Modification of G-C3N4 by the Surface Alkalinization Method and Its Photocatalytic Depolymerization of Lignin
Abstract
1. Introduction
2. Materials and Method
2.1. Chemicals
2.2. Materials Preparation
2.3. Characterization
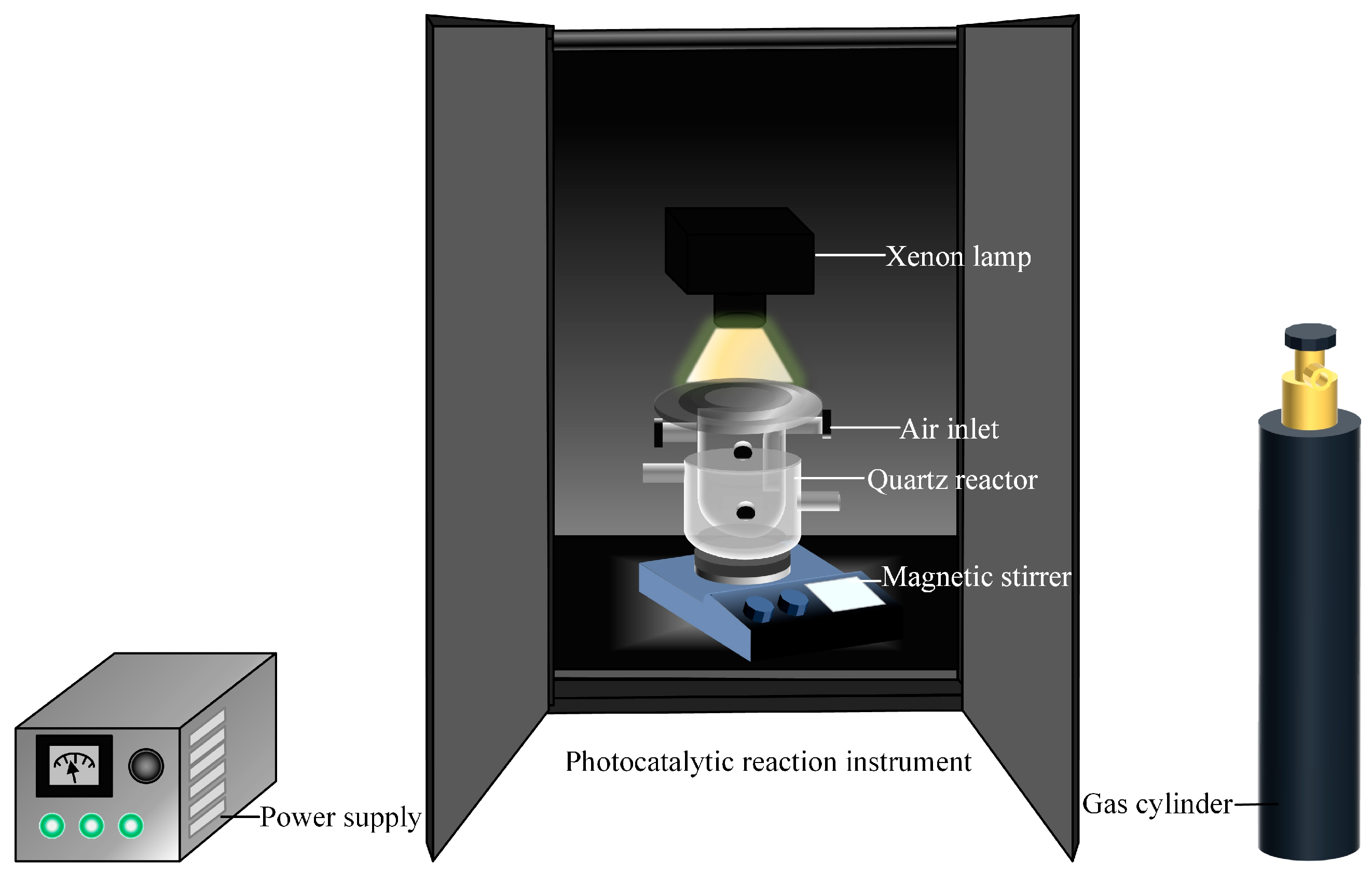
2.4. Photocatalytic Degradation of Lignin Model Compound
3. Results and Discussion
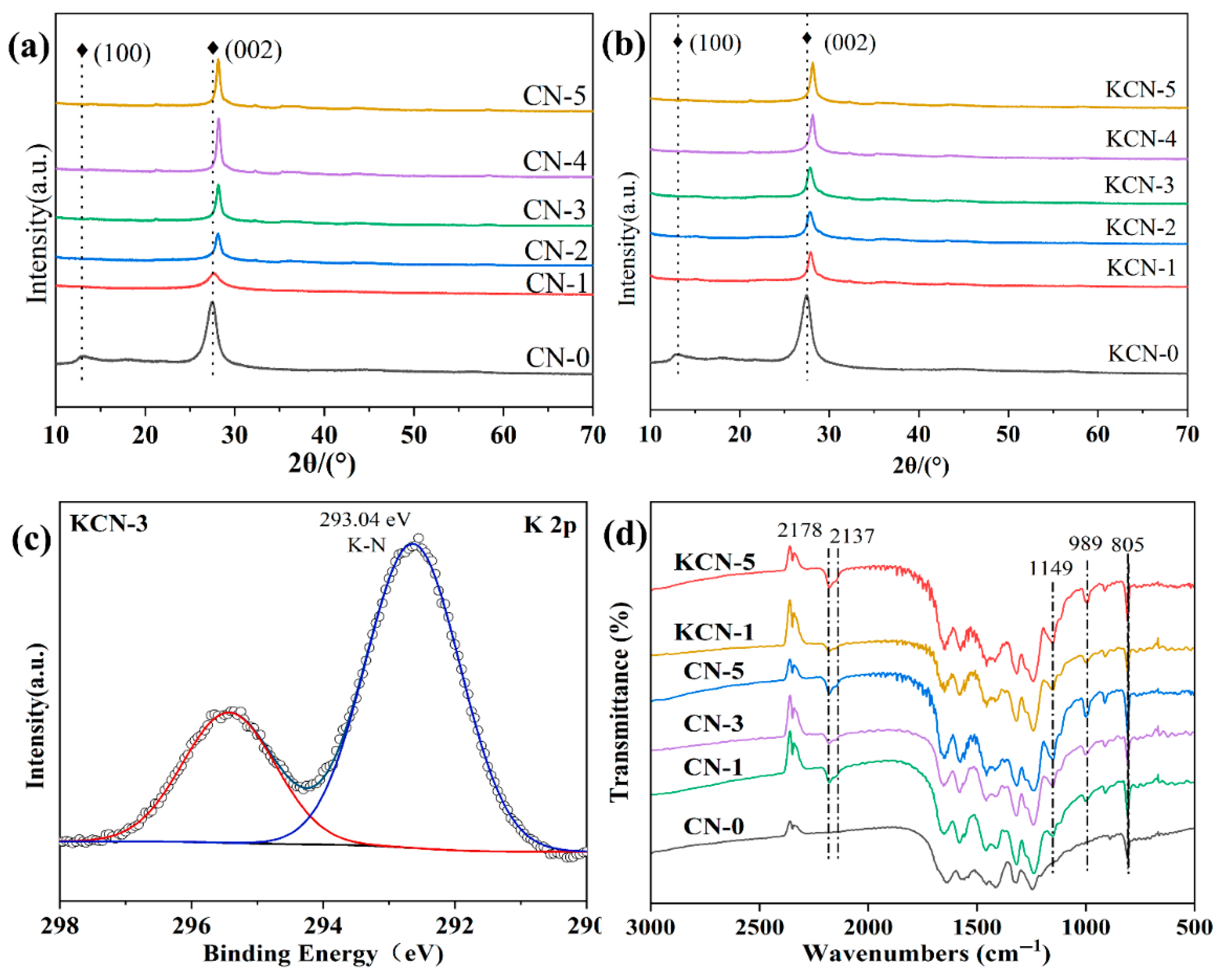
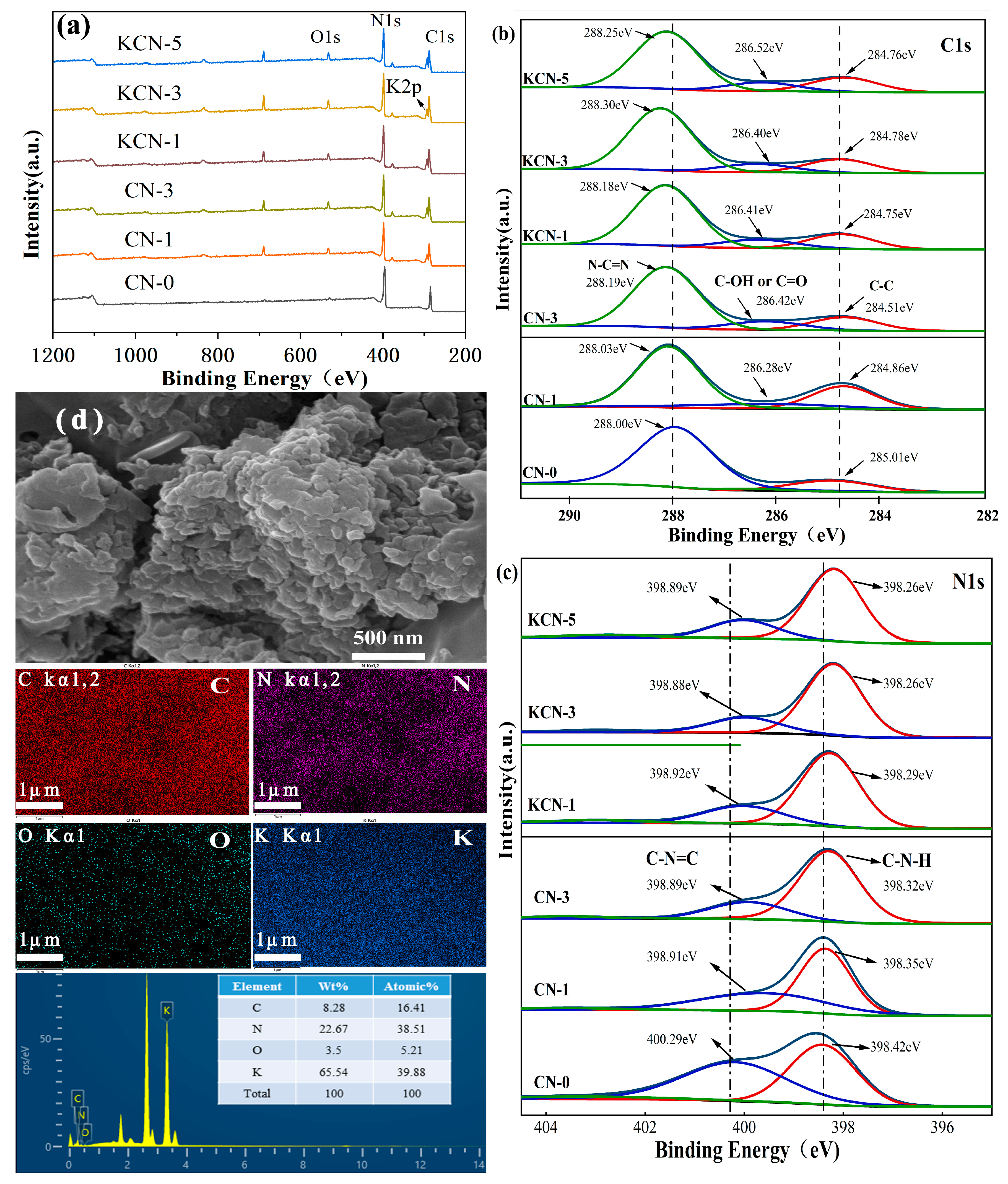
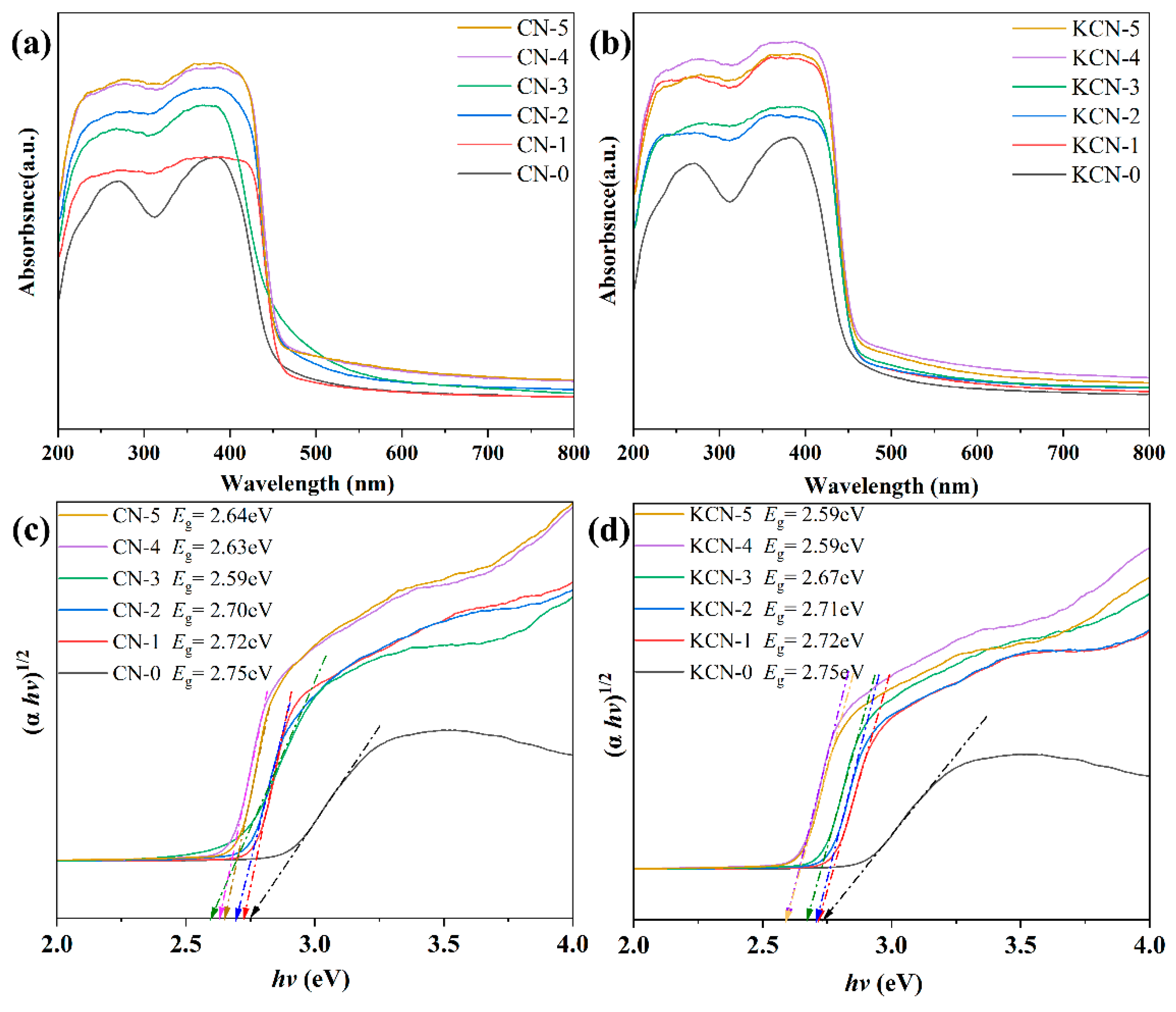
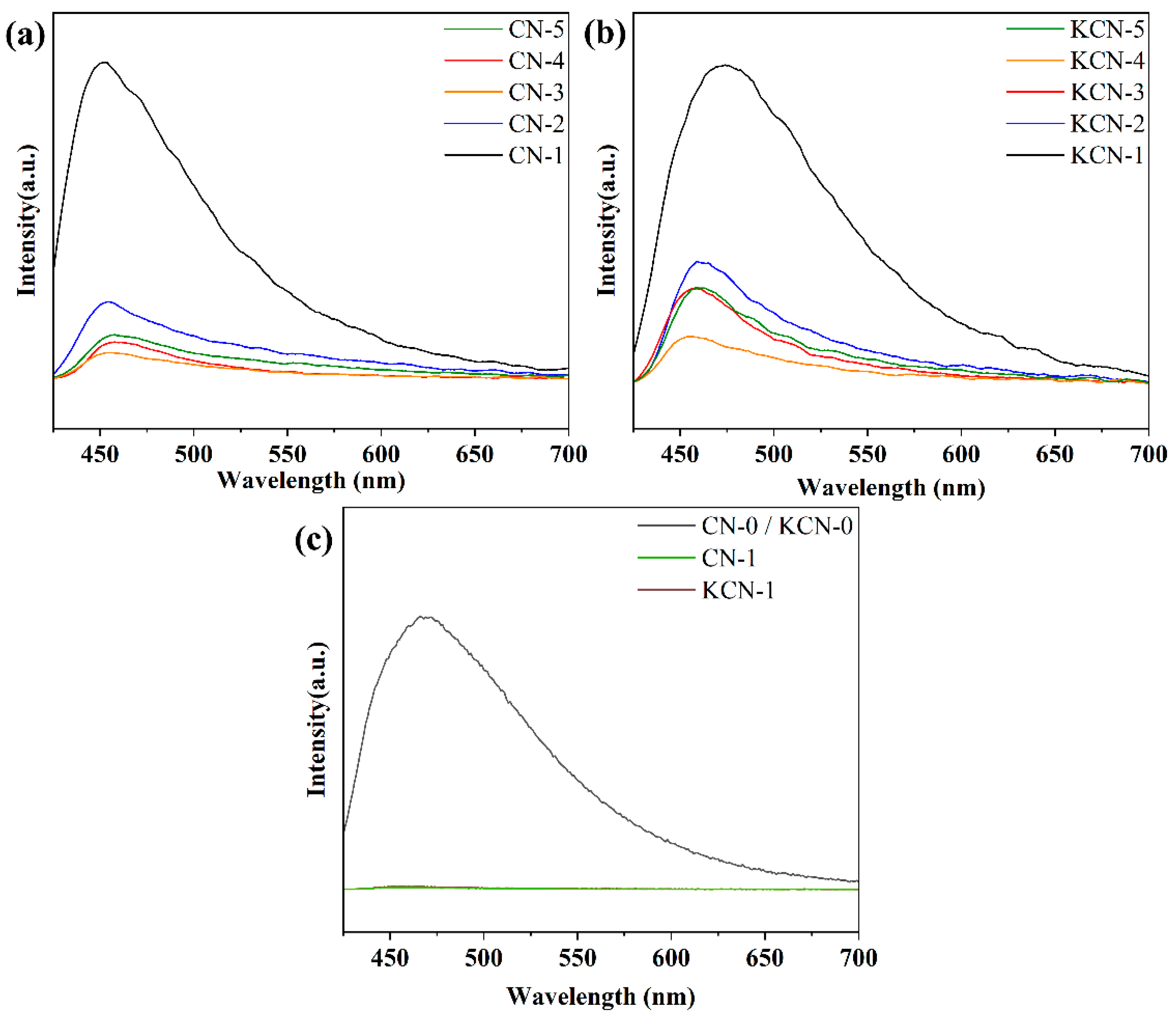
3.1. Characterization Results
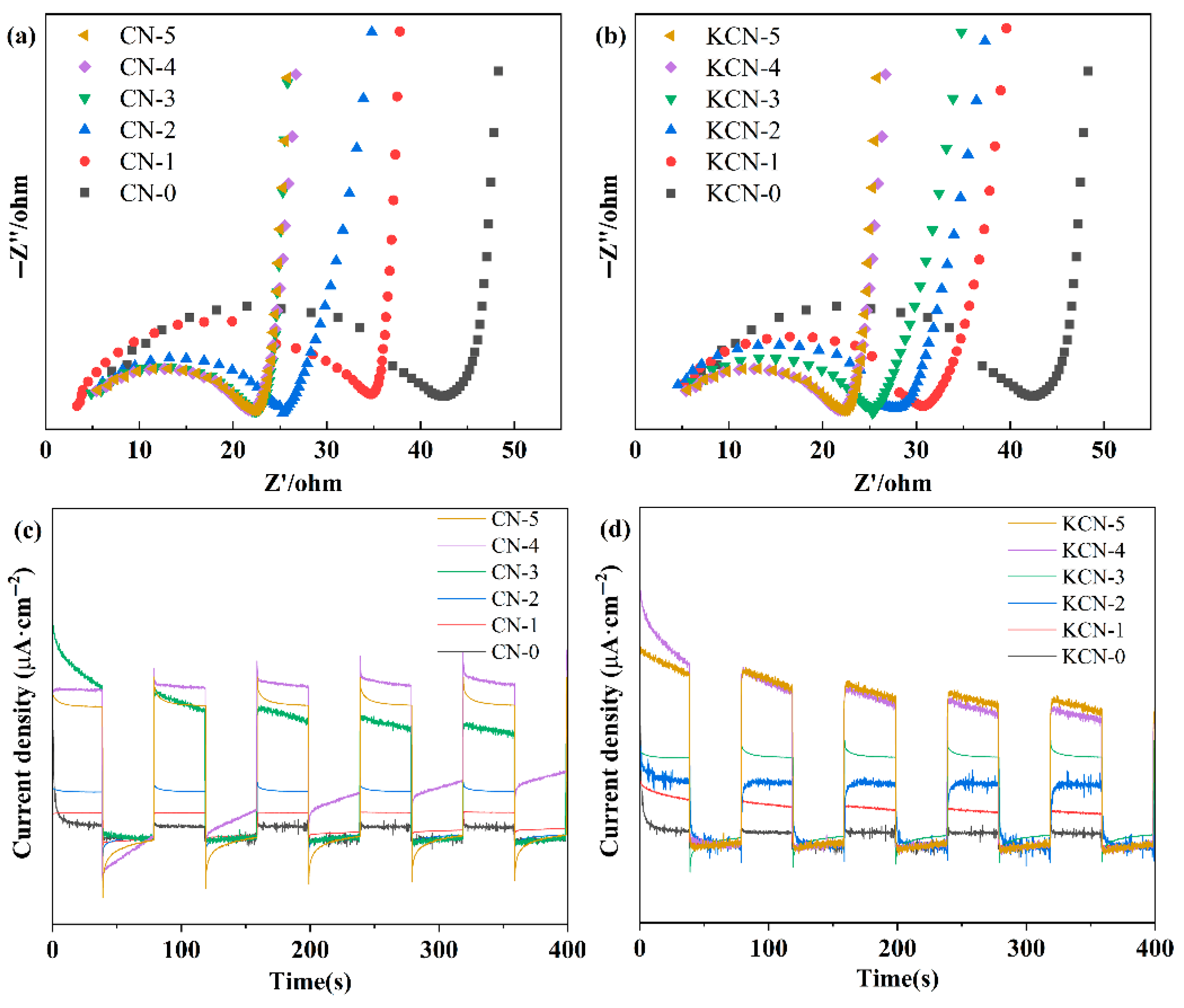
3.2. Photoelectric Performance Test Experiment
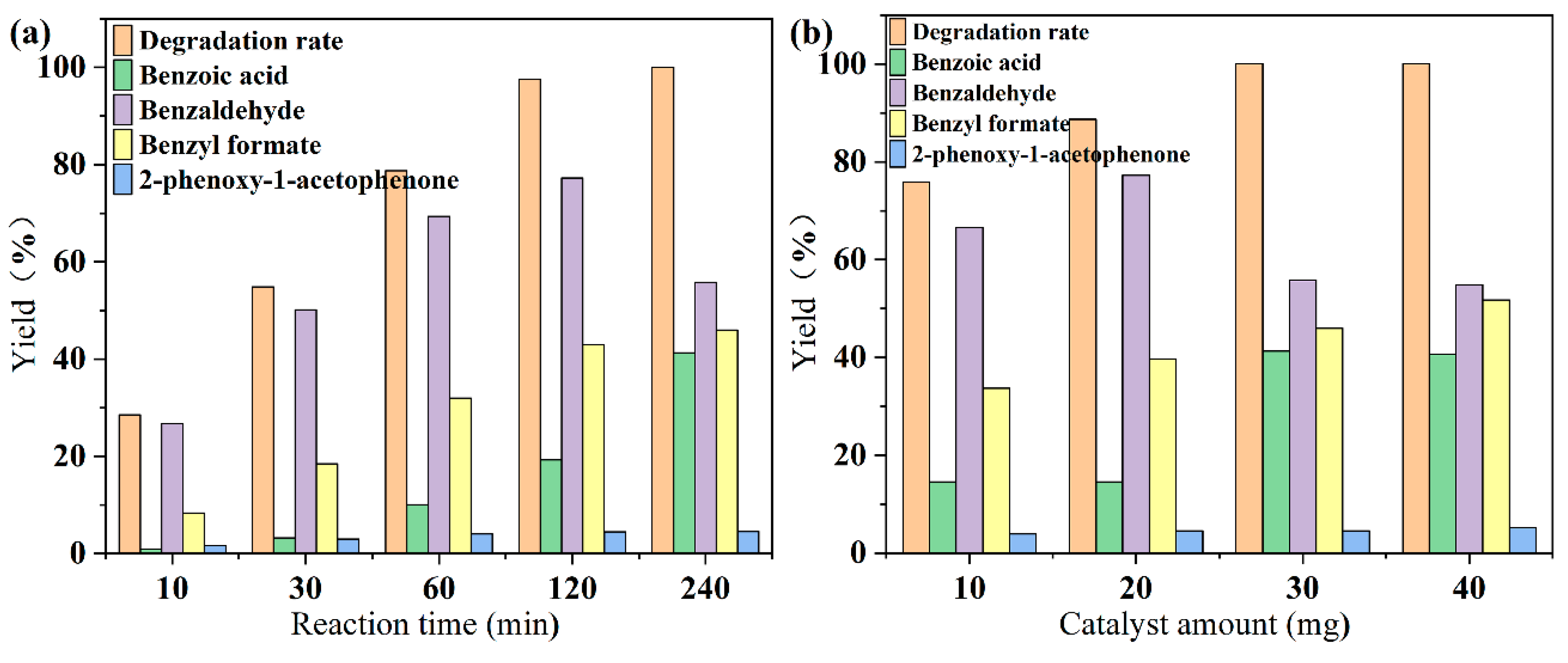
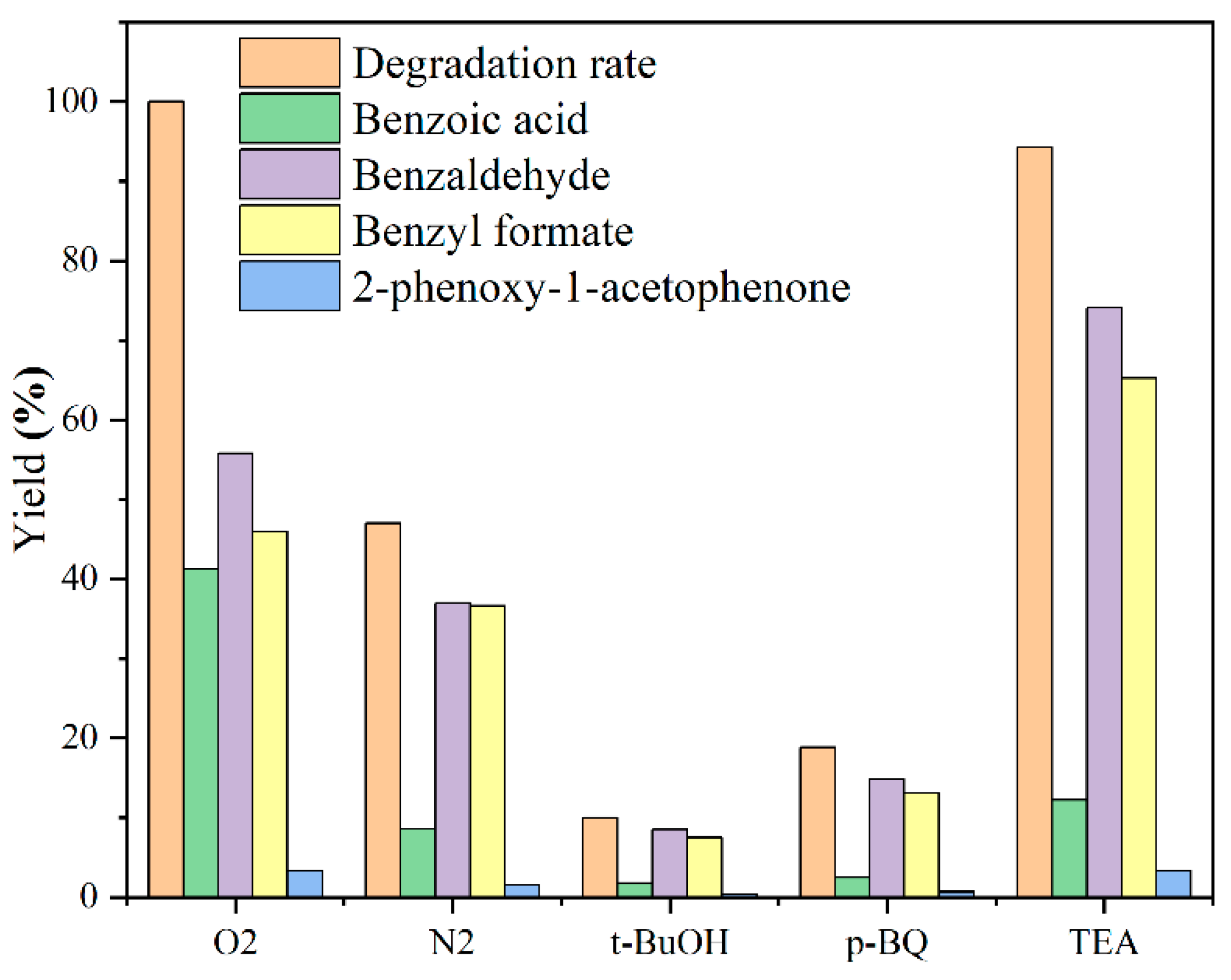
3.3. Catalytic Activity of Alkalinized G-C3N4 on Lignin Model Compound
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oza, G.; Olivito, F.; Rohokale, A.; Nardi, M.; Procopio, A.; Wan-Mohtar, W.A.A.Q.I.; Jagdale, P. Advancements in Catalytic Depolymerization Technologies. Polymers 2025, 17, 1614. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Park, J.-H. Advancement in technologies for the depolymerization of lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Wang, H.; Giardino, G.J.; Chen, R.; Yang, C.; Niu, J.; Wang, D. Photocatalytic depolymerization of native lignin toward chemically recyclable polymer networks. ACS Cent. Sci. 2022, 9, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Luo, N.; Wang, F. Visible-light-induced oxidative lignin C–C bond cleavage to aldehydes using vanadium catalysts. ACS Catal. 2019, 10, 632–643. [Google Scholar] [CrossRef]
- Shao, S.; Wang, X.; Li, W.; Zhang, Y.; Liu, S.; Xiao, W.; Yue, Z.; Lu, X.; Fan, X. A mini review on photocatalytic lignin conversion into monomeric aromatic compounds. Catal. Sci. Technol. 2025, 15, 962–987. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Zhang, Y. CeCl3-promoted simultaneous photocatalytic cleavage and amination of Cα–Cβ bond in lignin model compounds and native lignin. CCS Chem. 2020, 2, 107–117. [Google Scholar] [CrossRef]
- Magallanes, G.; Karkas, M.D.; Bosque, I.; Lee, S.; Maldonado, S.; Stephenson, C.R. Selective C–O bond cleavage of lignin systems and polymers enabled by sequential palladium-catalyzed aerobic oxidation and visible-light photoredox catalysis. ACS Catal. 2019, 9, 2252–2260. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; He, J.; Zhang, Y. Highly effective C–C bond cleavage of lignin model compounds. Green Chem. 2017, 19, 3135–3141. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Song, Y.; Yu, J.C.; Wu, L. Designing Efficient 2D Photocatalysts through Coordination Activation Mediated Surface Engineering. ChemCatChem 2024, 16, e202401037. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, W.; Li, Z.; Xiong, Y. Surface and interface engineering in photocatalysis. ChemNanoMat 2015, 1, 223–239. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Li, Z.; Xiong, Y. Facet-engineered surface and interface design of photocatalytic materials. Adv. Sci. 2017, 4, 1600216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, N.; Feng, Z.; Xiang, W.; Zhao, H.; Zhang, X. Constructing defect engineered 2D/2D MoO3/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic activity. J. Alloy. Compd. 2022, 926, 166964. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Z.; Zhao, J.; Liu, R. In-situ synthesis of Z-scheme V2O3/S-doped g-C3N4 heterojunction for enhanced photocatalytic hydrogen production. Appl. Surf. Sci. 2024, 654, 159518. [Google Scholar] [CrossRef]
- Zou, J.; Liao, G.; Jiang, J.; Xiong, Z.; Bai, S.; Wang, H.; Wu, P.; Zhang, P.; Li, X. In-situ construction of sulfur-doped g-C3N4/defective g-C3N4 isotype step-scheme heterojunction for boosting photocatalytic H2 evolution. Chin. J. Struct. Chem. 2022, 41, 2201025–2201033. [Google Scholar]
- Liu, H.; Yu, D.; Sun, T.; Du, H.; Jiang, W.; Muhammad, Y.; Huang, L. Fabrication of surface alkalinized g-C3N4 and TiO2 composite for the synergistic adsorption-photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2019, 473, 855–863. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, F.; Chen, Z.; Huang, S.; Wei, W.; Xie, M.; Xu, H.; Li, H. One-step synthesis of Fe-doped surface-alkalinized g-C3N4 and their improved visible-light photocatalytic performance. Appl. Surf. Sci. 2019, 469, 739–746. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Lu, D.; Wang, X.; Wang, D.; Ye, J. In situ surface alkalinized g-C3N4 toward enhancement of photocatalytic H2 evolution under visible-light irradiation. J. Mater. Chem. A 2016, 4, 2943–2950. [Google Scholar] [CrossRef]
- Feng, C.; Wu, Z.P.; Huang, K.W.; Ye, J.; Zhang, H. Surface modification of 2D photocatalysts for solar energy conversion. Adv. Mater. 2022, 34, 2200180. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, K.; Li, P.; Wang, F.; Kang, X.; Wu, G. The effect of hydroxyl group grafting on the photocatalytic phenolic compounds oxidation ability of g-C3N4 prepared by a novel H2O2-alkali hydrothermal method. Appl. Surf. Sci. 2020, 513, 145783. [Google Scholar] [CrossRef]
- Cao, M.; Wang, K.; Tudela, I.; Fan, X. Synthesis of Zn doped g-C3N4 in KCl-ZnCl2 molten salts: The temperature window for promoting the photocatalytic activity. Appl. Surf. Sci. 2020, 533, 147429. [Google Scholar] [CrossRef]
- Wang, X.L.; Fang, W.Q.; Wang, H.F.; Zhang, H.; Zhao, H.; Yao, Y.; Yang, H.G. Surface hydrogen bonding can enhance photocatalytic H2 evolution efficiency. J. Mater. Chem. A 2013, 1, 14089–14096. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.-K.; Jiang, Q.; Wang, Y.; Li, Y.-X. Facile alkali-assisted synthesis of g-C3N4 materials and their high-performance catalytic application in solvent-free cycloaddition of CO2 to epoxides. RSC Adv. 2016, 6, 55382–55392. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, D.; Zhang, L.; Wang, W. Alkaline modified g-C3N4 photocatalyst for high selective oxide coupling of benzyl alcohol to benzoin. Appl. Catal. B-Environ. Energy 2018, 220, 553–560. [Google Scholar] [CrossRef]
- Jasni, N.; Iqbal, A.; Norazmi Ahmad, M.; Pauzi, H.; Hussain, M.H. Synthesis and characterisation of Li-modified g-C3N4. Mater. Today 2022, 57, 1154–1161. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Z.; Cao, X.; Shen, Z.; Zhao, W.; Wang, F.; Cui, M.; Liang, C. Z-scheme heterojunction g-C3N4/CQD/CdZnS with high redox capability for enhancing visible light-driven photocatalytic depolymerization of lignin into aromatic monomers. Green Chem. 2024, 26, 1935–1948. [Google Scholar] [CrossRef]
- Yu, T.; Liu, Z.; Ma, J.; Tan, X. The Photocatalytic Oxidation of As (III) Enhanced by Surface Alkalinized g-C3N4. Trans. Tianjin Univ. 2020, 26, 40–48. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zhao, F.; Hu, C.; Zhao, Y.; Zhang, Z.; Chen, S.; Shi, G.; Qu, L. Monoatomic-thick graphitic carbon nitride dots on graphene sheets as an efficient catalyst in the oxygen reduction reaction. Nanoscale 2015, 7, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lai, H.; Ma, J.; Deng, G.; Long, B.; Song, T.; Liu, L.; Wang, X.; Tong, Y. Molten salt synthesis of KCl-preintercalated C3N4 nanosheets with abundant pyridinic-N as a superior anode with 10 K cycles in lithium ion battery. J. Colloid Interface Sci. 2022, 606, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, Z.-T.; Lin, K.-Y.A.; Wei, W.-H.; Wang, K.-H. Synergistic effect of KCl mixing and melamine/urea mixture in the synthesis of g-C3N4 for photocatalytic removal of tetracycline. J. Ind. Eng. Chem. 2022, 107, 118–125. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, G.; Yan, Y.; Jiao, Y.; Sun, B.; Cui, T. KCl-modified g-C3N4 coupled with ZnO to form S-scheme heterojunction photocatalytic materials rich in oxygen vacancies. J. Alloy. Compd. 2024, 980, 173655. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, P.; Geng, X.; Zhang, W.; Song, F.; Zhao, R.; Zhang, K.; Cui, H.; Chen, X.; Zou, J.-P. Visible light-driven selective cleavage of Cα-Cβ or Cβ-O bond in lignin β-O-4 model into high-value aromatic chemicals over surface modified 2D g-C3N4. Sep. Purif. Technol. 2025, 354, 128765. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Rabeah, J.; Brückner, A.; Cao, H. Visible-light photocatalytic ozonation using graphitic C3N4 catalysts: A hydroxyl radical manufacturer for wastewater treatment. Acc. Chem. Res. 2020, 53, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Qi, K.; Dong, G.; Wang, M.; Ho, W. The photocatalytic •OH production activity of g-C3N4 improved by the introduction of NO. Chin. Chem. Lett. 2022, 33, 4715–4718. [Google Scholar] [CrossRef]


| Series | KCl: Melamine (Mass Ratio) | KOH (mol/L) | Sample Label |
|---|---|---|---|
| Baseline | – | – | CN-0/KCN-0 |
| KCl Ratio | 0.1:1 * | 10 | CN-1 |
| 1:1 | 10 | CN-2 | |
| 3:1 | 10 | CN-3 | |
| 5:1 | 10 | CN-4 | |
| 7:1 | 10 | CN-5 | |
| KOH Concentration | 3:1 | 0.01 | KCN-1 |
| 3:1 | 0.1 | KCN-2 | |
| 3:1 | 0.5 | KCN-3 | |
| 3:1 | 2 | KCN-4 | |
| 3:1 | 10 | KCN-5 |
| Substrate | Degradation Rate (%) | Yield (%) | ||
|---|---|---|---|---|
| Benzoic Acid | Benzaldehyde | Benzoyl Formate | ||
| 2-phenoxy-1-acetophenone | 5.87 | 28.88 | 0.12 | 10.25 |
| Benzaldehyde | 100 | 100 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Zhang, L.; Zang, L.; Yu, F. Modification of G-C3N4 by the Surface Alkalinization Method and Its Photocatalytic Depolymerization of Lignin. Materials 2025, 18, 3350. https://doi.org/10.3390/ma18143350
Ma Z, Zhang L, Zang L, Yu F. Modification of G-C3N4 by the Surface Alkalinization Method and Its Photocatalytic Depolymerization of Lignin. Materials. 2025; 18(14):3350. https://doi.org/10.3390/ma18143350
Chicago/Turabian StyleMa, Zhongmin, Ling Zhang, Lihua Zang, and Fei Yu. 2025. "Modification of G-C3N4 by the Surface Alkalinization Method and Its Photocatalytic Depolymerization of Lignin" Materials 18, no. 14: 3350. https://doi.org/10.3390/ma18143350
APA StyleMa, Z., Zhang, L., Zang, L., & Yu, F. (2025). Modification of G-C3N4 by the Surface Alkalinization Method and Its Photocatalytic Depolymerization of Lignin. Materials, 18(14), 3350. https://doi.org/10.3390/ma18143350






