Abstract
This study investigated the effect of adding superhard ReB2 to atmospheric plasma sprayed (APS) coatings based on 60 wt% Al2O3 and 40 wt% ZrO2. The amorphous phases commonly present in such coatings are known to impair their performance. ReB2 was introduced as a crystallization nucleus due to its high melting point. ReB2 decomposes in the presence of moisture and oxygen into H3BO3, ReO3, HBO2, and HReO4. ReB2 was encapsulated with Al2O3 via metallothermic synthesis to improve moisture stability, yielding a powder with d90 = 15.1 μm. After milling, it was added at 20 wt% to the Al2O3-ZrO2 feedstock. Agglomeration parameters were optimized, and coatings were deposited under varying APS conditions onto 316L steel substrates with a NiAl bond coat. In the coating with the highest ReB2 content, the identified phases included ReB2 (2.6 wt%), Re (0.8 wt%), α-Al2O3 (30.9 wt%), η-Al2O3 (32.4 wt%), and monoclinic and tetragonal ZrO2. The nanohardness of the coating, measured using a Vickers indenter at 96 mN and calculated via the Oliver–Pharr method, was 9.2 ± 1.0 GPa. High abrasion resistance was obtained for the coating with a higher content of η-Al2O3 (48.7 wt%). The coefficient of friction, determined using a ball-on-disc test with a corundum ball, was 0.798 ± 0.03. After 15 months, the formation of (H3O)(ReO4) was observed, suggesting initial moisture-induced changes. The results confirm that Al2O3-encapsulated ReB2 can enhance phase stability and crystallinity in APS coatings.
1. Introduction
The development of new coating materials with high hardness and enhanced abrasion resistance, applicable via industry-standard methods such as Atmospheric Plasma Spraying (APS), remains a critical area of research [1]. APS coatings, particularly those based on Al2O3–ZrO2 mixtures, are commonly used for components exposed to abrasive, erosive, and corrosive conditions. However, these coatings often exhibit conventional lamellar microstructures with inherent defects, including pores, cracks, and partially or unmelted particles [2,3,4,5,6]. Moreover, APS processing of Al2O3–ZrO2 frequently results in the formation of an amorphous phase, which has been shown to reduce coating hardness [7,8,9,10,11].
The aim of the following studies was to introduce particles of ReB2 that will promote the crystallization of Al2O3–ZrO2 coating with a near-eutectic composition [12]. ReB2 is a superhard compound with a high melting point. ReB2 (P63/mmc), originally synthesized in 1962 [13], has demonstrated hardness values exceeding 40 GPa [14,15]. Its high resistance to elastic and plastic deformation arises from strong covalent B–B and Re–B bonds [16]. Among several polymorphic forms, the hexagonal P63/mmc structure is considered the most thermodynamically stable [16,17].
Despite its mechanical advantages, ReB2 is known to react with moisture, leading to degradation products such as H3BO3 and HBO4. These acids are hygroscopic and form a liquid layer on the surface of the ReB2 material, which further contributes to the destruction of the bulk material [18,19]. Recent studies have shown that passivation with aluminum or titanium, either through doping or encapsulation, can significantly enhance its chemical stability [20,21]. For instance, thin films of Re–Al alloys have demonstrated improved resistance to atmospheric degradation due to the formation of protective Al2O3 layers [20].
ReB2-based coatings have been produced using methods including Confined-Plume Chemical Deposition, magnetron sputtering, and pulsed laser deposition [20,21,22,23,24,25]. However, their integration into plasma-sprayed ceramic systems remains underexplored. The decreasing cost of rhenium and advances in recycling technologies further support the feasibility of incorporating ReB2 into industrial coating processes [26].
The present research addresses this gap by investigating the effect of ReB2 incorporation on the structure and properties of APS-deposited Al2O3-ZrO2 coatings. The study involved the preparation of composite feedstock powders, optimization of the spraying process, and characterization of the resulting microstructures and phase compositions. The primary objective was to develop a superhard, moisture-resistant Al2O3-ZrO2-ReB2 coating material with minimized amorphous content, suitable for use in wear-resistant ceramic coatings produced via thermal spraying.
2. Materials and Methods
2.1. Initial Materials—ReB2 + Al2O3 Powders Obtaining
In order to obtain a composite material consisting of rhenium boride and alumina, the process of metallothermic reduction of rhenium oxide using Al was used [27]. The starting materials for obtaining the ReB2 + Al2O3 composite powder at elevated temperature in an Ar gas shield were: ammonium perrhenate (APR)—NH4ReO4 (99.9%, INNOVATOR SP.z o.o., Gliwice, Poland) as a substrate to obtain ReO2 and Re precursor; aluminum powder (99.8%, Al BLS0072:APS7/99.8 AlATOMIZED:CJ01, Benda-Lutz Skawina Sp. z o.o., Skawina, Poland) as a stoichiometric reductant being consumed during the process for the metallothermic reaction; B2O3 powder (~420 μm, 98%, AcrosOrganics, Jinan, China) as a boron precursor; and Al2O3 powder (≥99.8%, Areoxide Alu C, BET: 85–115 m2/g, Evonic Industries AG, Essen, Germany) to slow down the reaction [28]. It was assumed that the metallothermic reaction would take place according to reaction (1):
Me can be substituted with a specific metal, e.g., Re.
3 MeO2 + 3 B2O3 + 10 Al → 3 MeB2 + 5 Al2O3
The synthesis of these materials is described in detail in [28]. ReO2, as the substrate used in reaction (1), was obtained by the thermal method from ammonium perrhenate, through two-stage thermal treatment, in a tube electric furnace, at a temperature of 350 °C, for 1.5 h, in an argon flow of 1 L/min (first stage) and 470 °C, for 1.5 h, in an argon flow of 1 L/min (second stage). Meanwhile, ReB2 from reaction (1) was obtained at a temperature of 1050 °C, for 1 h, in an argon flow of 4 L/min. Materials with a higher content of Al2O3 and Al produced in syntheses at a molar ratio B2O3/ReO2 = 1 are characterized by greater chemical stability and lower reactivity with water than materials with lower Al2O3 and Al content [28]. Quantitative and qualitative analysis of the phase composition of the composite powder was carried out. For X-ray diffraction measurements, an XRD7 Seifert-FPM diffractometer (Freiberger Präzisionsmechanik, FPM Holding GmbH, Freiberg, Germany) was employed using CuKα1 radiation with a wavelength of λ = 1.540598 Å and the Ni filter. Measurements were performed in the Bragg–Brentano geometry. The scan was performed with a step size of 0.04°. Phase identification was carried out using software Match! V.4.1. in conjunction with the ICDD PDF -5+ database (2025 update). The following reference cards were applied for phase analysis: ReB2—04-008-5058, 01-078-4108; Al2O3—04-006-9359; Re7B3—04-004-2746. A semi-quantitative evaluation of the phase composition was conducted in Match! 3 software using the Reference Intensity Ratio (RIR) method, comparing the intensity scaling factors of identified phases against a corundum standard.
The XRD phase composition of the ReB2 + Al2O3 powder measurements is presented in Figure 1.
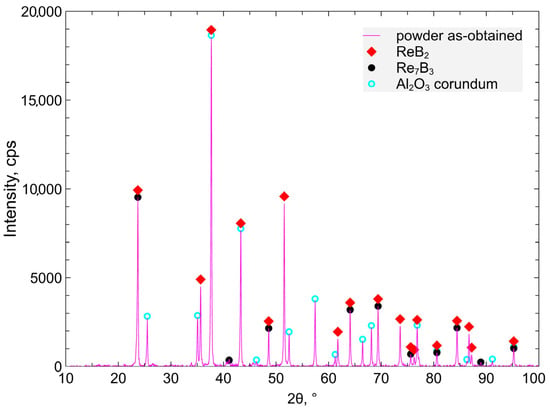
Figure 1.
The XRD diffractogram of the synthesized ReB2 + Al2O3 powders.
Synthesized powders were analyzed by Scanning Electron Microscopy (SEM) using JEOL JXA 8230 (X-Ray microprobe, JEOL Ltd., Tokyo, Japan). Figure 2 illustrates the morphology of composite powders Al2O3 + ReB2 after the crushing and milling processes.
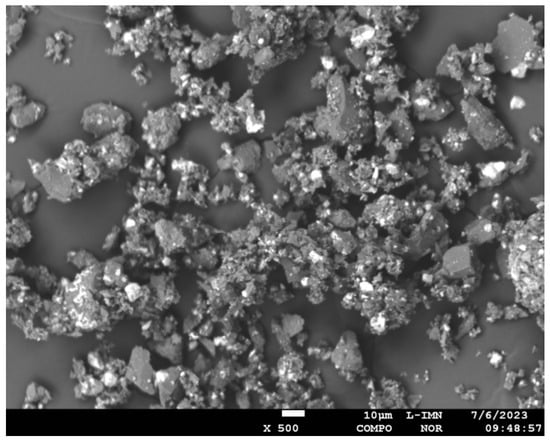
Figure 2.
Morphology of the composite ReB2 + Al2O3 powders, COMPO image, magnification 500×.
The milling was carried out in a planetary ball mill type PM400 by Retsch (Fisher Scientific, Waltham, MA, USA) in bowls (250 mL) with a ZrO2 lining, using 10 mm diameter ZrO2 balls. The powder fraction after the crushing process (<312 µm), with the addition of ethyl alcohol, was introduced into the mill bowl. The milling was carried out for 40 min, with a milling speed of 180 rpm. The ratio of the powder mass (subjected to a single milling operation during 40 min.) to the mass of the balls was 1:3. Densities were measured using an AccuPyC II 1340 gas pycnometer (Micromeritics, part of the Malvern Panalytical Ltd., Westborough, MA, USA). The specific surface area of the powders was measured by the BET method using Gemini 2360 (Micromeritics Instrument Corporation, Norcross, GA, USA) surface area analyzer, (Table 1).

Table 1.
Characteristics of the ReB2 + Al2O3 powder after crushing and grinding.
The powder was tested after 20 months of storage in a container (polypropylene) filled with argon. Boric acid H3BO3 and aluminum rhenium oxide hydrate Al(ReO4)3(H2O)8, along with the phases ReB2, corundum Al2O3, and Re7B3, were observed in the XRD analysis of this powder.
2.2. Agglomeration of Composite Powders
Studies were carried out on agglomerates using the so-called ceramic binding phases based on 80 wt% of Al2O3-ZrO2 powders (60:40, Amperit 750, fused, grain size 22/5 µm, d10 = 5.66 µm, d50 = 15.93 µm, d90 = 35.35 µm, prod. H.C. Strack, now Höganäs AB, Höganäs, Sweden), 20 wt% of the synthesized ReB2 + Al2O3 powder with the addition of a binder in the form of an aqueous solution—an aqueous solution of 10, 15, or 20 wt% hydroxypropylcellulose (HPC-SL Fine Powder, Nippon Soda Co., Ltd., Tokyo, Japan) with a concentration of 5%. The synthesized Al2O3 + ReB2 powder was characterized by a particle size similar to that of the commercial Al2O3-ZrO2 powder. Size enlargement by wet agglomeration is widely used in the powder processing industry. In this work, the wet shear agglomeration method was used, employing a high shear mixer type Eirich EL1 (Maschinenfabrik Gustaw Eirich GmbH & Co KG, Hardheim, Germany). The process consists of mixing, densifying, and agglomerating wet powder particles under the action of shearing and densifying forces exerted by the impeller in high-speed mixers [29]. Agglomeration is necessary for technological reasons of the APS process and helps to distribute the elements evenly in the coating. The criterion for selecting the amount of binder added was to create as many larger particles as possible in the agglomerates.
Figure 3 shows an example of agglomerates produced using a high-speed mixer (fraction immediately after agglomeration) and after the grinding and sieving process. In Figure 4, the elements distribution is shown.

Figure 3.
Morphology of the composite 80 wt% Al2O3-ZrO2 and 20 wt% ReB2 + Al2O3 powder < 100 µm (SEI, magnification 200×).
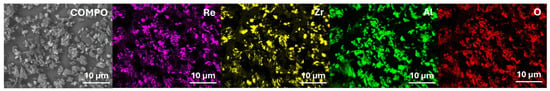
Figure 4.
Element distributions in agglomerates, obtained from 80 wt% Al2O3-ZrO2 + 20 wt% Al2O3 + ReB2 powder.
Table 2 presents results of agglomerate compositions for 80 wt% Al2O3-ZrO2, 20 wt% Al2O3 + ReB2 with 10 wt% of binder in the form of an aqueous solution of hydroxypropylcellulose.

Table 2.
Sieve analysis of the obtained agglomerates for 80 wt% Al2O3-ZrO2, 20 wt% Al2O3 + ReB2.
3. Results of the Atmospheric Plasma Spraying Process
The coating spraying process was carried out at the laboratory stand, using the AP 50 plasma spray system with an F4 plasma spray gun torch, equipped with the PF-50 powder feeder (FST-Flame Spray Technologies, Duiven, The Netherlands). The coatings were sprayed onto sheets made of 316L stainless steel with dimensions of 190 mm × 85 mm × 5 mm. The sheets were previously cut using the water-jet method to prepare smaller shapes in the sheet—samples in the form of squares with dimensions of 35 mm × 35 mm (which were cut out of the sheet after spraying the coating). Before spraying the coatings, the metal sheets were subjected to sandblasting to increase the adhesion of the substrate surface and spraying an intermediate coating, which was NiAl (Amperit 281—NiAl 95-5, gas atomized, 90 + 45 µm, H.C. Starck, now Höganäs AB, Höganäs, Sweden) powder material. Parameters for the process are presented in Table 3.

Table 3.
Parameters for APS—NiAl coatings.
APS processes for the 80 wt% Al2O3-ZrO2, 20 wt% Al2O3 + ReB2 coatings are realized using the following constant values: current intensity—600 A; distance of the torch nozzle from the steel substrate (on which the coatings were applied)—120 mm; number of powder spray repetitions on the substrate (i.e., torch passes)—four times; and powder feeder—5.5 rpm. The share of H2 and Ar during the atmospheric plasma spraying process is presented in Table 4.

Table 4.
Gase flows during the APS processes for 80 wt% Al2O3-ZrO2, 20 wt% Al2O3 + ReB2 coatings.
The Ar/H2 ratio affects, among other things, the plasma temperature and the shape of the plasma jet. In coating samples no. 2 and no. 3 (Table 4 and Table 5), which were sprayed using a higher flow of hydrogen as a process gas, losses in the form of splatters were observed on the coating surface. In these samples, the lowest content of the ReB2 phase and the α-Al2O3 phase, and the ZrO2—monoclinic phase were noted based on the XRD test results. These samples, however, were characterized by the highest content of the η-Al2O3 phase (over 60% by weight), which is a cubic form of alumina characterized by a large surface area, high hardness, and good thermal stability [30]. It is a metastable phase, meaning it can transform into the more stable alpha-alumina (α-alumina) under certain conditions. Sample no. 4 is characterized by a high content of the η-Al2O3 phase (about 48.7 wt%) and of ReB2 and Re, slightly lower than samples no. 5 and 6 (Table 5). A higher hydrogen content, H2, may affect ReB2 decomposition processes. The selection of the Ar/H2 ratio was based on obtaining a coating with the highest content of the ReB2 phase. Based on the results of quantitative XRD phase composition analysis, it was found that it is advantageous to use a larger flow of argon as the process gas relative to the flow of hydrogen.

Table 5.
Results of the phase composition measurements of coatings using the XRD method (quantitative measurement—Rietveld method, wt%).
3.1. Microstructure of APS Coatings
SEM surface microstructural studies of the coatings were realized using a Zeiss LEO Gemini 1525 electron microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with a Bruker Quantax XFlash® 5010 EDS SDD detector (Bruker, Rheinstetten, Germany) and an X-ray microprobe JXA 8230 JEOL (JEOL Ltd., Tokyo, Japan). SEM microstructural studies of cross-section coatings were realized using a Zeiss Evo MA10 electron microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with a Bruker Quantax XFlash® 6 Bruker Nano (EDS SD) detector (Bruker, Rheinstetten, Germany) and a JXA 8230 JEOL X-ray microprobe (JEOL Ltd., Tokyo, Japan).
The microstructure of the surface of coating no. 5 (Table 5) with the highest content of ReB2 and α-Al2O3 is presented in Figure 5.

Figure 5.
The surface of the 80 wt% Al2O3-ZrO2 with 20 wt% Al2O3 + ReB2 APS coating (magnification 2000×).
SEM and EDS microstructural examination of the cross-section of coating number 5 is presented in Figure 6 and Figure 7.
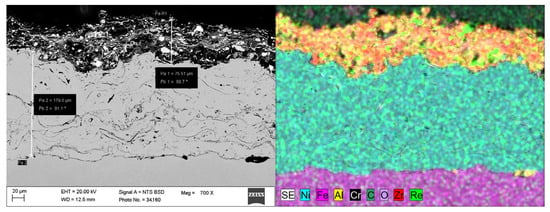
Figure 6.
The elements distribution (EDS) for the cross section of the 80 wt% Al2O3-ZrO2 with 20 wt% Al2O3 + ReB2 APS coating, sample number 5 (Table 5), magnification 700×.
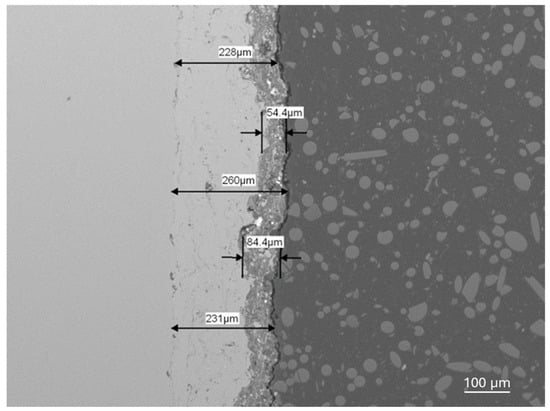
Figure 7.
The Al2O3 + ZrO2 + ReB2 + Re coating and the NiAl subcoating thicknesses for sample number 5 (Table 5), magnification 100×.
The thickness of coating no. 4 is about 100 μm. Coatings no. 5 and no. 6 (Table 5) have very similar phase compositions but differ in thickness. Coating no. 5 is approximately 80 μm thick, while coating no 6 (Table 5) is approximately 35 μm thick. The nanohardness tests were carried out using a KLA nanoindenter model G200 (Keysight Technologies, Santa Rosa, CA, USA) Hardness was calculated using the Oliver–Pharr method. Measurements were taken with a Vickers indenter with a maximum force of 96 mN. The nanohardness of coatings no. 4, no. 5, and no. 6 are, respectively, 937 ± 15, 919 ± 10, and 451 ± 10 (Table 5). The phase composition of samples No. 5 and No. 6 is very similar, but the coatings differ in thickness by more than two times. The relationship between hardness and coating thickness is clearly visible in this case. The HV0.1 nanohardnesses are lower than for the Al2O3 + ZrO2 coatings, which are about 1070, but the thickness of these the Al2O3 + ZrO2 coatings is about three times higher, which influences the stiffness of the system and the measurement result [11].
3.2. Ball-on-Disk Studies of the APS Coating
Ball-on-disk tribological tests (tribotester, THT CSM Instruments, part of the Anton Paar Group, Graz, Austria) were realized for the Al2O3 + ZrO2 + ReB2 + Re coatings (for samples no. 4 and no. 5). For comparison, studies were carried out for 316L (no. 1.4404, EN 10028-7 [31]) steel. Counter samples were Al2O3 balls, and the diameter of the balls was Ø6 mm. Sampling frequency during tests was 60 Hz, rub radius—6 mm, load 15 n, linear speed—10 cm/s, rotation speed—95.4 rpm, friction path—600 m. The roughnesses of the coating surfaces were measured using a Hommel-Etamic W20 profilometer (Jenoptic AG, Jena, Germany). The roughness coefficient Ra for sample no. 4 is 10.07 μm, and for sample no. 5, the Ra is 10.36 μm. Track analyses, along with the analysis of the wear profile of the samples after tribological tests, were performed on a Keyence VHX-7000 digital microscope (MC Keyence, Mechelen, Belgium). In Figure 8, the wear traces on coating surfaces and balls are presented.
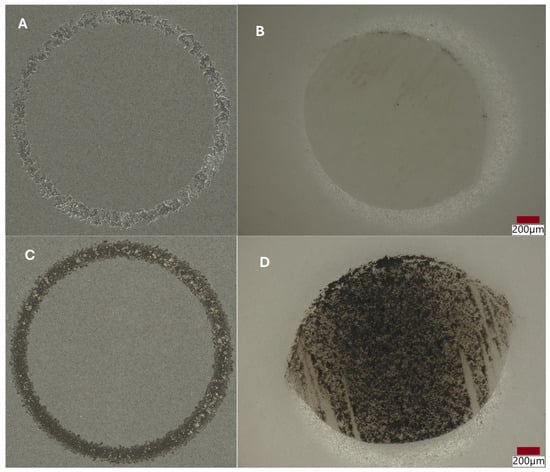
Figure 8.
Wear tracks of abrasions on the coatings and counter samples (balls), examined using an optical microscope after tribological tests: (A,B) coating no. 4; (C,D) coating no. 5.
Figure 9A,B presents the profiles for the wear tracks of sample no. 5 after the ball-on-disk dry tests with the Al2O3 counterpart.
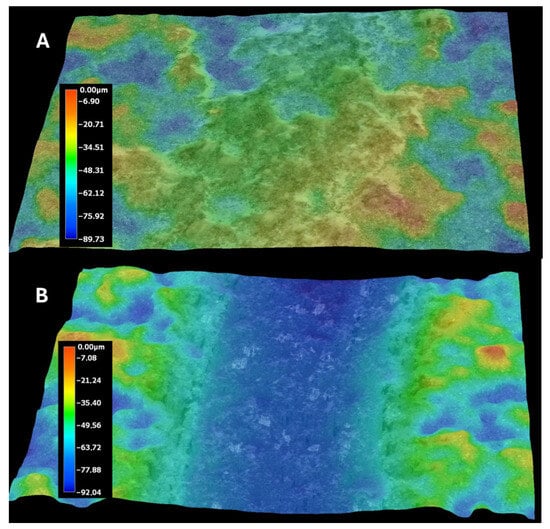
Figure 9.
Wear tracks of abrasions on the coatings: (A) sample no. 4; (B) sample no. 5.
Figure 10 presents the profiles for the wear tracks of sample no. 5 after the ball-on-disk dry tests with the Al2O3 counterpart.
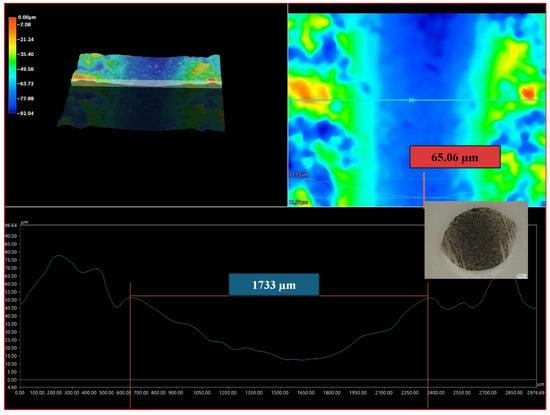
Figure 10.
Profiles for the wear track of sample no. 5, after the ball-on-disk dry test with the Al2O3 counterpart. The profile shown at the bottom of the figure corresponds to the line on the upper right side of the figure.
The wear process is influenced by the abrasive mechanism, mainly the micro-cutting sub-mechanism. Figure 11 clearly shows the scratches in the wear scar of sample no. 5.

Figure 11.
Microstructure of the wear scar of sample no. 5, after the ball-on-disk test.
Average coefficients of friction and samples mass loss for coatings no. 4 and 5 and the 316L steel are presented in Table 6.

Table 6.
Average coefficients of friction and samples mass loss for the Al2O3 + ZrO2 + ReB2 + Re coatings and the 316L steel.
3.3. Chemical Stability of the Al2O3 + ZrO2 + ReB2 + Re Coating
In Figure 12, the XRD phase composition of the Al2O3 + ZrO2 + ReB2 + Re coating after six months of storage at atmospheric pressure, in a plastic bag (polietylen PE-LD), is presented.
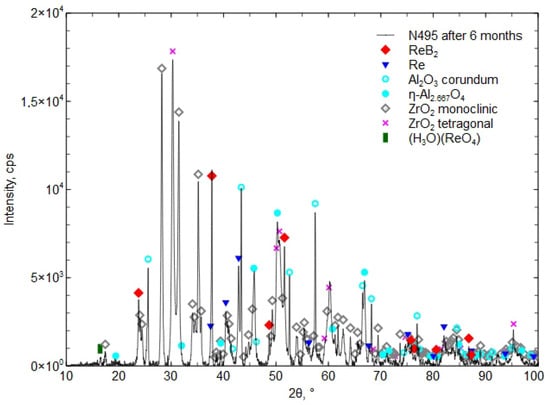
Figure 12.
The XRD phase composition of the Al2O3 + ZrO2 + ReB2 + Re coating after six months of storage in a plastic bag.
Figure 13 presents the XRD phase composition of the Al2O3 + ZrO2 + ReB2 + Re coating after 15 months of storage.
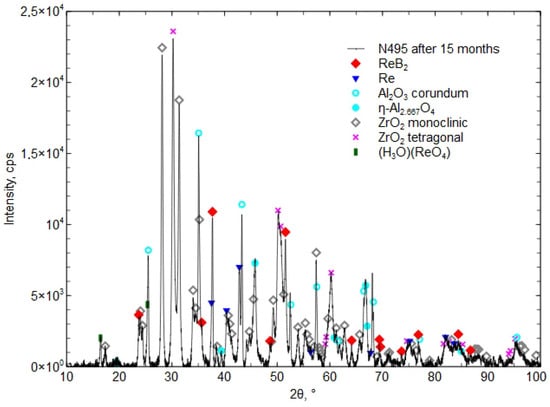
Figure 13.
The XRD phase composition of the Al2O3 + ZrO2 + ReB2 + Re coating after 15 months of storage in a plastic bag.
After 6 and 15 months, the coating was found to contain the hydronium rhenium oxide (H3O)(ReO4) [04-012-4121], which indicates a reaction with water. The presence of the boric acid H3BO3 phase was not detected, which indicates its small amount, below the detection limit of the XRD method (less than 2%).
4. Discussion
Research carried out by other scientists has shown that for the Al2O3-ZrO2 composite coatings obtained using APS, an amorphous phase was formed due to the rapid cooling and solidification of plasma spraying [11]. If the coating has a higher amorphous phase content, it always undergoes a greater volume reduction factor when operating at high temperatures, which is directly related to crystallization [32]. The change in the volume of the coatings during their operation contributes to a reduction in their mechanical properties. The introduction of crystallization nuclei, particularly high-melting particles of other phases, can facilitate the transformation of amorphous phases into crystalline structures. These nuclei act as centers for crystal growth, leading to the transformation of the surrounding amorphous material into a crystalline structure [33]. The X-ray diffraction pattern presented in Table 5 confirms the crystalline structure of the obtained coatings. The addition of high-melting rhenium boride in the present work was aimed at improving the mechanical properties of the coatings and introducing crystal nuclei into the applied coating in order to obtain the crystal structure of the entire Al2O3-ZrO2 coating. Rhenium diboride is a good candidate for nucleation of the crystallization process and improvement of the properties of Al2O3-ZrO2 coatings due to its high melting point, high hardness, and fracture toughness. However, it has the disadvantage of being hygroscopic. Reports in the literature indicate that encapsulation with Al2O3 can reduce or eliminate the reaction of ReB2 with water [20].
For the synthesis of Al2O3 + ReB2, the metallothermic reaction was used, with participation of aluminum as the stoichiometric reductant being consumed during the process. Past studies confirmed that the molar ratio B2O3/ReO2 = 1 guarantees the highest percentage of ReB2 in the product and better water resistance compared to syntheses with other B2O3/ReO2 ratios [28]. The composite material composition after synthesis is α Al2O3—75.8%, ReB2—23.2%, Re7B3—1% (Figure 1 and Figure 2, Table 1). After the material crushing and milling, the grain size d90 is 15.1 μm (90% of the particles have a diameter less than or equal to the indicated value).
Al2O3-ZrO2 is the initial matrix material of the APS coating, which constitutes 80 wt% of the coating material. The composition of this mixture was selected as 60 wt% Al2O3 and 40 wt% ZrO2. It is near the eutectic composition for the binary equilibrium phase diagram of the zirconia–alumina system [7]. According to previous studies, the flowability of the 60 wt% Al2O3 and 40 wt% ZrO2 melt was better than for other weight ratios for Al2O3 and ZrO2 APS composite coatings (35 wt% and 45 wt% of ZrO2) [11]. The addition of 20 wt% Al2O3—ReB2 composite powder was aimed at reducing the presence of the amorphous phase in the coating and increasing the abrasion resistance of the ReB2-containing coating.
Shear agglomeration was used to prepare agglomerates intended for the APS, which enables the transformation of fine, cohesive powders into strong, dense agglomerates. The quality of agglomerates depends on the quantity and viscosity of the binders and the grain size of the powders used [34]. Different amounts of binder—10 wt%, 15 wt%, and 20 wt%—were used in the form of hydroxypropylcellulose. The initial grain size of the commercial Al2O3-ZrO2 powders, d90, is 35.35 μm. The best agglomerate size distribution was obtained for 10 wt% of binder, which is shown in Table 2. Agglomerates are characterized by a growth characteristic of a layering mechanism, which is presented in Figure 3. The reason for the layered morphology of the agglomerates is the coarse powder. Despite this layered morphology, the agglomerates are characterized by a uniform distribution of elements, which is visible in Figure 4. In order to obtain better adhesion on steel substrates (316L), NiAl was deposited, and then the obtained agglomerates were deposited using different APS process parameters, as shown in Table 4. The choice of process parameters was determined by the largest share of ReB2 after the APS process. The results of the coating composition are presented in Table 5. The highest ReB2 content was characterized by samples 5 and 6. The thickness of the NiAl coating was about 230 μm (Figure 7). The share of ReB2 in coatings no. 5 and 6 is about 2.6 wt%. For these same materials, monoclinic and tetragonal ZrO2 were obtained. A commercial non-stabilized mixture was used for the tests; in future works, yttria-stabilized tetragonal ZrO2 (Y-TZP) should be used, which will be more beneficial for the phase stability of the coating. Y-TZP is outstanding in terms of low thermal conductivity and has good thermal shock resistance [35]. No amorphous phase was observed in the materials, which may have a positive effect on the abrasion resistance of new coatings (Table 6). The surface of the APS coating is very rough. Figure 5 confirms the presence of fully and partially melted powders. The elements distribution on the cross section confirms the presence of large rhenium or ReB2 particles (see Figure 6).
ReB2 has a very high melting point, about 2400 °C, and a very low self-diffusion coefficient [36]. The coatings are characterized by the porosity typical of APS coatings. Voids are visible in Figure 6. The larger amount of ReB2 particles accumulates at the bottom of the coating (green spots in Figure 6), although single unmelted particles are also visible on the surface. Pores occur in contact with the particles. The reason for the accumulation of pores near ReB2 may be the large unmelted ReB2 particles falling to the Al2O3-ZrO2 coating bottom (due to their high density).
Abrasion resistance tests were carried out. Because the microstructure of APS coatings is characterized by high porosity, a lamellar structure, and unmolten particles, the wear properties of APS coatings consequently depend strongly on spraying conditions (current intensity, distance of the torch nozzle from the substrate, share of gases, and gas flow) that affect the plasma temperature and on variables related to the injected powder (particle size, feeding rate), as well as the substrate’s mechanical properties [37]. The coatings were deposited under various conditions shown in Table 5. Coating no. 4 is characterized by the high ReB2 + Re content and a high η-Al2O3 content (1.2 wt%—ReB2, 1.5 wt%—Re, 14.2 wt%—α-Al2O3, 48.7 wt% η-Al2O3, 19.0 wt%—monoclinic ZrO2., 15.5 wt%—tetragonal—ZrO2). The η-Al2O3 phase has good mechanical properties [30], making coating no. 4 very abrasion resistant, as evidenced by the low mass loss in the ball-on-disk test, presented in Table 6 and the wear track abrasion profile presented in Figure 8A,B. The wear properties of coating no. 5 (2.6 wt%—ReB2, 0.8 wt%—Re, 30.9 wt%—α-Al2O3, 32.4 wt% η-Al2O3, 21.2 wt%—monoclinic ZrO2, 12.2 wt%—tetragonal—ZrO2) are completely different. η-Al2O3 content is 16.3% lower than in sample no. 5. The material is characterized by significant wear (see Table 6 and Figure 9B). The depth of wear is similar to the thickness of the layer. The micro-cutting abrasive mechanism is visible in Figure 10. However, taking into account the large amount of solid particles visible on the surface of the coatings, resulting in a roughness Ra of 10 μm, it is necessary to indicate a three-body abrasion mechanism in the first stage of the wear test, in which the material abrasion increases (Table 6). The wear mechanism in the first stage of the test should change, thanks to the reduction in the amount of large, hard ReB2 particles on the coating surface and consequently in the debris (during the wear process). This can be achieved by extending the milling time of the material immediately after the coating material synthesis process.
Investigations showed small phase changes in the coating after 6 months of storage. The XRD diffraction pattern in Figure 12 shows a small peak (H3O)(ReO4) [04-012-4121], which “is identical to phase called HReO4 in [38] (Database Comments). After 15 months, an increase in the presence of the phase (H3O)(ReO4) [04-012-4121] was found, which confirmed the reaction with water from moisture (Figure 12 and Figure 13). The presence of H3BO3 was not confirmed by the XRD method. The proposed concept, involving a composite coating composed of a matrix forming a eutectic that melts at a lower temperature and encapsulates ReB2 grains during atmospheric plasma spraying (APS), does not fully prevent ReB2 from interacting with atmospheric moisture. Nevertheless, it contributes to delaying the decomposition of both ReB2 and residual metallic rhenium. After 15 months of storage in a plastic bag, the phase composition revealed the presence of (H3O)(ReO4) (chemically equivalent to HReO4), while ReB2 and residual rhenium remained detectable. Researchers in [19] reported that after 12 months of storing ReB2 powder (produced by the mechanochemical method) in air without a protective atmosphere, the phase composition revealed the presence of HReO4 and boric acid in addition to ReB2. Other researchers [38] noticed that the composition of ReB2 powder (obtained by mechanical milling), after 2 months stored in ambient conditions in the presence of humidity and oxygen, contained the phases boric acid, ReO3, and HReO4. After 26 months, the powder completely decomposed [39].
The porosity of the coating has a decisive influence on the ReB2 reaction process. The ReB2 milling, greater dispersion of small particles, separation from pores, and greater densification of the coating material can result in improved chemical resistance of coatings. The issue is very similar to the presence of Al4C3 in steels and cast irons, where cutting off the compound from the porosity also improves the material properties [40].
5. Conclusions
The introduction of rhenium boride and rhenium as nucleation centers affected the crystallization process of the amorphous phase in the Al2O3 + ZrO2 + ReB2 + Re coating, as a result of which there is no amorphous phase in the APS coating.
The phase composition of APS coatings depends strongly on spraying conditions. The Al2O3 + ZrO2 + ReB2 + Re coating, with a high content of η-Al2O3 (about 50 wt%), is characterized by very high abrasion resistance.
ReB2 does not melt, which affects the higher roughness of the coating, and its initial particle size should be below 15 μm. The lower grain size of ReB2 particles in the Al2O3 + ReB2 phase could eliminate ReB2 clusters and reduce the porosity of APS coatings.
Chemical stability studies of the encapsulated ReB2 in Al2O3 indicate the presence of a small amount of reaction with water (from moisture) products. Reducing the number of pores in the coating can limit or even eliminate the ReB2 reaction with water.
Author Contributions
Conceptualization, A.W., K.C. and L.J.; methodology, K.C., K.B., M.C., A.C., M.L. and A.B.; software, G.M., A.C., and G.M.; validation, A.W., K.C., and L.J.; investigation, K.C., K.B., M.C., A.C., M.L. and A.B.; data curation, K.C., M.C., A.B. and A.C.; writing—original draft preparation, L.J. and K.C.; writing—review and editing, L.J. and K.C.; visualization, M.C., K.B. and G.M.; supervision; L.J. and A.W.; project administration, K.C.; funding acquisition, A.W. and K.C. All authors have read and agreed to the published version of the manuscript.
Funding
The submission of the article was financed by a subsidy from the Ministry of Education and Science of the Republic of Poland. Part of the study was carried out as part of the Implementation Doctorate Program in the Łukasiewicz Research Network—Institute of Non-Ferrous Metals.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APS | Atmospheric plasma spray |
| CPCD | Confined-Plume Chemical Deposition |
| PLD | Pulsed laser deposition |
References
- Prashar, G.; Vasudev, H.; Thakur, L. Thermal spraying fundamentals. Process applications, challenges, and future market. In Thermal Spray Coatings, 1st ed.; Thakur, L., Vasudev, H., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 2–36. [Google Scholar]
- Sathish, M.; Radhika, N.; Saleh, B. Microstructure and dry sliding wear evaluation of functionally graded coating deposited via atmospheric plasma spray. Sci. Rep. 2024, 14, 22272. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, F.; Wu, X.; He, J.; Li, S. Microstructure and phase formation of atmospheric plasma sprayed YAG coatings. Surf. Coat. Technol. 2012, 466, 129614. [Google Scholar] [CrossRef]
- Heberlein, J.; Fauchais, P.; Boulos, M. Thermal Spray Fundamentals: From Powder to Part, 1st ed.; Springer: New York, NY, USA, 2014; pp. 12–70. [Google Scholar]
- Dwivedi, G.; Viswanathan, V.; Sampath, S.; Shyam, A.; Lara-Curzio, E. Fracture toughness of plasma-sprayed thermal barrier ceramics: Influence of processing, microstructure, and thermal aging. J. Am. Ceram. Soc. 2014, 97, 2736–2744. [Google Scholar] [CrossRef]
- Shan, X.; Huang, T.; Luo, L.; Lu, J.; Cai, H.; Zhao, J.; Sheng, G.; Zhao, X. Automatic Recognition of Microstructures of Air-Plasma-Sprayed Thermal Barrier Coatings Using a Deep Convolutional Neural Network. Coatings 2023, 13, 29. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.J. Amorphous phase formation of the pseudo-binary Al2O3–ZrO2 alloy during plasma spray processing. J. Mater. Sci. 1999, 34, 29–33. [Google Scholar] [CrossRef]
- Sodeoka, S.; Suzuki, M.; Inoue, T. Thermal stability and mechanical properties of plasma sprayed Al2O3/ZrO2 nano-composite coating. Key Eng. Mater. 2006, 317–318, 513–516. [Google Scholar] [CrossRef]
- Tarasi, F.; Medraj, M.; Dolatabadi, A.; Oberste-Berghaus, J.; Moreau, C. Amorphous and crystalline phase formation during suspension plasma spraying of the alumina–zirconia composite. J. Eur. Ceram. Soc. 2011, 31, 2903–2913. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Lai, X.; Zheng, X.; Jia, R.; Yuan, X. Influence of Different Heat Treatment Temperatures on the Microstructure, Corrosion, and Mechanical Properties Behavior of Fe-Based Amorphous/Nanocrystalline Coatings. Coatings 2019, 9, 858. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Yang, Y.; Chu, Z.; Chen, X.; Wang, L.; Liu, Z.; Dong, Y.; Yan, D.; Zhang, J.; Kang, Z. Microstructure and properties of Al2O3-ZrO2 composite coatings prepared by air plasma spraying. Appl. Surf. Sci. 2018, 431, 93–100. [Google Scholar] [CrossRef]
- Chraska, T.; Neufuss, K.; Dubský, J.; Ctibor, P.; Rohan, P. Fabrication of bulk nanocrystalline alumina–zirconia materials. Ceram. Int. 2008, 34, 1229–1236. [Google Scholar] [CrossRef]
- La Placa, S.J.; Post, B. The crystal structure of rhenium diboride. Acta Crystallogr. 1962, 15, 97–99. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Cumberland, R.W.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Korsukova, M.M.; Aizawa, T. High-temperature hardness of ReB2 single crystals. J. Alloys Compd. 2009, 477, L28–L29. [Google Scholar] [CrossRef]
- Long, R.; Dai, Y.; Jin, H.; Huang, B. Structural, elastic, and electronic properties of ReB2: A first -principles calculation. Phys. Res. Int. 2008, 2008, 293517. [Google Scholar] [CrossRef]
- Portnoi, K.I.; Romashov, V.M. State diagram of the rhenium-boron system. Porosh. Met. 1968, 8, 41–44. [Google Scholar]
- Maździarz, M.; Mościcki, T. Structural, mechanical, optical, thermodynamical and phonon properties of stable ReB2 polymorphs from density functional calculations. J. Alloys Compd. 2016, 657, 878–888. [Google Scholar] [CrossRef]
- Orlovskaya, N.; Xie, Z.; Klimov, M.; Heinrich, H.; Restrepo, D.; Blair, R.; Suryanarayana, C. Mechanochemical synthesis of ReB2 powder. J. Mater. Res. 2011, 26, 2772. [Google Scholar] [CrossRef]
- Bliem, P.; Mráz, S.; Sen, S.; Hunold, O.; Schneider, J.M. Self-passivating (Re,Al)B2 coatings synthesized by magnetron sputtering. Sci. Rep. 2018, 8, 15570. [Google Scholar] [CrossRef] [PubMed]
- Wicher, B.; Chodun, R.; Trzciński, M.; Lachowski, A.; Nowakowska-Langier, K.; Ibrahim, S.H.; Jaroszewicz, J.; Kubiś, M.; Grzanka, E.; Zdunek, K. Application of the plasma Surface sintering conditions in the synthesis of ReBx-Ti targets for hard films deposition in magnetron sputtering technique. Int. J. Refract. Met. Hard Mater. 2022, 103, 105756. [Google Scholar] [CrossRef]
- Ivanov, B.L.; Wellons, M.S.; Lukehart, C.M. Confined-Plume Chemical Deposition: Rapid Synthesis of Crystalline Coatings of Known Hard or Superhard Materials on Inorganic or Organic Supports by Resonant IR Decomposition of Molecular Precursors. J. Am. Chem. Soc. 2009, 131, 11744–11750. [Google Scholar] [CrossRef] [PubMed]
- Rylski, A. ReB2 coating on carbide and high speed steel substrates. Inżynieria Mater. (Mater. Eng.) 2013, 6, 833–836. (In Polish) [Google Scholar]
- Latini, A.; Barinov, S.M.; Rau, J.V.; Ferro, D.; Teghil, R.; Albertini, V.R.; Barinov, S.M. Superhard Rhenium Diboride Films: Preparation and Characterization. Chem. Mater. 2008, 20, 4507–4511. [Google Scholar] [CrossRef]
- Chrzanowska, J.; Hoffman, J.; Denis, P.; Giżyński, M.; Mościcki, T. The effect of process parameters on rhenium diboride films deposited by PLD. Surf. Coat. Technol. 2015, 277, 15–22. [Google Scholar] [CrossRef]
- Shen, L.; Tesfaye, F.; Li, X.; Lindberg, D.; Taskinen, P. Review of rhenium extraction and recycling technologies from primary and secondary resources. Min. Eng. 2021, 161, 106719. [Google Scholar] [CrossRef]
- Nishiyama, K.; Nakamur, T.; Utsumi, S.; Sakai, H.; Abe, M. Preparation of ultrafine boride powders by metallothermic reduction method 16th International Symposium on Boron, Borides and Related Materials. J. Phys. Conf. Ser. 2009, 176, 012043. [Google Scholar] [CrossRef]
- Czechowska, K.; Wrona, A.; Onderka, B.; Krzywiecki, M.; Warski, T.; Pęcak, K.; Czerny, M.; Jaworska, L. Synthesis of composite powders and chemical resistance of the products to moisture. Int. J. Refract. Met. Hard Mater. 2023, 113, 106175. [Google Scholar] [CrossRef]
- Chitu, T.M.; Oulahna, D.; Hemati, M. Wet granulation in laboratory scale high shear mixers: Effect of binder properties. Powder Technol. 2011, 206, 25–33. [Google Scholar] [CrossRef]
- Baronskiy, M.G.; Tsybulya, S.V.; Kostyukov, A.I.; Zhuzhgov, A.V.; Snytnikov, V.N. Structural properties investigation of different alumina polymorphs (η-, γ-, χ-, θ-, α-Al2O3) using Cr3+ as a luminescent probe. J. Lumin. 2022, 242, 118554. [Google Scholar] [CrossRef]
- EN 10028-7; Flat Products Made of Steels for Pressure purposes—Part 7: Stainless Steels. CEN European Committee for Standardization: Brussels, Belgium, 2000.
- Sun, J.; Wang, J.; Zhou, X.; Dong, S.; Deng, L.; Jiang, J.; Cao, X. Microstructure and thermal cycling behavior of plasma-sprayed LaMgAl11O19 coatings. Ceram. Int. 2018, 44, 5572–5580. [Google Scholar] [CrossRef]
- Myerson, A.S.; Erdemir, D.; Lee, A.Y. Crystal nucleation. In Handbook of Industrial Crystallization; Cambridge University Press: Cambridge, UK, 2019; pp. 76–114. [Google Scholar] [CrossRef]
- Johansen, A.; Schæfer, T. Effects of interactions between powder particle size and binder viscosity on agglomerate growth mechanisms in a high shear mixer. Eur. J. Pharm. Sci. 2001, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Nettleship, I.; Stevens, R. Tetragonal zirconia polycrystal (TZP)—A review. Int. J. High Technol. Ceram. 1987, 3, 297–309. [Google Scholar] [CrossRef]
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on ultra-high temperature boride ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Psyllaki, P.; Alexopoulos, N. Tribological behaviour of plasma-sprayed Al2O3 coatings under se-vere wear conditions. Wear 2000, 237, 197–204. [Google Scholar] [CrossRef]
- Wltschek, G.; Svoboda, I.; Fuess, H. The Crystal Structure of Solid Perrhenic Acid Monohydrate. Z. Anorg. Allg. Chem. 1993, 619, 1679–1681. [Google Scholar] [CrossRef]
- Granados -Fitch, M.G.; Quintana-Melgoza, J.M.; Juarez-Arellano, E.A.; Avalos-Borja, M. Chemical stability of superhard rhenium diboride at oxygen and moisture ambient environmental conditions prepared by mechanical milling. J. Am. Ceram. Soc. 2018, 101, 3148–3155. [Google Scholar] [CrossRef]
- Gilewski, R.; Kopyciński, D.; Guzik, E.; Szczęsny, A. Shaping the microstructure of high-aluminum cast iron in terms of the phenomenon of spontaneous decomposition generated by the presence of aluminum carbide. Materials 2021, 14, 5993. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).