered asymmetric seawater electrolysis. Chem. Eng. J. 2024, 494, 153094. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Chen, Y.; Zhang, Y.; Feng, Y.; Zhang, G. Screened d-p Orbital Hybridization in Turing Structure of Confined Nickel for Sulfion Oxidation Accelerated Hydrogen Production. Angew. Chem. Int. Ed. 2024, 64, e202419572. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Zhou, B.; Meng, F.; Wang, Y.; Wen, G. Recent advance of layered double hydroxides materials: Structure, properties, synthesis, modification and applications of wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 111191. [Google Scholar] [CrossRef]
- Zhai, Y.; Ren, X.; Gan, T.; She, L.; Guo, Q.; Yang, N.; Wang, B.; Yao, Y.; Liu, S. Deciphering the Synergy of Multiple Vacancies in High-Entropy Layered Double Hydroxides for Efficient Oxygen Electrocatalysis. Adv. Energy Mater. 2025, 2502065. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Tian, Y.; Xiao, T.; Zhang, J.; Zhang, C.; Jiang, J. Energy-saving hydrogen production from sulfion oxidation-hybrid seawater splitting enabled by superwettable corrosion-resistant NiFe layered double hydroxide/FeNi2S4 heterostructured nanoarrays. J. Colloid Interface Sci. 2024, 673, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tian, Y.; Zhang, J.; Zhang, C.; Ai, L. Metallic Cu-incorporated NiFe layered double hydroxide nanosheets enabling energy-saving hydrogen generation from chlorine-free seawater electrolysis coupled with sulfion upcycling. Fuel 2024, 367, 131506. [Google Scholar] [CrossRef]
- Li, Z.; Lin, G.; Wang, L.; Lee, H.; Du, J.; Tang, T.; Ding, G.; Ren, R.; Li, W.; Cao, X.; et al. Seed-assisted formation of NiFe anode catalysts for anion exchange membrane water electrolysis at industrial-scale current density. Nat. Catal. 2024, 7, 944–952. [Google Scholar] [CrossRef]
- Xie, C.; Chen, W.; Wang, Y.; Yang, Y.; Wang, S. Dynamic evolution processes in electrocatalysis: Structure evolution, characterization and regulation. Chem. Soc. Rev. 2024, 53, 10852–10877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Wang, H.-Y.; Feng, L.; Zhu, J.-Z.; Liu, J.-X.; Li, W.-X. Crystal-Phase Engineering in Heterogeneous Catalysis. Chem. Rev. 2023, 124, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, C.; Ma, Y.; Huang, S.; Ren, Y.; Ding, F.; Li, F.; Yang, Y.; Gu, J.; Tang, S.; et al. NiO/RuO2p-n Heterojunction Nanofoam as a High-Performance Electrocatalyst for Desulfurization and Concurrent Hydrogen Evolution. Lnorg. Chem. 2024, 63, 12604–12614. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, W.; Mao, Q.; Deng, K.; Xu, Y.; Wang, Z.; Li, X.; Wang, H.; Wang, L. Electrocatalytic sulfion recycling assisted energy-saving hydrogen production using CuCo-based nanosheet arrays. J. Mater. Chem. A 2023, 11, 2218–2224. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, H.; Li, W.; Chen, C.; Zhang, Z.; Dong, J.; Lu, W.; Feng, Q.; Jia, B.; Song, K. Effect of trace Sc on corrosion behavior of titanium material in cathode roller for electrolytic copper foil. Corros. Sci. 2025, 246, 112722. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, C.; Zhou, J.; Ma, C.; Shi, P.; Wu, H.; Yin, P.; Cao, W.; Xia, J.; Zhu, L.; et al. Atomically dispersed ruthenium sites with electron-rich environments in intermetallic compounds for high-current-density hydrogen evolution. J. Mater. Chem. A 2023, 11, 10328–10336. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.-I. Carbon-supported bimetallic Pd–Ir catalysts for alkaline sulfide oxidation in direct alkaline sulfide fuel cell. J. Appl. Electrochem. 2015, 45, 533–539. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.-I. Carbon supported bimetallic Pd–Co catalysts for alkaline sulfide oxidation in direct alkaline sulfide fuel cell. Int. J. Hydrogen Energy 2015, 40, 4567–4572. [Google Scholar] [CrossRef]
- Kim, K.; Son, J.; Han, J.-I. Metal sulfides as anode catalysts in direct alkaline sulfide fuel cell. Int. J. Hydrogen Energy 2014, 39, 10493–10497. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Kim, K.; Lee, J.H.; Han, J.-I.; Oh, Y.-K. Biomimetically Synthesized Hierarchical TiO2-Graphitic Carbon as Anodic Catalysts for Direct Alkaline Sulfide Fuel Cell. ACS Sustain. Chem. Eng. 2015, 3, 1764–1770. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, G.; Tian, P.; Li, X.; Deng, K.; Yu, H.; Xu, Y.; Wang, H.; Wang, L. Hydrophilic functionalization of rhodium metallene for saving-energy hydrogen production and sulfur recovery. Chem. Eng. J. 2023, 473, 145147. [Google Scholar] [CrossRef]
- Yi, L.; Ji, Y.; Shao, P.; Chen, J.; Li, J.; Li, H.; Chen, K.; Peng, X.; Wen, Z. Scalable Synthesis of Tungsten Disulfide Nanosheets for Alkali-Acid Electrocatalytic Sulfion Recycling and H2 Generation. Angew. Chem. Int. Ed. 2021, 60, 21550–21557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, Y.; Zhang, K.; Yu, W. Efficient anodic chemical conversion to boost hydrogen evolution with low energy consumption over cobalt-doped nickel sulfide electrocatalyst. Chem. Eng. J. 2022, 433, 134472. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Shen, Z.; Jin, X.; Zhang, Y.; Shi, M.; Zhou, J.; Liu, J.; Lu, Z.; Zhou, Y.N.; et al. Sulfophobic and Vacancy Design Enables Self-Cleaning Electrodes for Efficient Desulfurization and Concurrent Hydrogen Evolution with Low Energy Consumption. Adv. Funct. Mater. 2021, 31, 2101922. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, L.; Wei, Z.; Wang, Z.; Liu, Q.; Hu, G.; Luo, J.; Liu, X. Coupling Nitrate-to-Ammonia Conversion and Sulfion Oxidation Reaction Over Hierarchical Porous Spinel MFe2O4 (M = Ni, Co, Fe, Mn) in Wastewater. Small 2025, 21, 2411317. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yu, Z.; Zhang, C.; Lin, F.; Ma, S.; Huang, H.; Li, H.; Xiong, D.; Liu, L. Self-supported NiTe@NiMo electrodes enabling efficient sulfion oxidation reaction toward energy-saving and chlorine-free hybrid seawater electrolysis at high current densities. Energy Environ. Sci. 2024, 18, 1440–1451. [Google Scholar] [CrossRef]
- Yang, M.; Ding, J.; Wang, Z.; Zhang, J.; Peng, Z.; Liu, X. NiMo-based alloy and its sulfides for energy-saving hydrogen production via sulfion oxidation assisted alkaline seawater splitting. Chin. Chem. Lett. 2025, 25, 110861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Nian, Y.; Du, H.; Li, P.; Wang, M.; Ma, G. Multiphase NiCoFe-Based LDH for Electrocatalytic Sulfion Oxidation Reaction Assisting Efficient Hydrogen Production. Materials 2025, 18, 3377. https://doi.org/10.3390/ma18143377
Liang Z, Nian Y, Du H, Li P, Wang M, Ma G. Multiphase NiCoFe-Based LDH for Electrocatalytic Sulfion Oxidation Reaction Assisting Efficient Hydrogen Production. Materials. 2025; 18(14):3377. https://doi.org/10.3390/ma18143377
Chicago/Turabian StyleLiang, Zengren, Yong Nian, Hao Du, Peng Li, Mei Wang, and Guanshui Ma. 2025. "Multiphase NiCoFe-Based LDH for Electrocatalytic Sulfion Oxidation Reaction Assisting Efficient Hydrogen Production" Materials 18, no. 14: 3377. https://doi.org/10.3390/ma18143377
APA StyleLiang, Z., Nian, Y., Du, H., Li, P., Wang, M., & Ma, G. (2025). Multiphase NiCoFe-Based LDH for Electrocatalytic Sulfion Oxidation Reaction Assisting Efficient Hydrogen Production. Materials, 18(14), 3377. https://doi.org/10.3390/ma18143377






