Method for Elucidating the Structural Evolution of a Nanoscale Release Layer in Double Copper Foils Under Thermal Exposure
Abstract
1. Introduction
2. Experimental Section
2.1. Material
2.2. Characterization
3. Results and Discussion
3.1. Peeling Testing
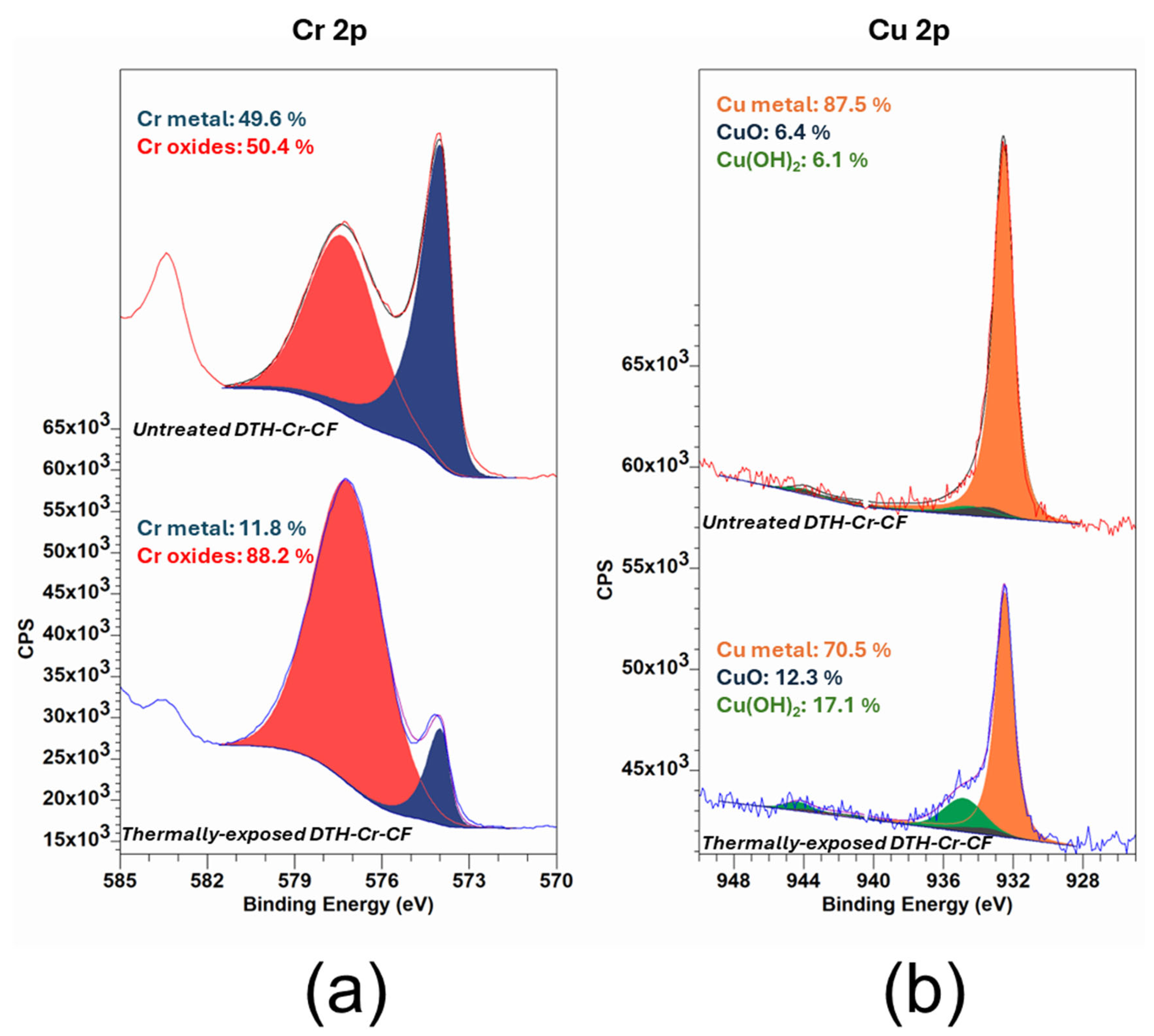
3.2. XPS Analysis
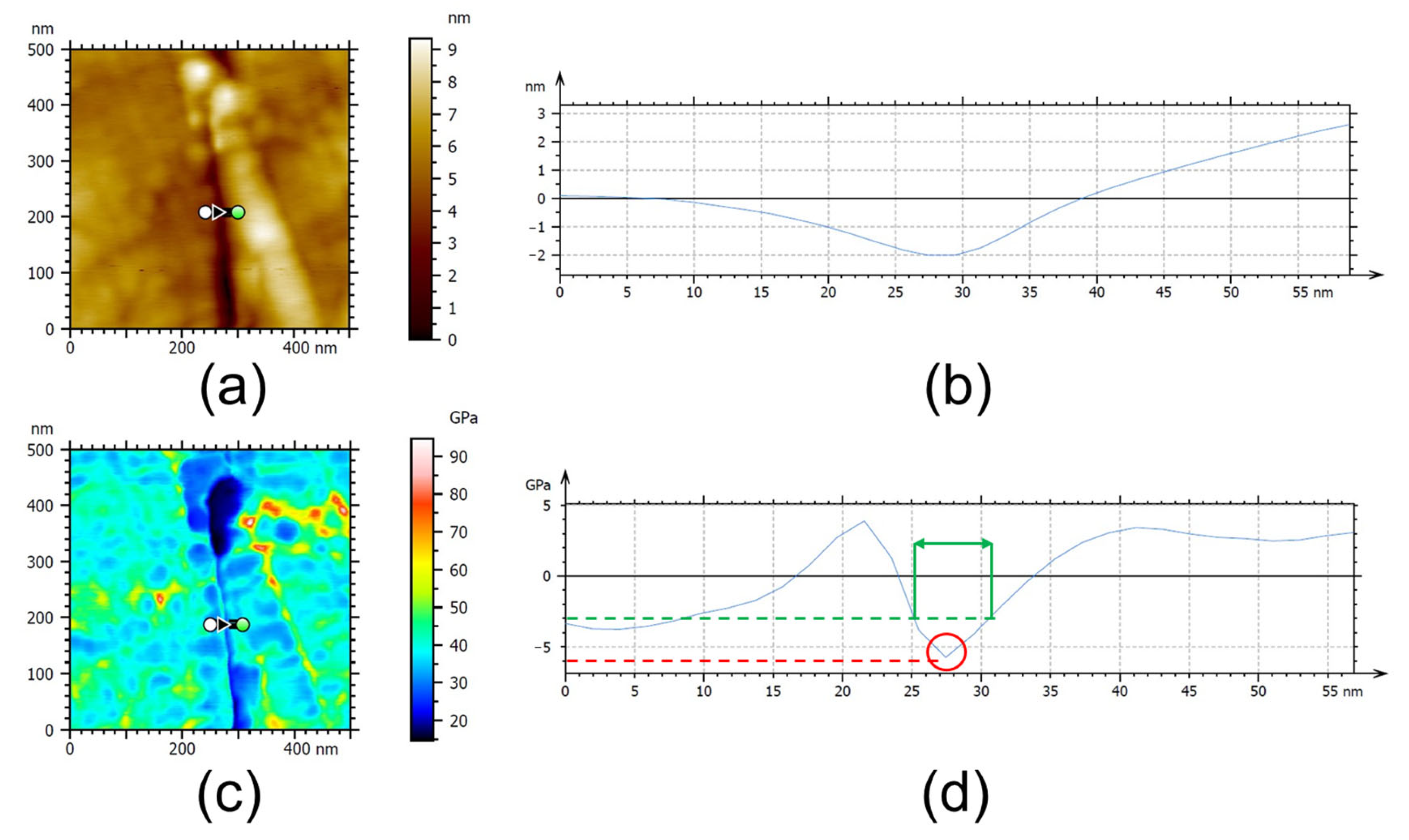
3.3. AFM Analysis
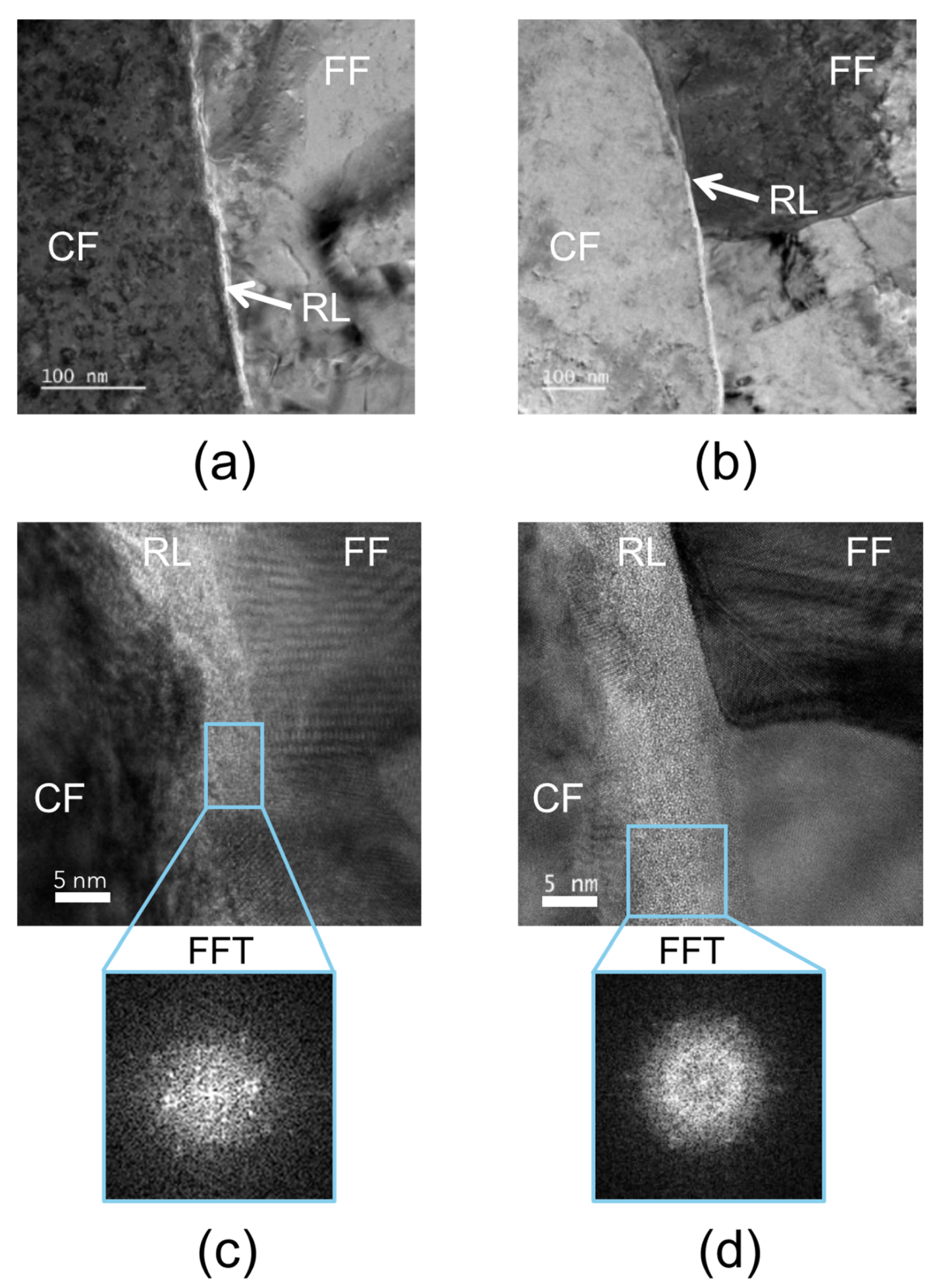
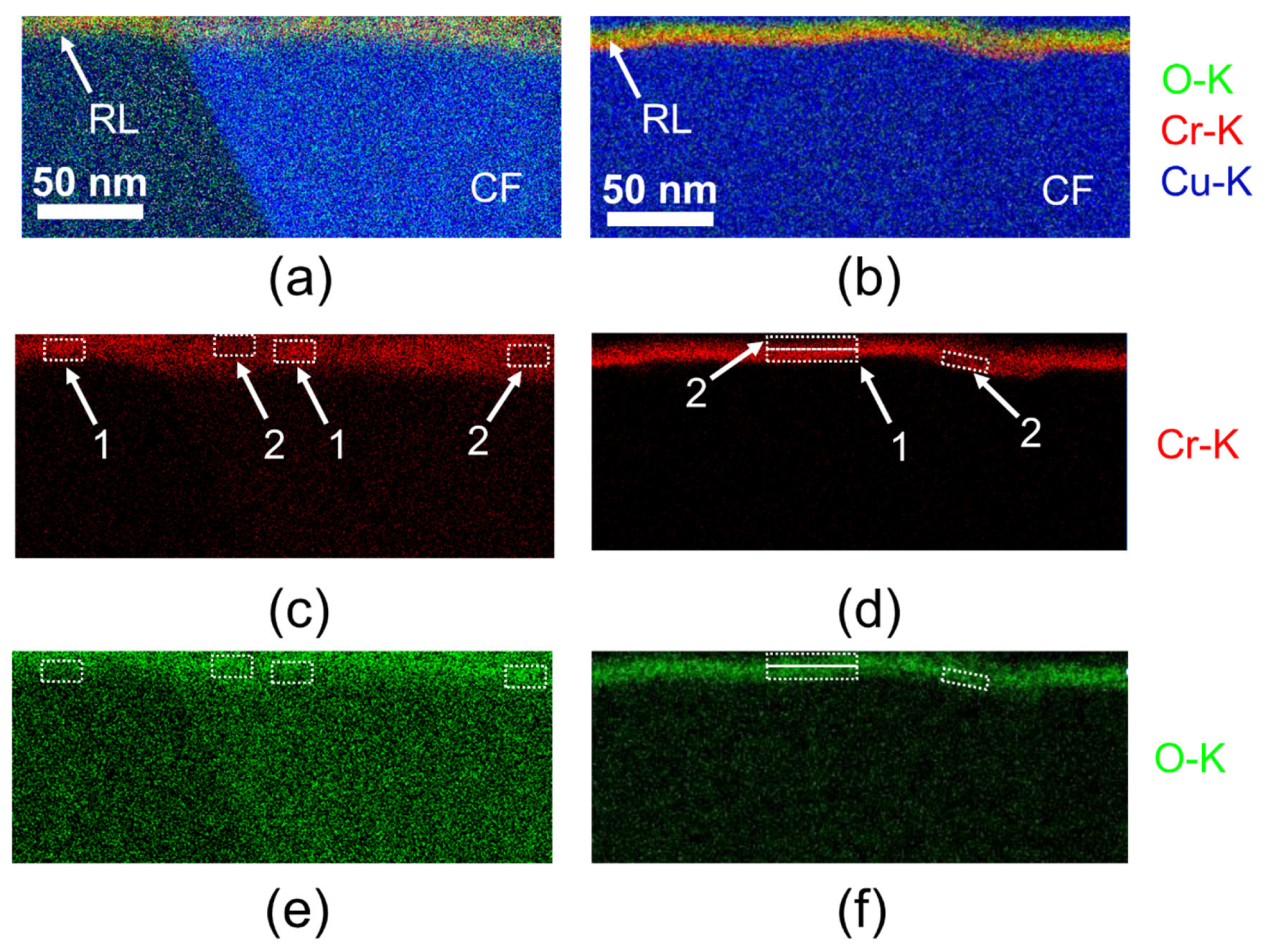
3.4. TEM Analysis
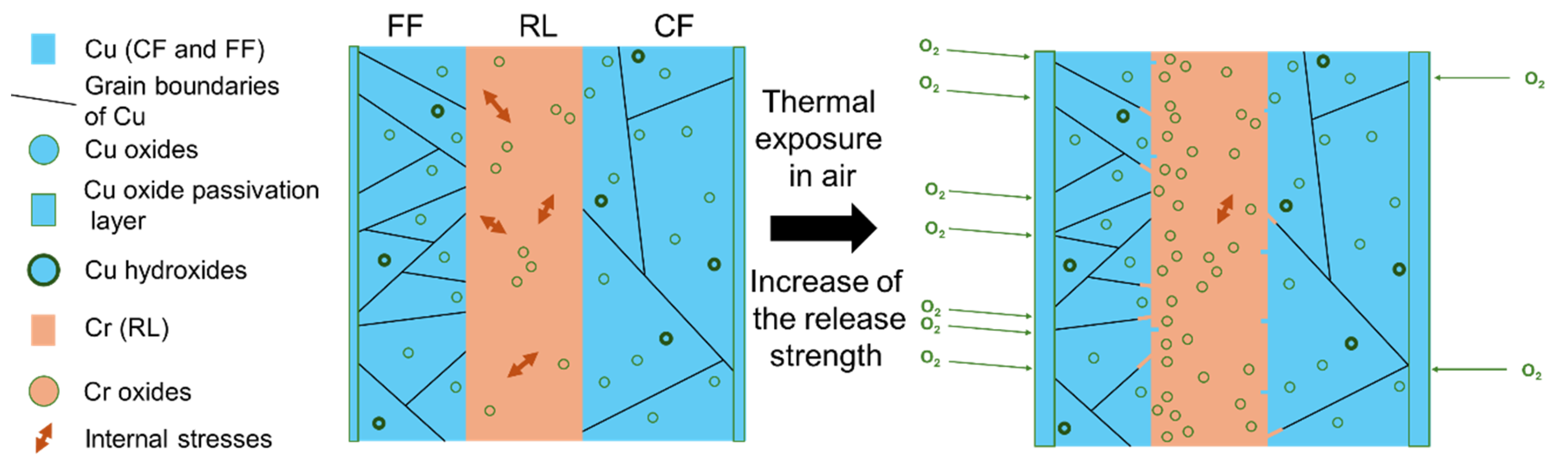
3.5. Summary of the Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Lo, Y.-L.; Chen, Y.-C.; Lee, H.-K.; Lo, S.-Y.; Cheng, T.-P. Two-Layered Thin Carrier-Attached Copper Foil. In Proceedings of the 2012 7th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taiwan, China, 24–26 October 2012; pp. 277–279. [Google Scholar] [CrossRef]
- Moon, H.; Kim, S.; Kim, S. Double Layered Electrolytic Copper Foil and Manufacturing Method Thereof. EP4202084A3, 4 October 2023. [Google Scholar]
- Kaidi, Z.; Devahif, T.; Kersten, A.; Streel, M. Composite Copper Foil and Method of Fabricating the Same. US11639557B, 28 April 2022. [Google Scholar]
- Ryu, J.H.; Yang, C.Y.; Kang, J.A. Carrier-Attached Ultrathin Copper Foil and Method for Manufacturing Same. WO2016117587A1, 28 July 2016. [Google Scholar]
- Yamamoto, H.; Okada, T.; Sato, Y.; Tanaka, K. Carrier-Attached Ultrathin Copper Foil and Method for Manufacturing Same. US8530749B2, 10 September 2013. [Google Scholar]
- Kim, H.C.; Lee, S.H.; Choi, E.S.; Song, K.D.; Beom, W.J. Carrier-Foil-Attached Ultra-Thin Copper Foil. WO2018131962A1, 19 July 2018. [Google Scholar]
- Tzou, M.-J.; Chen, K.-C.; Lin, Y.-M. Copper Foil Structure Having Blackened Ultra-Thin Foil and Manufacturing Method Thereof. US2014141274A1, 22 May 2014. [Google Scholar]
- Suzuki, Y.; Moteki, T.; Hoshino, K.; Fujisawa, S.; Kawakami, A. Ultrathin Copper Foil with Carrier and Printed Circuit Board Using Same. US7892655B2, 5 July 2007. [Google Scholar]
- Kaidi, Z.; Scholzen, S.; Kersten, A.; Streel, M. Method for Producing a Composite Copper Foil and Composite Copper Foil Obtained Therewith. WO2024132423A1, 27 June 2024. Available online: https://patents.google.com/patent/WO2024132423A1/ (accessed on 9 July 2025).
- Chen, S.; Fister, J.; Vacco, A.; Yukov, N.; Brock, A.J. Copper Foil Composite Including a Release Layer. US6346335B1, 12 February 2002. [Google Scholar]
- Wu, Y.; Han, J.; Wang, H.; Liu, X. Preparation Method Of Ultrathin Copper Foil Based on Crystalline-Amorphous Composite Stripping Layer. CN115948773A, 11 April 2023. [Google Scholar]
- Addiego, F.; Bhusari, R.; Bardon, J.; Scholzen, S.; Kaidi, Z. Nanoscale Cross-Sectional Characterization of Thin Layers in Material Assemblies. Nanomaterials 2025, 15, 840. [Google Scholar] [CrossRef] [PubMed]
- Kocun, M.; Labuda, A.; Meinhold, W.; Revenko, I.; Proksch, R. Fast, High Resolution, and Wide Modulus Range Nanomechanical Mapping with Bimodal Tapping Mode. ACS Nano 2017, 11, 10097–10105. [Google Scholar] [CrossRef]
- Hoshino, S.; Laitinen, H.A.; Hoflund, G.B. The Electrodeposition and Properties of Amorphous Chromium Films Prepared from Chromic Acid Solutions. J. Electrochem. Soc. 1986, 133, 681–685. [Google Scholar] [CrossRef]
- El-Sharif, M.; Chisholm, C.U. Characteristics of Electrodeposited Chromium. Trans. IMF 1997, 75, 208–212. [Google Scholar] [CrossRef]
- Baglin, J.E.E.; Brusic, V.; Alessandrini, E.; Ziegler, J. Thin Film Interdiffusion of Chromium and Copper. In Applications of Ion Beams to Metals; Picraux, S.T., EerNisse, E.P., Vook, F.L., Eds.; Springer: Boston, MA, USA, 1974; pp. 169–178. ISBN 9781468420814. [Google Scholar]
- O’Neill, J.J.; Vossen, J.L. Cr–Cu and Cr–Cu–Cr Thin Film Metallization. J. Vac. Sci. Technol. 1973, 10, 533–538. [Google Scholar] [CrossRef]
- Borges, J.N.; Belmonte, T.; Guillot, J.; Duday, D.; Moreno-Couranjou, M.; Choquet, P.; Migeon, H. Functionalization of Copper Surfaces by Plasma Treatments to Improve Adhesion of Epoxy Resins. Plasma. Process. Amp. Polym. 2009, 6, S490–S495. [Google Scholar] [CrossRef]
- Choudhary, S.; Sarma, J.V.N.; Pande, S.; Ababou-Girard, S.; Turban, P.; Lepine, B.; Gangopadhyay, S. Oxidation Mechanism of Thin Cu Films: A Gateway towards the Formation of Single Oxide Phase. AIP Adv. 2018, 8, 055114. [Google Scholar] [CrossRef]
- Perinet, F.; Le Duigou, J.; Monty, C. Oxygen Self-Diffusion in Volume and in Grain-Boundaries of Cu2−xO. In Non-Stoichiometric Compounds: Surfaces, Grain Boundaries and Structural Defects; Nowotny, J., Weppner, W., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 387–397. ISBN 9789400909434. [Google Scholar]
- Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes; Springer series in solid state science; Springer: Berlin, Germany; New York, NY, USA, 2007; ISBN 9783540714866. [Google Scholar]
- Urata, S.; Yoshino, H.; Ono, M.; Miyasaka, S.; Ando, R.; Hayashi, Y. Adhesion between Copper and Amorphous Silica: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2018, 122, 28204–28214. [Google Scholar] [CrossRef]
- DelRio, F.W.; Kracum, M.R.; Lu, P.; Winter, I.S.; Chandross, M.; Hardin, T.J. Surface Oxide and Impact Ratio Dictate Interfacial Adhesion of Cold Spray Bulk Metallic Glass Single Splats. Surf. Coat. Technol. 2024, 489, 131057. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, H.; Zhang, J.; Li, Q.; Wang, Y.; Huang, T.; Liu, J.; Wang, X. Interfacial Bonding of CuZr Metallic Glass via Oxide: A Molecular Dynamics Study. Corros. Sci. 2021, 182, 109275. [Google Scholar] [CrossRef]
- Lee, H.J.; Yu, J. Study on the Effects of Copper Oxide Growth on the Peel Strength of Copper/Polyimide. J. Elec. Mater. 2008, 37, 1102–1110. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Grogan, A.L.; Asbury, D.A.; Laitinen, H.A.; Hoshino, S. A Characterization Study of Amorphous Chromium Films Electrodeposited from Chromic Acid Solutions. II. Appl. Surf. Sci. 1987, 28, 224–234. [Google Scholar] [CrossRef]
- Sziráki, L.; Kuzmann, E.; Papp, K.; Chisholm, C.U.; El-Sharif, M.R.; Havancsák, K. Electrochemical Behaviour of Amorphous Electrodeposited Chromium Coatings. Mater. Chem. Phys. 2012, 133, 1092–1100. [Google Scholar] [CrossRef]
- Masumoto, T.; Hashimoto, K. Corrosion properties of amorphous metals. J. Phys. Colloq. 1980, 41, C8-894–C8-900. [Google Scholar] [CrossRef]
- Raja, P.M.V.; Esquenazi, G.L.; Gowenlock, C.E.; Jones, D.R.; Li, J.; Brinson, B.; Barron, A.R. Electrodeposition of Cu–SWCNT Composites. C 2019, 5, 38. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chuang, R.W. Study on Annealing Process of Aluminum Oxide Passivation Layer for PERC Solar Cells. Coatings 2021, 11, 1052. [Google Scholar] [CrossRef]
- Enganati, S.K.; Addiego, F.; Fernandes, J.P.C.; Koutsawa, Y.; Zielinski, B.; Ruch, D.; Mertz, G. Multiscale Characterization of the Interfacial Region in Flexible Rubber Composites: Initial Structure and Evolution upon Thermal Treatment. Polym. Test. 2021, 98, 107203. [Google Scholar] [CrossRef]
- Salvati, E.; Romano-Brandt, L.; Mughal, M.Z.; Sebastiani, M.; Korsunsky, A.M. Generalised Residual Stress Depth Profiling at the Nanoscale Using Focused Ion Beam Milling. J. Mech. Phys. Solids 2019, 125, 488–501. [Google Scholar] [CrossRef]
- Stratton, P. Ellingham Diagrams—Their Use and Misuse. Int. Heat. Treat. Surf. Eng. 2013, 7, 70–73. [Google Scholar] [CrossRef]
- Yu, S.; Gong, Z.; Gao, M.; Li, J.; Xie, W.; Wei, Y.; Li, D.; Yang, L.; Chen, D.; Li, Y.; et al. In-Situ Construction of Nano-Multifunctional Interlayer to Obtain Intimate Li/Garnet Interface for Dendrite-Free All Solid-State Battery. J. Mater. Sci. Technol. 2025, 206, 248–256. [Google Scholar] [CrossRef]
- Guan, S.; Li, Y.; Xu, C.; Yin, N.; Xu, C.; Wang, C.; Wang, M.; Xu, Y.; Chen, Q.; Wang, D.; et al. Self-Assembled Interlayer Enables High-Performance Organic Photovoltaics with Power Conversion Efficiency Exceeding 20%. Adv. Mater. 2024, 36, 2400342. [Google Scholar] [CrossRef]
- Available online: https://www.doitpoms.ac.uk/tlplib/ellingham_diagrams/interactive.php (accessed on 11 June 2025).
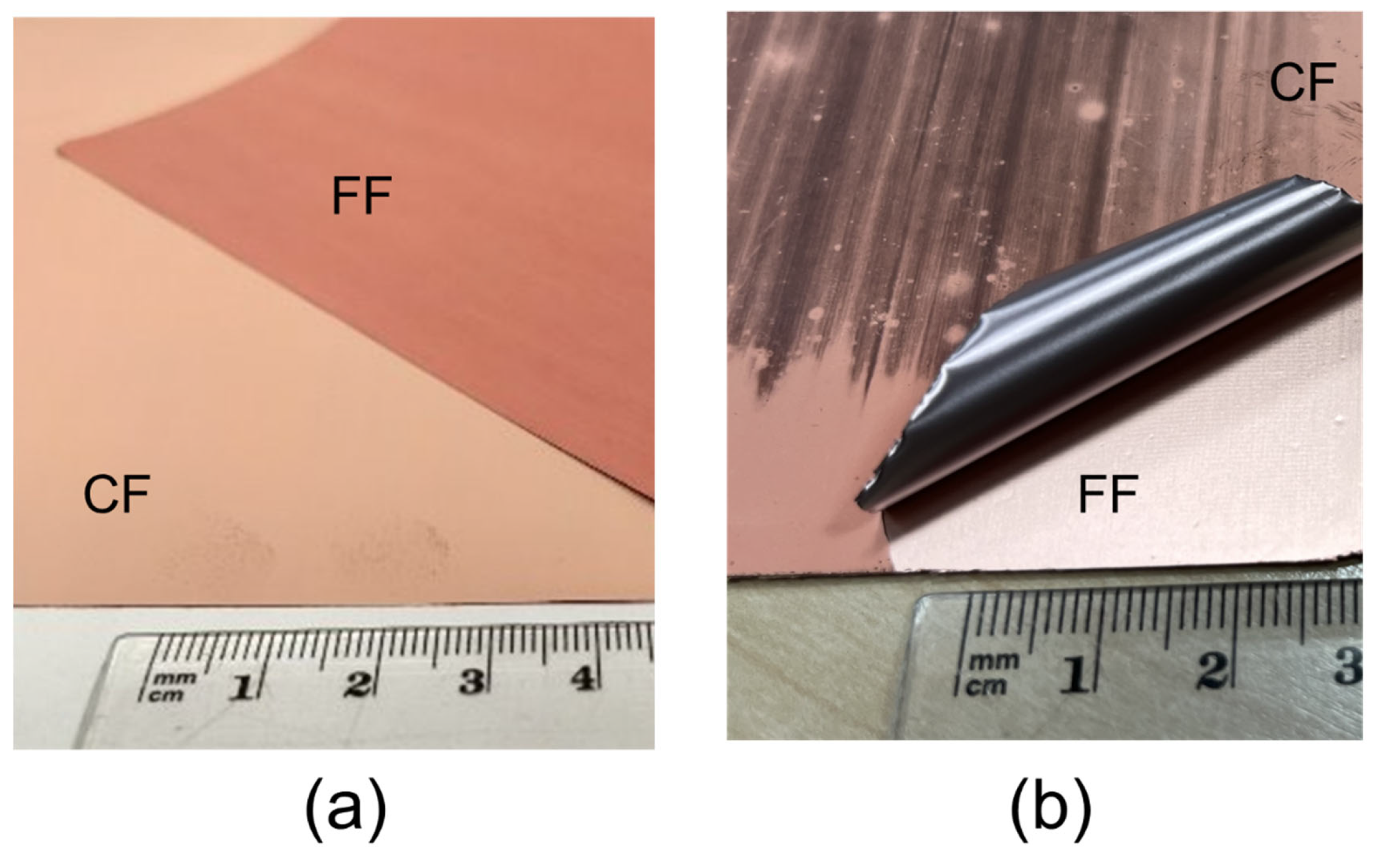

| Sample | Release Strength (N/m) |
|---|---|
| Untreated DTH-Cr | 5.9 |
| Thermally exposed DTH-Cr at 230 °C | 163.0 |
| Sample | Elemental Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (at%) | Cr (at%) | Cu (at%) | N (at%) | Na (at%) | O (at%) | P (at%) | S (at%) | Si (at%) | O/(Cr + Cu) (-) | |
| Untreated DTH-Cr-CF | 27.8 | 22.2 | 2.0 | 0.7 | <0.5 | 43.3 | 3.3 | <0.5 | <0.5 | 1.79 |
| Untreated DTH-Cr-FF | 31.7 | 1.8 | 33.9 | <0.5 | <0.5 | 30.1 | 2.4 | <0.5 | <0.5 | 0.84 |
| Thermally exposed DTH-Cr-CF at 230 °C | 21.1 | 20.0 | 1.2 | <0.5 | 0.6 | 52.5 | 3.5 | <0.5 | <0.5 | 2.48 |
| Sample | Composition in Cr Species from Cr 2p | |
|---|---|---|
| Cr0 (%) | Cr Oxides (%) | |
| Untreated DTH-Cr-CF | 49.6 | 50.4 |
| Thermally exposed DTH-Cr-CF at 230 °C | 11.8 | 88.2 |
| Sample | Composition in Cu Species from Cu 2p | ||
|---|---|---|---|
| Cu0 (%) | Cu(OH)2 (%) | CuO (%) | |
| Untreated DTH-Cr-CF | 87.5 | 6.1 | 6.4 |
| Thermally exposed DTH-Cr-CF at 230 °C | 70.5 | 17.1 | 12.3 |
| Sample | AFM Analysis of RL | |
|---|---|---|
| Minimum Elastic Modulus (GPa) | Thickness (nm) | |
| Untreated DTH-Cr | 27.1 ± 8.2 | 13.8 ± 2.8 |
| Thermally exposed DTH-Cr at 230 °C | 27.2 ± 5.1 | 15.3 ± 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhusari, R.; Bardon, J.; Guillot, J.; Philippe, A.-M.; Scholzen, S.; Kaidi, Z.; Addiego, F. Method for Elucidating the Structural Evolution of a Nanoscale Release Layer in Double Copper Foils Under Thermal Exposure. Materials 2025, 18, 3316. https://doi.org/10.3390/ma18143316
Bhusari R, Bardon J, Guillot J, Philippe A-M, Scholzen S, Kaidi Z, Addiego F. Method for Elucidating the Structural Evolution of a Nanoscale Release Layer in Double Copper Foils Under Thermal Exposure. Materials. 2025; 18(14):3316. https://doi.org/10.3390/ma18143316
Chicago/Turabian StyleBhusari, Rutuja, Julien Bardon, Jérôme Guillot, Adrian-Marie Philippe, Sascha Scholzen, Zainhia Kaidi, and Frédéric Addiego. 2025. "Method for Elucidating the Structural Evolution of a Nanoscale Release Layer in Double Copper Foils Under Thermal Exposure" Materials 18, no. 14: 3316. https://doi.org/10.3390/ma18143316
APA StyleBhusari, R., Bardon, J., Guillot, J., Philippe, A.-M., Scholzen, S., Kaidi, Z., & Addiego, F. (2025). Method for Elucidating the Structural Evolution of a Nanoscale Release Layer in Double Copper Foils Under Thermal Exposure. Materials, 18(14), 3316. https://doi.org/10.3390/ma18143316








