Nanocomposite Polysulfone/CB Modified by Melt Extrusion and Solution Mixing for Enhanced Removal of Uremic Toxins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Carbon Black
2.3. Preparation of Nanocomposites
2.3.1. Melt-Extrusion Process
2.3.2. Mixing Solution
2.4. Characterization
2.5. Urea Adsorption
2.6. Hemolysis Assay
3. Results and Discussions
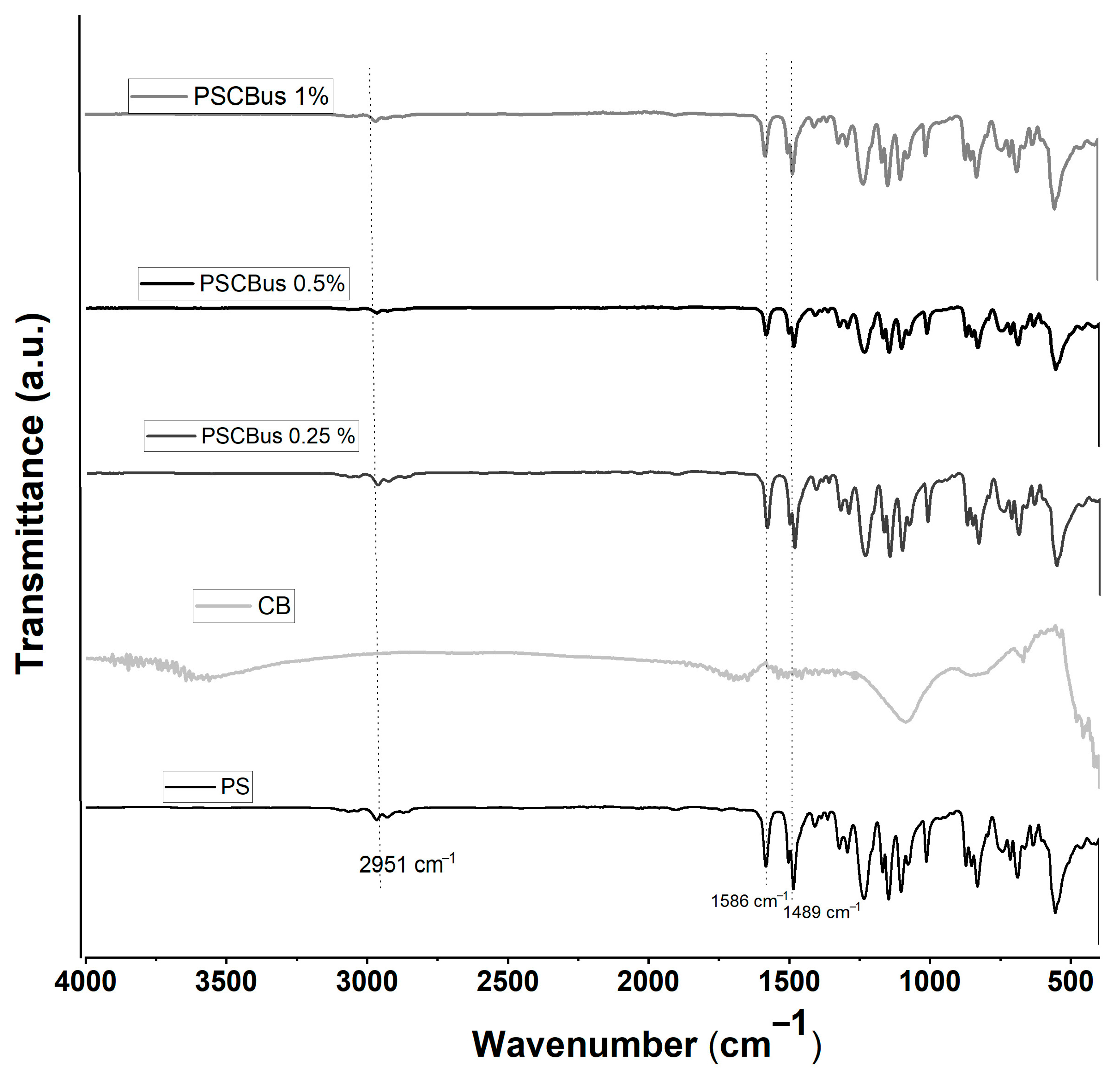
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
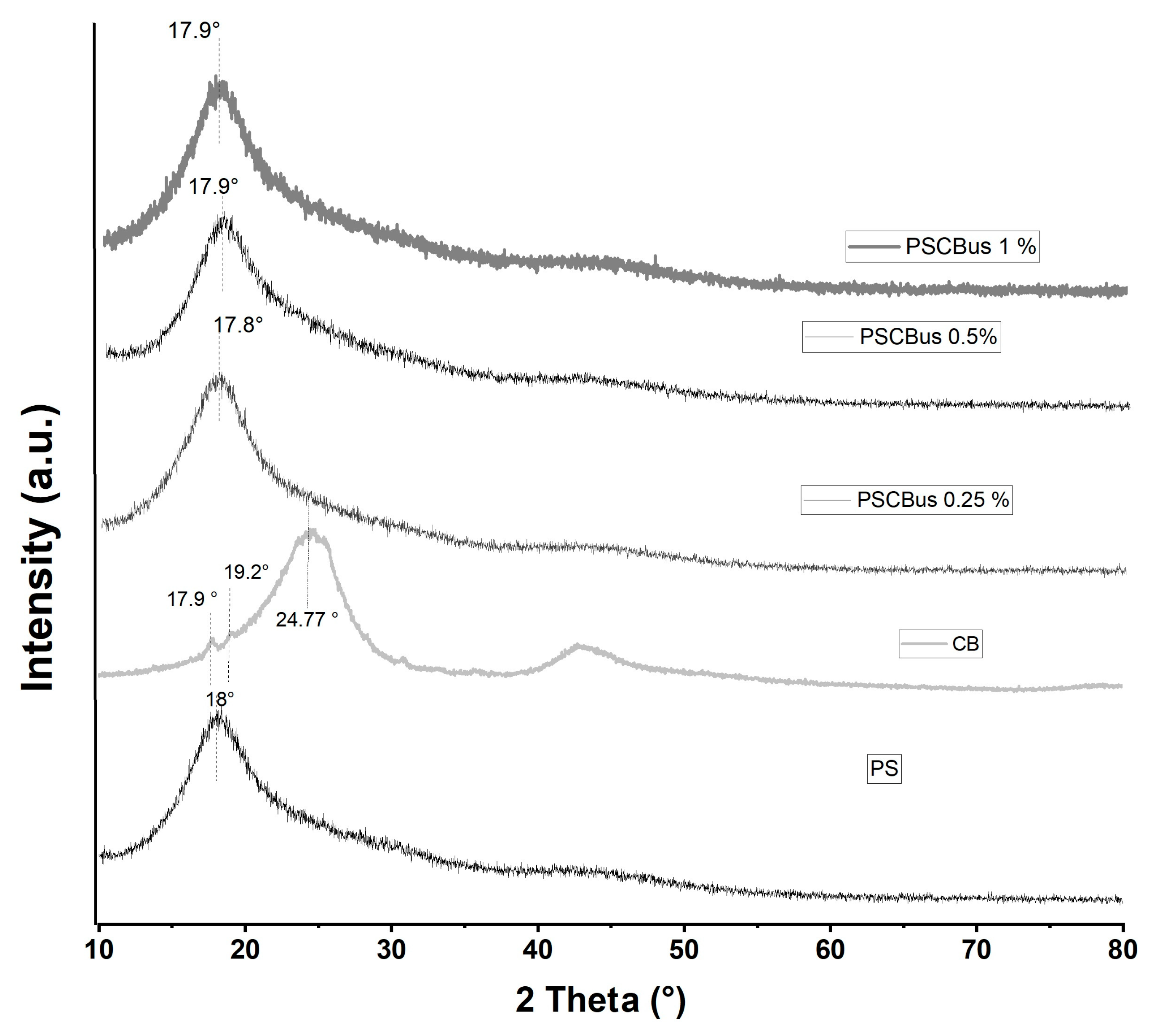
3.2. X-Ray Diffraction (XRD)
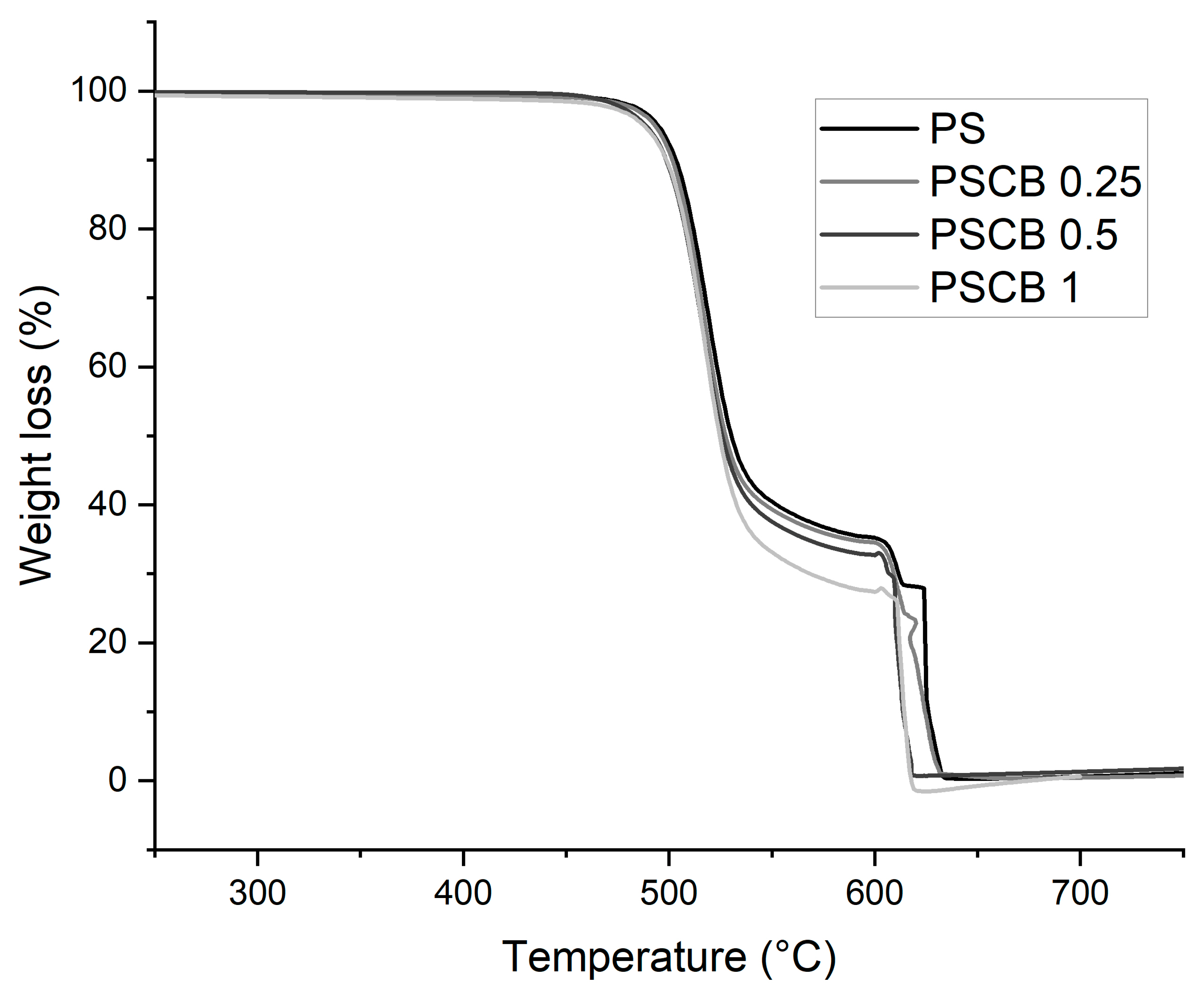
3.3. Thermogravimetric Analysis (TGA)
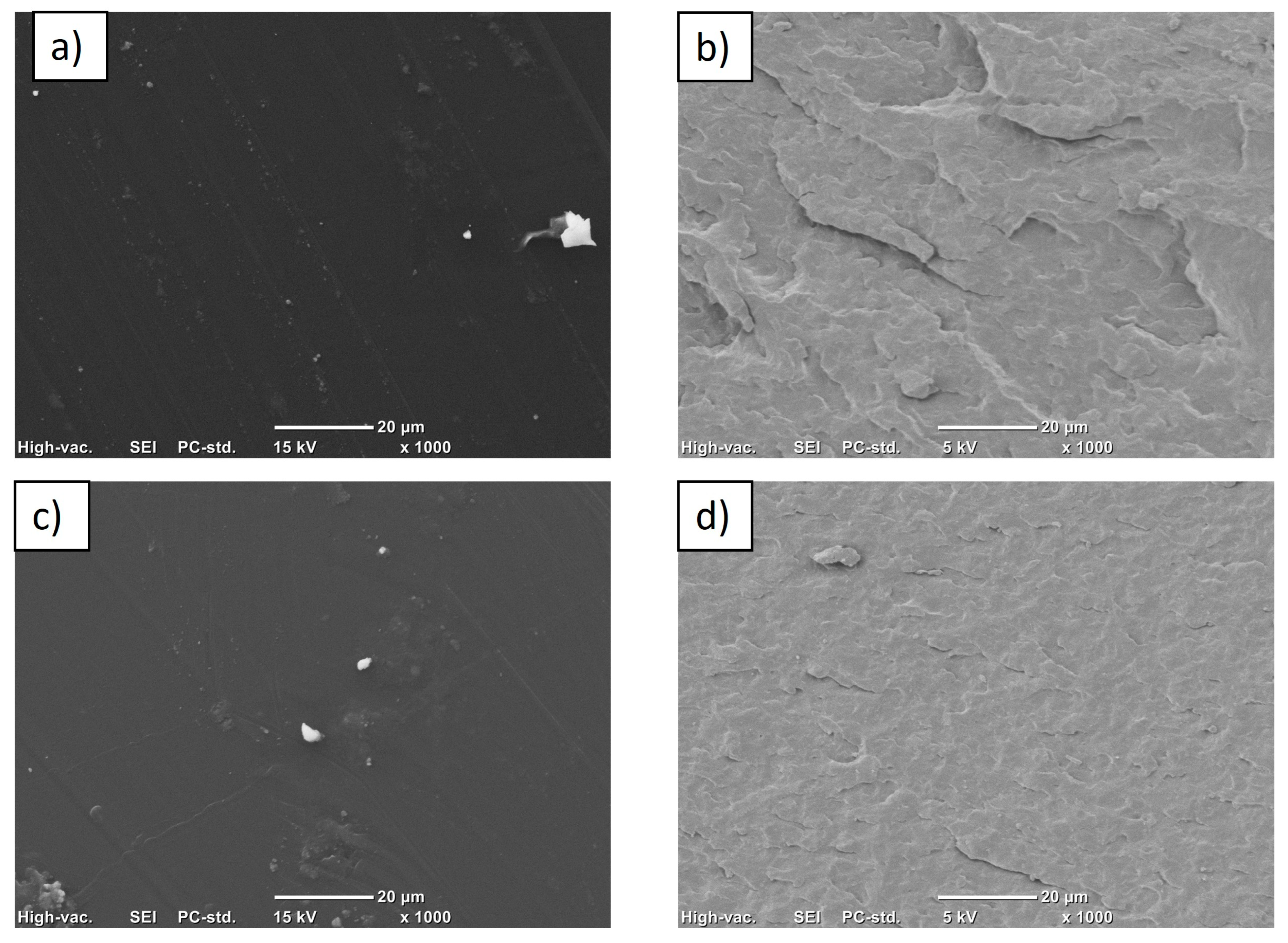
3.4. Scanning Electron Microscopy (SEM)
3.5. Urea Adsorption
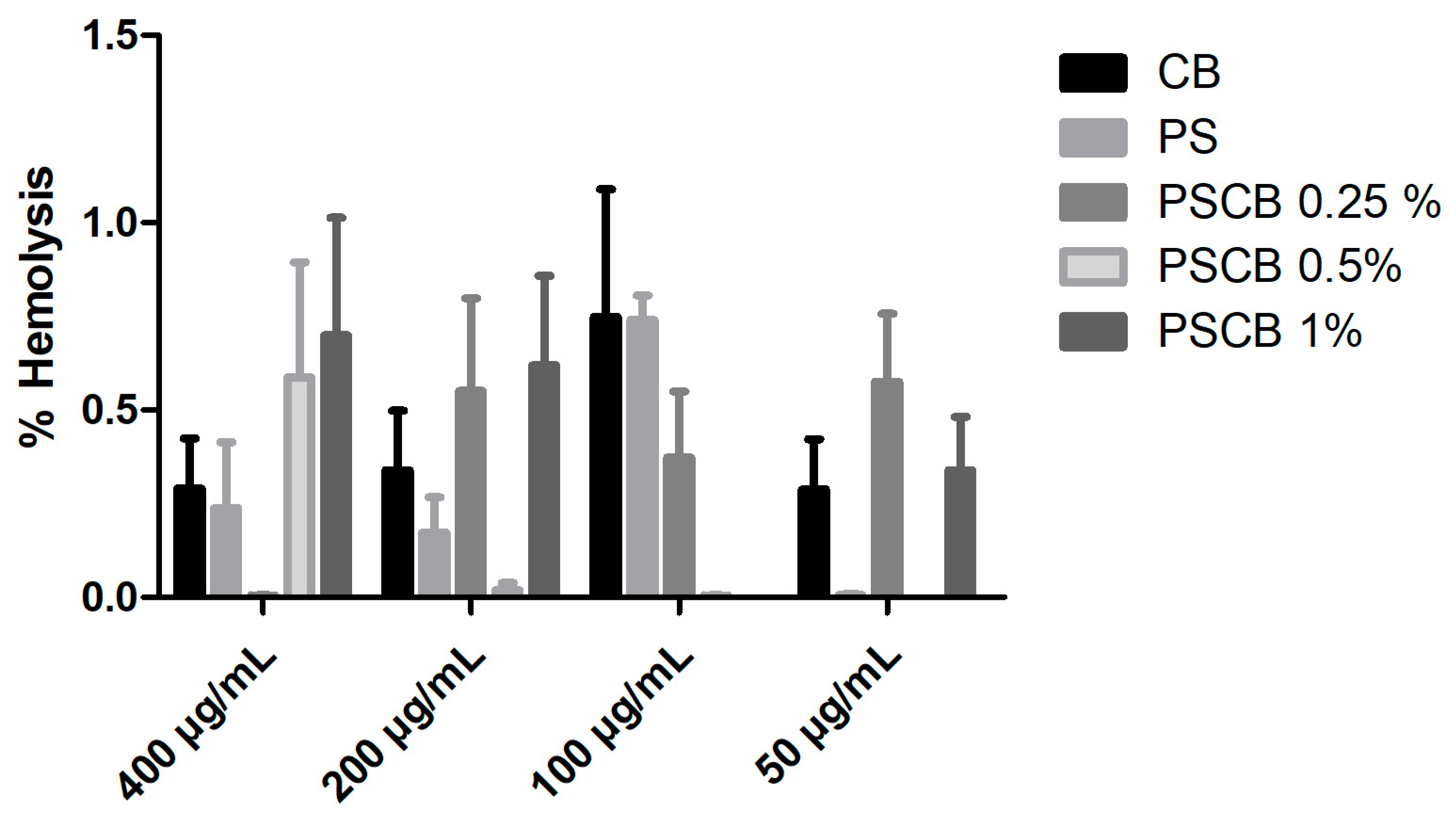
3.6. Hemolysis Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agudelo-Botero, M.; Valdez-Ortiz, R.; Giraldo-Rodríguez, L.; González-Robledo, M.C.; Mino-León, D.; Rosales-Herrera, M.F.; Cahuana-Hurtado, L.; Rojas-Russell, M.E.; Dávila-Cervantes, C.A. Overview of the burden of chronic kidney disease in Mexico: Secondary data analysis based on the Global Burden of Disease Study 2017. BMJ Open 2020, 10, e035285. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Durán, A.; Méndez-Bueno, J.F.; Tapia-Yáñez, T.; Montes, A.M.; Aguilar-Sánchez, L. Epidemiología de la insuficiencia renal crónica en México. Diálisis Traspl. 2010, 31, 7–11. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Thajudeen, B.; Issa, D.; Roy-Chaudhury, P. Advances in hemodialysis therapy. Fac. Rev. 2023, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Stasi, A.; Caggiano, G.; Squiccimarro, E.; Losappio, V.; Fiorentino, M.; Alfieri, C.; Stallone, G.; Gesualdo, L.; Castellano, G. Enhancing Immune Protection in Hemodialysis Patients: Role of the Polymethyl Methacrylate Membrane. Blood Purif. 2023, 52 (Suppl. 1), 49–61. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Rodrigue, D. A review on porous polymeric membrane preparation. Part I: Production techniques with polysulfone and poly (vinylidene fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J. Polymeric membranes for biomedical applications. Phys. Sci. Rev. 2023, 8, 1181–1211. [Google Scholar] [CrossRef]
- Zaman, S.U.; Rafiq, S.; Ali, A.; Mehdi, M.S.; Arshad, A.; Rehman, S.-U.; Muhammad, N.; Irfan, M.; Khurram, M.S.; Zaman, M.K.U.; et al. Recent advancement challenges with synthesis of biocompatible hemodialysis membranes. Chemosphere 2022, 307, 135626. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.I.; Sandru, M. Composite Hybride Membrane Materials for Artificial Organs. In Handbook of Bioceramics and Biocomposites; Springer: Cham, Switzerland, 2015; pp. 407–429. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; San-Miguel, V.; Baselga, J.; Fernández-Blázquez, J.P.; Gedler, G.; Ozisik, R.; Cabanelas, J.C. Effect of polysulfone brush functionalization on thermo-mechanical properties of melt extruded graphene/polysulfone nanocomposites. Carbon 2019, 151, 84–93. [Google Scholar] [CrossRef]
- Radu, E.R.; Voicu, S.I. Functionalized Hemodialysis Polysulfone Membranes with Improved Hemocompatibility. Polymers 2022, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Cheng, C. Morphology Control of Polysulfone Membranes in Filtration Processes: A Critical Review. ChemBioEng Rev. 2015, 2, 22–43. [Google Scholar] [CrossRef]
- Pandele, A.M.; Serbanescu, O.S.; Voicu, S.I. Polysulfone Composite Membranes with Carbonaceous Structure. Synthesis and Applications. Coatings 2020, 10, 609. [Google Scholar] [CrossRef]
- Nechifor, G.; Voicu, S.; Nechifor, A.; Garea, S. Nanostructured hybrid membrane polysulfone-carbon nanotubes for hemodialysis. Desalination 2009, 241, 342–348. [Google Scholar] [CrossRef]
- Pandele, A.M.; Oprea, M.; Dutu, A.A.; Miculescu, F.; Voicu, S.I. A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis. Polymers 2021, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Abidin, M.N.Z.; Goh, P.S.; Ismail, A.F.; Othman, M.H.D.; Hasbullah, H.; Said, N.; Kadir, S.H.S.A.; Kamal, F.; Abdullah, M.S.; Ng, B.C. Antifouling polyethersulfone hemodialysis membranes incorporated with poly (citric acid) polymerized multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 68, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Gray, C.A.; Reznek, S.A.; Mahmud, K.; Kutsovsky, Y. Carbon Black. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Kausar, A. Contemporary applications of carbon black-filled polymer composites: An overview of essential aspects. J. Plast. Film Sheeting 2018, 34, 256–299. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Zubkiewicz, A.; Subocz, J.; Stanik, R.; Szczepaniak, J. Enhanced Functional Properties of Low-Density Polyethylene Nanocomposites Containing Hybrid Fillers of Multi-Walled Carbon Nanotubes and Nano Carbon Black. Polymers 2020, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Toghchi, M.J.; Campagne, C.; Cayla, A.; Bruniaux, P.; Loghin, C.; Cristian, I.; Burgnies, L.; Chen, Y. Electrical conductivity enhancement of hybrid PA6,6 composite containing multiwall carbon nanotube and carbon black for shielding effectiveness application in textiles. Synth. Met. 2019, 251, 75–84. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, S.; Zhang, Y.; Zhang, Y. Effect of different carbon fillers on the properties of PP composites: Comparison of carbon black with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2006, 102, 4823–4830. [Google Scholar] [CrossRef]
- Nayak, L.; Chaki, T.K.; Khastgir, D. Super Heat-Resistant Conductive Nanocomposites Based on Polysulfone–Carbon Nanofillers. Polym. Plast. Technol. Eng. 2015, 54, 315–323. [Google Scholar] [CrossRef]
- Khan, A.; Sherazi, T.A.; Khan, Y.; Li, S.; Naqvi, S.A.R.; Cui, Z. Fabrication and characterization of polysulfone/modified nanocarbon black composite antifouling ultrafiltration membranes. J. Membr. Sci. 2018, 554, 71–82. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Cabello-Alvarado, C.J.; Pérez-Alvarez, M.; Reyes-Rodríguez, P.; Inam, F.; Cortés-Hernández, D.A.; Quiñones-Jurado, Z.V. Synthesis of Nylon 6/Modified Carbon Black Nanocomposites for Application in Uric Acid Adsorption. Materials 2020, 13, 5173. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-T.; Wang, C.-C.; Lee, H.-T.; Wu, C.-L.; Gu, J.-H.; Suen, M.-C. Preparation and Characterization of Polysulfone/Nanosilver-Doped Activated Carbon Nanocomposite. Polym. Sci. Ser. A 2018, 60, 90–101. [Google Scholar] [CrossRef]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Alosaimi, A.M. Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions. Polymers 2021, 13, 2792. [Google Scholar] [CrossRef] [PubMed]
- Bouchareb, S.; Doufnoune, R.; Riahi, F.; Cherif-Silini, H.; Belbahri, L. High performance of polysulfone/graphene oxide-silver nanocomposites with excellent antibacterial capability for medical applications. Mater. Today Commun. 2021, 27, 102297. [Google Scholar] [CrossRef]
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B Eng. 2015, 72, 108–115. [Google Scholar] [CrossRef]
- Song, J.-P.; Tian, K.-Y.; Ma, L.-X.; Li, W.; Yao, S.-C. The effect of carbon black morphology to the thermal conductivity of natural rubber composites. Int. J. Heat Mass Transf. 2019, 137, 184–191. [Google Scholar] [CrossRef]
- Nayak, L.; Khastgir, D.; Chaki, T.K. A mechanistic study on electromagnetic shielding effectiveness of polysulfone/carbon nanofibers nanocomposites. J. Mater. Sci. 2013, 48, 1492–1502. [Google Scholar] [CrossRef]
- Khvatov, A.V.; Brevnov, P.N.; Shilkina, N.G.; Lomakin, S.M. Thermal and Physical and Mechanical Properties of Polysulfone Composites with Carbon Nanotubes. Russ. J. Phys. Chem. B 2019, 13, 519–524. [Google Scholar] [CrossRef]
- Nayak, L.; Rahaman, M.; Khastgir, D.; Chaki, T.K. Thermal and electrical properties of carbon nanotubes based polysulfone nanocomposites. Polym. Bull. 2011, 67, 1029–1044. [Google Scholar] [CrossRef]
- Mohammad, H.; Stepashkin, A.A.; Tcherdyntsev, V.V. Formation of Conductive Networks in Polysulfone Filled with Graphite-Derived Materials. Appl. Sci. 2024, 14, 2756. [Google Scholar] [CrossRef]
- Zailani, M.Z.; Ismail, A.F.; Goh, P.S.; Kadir, S.H.S.A.; Othman, M.H.D.; Hasbullah, H.; Abdullah, M.S.; Ng, B.C.; Kamal, F.; Mustafar, R. Immobilizing chitosan nanoparticles in polysulfone ultrafiltration hollow fibre membranes for improving uremic toxins removal. J. Environ. Chem. Eng. 2021, 9, 106878. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Medellin-Banda, D.; Ávila-Orta, C.; Cadenas-Pliego, G.; Sáenz-Galindo, A.; Radillo-Radillo, R.; Lara-Sánchez, J.; Melo-Lopez, L. Non-woven fabrics based on Nylon 6/carbon black-graphene nanoplatelets obtained by melt-blowing for adsorption of urea, uric acid and creatinine. Mater. Lett. 2022, 320, 132382. [Google Scholar] [CrossRef]
- Pajnič, M.; Drašler, B.; Šuštar, V.; Krek, J.L.; Štukelj, R.; Šimundić, M.; Kononenko, V.; Makovec, D.; Hägerstrand, H.; Drobne, D.; et al. Effect of carbon black nanomaterial on biological membranes revealed by shape of human erythrocytes, platelets and phospholipid vesicles. J. Nanobiotechnol. 2015, 13, 28. [Google Scholar] [CrossRef] [PubMed]






| Scheme | Method | Description |
|---|---|---|
| PS | Extrusion and mix solution | Pristine PS |
| CB-AC | Extrusion and mix solution | Carbon black |
| PSCB-0.25% | Extrusion | PS with 0.25% of carbon black modified with citric acid |
| PSCB-0.5% | Extrusion | PS with 0.5% of carbon black modified with citric acid |
| PSCB-1% | Extrusion | PS with 1% of carbon black modified with citric acid |
| PSCBus-0.25% | Ultrasound | PS with 0.25% of carbon black modified with citric acid |
| PSCBus-0.5% | Ultrasound | PS with 0.5% of carbon black modified with citric acid |
| PSCBus-1% | Ultrasound | PS with 1% of carbon black modified with citric acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Guel, M.; Cabello-Alvarado, C.J.; Nery-Flores, S.D.; Cadenas-Pliego, G.; Avila-Orta, C.; Pérez-Alvarez, M.; Martínez-Carrillo, D.; Quiñones-Jurado, Z.V.; Caero, L.C. Nanocomposite Polysulfone/CB Modified by Melt Extrusion and Solution Mixing for Enhanced Removal of Uremic Toxins. Materials 2025, 18, 3352. https://doi.org/10.3390/ma18143352
Andrade-Guel M, Cabello-Alvarado CJ, Nery-Flores SD, Cadenas-Pliego G, Avila-Orta C, Pérez-Alvarez M, Martínez-Carrillo D, Quiñones-Jurado ZV, Caero LC. Nanocomposite Polysulfone/CB Modified by Melt Extrusion and Solution Mixing for Enhanced Removal of Uremic Toxins. Materials. 2025; 18(14):3352. https://doi.org/10.3390/ma18143352
Chicago/Turabian StyleAndrade-Guel, Marlene, Christian J. Cabello-Alvarado, Sendar Daniel Nery-Flores, Gregorio Cadenas-Pliego, Carlos Avila-Orta, Marissa Pérez-Alvarez, Diego Martínez-Carrillo, Zoe V. Quiñones-Jurado, and Luis Cedeño Caero. 2025. "Nanocomposite Polysulfone/CB Modified by Melt Extrusion and Solution Mixing for Enhanced Removal of Uremic Toxins" Materials 18, no. 14: 3352. https://doi.org/10.3390/ma18143352
APA StyleAndrade-Guel, M., Cabello-Alvarado, C. J., Nery-Flores, S. D., Cadenas-Pliego, G., Avila-Orta, C., Pérez-Alvarez, M., Martínez-Carrillo, D., Quiñones-Jurado, Z. V., & Caero, L. C. (2025). Nanocomposite Polysulfone/CB Modified by Melt Extrusion and Solution Mixing for Enhanced Removal of Uremic Toxins. Materials, 18(14), 3352. https://doi.org/10.3390/ma18143352











