SiOx-Based Anode Materials with High Si Content Achieved Through Uniform Nano-Si Dispersion for Li-Ion Batteries
Abstract
1. Introduction
2. Experimental
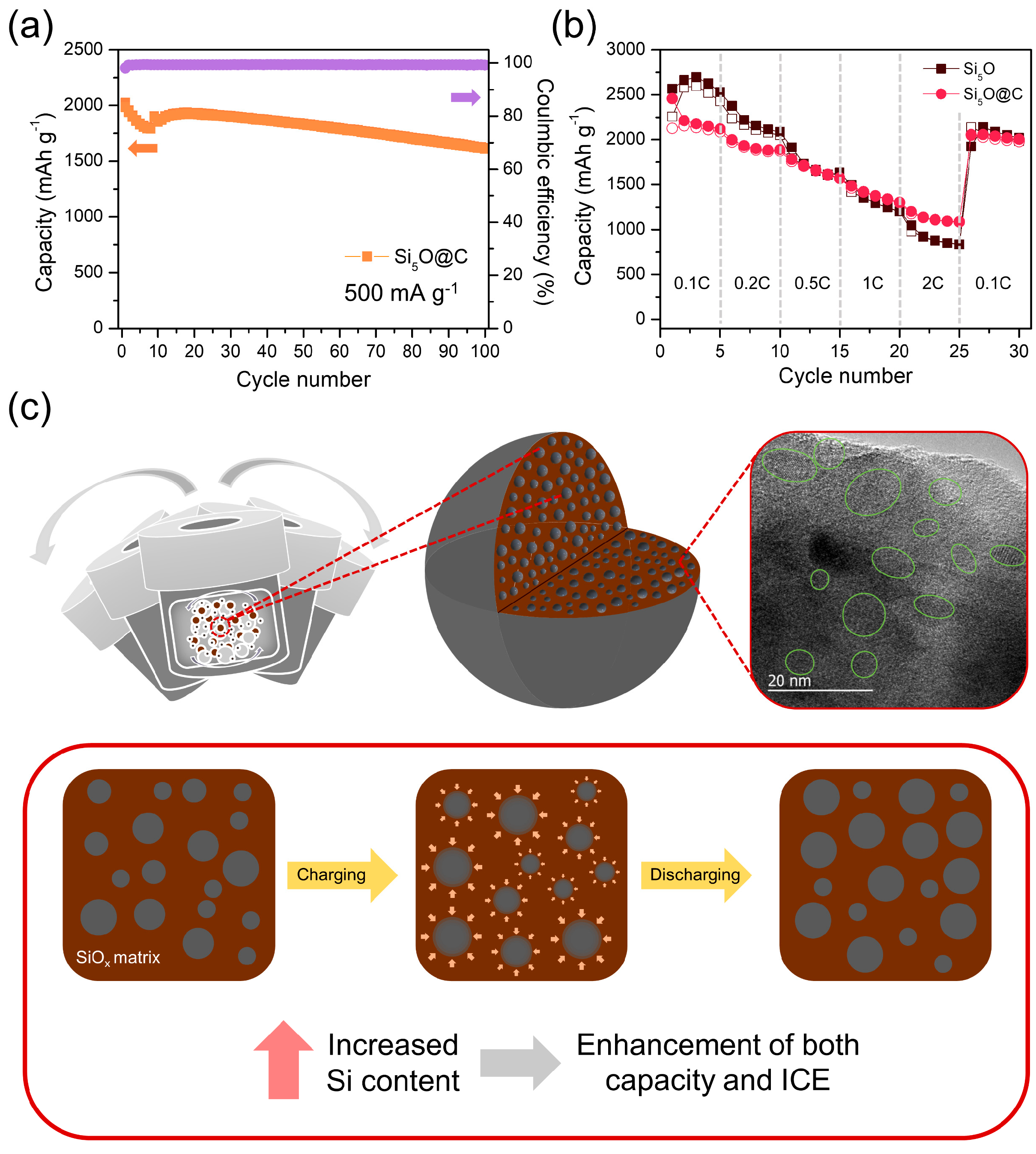
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhao, Q.; Huang, X.; Jia, S.; Ma, J.; Ren, Y. Si-Based Anodes: Advances and Challenges in Li-Ion Batteries for Enhanced Stability. Electrochem. Energy Rev. 2024, 7, 11. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, G.; Kim, Y.-U.; Kim, J.-H.; Park, C.-M.; Sohn, H.-J. Metallic anodes for next generation secondary batteries. Chem. Soc. Rev. 2013, 42, 9011–9034. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Yoon, J.-M.; Park, C.-M. Recent Progress of Alloy-Based All-Solid-State Li-Ion Battery Anodes. Corros. Sci. Technol. 2023, 22, 466–477. [Google Scholar]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Zhang, W.-J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 877–885. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.H.; Lee, Y.M.; Kong, B.-S.; Seo, J.-S.; Choi, J.W. Electrospun Core–Shell Fibers for Robust Silicon Nanoparticle-Based Lithium Ion Battery Anodes. Nano Lett. 2012, 12, 802–807. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Song, T.; Xia, J.; Lee, J.-H.; Lee, D.H.; Kwon, M.-S.; Choi, J.-M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of Sealed Silicon Nanotubes As Anodes for Lithium Ion Batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Wei, C.; Jiang, H.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Scalable and Physical Synthesis of 2D Silicon from Bulk Layered Alloy for Lithium-Ion Batteries and Lithium Metal Batteries. ACS Nano 2019, 13, 13690–13701. [Google Scholar] [CrossRef]
- Kim, J.S.; Pfleging, W.; Kohler, R.; Seifert, H.J.; Kim, T.Y.; Byun, D.; Jung, H.-G.; Choi, W.; Lee, J.K. Three-dimensional silicon/carbon core–shell electrode as an anode material for lithium-ion batteries. J. Power Sources 2015, 279, 13–20. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zheng, S.; Xu, K.; Wu, J.; Chen, S.; Liang, J.; Shi, A.; Wang, Z. A submicron Si@C core-shell intertwined with carbon nanowires and graphene nanosheet as a high-performance anode material for lithium ion battery. Energy Storage Mater. 2021, 39, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Yang, Z.; Yang, L.; Gong, J.; Liu, Y.; Wang, G.; Zheng, Z.; Zhong, B.; Song, Y.; et al. SiOx Anode: From Fundamental Mechanism toward Industrial Application. Small 2021, 17, 2102641. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon oxides for Li-ion battery anode applications: Toward long-term cycling stability. J. Power Sources 2023, 559, 232660. [Google Scholar] [CrossRef]
- Li, Z.; Du, M.; Guo, X.; Zhang, D.; Wang, Q.; Sun, H.; Wang, B.; Wu, Y.A. Research progress of SiOx-based anode materials for lithium-ion batteries. Chem. Eng. J. 2023, 473, 145294. [Google Scholar] [CrossRef]
- Schulmeister, K.; Mader, W. TEM investigation on the structure of amorphous silicon monoxide. J. Non-Cryst. Solids 2003, 320, 143–150. [Google Scholar] [CrossRef]
- Hohl, A.; Wieder, T.; van Aken, P.A.; Weirich, T.E.; Denninger, G.; Vidal, M.; Oswald, S.; Deneke, C.; Mayer, J.; Fuess, H. An interface clusters mixture model for the structure of amorphous silicon monoxide (SiO). J. Non-Cryst. Solids 2003, 320, 255–280. [Google Scholar] [CrossRef]
- Park, C.-M.; Choi, W.; Hwa, Y.; Kim, J.-H.; Jeong, G.; Sohn, H.-J. Characterizations and electrochemical behaviors of disproportionated SiO and its composite for rechargeable Li-ion batteries. J. Mater. Chem. 2010, 20, 4854–4860. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, K.; Zaccaria, R.P.; Huang, H.; Xia, Y.; Liu, Z. Two-dimensional silicon suboxides nanostructures with Si nanodomains confined in amorphous SiO2 derived from siloxene as high performance anode for Li-ion batteries. Nano Energy 2017, 39, 546–553. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, B.; Shao, J.; Zhang, Q.; Wan, Y.; Zhong, C.; Lu, J. Fundamental Mechanisms and Promising Strategies for the Industrial Application of SiOx Anode. Adv. Funct. Mater. 2023, 33, 2213363. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, K.; Jung, W.-S.; Choi, H.; Kim, J.-H. Facile and scalable synthesis of SiOx materials for Li-ion negative electrodes. J. Power Sources 2019, 436, 226883. [Google Scholar] [CrossRef]
- Yang, H.-R.; Hwang, J.; Seo, H.; Kim, K.; Kim, J.-H. Nano-spatially stable Si2O composite and its balanced electrochemical performance for Li rechargeable batteries. J. Power Sources 2022, 519, 230777. [Google Scholar] [CrossRef]
- Cao, Y.; Bennett, J.C.; Dunlap, R.A.; Obrovac, M.N. A Simple Synthesis Route for High-Capacity SiOx Anode Materials with Tunable Oxygen Content for Lithium-Ion Batteries. Chem. Mater. 2018, 30, 7418–7422. [Google Scholar] [CrossRef]
- Kwon, H.-T.; Park, A.-R.; Lee, S.-S.; Cho, H.; Jung, H.; Park, C.-M. Nanostructured Si-FeSi2-Graphite-C Composite: An Optimized and Practical Solution for Si-Based Anodes for Superior Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A2221. [Google Scholar] [CrossRef]
- Mélinon, P.; Kéghélian, P.; Prével, B.; Dupuis, V.; Perez, A.; Champagnon, B.; Guyot, Y.; Pellarin, M.; Lermé, J.; Broyer, M.; et al. Structural, vibrational, and optical properties of silicon cluster assembled films. J. Chem. Phys. 1998, 108, 4607–4613. [Google Scholar] [CrossRef]
- Hwa, Y.; Park, C.-M.; Sohn, H.-J. Modified SiO as a high performance anode for Li-ion batteries. J. Power Sources 2013, 222, 129–134. [Google Scholar] [CrossRef]
- Zhao, X.; Lehto, V.-P. Challenges and prospects of nanosized silicon anodes in lithium-ion batteries. Nanotechnology 2021, 32, 042002. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Hatchard, T.D.; Dahn, J.R. In Situ XRD and Electrochemical Study of the Reaction of Lithium with Amorphous Silicon. J. Electrochem. Soc. 2004, 151, A838. [Google Scholar] [CrossRef]
- Kim, T.; Park, S.; Oh, S.M. Solid-State NMR and Electrochemical Dilatometry Study on Li+ Uptake/Extraction Mechanism in SiO Electrode. J. Electrochem. Soc. 2007, 154, A1112–A1117. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, C.-M.; Kim, H.; Kim, Y.-J.; Sohn, H.-J. Electrochemical behavior of SiO anode for Li secondary batteries. J. Electroanal. Chem. 2011, 661, 245–249. [Google Scholar] [CrossRef]
- Lai, S.Y.; Knudsen, K.D.; Sejersted, B.T.; Ulvestad, A.; Mæhlen, J.P.; Koposov, A.Y. Silicon Nanoparticle Ensembles for Lithium-Ion Batteries Elucidated by Small-Angle Neutron Scattering. ACS Appl. Energy Mater. 2019, 2, 3220–3227. [Google Scholar] [CrossRef]
- Yu, B.-C.; Hwa, Y.; Kim, J.-H.; Sohn, H.-J. Carbon coating for Si nanomaterials as high-capacity lithium battery electrodes. Electrochem. Commun. 2014, 46, 144–147. [Google Scholar] [CrossRef]
- Kim, K.; Choi, H.; Kim, J.-H. Effect of carbon coating on nano-Si embedded SiOx-Al2O3 composites as lithium storage materials. Appl. Surf. Sci. 2017, 416, 527–535. [Google Scholar] [CrossRef]
- Yan, M.-Y.; Liu, Z.; Lu, Z.-Y.; Huang, L.-B.; Jiang, K.-C.; Li, H.-L.; Xin, S.; Xu, Q.; Guo, Y.-G. Stable Li storage in micron-sized SiOx particles with rigid-flexible coating. J. Energy Chem. 2022, 64, 309–314. [Google Scholar] [CrossRef]
- Xiao, Z.; Lin, X.; Zhang, C.; Shen, J.; Zhang, R.; He, Z.; Lin, Z.; Jiang, H.; Wei, F. Insights into the Coating Integrity and its Effect on the Electrochemical Performance of Core–Shell Structure SiOx@C Composite Anodes. Small Methods 2023, 7, 2201623. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Yu, R.; Wang, Y.; Zhou, L. Realizing Sub-5 nm Red Phosphorus Dispersion in a SiOx/C Matrix for Enhanced Lithium Storage. ACS Appl. Mater. Interfaces 2022, 14, 26775–26781. [Google Scholar] [CrossRef]
- Yu, R.; Pan, Y.; Jiang, Y.; Zhou, L.; Zhao, D.; Van Tendeloo, G.; Wu, J.; Mai, L. Regulating Lithium Transfer Pathway to Avoid Capacity Fading of Nano Si Through Sub-Nano Scale Interfused SiOx/C Coating. Adv. Mater. 2023, 35, 2306504. [Google Scholar] [CrossRef]
- Hernandha, R.F.H.; Rath, P.C.; Umesh, B.; Patra, J.; Huang, C.-Y.; Wu, W.-W.; Dong, Q.-F.; Li, J.; Chang, J.-K. Supercritical CO2-Assisted SiOx/Carbon Multi-Layer Coating on Si Anode for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2104135. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Q.; Cai, Z.; Ma, Z.; Ahsan, Z.; Li, Y.; Ma, Y.; Song, G.; Yang, W.; Wen, C. Dual-Phase SiC + C Coated Microsize Si@SiOx Powder as Anode Material for Li-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 9788–9797. [Google Scholar] [CrossRef]
- Tao, J.; Yan, Z.; Yang, J.; Li, J.; Lin, Y.; Huang, Z. Boosting the cell performance of the SiOx@C anode material via rational design of a Si-valence gradient. Carbon Energy 2022, 4, 129–141. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Chen, D.; Zhao, R. Hybrid MnO-SiOx@C microspheres with a hierarchical mesoporous structure for advanced lithium-ion battery anodes. J. Alloys Compd. 2022, 899, 163251. [Google Scholar] [CrossRef]
- Wang, J.-H.; Chung, C.-H.; Chi, P.-W.; Paul, T.; Chandan, P.; Yeh, K.-W.; Chang, C.-C.; Prakoso, S.P.; Chiu, Y.-C.; Wu, M.-K. Si@C Core–Shell Nanostructure-Based Anode for Li-Ion Transport. ACS Appl. Nano Mater. 2023, 6, 12578–12587. [Google Scholar] [CrossRef]
- Helmiyati, H.; Suci, R.P. Nanocomposite of Cellulose-ZnO/SiO2 as Catalyst Biodiesel Methyl Ester from Virgin Coconut Oil. AIP Conf. Proc. 2019, 2168, 020007. [Google Scholar]
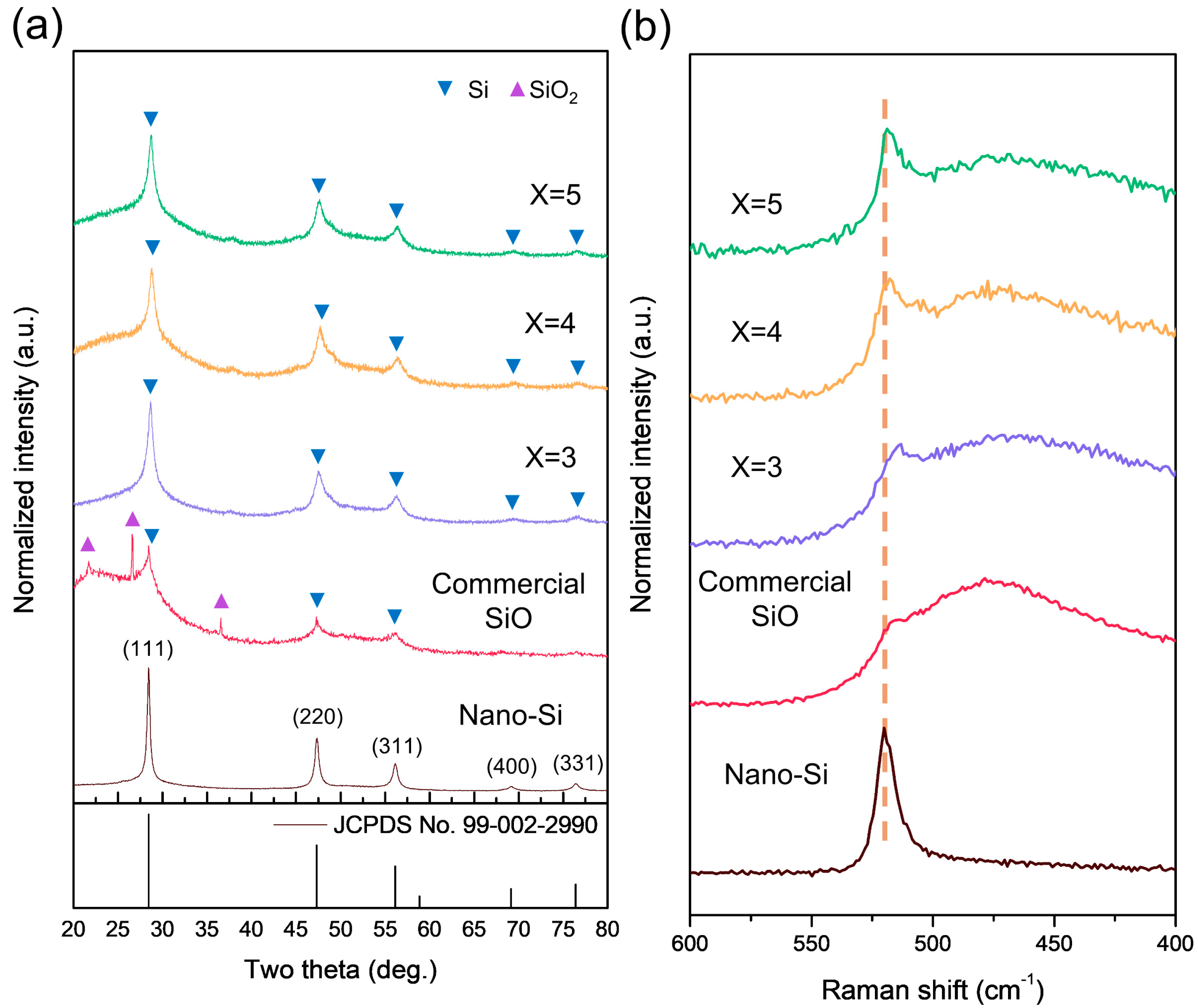
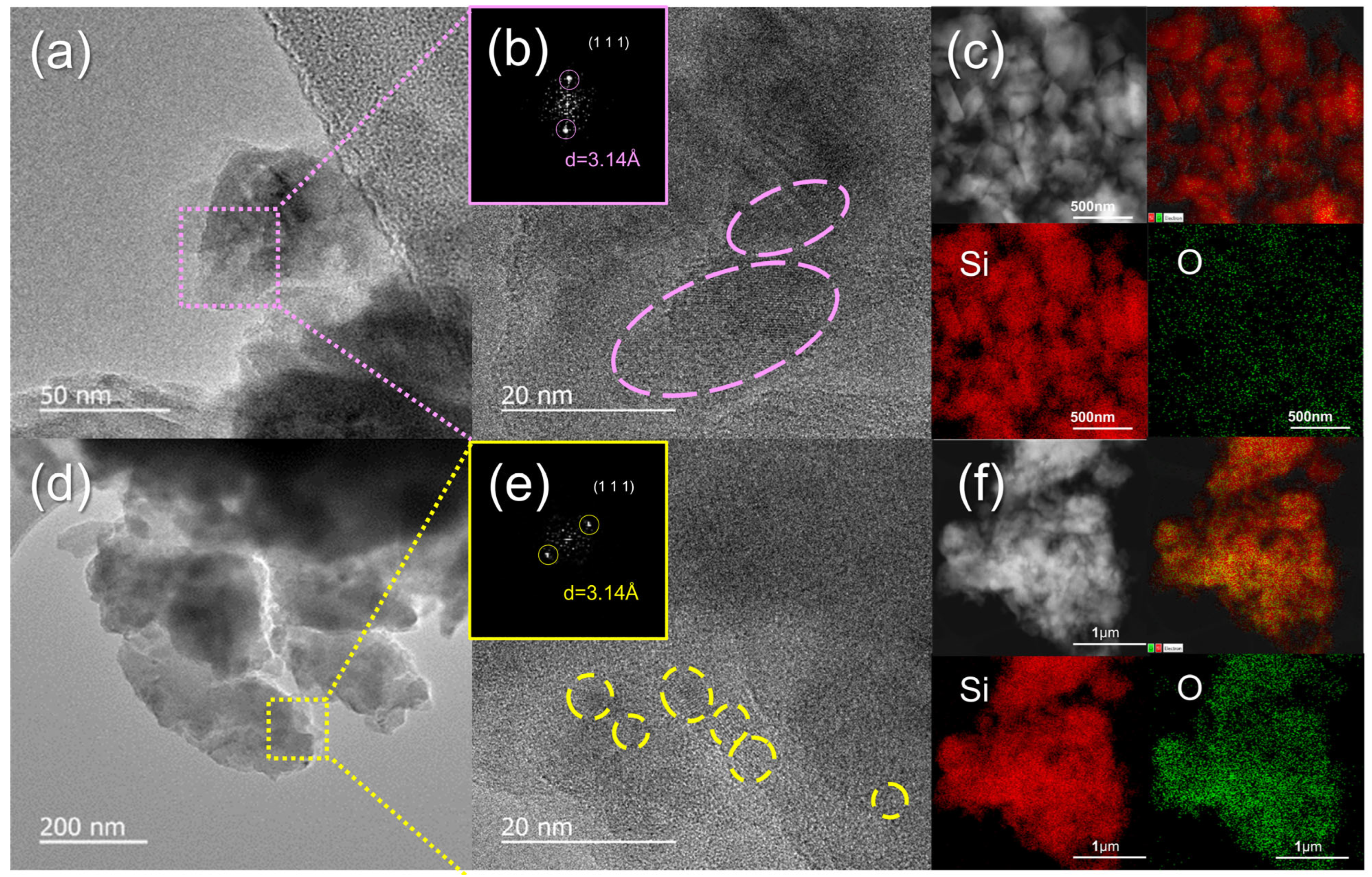
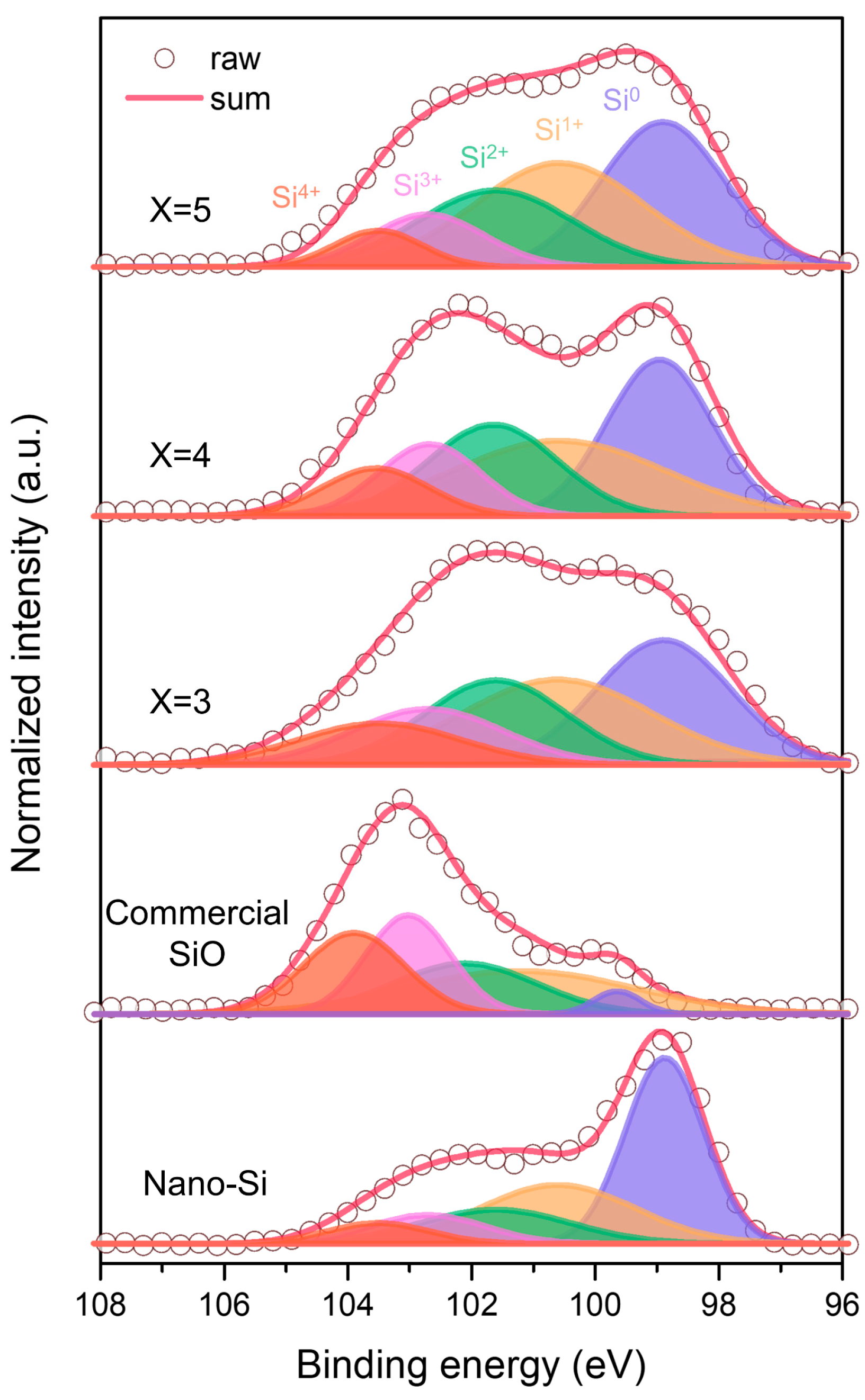
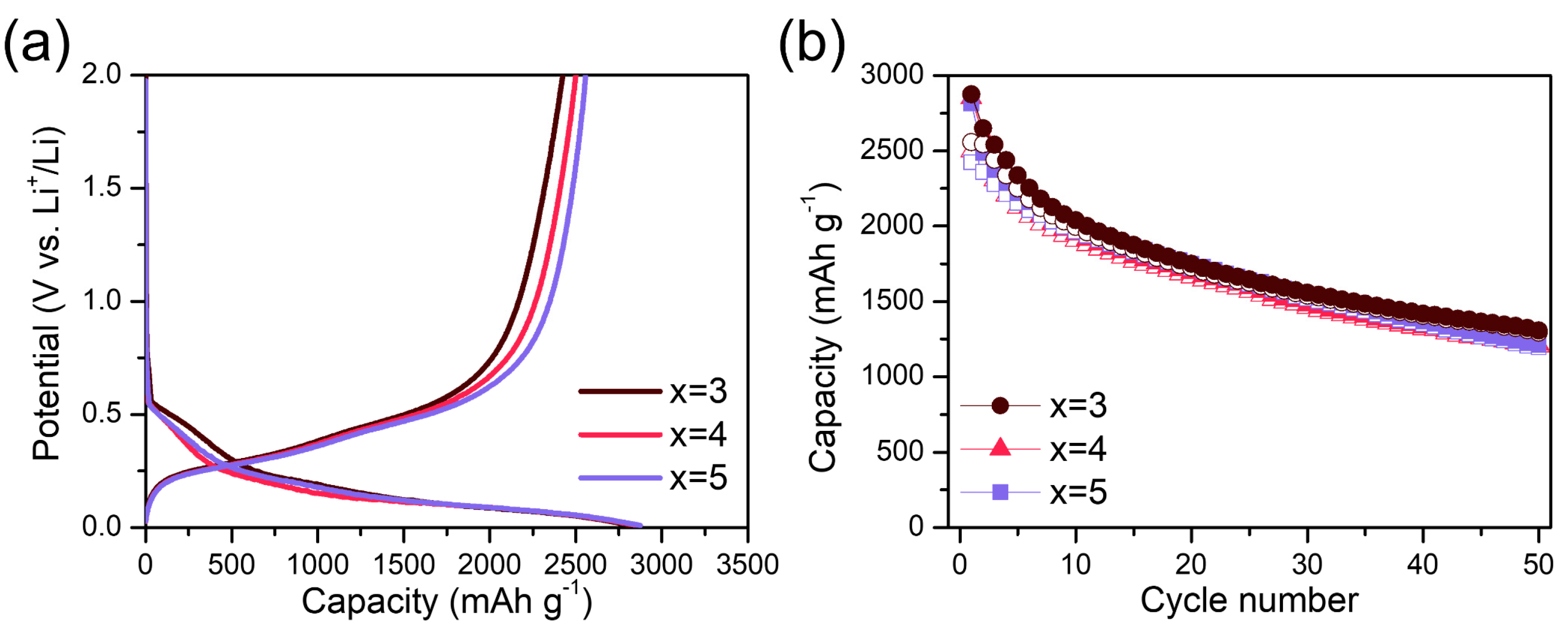

| Sample | Si0 (%) | Si1+ (%) | Si2+ (%) | Si3+ (%) | Si4+ (%) |
|---|---|---|---|---|---|
| Si5O | 31.6 | 31.1 | 21.1 | 10.6 | 5.6 |
| Si4O | 29.1 | 28.1 | 20.9 | 12.9 | 9.0 |
| Si3O | 27.9 | 26.8 | 20.0 | 14.1 | 11.2 |
| Commercial SiO | 3.1 | 28.4 | 20.9 | 24.0 | 23.6 |
| Nano Si | 42.9 | 26.5 | 14.9 | 9.6 | 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Kim, J.-H. SiOx-Based Anode Materials with High Si Content Achieved Through Uniform Nano-Si Dispersion for Li-Ion Batteries. Materials 2025, 18, 3272. https://doi.org/10.3390/ma18143272
Jang S, Kim J-H. SiOx-Based Anode Materials with High Si Content Achieved Through Uniform Nano-Si Dispersion for Li-Ion Batteries. Materials. 2025; 18(14):3272. https://doi.org/10.3390/ma18143272
Chicago/Turabian StyleJang, Seunghyeok, and Jae-Hun Kim. 2025. "SiOx-Based Anode Materials with High Si Content Achieved Through Uniform Nano-Si Dispersion for Li-Ion Batteries" Materials 18, no. 14: 3272. https://doi.org/10.3390/ma18143272
APA StyleJang, S., & Kim, J.-H. (2025). SiOx-Based Anode Materials with High Si Content Achieved Through Uniform Nano-Si Dispersion for Li-Ion Batteries. Materials, 18(14), 3272. https://doi.org/10.3390/ma18143272







