Impact of Mixed-In Polyacrylic- and Phosphonate-Based Additives on Lime Mortar Microstructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Microstructural and Mineralogical Characterization
2.1.1. Nano-Scale Analysis
2.1.2. Microstructural Characterization
2.1.3. Mineralogical Analysis
2.2. Porosity and Pore Size Distribution
2.3. Natural Carbonation Rate and Mechanical Performance
3. Results and Discussion
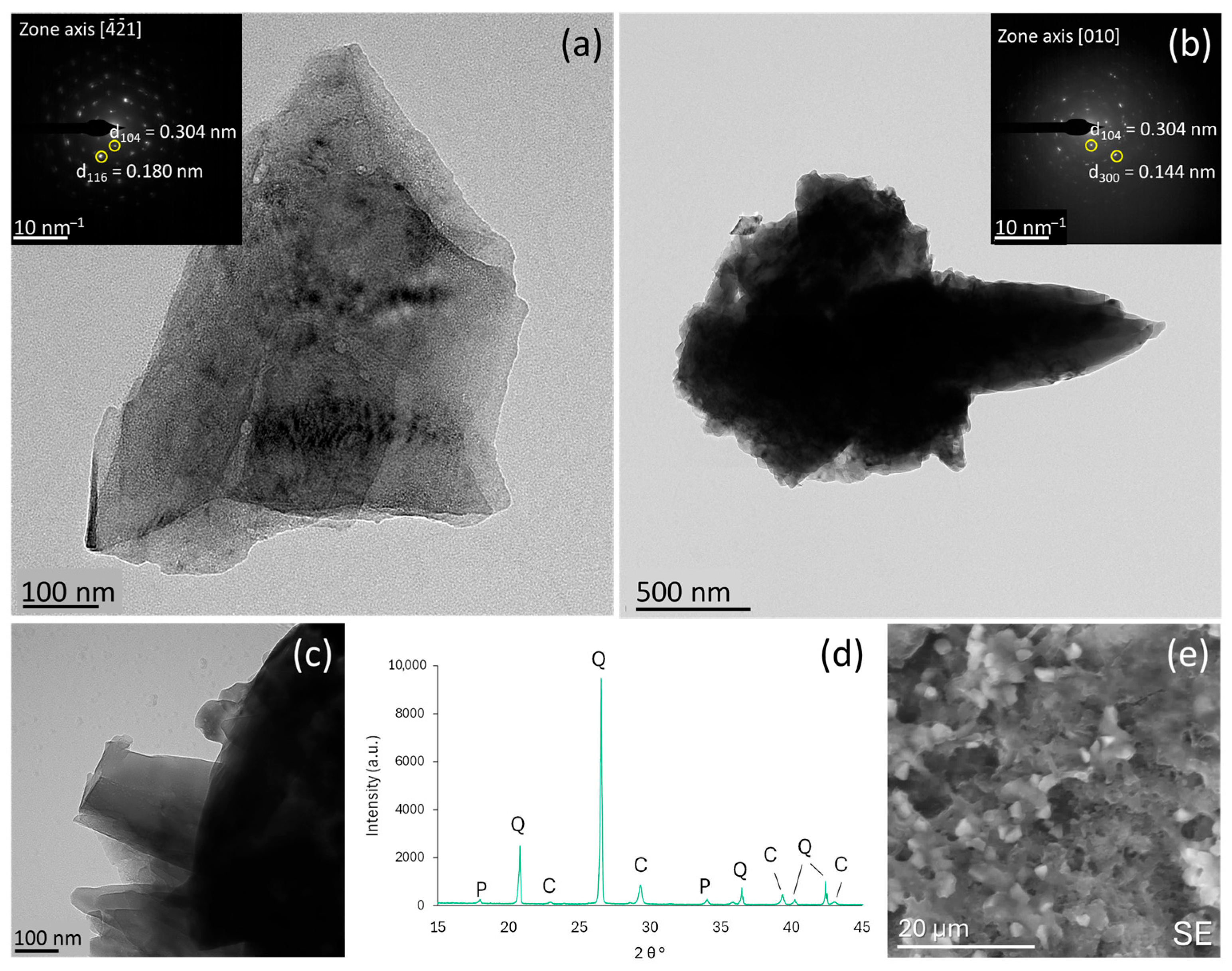
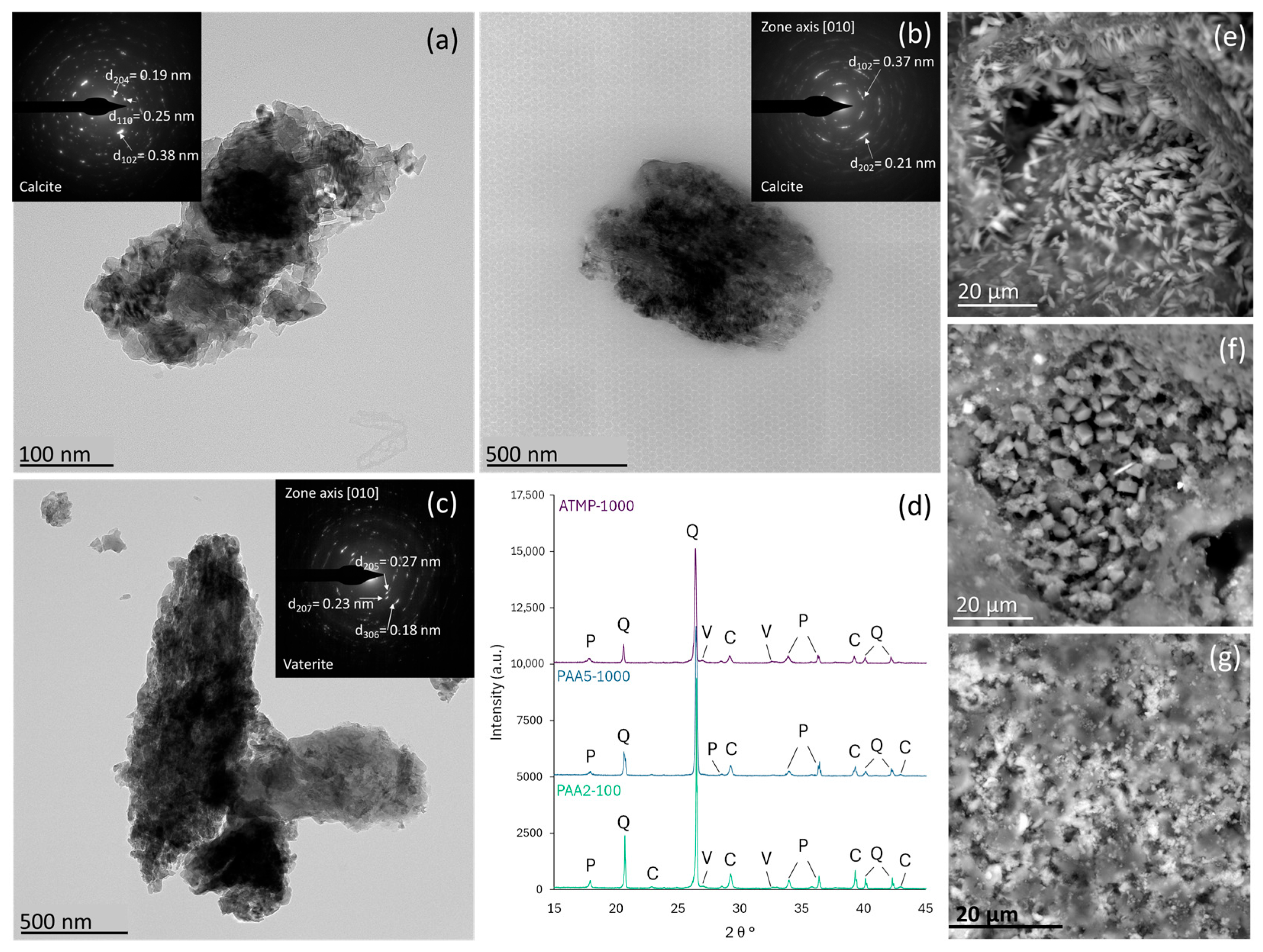
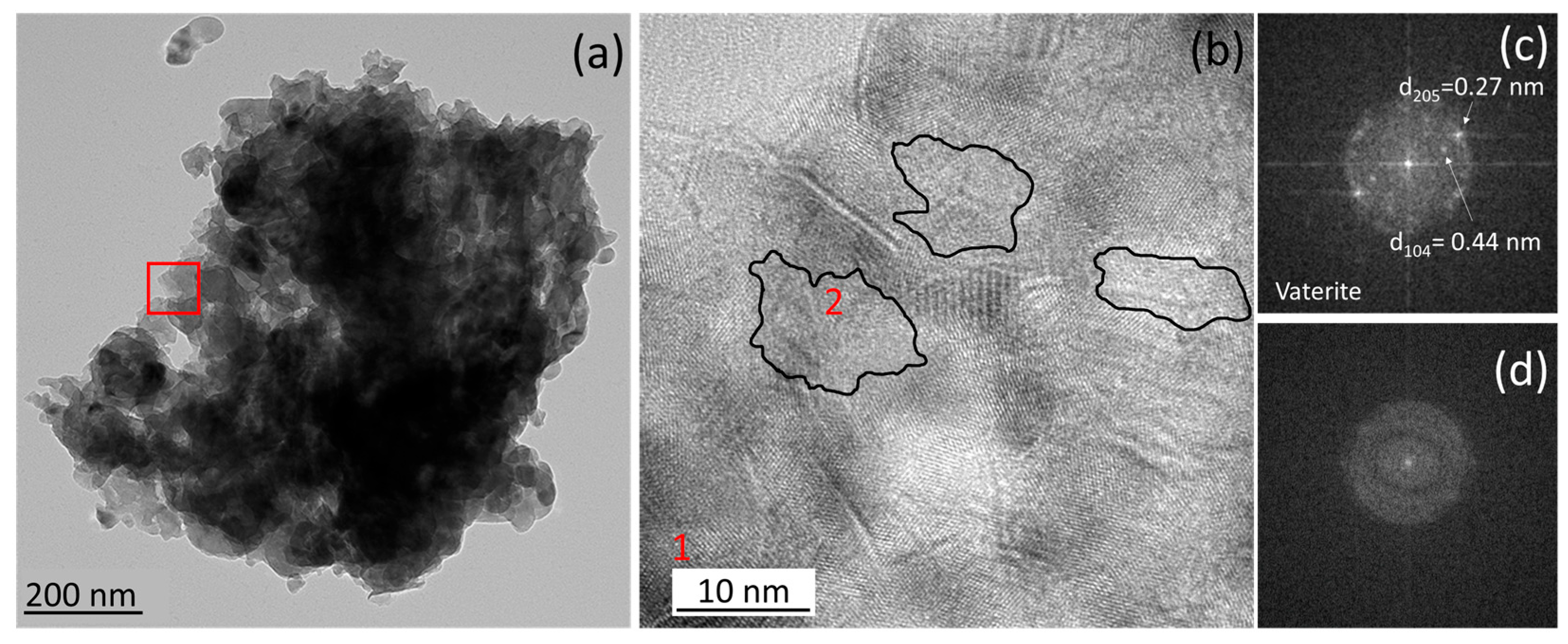
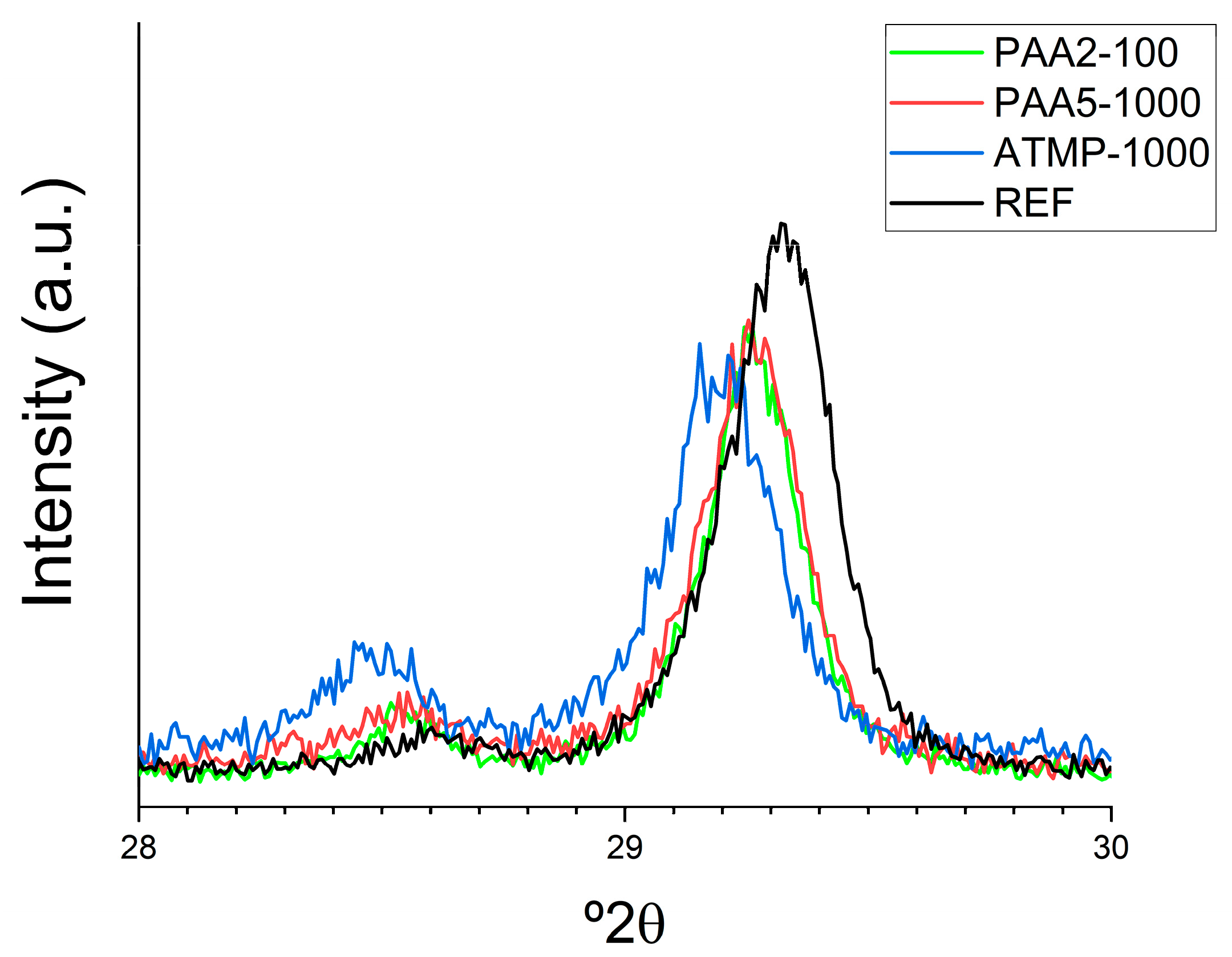
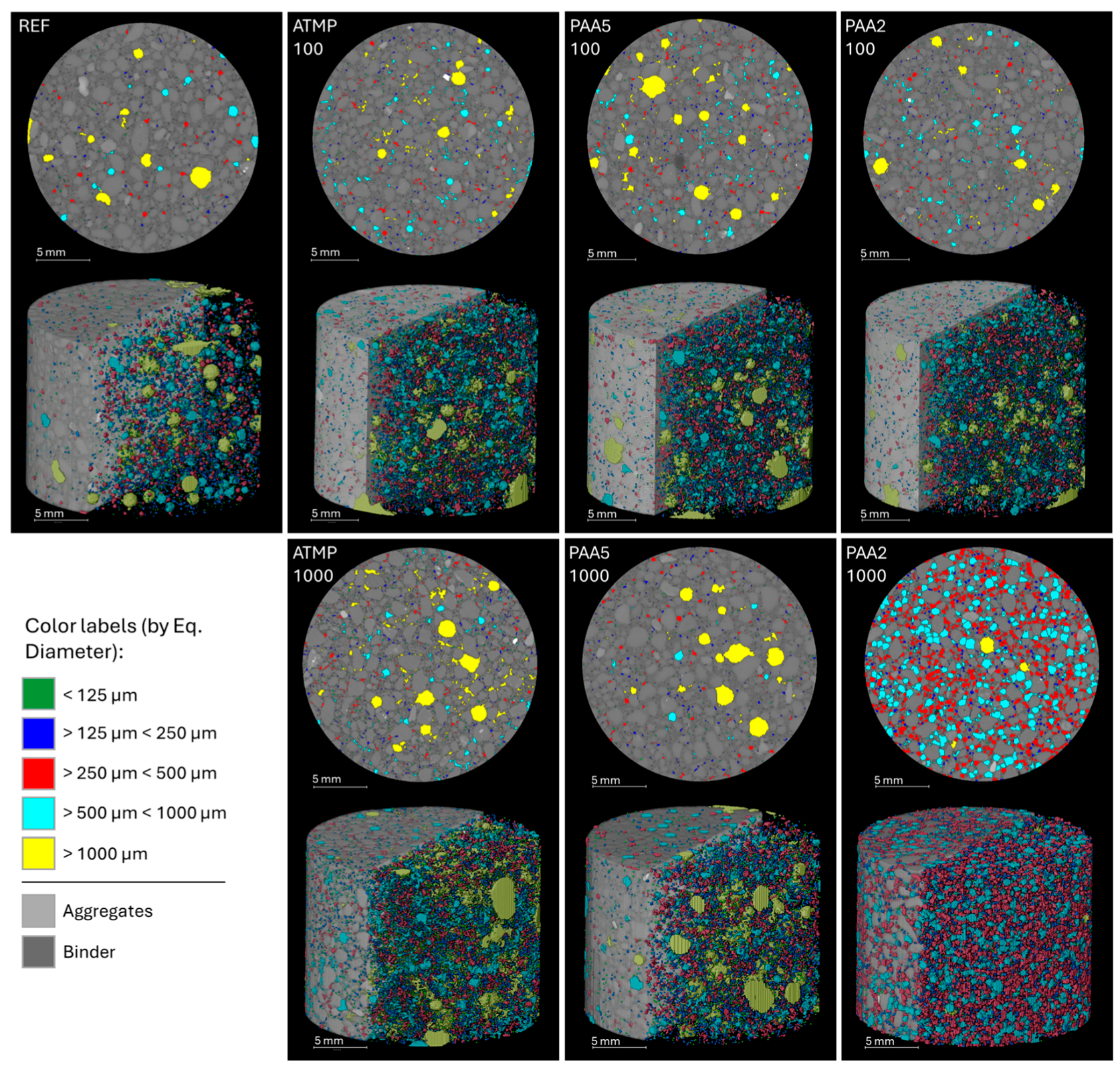
3.1. Impact of Additives on Mortar Microstructure and Mineralogy
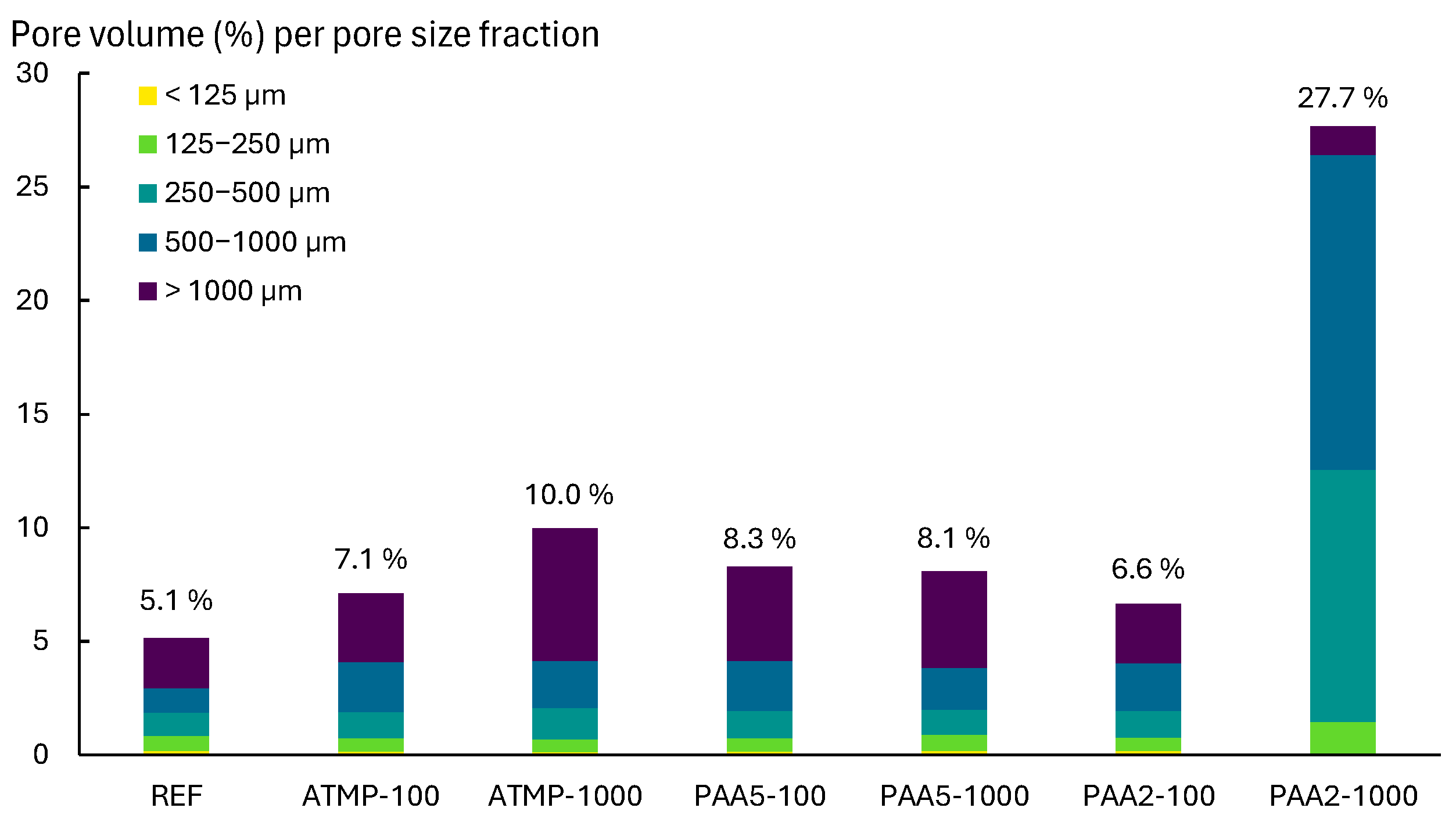
3.2. Porosity and Pore Size Distribution Results
3.3. Natural Carbonation Rate Evolution
3.4. Mechanical Performance
3.5. Effect of Additives on Lime Mortars’ Microstructure and Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATMP | Aminotris(methylene phosphonic acid) |
| PAA | Poly(acrylic acid) sodium salt |
| STEM | Scanning Transmission Electron Microscopy |
| SAED | Selected area electron diffraction |
| FEG-SEM-EDX | Field Emission Gun–Scanning Electron Microscopy coupled with Energy-Dispersive X-ray Spectroscopy |
| XRD | X-ray diffraction |
| MIP | Mercury intrusion porosimetry |
| Micro-CT | X-ray micro-computed tomography |
| NCR | Natural carbonation rate |
| HRTEM | High-resolution transmission electron microscopy |
| PBCs | Periodic Boundary Conditions |
| FFT | Fast Fourier Transform |
| ACC | Amorphous calcium carbonate |
References
- Snow, J.; Torney, C. Lime Mortars in Traditional Buildings; Historic Scotland: Edinburgh, UK, 2014; ISBN 9781849171775.
- Faria, P.; Henriques, F.; Rato, V. Comparative Evaluation of Lime Mortars for Architectural Conservation. J. Cult. Herit. 2008, 9, 338–346. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Monasterio-Guillot, L.; Burgos-Ruiz, M.; Ruiz-Agudo, E.; Elert, K. Unveiling the Secret of Ancient Maya Masons: Biomimetic Lime Plasters with Plant Extracts. Sci. Adv. 2023, 9, eadf6138. [Google Scholar] [CrossRef] [PubMed]
- Thirumalini, S.; Ravi, R.; Rajesh, M. Experimental Investigation on Physical and Mechanical Properties of Lime Mortar: Effect of Organic Addition. J. Cult. Herit. 2018, 31, 97–104. [Google Scholar] [CrossRef]
- Zhang, K.; Sui, Y.; Wang, L.; Tie, F.; Yang, F.; Liu, Y.; Zhang, Y. Effects of Sticky Rice Addition on the Properties of Lime-Tile Dust Mortars. Herit. Sci. 2021, 9, 4. [Google Scholar] [CrossRef]
- Kontić, A.; Vasconcelos, G.; Briceño Melendez, C.; Azenha, M.; Sokolović, N. Influence of Air Entrainers on the Properties of Hydrated Lime Mortars. Constr. Build. Mater. 2023, 403, 132968. [Google Scholar] [CrossRef]
- Nowak-Michta, A. Impact Analysis of Air-Entraining and Superplasticizing Admixtures on Concrete Compressive Strength. In Proceedings of the Procedia Structural Integrity; Elsevier, B.V.: Amsterdam, The Netherlands, 2019; Volume 23, pp. 77–82. [Google Scholar]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A.; Candeias, A. Suitability of Different Surfactants as Air-Entraining Admixtures for Lime Mortars. Constr. Build. Mater. 2020, 256, 118986. [Google Scholar] [CrossRef]
- Granneman, S. Mitigating Salt Damage in Lime-Based Mortars by Built-In Crystallization Modifiers. Ph.D. Thesis, TUDelft, Delft, The Netherlands, 2019. [Google Scholar]
- Granneman, S.J.C.; Lubelli, B.; Van Hees, R.P.J. Characterization of Lime Mortar Additivated with Crystallization Modifiers. Int. J. Archit. Herit. 2018, 12, 849–858. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Ibañez-Velasco, A.; Ruiz-Agudo, C.; Bonilla-Correa, S.; Elert, K.; Rodríguez-Navarro, C. Damage of Porous Building Stone by Sodium Carbonate Crystallization and the Effect of Crystallization Modifiers. Constr. Build. Mater. 2024, 411, 134591. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V.; Pel, L.; Rodriguez-Navarro, C. Template-Assisted Crystallization of Sulfates onto Calcite: Implications for the Prevention of Salt Damage. Cryst. Growth Des. 2013, 13, 40–51. [Google Scholar] [CrossRef]
- Andreotti, S.; Franzoni, E.; Ruiz-Agudo, E.; Scherer, G.W.; Fabbri, P.; Sassoni, E.; Rodriguez-Navarro, C. New Polymer-Based Treatments for the Prevention of Damage by Salt Crystallization in Stone. Mater. Struct. 2019, 52, 17. [Google Scholar] [CrossRef]
- Saleh, M.M.; Darwish, S.S.; Elzoghby, M. The Effectiveness of Some Crystallization Inhibitors in Preventing Salt Damage to Limestone. J. Cryst. Growth 2022, 585, 126606. [Google Scholar] [CrossRef]
- Huang, S.C.; Naka, K.; Chujo, Y. Effect of Molecular Weights of Poly(Acrylic Acid) on Crystallization of Calcium Carbonate by the Delayed Addition Method. Polym. J. 2008, 40, 154–162. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Morgan, D.J.; Peacock, C.L.; Benning, L.G. Effectiveness of Green Additives vs. Poly(Acrylic Acid) in Inhibiting Calcium Sulfate Dihydrate Crystallization. Ind. Eng. Chem. Res. 2019, 58, 1561–1569. [Google Scholar] [CrossRef]
- Ma, B.; Peng, Y.; Tan, H.; Lv, Z.; Deng, X. Effect of Polyacrylic Acid on Rheology of Cement Paste Plasticized by Polycarboxylate Superplasticizer. Materials 2018, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.; Pinto, F.; Gomes, A.; Silva, B.A.; Silva, B.; Candeias, A.; Do Vale, F. Role of Different Admixtures on the Properties of an Aerial Lime Mortar. In Proceedings of the 3rd International Conference on Protection of Historical Constructions, Lisbon, Portugal, 12–15 July 2017. [Google Scholar]
- Bracciale, M.P.; Sammut, S.; Cassar, J.A.; Santarelli, M.L.; Marrocchi, A. Molecular Crystallization Inhibitors for Salt Damage Control in Porous Materials: An Overview. Molecules 2020, 25, 1873. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jia, Z.; Li, L. Biomineralized Materials as Model Systems for Structural Composites: Intracrystalline Structural Features and Their Strengthening and Toughening Mechanisms. Adv. Sci. 2022, 9, 2103524. [Google Scholar] [CrossRef] [PubMed]
- Rianasari, I.; Benyettou, F.; Sharma, S.K.; Blanton, T.; Kirmizialtin, S.; Jagannathan, R. A Chemical Template for Synthesis of Molecular Sheets of Calcium Carbonate. Sci. Rep. 2016, 6, 25393. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Hu, Q.; Huang, J.; Guo, X.; Li, P.; Liu, X.; Bai, J. Evaluation of Mechanical Properties and Potential Environmental Applications of Biomimetic Mineralized Composites. Constr. Build. Mater. 2024, 438, 136976. [Google Scholar] [CrossRef]
- Ouhenia, S.; Chateigner, D.; Belkhir, M.A.; Guilmeau, E.; Krauss, C. Synthesis of Calcium Carbonate Polymorphs in the Presence of Polyacrylic Acid. J. Cryst. Growth 2008, 310, 2832–2841. [Google Scholar] [CrossRef]
- Xu, A.W.; Dong, W.F.; Antonietti, M.; Cölfen, H. Polymorph Switching of Calcium Carbonate Crystals by Polymer-Controlled Crystallization. Adv. Funct. Mater. 2008, 18, 1307–1313. [Google Scholar] [CrossRef]
- Wada, N.; Horiuchi, N.; Nakamura, M.; Nozaki, K.; Hiyama, T.; Nagai, A.; Yamashita, K. Controlled Calcite Nucleation on Polarized Calcite Single Crystal Substrates in the Presence of Polyacrylic Acid. J. Cryst. Growth 2015, 415, 7–14. [Google Scholar] [CrossRef]
- Hu, X.; He, P.; Shi, C. Carbonate Binders: Historic Developments and Perspectives. Cem. Concr. Res. 2024, 175, 107352. [Google Scholar] [CrossRef]
- Määttänen, A.; Ihalainen, P.; Bollström, R.; Wang, S.; Toivakka, M.; Peltonen, J. Enhanced Surface Wetting of Pigment Coated Paper by UVC Irradiation. Ind. Eng. Chem. Res. 2010, 49, 11351–11356. [Google Scholar] [CrossRef]
- Chen, C.; Lei, W.; Xia, M.; Wang, F.; Gong, X. Molecular Modeling of Several Phosphonates onto the Stepped Calcite (011) Surface. Desalination 2013, 309, 208–212. [Google Scholar] [CrossRef]
- Sawada, K.; Abdel-Aal, N.; Sekino, H.; Satoh, K. Adsorption of Inorganic Phosphates and Organic Polyphosphonate on Calcite. Dalton Trans. 2003, 3, 342–347. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.; Tomson, M.B. Adsorption and Precipitation of an Aminoalkylphosphonate onto Calcite. J. Colloid Interface Sci. 2005, 281, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, L.; Fu, W.; Chi, R.; Li, H.; Yang, S. Investigations of Amino Trimethylene Phosphonic Acid as a Green and Efficient Depressant for the Flotation Separation of Apatite from Calcite. Miner. Eng. 2022, 181, 107552. [Google Scholar] [CrossRef]
- CEN NBN EN 1015-11; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. iTeh Standards: San Francisco, CA, USA, 2019.
- CEN NBN EN 1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). iTeh Standards: San Francisco, CA, USA, 1999.
- CEN NBN EN 14630; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Carbonation Depth in Hardened Concrete by the Phenolphthalein Method. iTeh Standards: San Francisco, CA, USA, 2007.
- Van Den Heede, P.; De Schepper, M.; De Belie, N. Accelerated and Natural Carbonation of Concrete with High Volumes of Fly Ash: Chemical, Mineralogical and Microstructural Effects. R. Soc. Open Sci. 2019, 6, 181665. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Cazalla, O.; Elert, K.; Sebastian, E. Liesegang Pattern Development in Carbonating Traditional Lime Mortars. Proc. R. Soc. A 2002, 458, 2261–2273. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E.; Huertas, M.O. Forced and Natural Carbonation of Lime-Based Mortars with and without Additives: Mineralogical and Textural Changes. Cem. Concr. Res. 2005, 35, 2278–2289. [Google Scholar] [CrossRef]
- Cizer, Ö.; Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Elsen, J.; Van Gemert, D.; Van Balen, K. Phase and Morphology Evolution of Calcium Carbonate Precipitated by Carbonation of Hydrated Lime. J. Mater. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ilić, T.; Ruiz-Agudo, E.; Elert, K. Carbonation Mechanisms and Kinetics of Lime-Based Binders: An Overview. Cem. Concr. Res. 2023, 173, 107301. [Google Scholar] [CrossRef]
- Singh, M.; Vinodh Kumar, S.; Waghmare, S.A.; Sabale, P.D. Aragonite-Vaterite-Calcite: Polymorphs of CaCO3 in 7th Century CE Lime Plasters of Alampur Group of Temples, India. Constr. Build. Mater. 2016, 112, 386–397. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A.; Candeias, A. Effects of Natural and Accelerated Carbonation on the Properties of Lime-Based Materials. J. CO2 Util. 2021, 49, 101552. [Google Scholar] [CrossRef]
- You, X.; Hu, X.; Xiao, Z.; Saleh Bairq, Z.A.; Chen, W.; Shi, C. Thermodynamic Modelling of CaCO3 Polymorphs during CO2 Sequestration by Cement Slurry with the Addition of MgCl2. J. Clean. Prod. 2023, 410, 137294. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially Mediated Mineralization of Vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Riyazi, S.; Kevern, J.T.; Mulheron, M. Super Absorbent Polymers (SAPs) as Physical Air Entrainment in Cement Mortars. Constr. Build. Mater. 2017, 147, 669–676. [Google Scholar] [CrossRef]
- Valdez Madrid, D.E.; Winardhi, C.W.; de Belie, N.; Cnudde, V. Exploring Lime Mortar Microstructure: Investigating the Impact of Mixed-in Additives and their Role in Salt Efflorescence Inhibition. MATEC Web Conf. 2024, 403, 03006. [Google Scholar] [CrossRef]
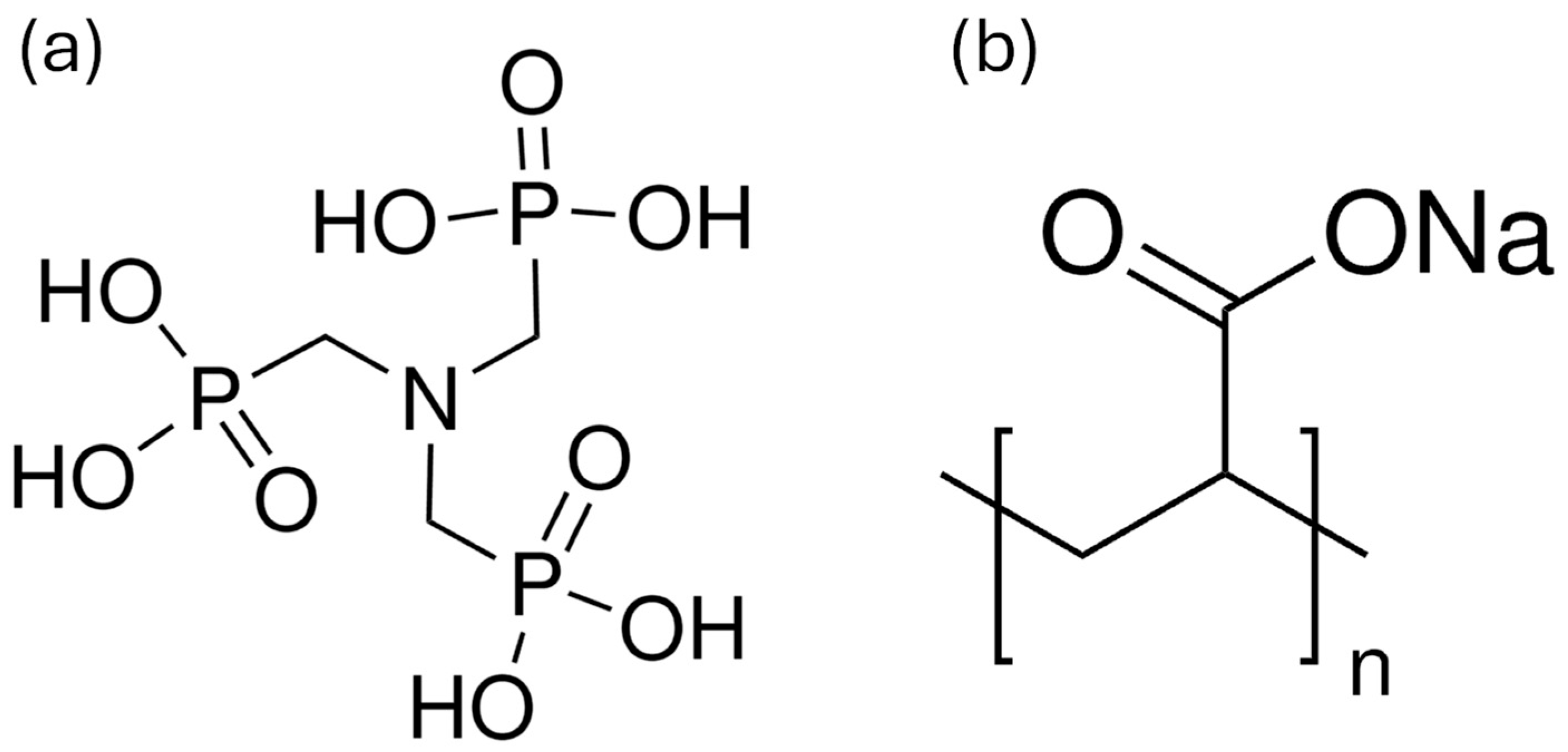





| Sample ID | Additive Type | Additive (ppm) | Additive (g) | Lime (g) | Sand (g) | Water (g) | w/b Ratio (by Mass) |
|---|---|---|---|---|---|---|---|
| REF | - | - | 113 | 1350 | 224 | 1.98 | |
| ATMP-100 | N[CH2PO(OH)2]3 Mw: 299 g/mol | 100 | 0.15 | 113 | 1350 | 224 | 1.98 |
| ATMP-500 | 500 | 0.73 | 113 | 1350 | 224 | 1.98 | |
| ATMP-1000 | 1000 | 1.46 | 113 | 1350 | 224 | 1.98 | |
| PAA5-100 | C3H3NaO2 Mw: 5100 g/mol | 100 | 0.15 | 113 | 1350 | 224 | 1.98 |
| PAA5-500 | 500 | 0.73 | 113 | 1350 | 224 | 1.98 | |
| PAA5-1000 | 1000 | 1.46 | 113 | 1350 | 210 | 1.86 | |
| PAA2-100 | C3H3NaO2 Mw: 2100 g/mol | 100 | 0.15 | 113 | 1350 | 224 | 1.98 |
| PAA2-500 | 500 | 0.73 | 113 | 1350 | 210 | 1.86 | |
| PAA2-1000 | 1000 | 1.46 | 113 | 1350 | 196 | 1.73 |
| Additive | Dosage (ppm) | Microstructural Features | Porosity 1 | NCR 2 | Mechanical Performance 3 |
|---|---|---|---|---|---|
| REF | - | Rhombohedral and scalenohedral calcite + portlandite | Moderate | Baseline | Baseline |
| ATMP | 100 | Calcite + vaterite | Moderate | Significantly decreased | Improved |
| 500 | Calcite + vaterite | Slight decrease | Significantly decreased | Significantly improved | |
| 1000 | Calcite + vaterite Mesocrystals via ACC precursor | High + predominance of >1000 µm | Increased | Improved | |
| PAA5 | 100 | Calcite + vaterite Mesocrystals via ACC precursor | Moderate | Decreased | Baseline |
| 500 | Calcite + vaterite | Moderate + shift from 10 to 7 µm | Decreased | Baseline | |
| 1000 | Calcite + vaterite Mesocrystals via ACC precursor Increase in big spherical air voids and microcracks in the binder | High + predominance of >1000 µm | Increased | Baseline | |
| PAA2 | 100 | Calcite + vaterite Mesocrystals via ACC precursor Discontinuities due to organic occlusions | Moderate | Decreased | Improved |
| 500 | Calcite + vaterite | Moderate increase | Decreased | Baseline | |
| 1000 | Calcite + vaterite Mesocrystals via ACC precursor Spherical air voids with high pore interconnectivity | Very high + predominance of 250–1000 µm | Significantly increased | Decreased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez Madrid, D.E.; Ruiz-Agudo, E.; Bonilla-Correa, S.; De Belie, N.; Cnudde, V. Impact of Mixed-In Polyacrylic- and Phosphonate-Based Additives on Lime Mortar Microstructure. Materials 2025, 18, 3322. https://doi.org/10.3390/ma18143322
Valdez Madrid DE, Ruiz-Agudo E, Bonilla-Correa S, De Belie N, Cnudde V. Impact of Mixed-In Polyacrylic- and Phosphonate-Based Additives on Lime Mortar Microstructure. Materials. 2025; 18(14):3322. https://doi.org/10.3390/ma18143322
Chicago/Turabian StyleValdez Madrid, Dulce Elizabeth, Encarnación Ruiz-Agudo, Sarah Bonilla-Correa, Nele De Belie, and Veerle Cnudde. 2025. "Impact of Mixed-In Polyacrylic- and Phosphonate-Based Additives on Lime Mortar Microstructure" Materials 18, no. 14: 3322. https://doi.org/10.3390/ma18143322
APA StyleValdez Madrid, D. E., Ruiz-Agudo, E., Bonilla-Correa, S., De Belie, N., & Cnudde, V. (2025). Impact of Mixed-In Polyacrylic- and Phosphonate-Based Additives on Lime Mortar Microstructure. Materials, 18(14), 3322. https://doi.org/10.3390/ma18143322








