A Critical Review of Membrane Distillation Using Ceramic Membranes: Advances, Opportunities and Challenges
Highlights
- The correlation between ceramic membrane properties (e.g., porosity, pore size, thermal conductivity) and antiwetting performance in membrane distillation is critically analyzed.
- Surface modification techniques and materials—including chemical grafting and physical coatings—for hydrophobizing ceramic membranes are discussed.
- Emerging strategies such as hierarchical structuring and fluorine-free coatings are explored to enhance membrane wetting resistance and sustainability.
- The review outlines the main challenges (e.g., high cost, fragility, wetting) and provides comprehensive guidelines for the development of ceramic membranes in membrane distillation applications.
Abstract
1. Introduction
2. Ceramic Membrane Properties
2.1. Liquid Entry Pressure
2.2. Pore Size, Porosity, and Thickness
| Year | Membrane Material | Membrane Configuration | Pore Size (μm) | Porosity (%) | Thickness (μm) | Contact Angle (°) | LEP (kPa) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2004 | Alumina | Tubular | 0.2 | N.A. | N.A. * | 123–143 | N.A. | [46] |
| Zirconia | 0.05 | N.A. | N.A. | 116–145 | N.A. | |||
| 2011 | Tunisian clay | Tubular | 0.18 | N.A. | 35 | 177–179 | N.A. | [47] |
| 2014 | Titania | Tubular | 0.170–0.175 | N.A. | N.A. | 135–145 | 300–900 | [48] |
| 2015 | Alumina | Flat sheet | 0.5–1 | 59 | 780 | 133 | 200 | [49] |
| 2016 | Almina | Tubular | 0.02–0.04 | 30 | N.A. | 120 | 100–500 | [50] |
| Titania | Flat sheet | 0.2 | 40 | N.A. | 148 | N.A. | ||
| 2016 | Alumina | Flat sheet | 1–2 | 62 | 45 | 135–137 | 220–230 | [51] |
| 2016 | β-sialon | Hollow fiber | 0.80 | 45–60 | 250 | 125 | 310 | [52] |
| 2018 | Alumina | Tubular | 0.333 | 23.3 | 200 | 138 | N.A. | [53] |
| Alumina | Hollow fiber | 0.22 | 55 | 200 | 137 | N.A. | ||
| 2018 | Alumina | Hollow fiber | N.A. | N.A. | N.A. | 140 | 235 | [54] |
| 2019 | Alumina/ zinc oxide | Hollow fiber | 0.90 | 26.64 | 201 | 134 | 110 | [55] |
| 2021 | Mullite-kaolite | Hollow fiber | N.A. | 10.8 | 223 | 49.5 | N.A. | [56] |
| 2021 | Palm oil fuel shell | Hollow fiber | 1.05 | 39.2 | N.A. | 149 | 289.6 | [57] |
| 2022 | Ball clay | Hollow fiber | 0.1–1.2 | N.A. | N.A. | >150 | 33–133 | [58] |
| 2023 | Coal fly ash | Tubular | 0.18 | N.A. | 40–100 | 123.1–125.5 | >60 | [59] |
| 2025 | Wollastonite | Planar | 0.51 | 27.70 | N.A. | 150 | 125 | [60] |
2.3. Thermal Conductivity
2.4. Chemical and Thermal Stability
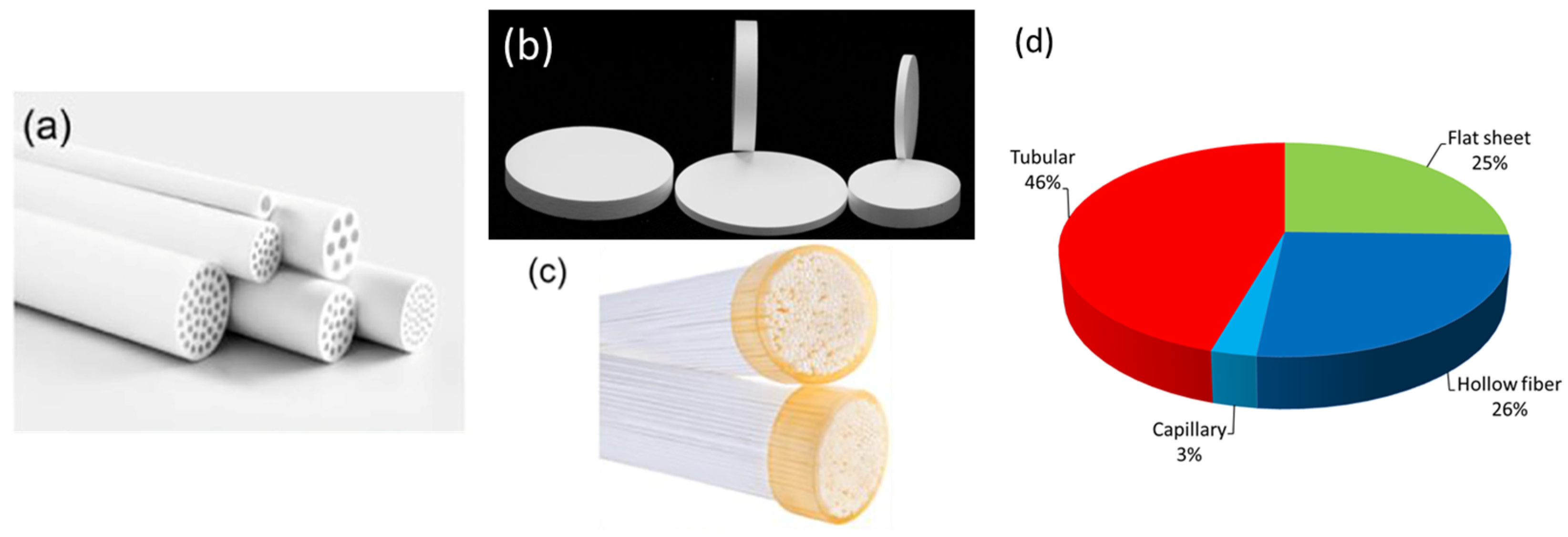
2.5. Membrane Configuration
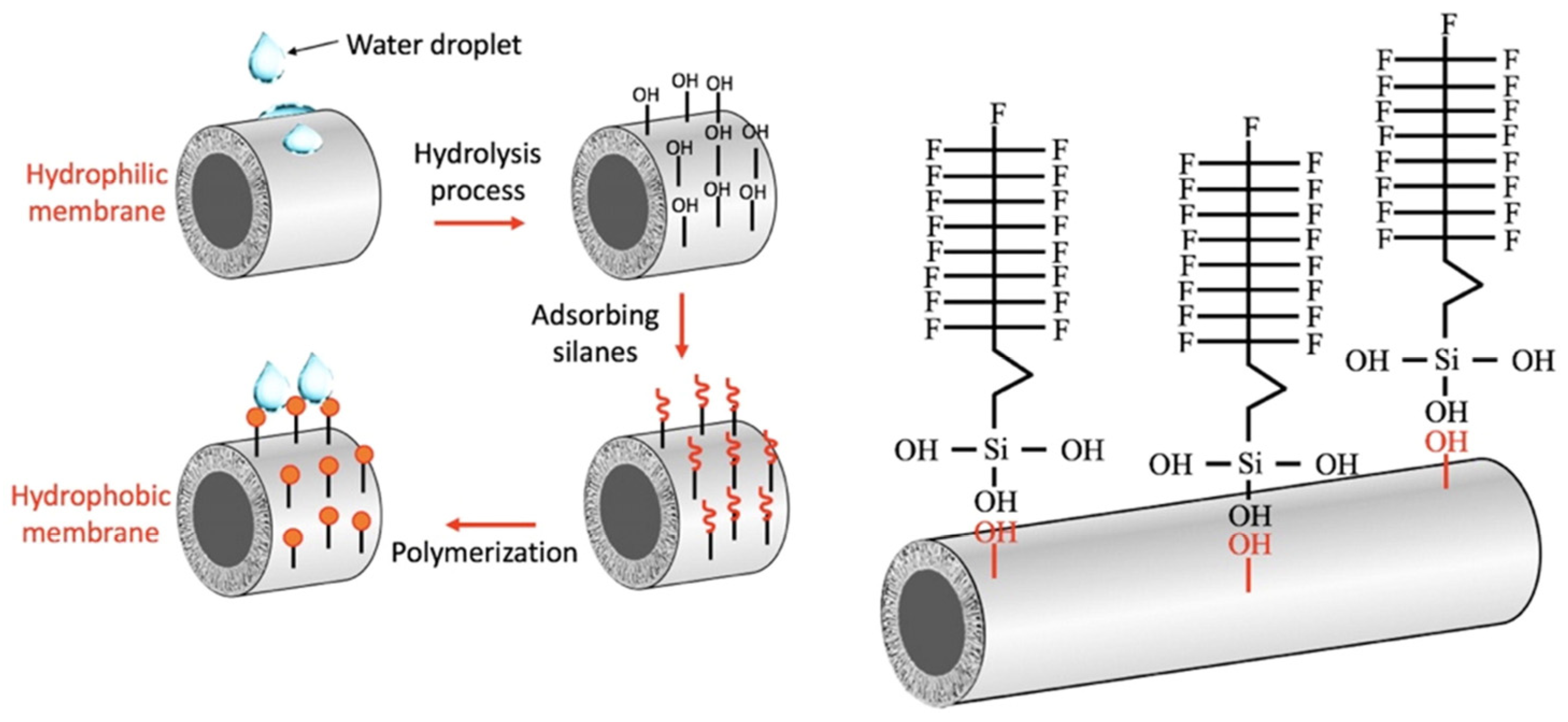
3. Surface Modification of Ceramic Membranes: From Intrinsic Hydrophilic to Functional Hydrophobic
4. Ceramic Membranes in MD Applications
| No. | Year | Material | Grafting Agent | WCA (°) | Membrane Configuration | MD Configuration | Application | Feed Solution | Feed Temp (°C) | Permeate Side | Permeate Flux (Lm2h−1) | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2004 | Zirconia/alumina | 1H,1H,2H,2H-Perfluorodecyltriehoxysilane (PFDTES) | 145 | Tubular | DCMD | Desalination | 0.001–2.9 M | 60–95 | 5 °C | 0.87–5.4 | ~100 | [46] |
| 2 | 2006 | Zirconia/alumina | PFDTES | N.A. * | Tubular | AGMD | Desalination | 1 M NaCl | 95 | 5 °C | 5.42 | 100 | [85] |
| 3 | 2007 | Zirconia | Tridecafluoro-1,1,2,2-tetrahydrooctyltriethoxysilane (T-PES) | N.A | Tubular | AGMD | Desalination | 0.5–2 M NaCl | 95 | 5 °C | 12.62 | >95 | [86] |
| 4 | 2009 | Zirconia | T-PES | 160 | Tubular | VMD/AGMD/DCMD | Desalination | 0.5–1 M NaCl | 75–95 | Vacuum 3 mbar/5 °C | 7.5/4.7/4 | 99.8/99.8/96.1 | [87] |
| 5 | 2011 | Tunisian clay | FAS | 180 | Planar | AGMD | Desalination | 1 M NaCl | 75–95 | 5 °C | 3.2–6.45 | 99 | [47] |
| 6 | 2014 | Silicon nitride | Tridecafluoro-1,1,2,2-tetrahydrooctyltriethoxysilane (T-PFOS) | 136 | Hollow fiber | VMD | Desalination | 0.5, 2, 4, 6 wt% NaCl | 50–80 | 0.02 bar | 15.1–22.25 | 99–100 | [88] |
| 7 | 2014 | Titania | FAS | 132 | Tubular | AGMD | Desalination | 0.5 M NaCl | 90 | 5 °C | 1.2 | N.A. | [89] |
| 8 | 2015 | Alumina | T-PFOS | 133 | Planar | DCMD | Desalination | 2 wt% NaCl | 80 | 20 °C | 19.1 | 99.5 | [49] |
| 9 | 2016 | β-sialon | T-PFOS | 125 | Hollow fiber | DCMD/VMD | Desalination | 2 wt% 4 wt% | 50–80 | 20 °C/0.02 bar | 7.92–10.75 | 99–100 | [52] |
| 10 | 2016 | Clay-alumina | Tridecafluoro-1,1,2,2-tetrahydrooctyltrimethoxysilane (T-PFS) | 145 | Capillary | AGMD | Desalination | 0.5 M NaCl | 60 | feed pressure of 0.85 bar | 4.1 | 99.96 | [90] |
| 11 | 2017 | β-sialon | PDMS | 145 | Flat sheet | SGDM | Desalination | 4 wt% NaCl | 90 | N.A. | 14.08 | >99 | [91] |
| 12 | 2017 | Alumina | T-PFS | 137 | Hollow fiber | VMD | Desalination | 3.5 wt% NaCl | 70 | 0.03 bar | 60 | >9.9 | [92] |
| 13 | 2018 | Silica/alumina | T-PFOS | 158 | Hollow fiber | VMD | Desalination | 3.5 wt% NaCl | 70 | 98 kPa | 29.3 | 99.9 | [93] |
| 14 | 2018 | Rice husk ash | T-PFS | >150 | Hollow fiber | DCMD | Desalination | 3.5 wt% NaCl | 65 | 25 °C | 38.20 | >99.9 | [94] |
| 15 | 2019 | Yttrium | Dimethyldichlorosilane (DMDCS)/dichloromethy-Isilane | 132 | Flat sheet | SGMD | Desalination | 20 wt% NaCl | 90 | 5 °C | 10.07 | 99.9 | [63] |
| 16 | 2019 | Kaolin | FAS | 145 | Hollow fiber | DCMD | Arsenic removal | As(V) | 60 | 15 °C | 28 | 100 | [95] |
| 17 | 2020 | Alumina | Methyltrichlorosilane (MTCS) | 145 | Tubular | VMD | Desalination | 9 wt% NaCl | 70 | 20 mbar | 31.2 | 100 | [96] |
| 18 | 2020 | Alumina/Titania | 1H,1H,2H,2H-perfluorodecylsilane-triethoxy (PDTS) | 152 | Hollow fiber | VMD | Desaliation | 10 wt% NaCl | 65 | 0.085 kPa | 5.81 | 100 | [97] |
| 19 | 2020 | Kaolin | FAS | 146 | Hollow fiber | DCMD | Arsenic removal | As | 60 | 10 °C | 23.3 | 100 | [98] |
| 20 | 2021 | Alumina, titania, cordierite | Tridecafluoro-1,1,2,2,-tetrahydrooctyltriethoxysilane (TFTES) | N.A | Tubular | DCMD | ZLD | 30 g NaCl/kg H2O | 60 | 20 °C | 25 | >99.9 | [99] |
| 21 | 2021 | Red clay | N.A. | 95.4 | Tubular | VMD | Desalination | 1 wt% NaCl | 70 | 3.5 mbar | 13.10 | 98.96 | [100] |
| 22 | 2022 | Ball clay | ZnO nanoparticles with T-PFOS | >150 | Hollow fiber | DCMD | Desalination | 1 wt% NaCl | 80 | 10 °C | 6.2 | >99.8 | [72] |
| 23 | 2022 | Cenosphere | Poly(dimethyl siloxane) | 119.2 | N.A. | DCMD | Desalination | 2 wt% NaCl | 90 | 5 °C | 13 | 99 | [101] |
| 24 | 2022 | β-sialon | Boron nitride | 145 | Planar | SGMD | Desalination | 2 wt% NaCl | 80 | Nitrogen | 7.7 | 99.9 | [76] |
| 25 | 2022 | Metakaolin | 1H,1H,2H,2H-perfluorooctyltriethoxysilane (FOTS) | 143.3 | Flat sheet | DCMD | Desalination | 3.5 wt% NaCl | 80 | Flow rate of 40 L/h | 6.58 | >95 | [102] |
| 26 | 2022 | Si2N2O | Dichloromethylsilane (DMDCS) | 152 | Planar | SGMD | Desalination | 4 wt% NaCl | 70 | Dry air | 15.6 | >99.9 | [103] |
| 27 | 2022 | Silica sand | 1H,1H,2H,2H-Perfluorodecyltriethoxysilane (FAS17) | 142.5 | Hollow fiber | VMD | Desalination | 0.8 wt% NaCl | 80 | 25 °C water | 35 | 100 | [69] |
| 28 | 2023 | Al2O3/TiO2 | C16 | 143.3–144.4 | Tubular | PVMD | Desalination | Produced water (TDS di 135 g/L, TDS di 175 g/L) | 68.5 | vacuum 11 kPa | 2.88–3.02 | 99.9 | [104] |
| 29 | 2023 | Al2O3/TiO2 | Alkyl triethoxysilane | 140 | Tubular | VMD | ZLD | ~26 wt% NaCl | 75 | vacuum 7.5 kPa | 35 | 99.98 | [105] |
| 30 | 2023 | Coal fly ash | 1H,1H,2H,2H-Perfluorodecyltrichlorosilane (PFDTS) | >123 | Tubular | VMD | Desalination | 1 wt% NaCl | 55 | vacuum 40 kPa | 9.54 | 98.36 | [59] |
| 32 | 2024 | Kaolin | 1H,1H,2H,2H-Perfluorododecyltrichlorosilane (PFDDoTS) | 160 | Flat-sheet | AGMD | Desalination | 7 wt% NaCl | 70 | Air gap/20 °C cold | 7/9.3 | >99.99 | [106] |
| 33 | 2024 | Mullite | Hexadecyltrimethoxysilane (HDTMS) | 160 | Tubular | DCMD | Desalination | 3.5 wt% NaCl | 68 | 15 | 3.15 | 99.6 | [107] |
| 34 | 2024 | SIC/alumina | PFDTES | 152.4 | Disc | VMD | Desalination | 3.5 wt% NaCl | 70 | vacuum 90 kPa | 11.1 | 99.9 | [73] |
| 35 | 2024 | Mullite/kaolin | PFDTES | 155.9 | Hollow fiber | DCMD | Desalination | 3.5 wt% NaCl | 70 | 10 °C water | 24.3 | 99.91 | [75] |
| 36 | 2024 | Wollastonite | Methylphenyl Silicone Resin | 150 | Planar | VMD | Desalination | 3.5 wt% NaCl | 80 | vacuum 100 kPa | 35.2 | 99.9 | [60] |
| 37 | 2025 | Cordierite clay | PFDTES | 145.5 | Capillary | AGMD | Desalination | 3.5 wt% NaCl | 85 | Air gap/5 °C cold | 3 | 95.0 | [108] |
5. Critical Challenges and Emerging Strategies for Ceramic Membranes Development in MD
5.1. High Fabrication Cost
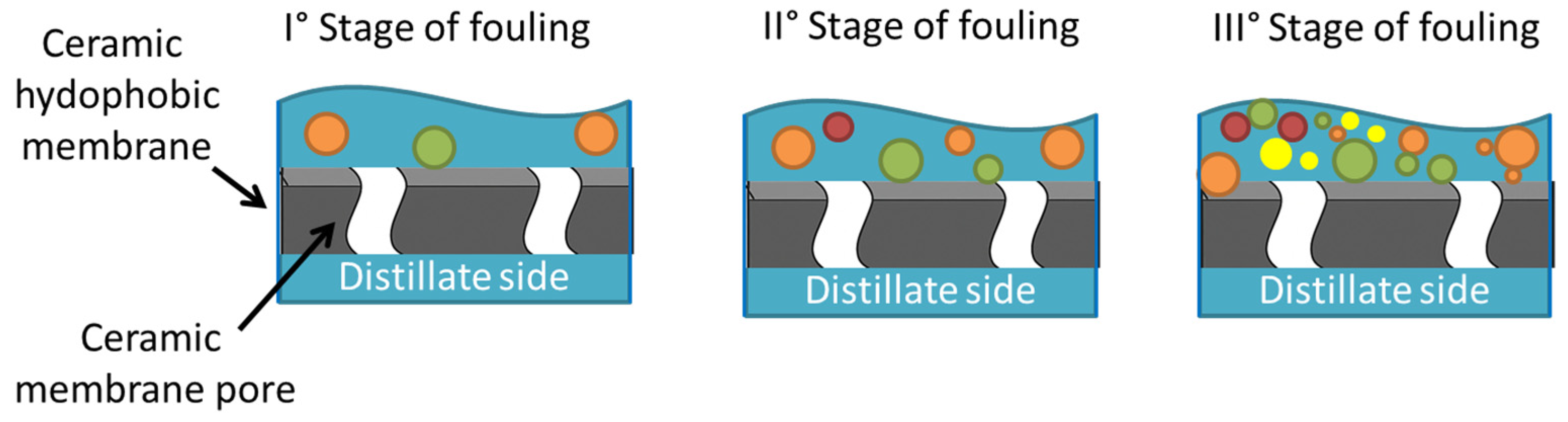
5.2. Wetting and Fouling
5.3. Fragility
6. Emerging Applications of Ceramic Membrane in MD
7. Conclusions and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Abejón, R.; Romero, J.; Quijada-Maldonado, E. Potential of membrane distillation for water recovery and reuse in water stress scenarios: Perspective from a bibliometric analysis. Desalination 2024, 591, 117989. [Google Scholar] [CrossRef]
- Shemer, H.; Wald, S.; Semiat, R. Challenges and solutions for global water scarcity. Membranes 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Xie, M.; Li, J.; Shon, H.; Zheng, C.; Zhao, S. Polyaniline-based adsorbents for aqueous pollutants removal: A review. Chem. Eng. J. 2021, 418, 129425. [Google Scholar] [CrossRef]
- Tene, T.; Bellucci, S.; Guevara, M.; Viteri, E.; Arias Polanco, M.; Salguero, O.; Vera-Guzmán, E.; Valladares, S.; Scarcello, A.; Alessandro, F.; et al. Cationic pollutant removal from aqueous solution using reduced graphene oxide. Nanomaterials 2022, 12, 309. [Google Scholar] [CrossRef]
- Fu, J.; Gao, P.; Wang, L.; Zhang, Y.; Deng, Y.; Huang, R.; Zhao, S.; Yu, Z.; Wei, Y.; Wang, G.; et al. Regulating Regulating crystal facets of MnO2 for enhancing peroxymonosulfate activation to degrade pollutants: Performance and mechanism. Catalysts 2022, 12, 342. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Y.; Zhang, Q.; Hu, J.; Wen, Y.; Wang, J.; Wang, R.; Zhao, S.; Zhao, S. Activity and mechanism of vanadium sulfide for organic contaminants oxidation with peroxymonosulfate. J. Colloid Interface Sci. 2023, 635, 358–369. [Google Scholar] [CrossRef]
- Minier-Matar, J.; AlShamari, E.; Raja, M.; Khan, F.; Al-Maas, M.; Hussain, A.; Adham, S. Detailed organic characterization of process water to evaluate reverse osmosis membrane fouling in industrial wastewater treatment. Desalination 2024, 572, 117128. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Y.; Bai, Y.; Lv, H.; Zhou, W.; Liu, Y.; Yu, D.G. Progress in defect engineering strategies to enhance piezoelectric catalysis for efficient water treatment and energy regeneration. Sep. Purif. Technol. 2024, 330, 125247. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of heterogeneous catalytic ozonation in wastewater treatment: An overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Sun, K.; Lyu, Q.; Zheng, X.; Liu, R.; Tang, C.Y.; Zhao, M.; Dong, Y. Enhanced water treatment performance of ceramic-based forward osmosis membranes via MOF interlayer. Water Res. 2024, 254, 121395. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Ye, J.; Li, E.; Xu, S.; Huang, M. Enhanced anti-fouling of forward osmosis membrane by pulsatile flow operation in textile wastewater treatment. Desalination 2023, 565, 116878. [Google Scholar] [CrossRef]
- Alessandro, F.; Macedonio, F.; Frappa, M.; Drioli, E. Freshwater and minerals recovery from synthetic produced water by membrane distillation/membrane crystallization processes. Appl. Water Sci. 2024, 14, 104. [Google Scholar] [CrossRef]
- Santoro, S.; Occhiuzzi, J.; Aquino, M.; Politano, A.; Straface, S.; D’Andrea, G.; Carrillo, C.; Mallada, R.; Garcia, A.; Estay, H.; et al. Green photocatalytic mixed matrix membranes for simultaneous arsenic photo-oxidation and water recovery via membrane distillation. Sep. Purif. Technol. 2024, 342, 127042. [Google Scholar] [CrossRef]
- Dow, N.; Gray, S.; Li, J.D.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot trial of membrane distillation driven by low grade waste heat: Membrane fouling and energy assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Boukhriss, M.; Timoumi, M.; Bacha, H.B. Experimental of membrane distillation unit coupled with a DCMD using solar energy. Solar Compass 2023, 7, 100055. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Du, F.; Han, J.; Hao, G.; Li, L.; Ma, Q. Geothermal direct contact membrane distillation system for purifying brackish water. Desalination 2021, 500, 114887. [Google Scholar] [CrossRef]
- Memon, S.; Lee, H.S.; Kim, W.S.; Kim, Y.D. Parametric investigation of modular configuration of multi-stage direct contact membrane distillation powered by waste heat of wind turbine. Desalination 2022, 533, 115770. [Google Scholar] [CrossRef]
- Basile, A.; Cassano, A.; Rastogi, N.K. Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 411–441. [Google Scholar]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Newnes: Amsterdam, The Netherlands, 2010; Volumes 1 and 4, pp. 1–44. [Google Scholar]
- Curcio, E.; Di Profio, G. The Handbook of Continuous Crystallization; The Royal Society of Chemistry: London, UK, 2020; pp. 321–352. [Google Scholar]
- Shen, L.; Dang, M.; Han, X. Recent advances in membrane crystallization. CrystEngComm 2023, 25, 2503–2517. [Google Scholar] [CrossRef]
- Huang, S.M.; Chen, Y.H.; Yuan, W.Z.; Zhao, S.; Hong, Y.; Ye, W.B.; Yang, M. Heat and mass transfer in a hollow fiber membrane contactor for sweeping gas membrane distillation. Sep. Purif. Technol. 2019, 220, 334–344. [Google Scholar] [CrossRef]
- Santoro, S.; Vidorreta, I.M.; Sebastian, V.; Moro, A.; Coelhoso, I.M.; Portugal, C.A.; Desiderio, G.; Lombardo, G.; Drioli, E.; Mallada, R.; et al. A non-invasive optical method for mapping temperature polarization in direct contact membrane distillation. J. Membr. Sci. 2017, 536, 156–166. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Christie, K.S.; Horseman, T.; Lin, S. Energy efficiency of membrane distillation: Simplified analysis, heat recovery, and the use of waste-heat. Environ. Int. 2020, 138, 105588. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zeid, M.A.E.R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Xia, L.; Guan, K.; He, S.; Luo, P.; Matsuyama, H.; Zhong, Z.; Zou, D. Engineering high-flux poly (vinylidene fluoride) membranes with symmetric structure for membrane distillation via delayed phase inversion. Sep. Purif. Technol. 2024, 338, 126499. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process–A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef]
- Arjmand, E.; Mansourizadeh, A.; Ghaedi, A.M.; Rahbari-Sisakht, M. Surface modification of porous polyvinylidene fluoride hollow fiber membrane by sulfonated poly (ether ether ketone) coating for membrane distillation of oily wastewater. J. Appl. Polym. Sci. 2022, 139, e53196. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, W.; Zhang, X.; Li, J.; Ma, J.; Zhang, T. Engineering a protective surface layer to resist membrane scaling and scale-induced wetting in membrane distillation for the treatment of hypersaline wastewater. J. Chem. Eng. 2023, 452, 139167. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, G.; An, X.; Hu, Y. Robust reduced graphene oxide composite membranes for enhanced anti-wetting property in membrane distillation. Desalination 2022, 526, 115549. [Google Scholar] [CrossRef]
- Yang, H.R.; Huang, Y.H.; Wang, C.F.; Chung, T.S. Green fabrication of PVDF superhydrophobic membranes using a green solvent triethyl phosphate (TEP) for membrane distillation. Desalination 2023, 566, 116934. [Google Scholar] [CrossRef]
- Eryildiz, B.; Ozbey-Unal, B.; Menceloglu, Y.Z.; Keskinler, B.; Koyuncu, I. Development of robust superhydrophobic PFA/TMI/PVDF membrane by electrospinning/electrospraying techniques for air gap membrane distillation. J. Appl. Polym. Sci. 2023, 140, e53635. [Google Scholar] [CrossRef]
- Capizzano, S.; Frappa, M.; Drioli, E.; Alessandro, F.; Macedonio, F. Chemical interaction between PVDF and Li cations during LiCl crystallization in VMCr. J. Membr. Sci. 2022, 658, 120733. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Tai, Z.S.; Othman, M.H.D.; Harun, Z.; Jamalludin, M.R.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Hydrophobic ceramic membrane for membrane distillation: A mini review on preparation, characterization, and applications. Sep. Purif. Technol. 2019, 217, 71–84. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Kurniawan, T.A.; El-badawy, T.; Goh, P.S.; Othman, N.H.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Bottlenecks and recent improvement strategies of ceramic membranes in membrane distillation applications: A review. J. Eur. Ceram. 2022, 42, 5179–5194. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Tian, R.; Yin, H.; Qiu, Y.; Wang, F. Optimization of preparation process and characterization for hydrophobic a-Al2O3 ceramic membrane. Mater. Chem. Phys. 2022, 276, 125280. [Google Scholar] [CrossRef]
- Ferreira, R.K.M.; Ramlow, H.; Marangoni, C.; Machado, R.A.F. A review on the manufacturing techniques of porous hydrophobic ceramic membranes applied to direct contact membrane distillation. Adv Appl Ceram. 2021, 120, 336–357. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How to optimize the membrane properties for membrane distillation: A review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Ni, T.; Lin, J.; Kong, L.; Zhao, S. Omniphobic membranes for distillation: Opportunities and challenges. Chin. Chem. Lett. 2021, 32, 3298–3306. [Google Scholar] [CrossRef]
- Hendren, Z.D.; Brant, J.; Wiesner, M.R. Surface modifi cation of nanostructured ceramic membranes for direct contact mem brane distillation. J. Membr. Sci. 2009, 331, 1–10. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Khemakhem, S.; Amar, R.B. Modification of Tunisian clay membrane surface by silane grafting: Application for desalination with Air Gap Membrane Distillation process. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 79–85. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Koter, S.; Kujawski, W. Highly efficient hydrophobic titania ceramic membranes for water desalination. ACS Appl. Mater. Interfaces 2014, 6, 14223–14230. [Google Scholar] [CrossRef]
- Ren, C.; Fang, H.; Gu, J.; Winnubst, L.; Chen, C. Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J. Eur. Ceram. Soc. 2015, 35, 723–730. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Bryjak, M.; Kujawski, J. How to functionalize ceramics by perfluoroalkylsilanes for membrane separation process? Properties and application of hydrophobized ceramic membranes. ACS Appl. Mater. Interfaces 2016, 8, 7564–7577. [Google Scholar] [CrossRef]
- Gu, J.; Ren, C.; Zong, X.; Chen, C.; Winnubst, L. Preparation of alumina membranes comprising a thin separation layer and a support with straight open pores for water desalination. Ceram. Int. 2016, 42, 12427–12434. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, L.; Zhang, J.W.; Xu, X.; Chen, C.S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Ko, C.C.; Ali, A.; Drioli, E.; Tung, K.L.; Chen, C.H.; Chen, Y.R.; Macedonio, F. Performance of ceramic membrane in vacuum membrane distillation and in vacuum membrane crystallization. Desalination 2018, 440, 48–58. [Google Scholar] [CrossRef]
- Chen, Y.R.; Chen, L.H.; Chen, C.H.; Ko, C.C.; Huang, A.; Li, C.L.; Chuang, C.J.; Tung, K.L. Hydrophobic alumina hollow fiber membranes for sucrose concentration by vacuum membrane distillation. J. Membr. Sci. 2018, 555, 250–257. [Google Scholar] [CrossRef]
- Wang, T.; Yun, Y.; Wang, M.; Li, C.; Liu, G.; Yang, W. Superhydrophobic ceramic hollow fiber membrane planted by ZnO nanorod-array for high-salinity water desalination. J. Taiwan Inst. Chem. Eng. 2019, 105, 17–27. [Google Scholar] [CrossRef]
- Twibi, M.F.; Othman, M.H.D.; Hubadillah, S.K.; Alftessi, S.A.; Kurniawan, T.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O. Development of high strength, porous mullite ceramic hollow fiber membrane for treatment of oily wastewater. Ceram. Int. 2021, 47, 15367–15382. [Google Scholar] [CrossRef]
- Tai, Z.S.; Othman, M.H.D.; Mustafa, A.; Ravi, J.; Wong, K.C.; Koo, K.N.; Hubadillah, S.K.; Azali, M.A.; Alias, N.H.; Ng, B.C.; et al. Development of hydrophobic polymethylhydrosiloxane/tetraethylorthosilicate (PMHS/TEOS) hybrid coating on ceramic membrane for desalination via membrane distillation. J. Membr. Sci. 2021, 637, 119609. [Google Scholar] [CrossRef]
- Abd Aziz, M.H.; Pauzan, M.A.B.; Hisam, N.A.S.M.; Othman, M.H.D.; Adam, M.R.; Iwamoto, Y.; Puteh, M.H.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; et al. Superhydrophobic ball clay based ceramic hollow fibre membrane via universal spray coating method for membrane distillation. Sep. Purif. Technol. 2022, 288, 120574. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Qi, R.; Huang, J.; Chen, H.; Zhang, H. Development of hydrophobic coal-fly-ash-based ceramic membrane for vacuum membrane distillation. Materials 2023, 16, 3153. [Google Scholar] [CrossRef]
- Lv, H.; Chen, W.; Su, L.; Di, R.; Liu, C.; Li, Q.; Nie, Z.; Wei, Q. Slag-derived asymmetric porous wollastonite membranes hydrophobized by methylphenyl silicone resin for desalination. Ceram. Int. 2025, 51, 6567–6578. [Google Scholar] [CrossRef]
- Li, L.; Sirkar, K.K. Influence of microporous membrane properties on the desalination performance in direct contact membrane distillation. J. Membr. Sci. 2016, 513, 280–293. [Google Scholar] [CrossRef]
- Tai, Z.S.; Abd Aziz, M.H.; Othman, M.H.D.; Mohamed Dzahir, M.I.H.; Hashim, N.A.; Koo, K.N.; Hubadillah, S.K.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Ceramic membrane distillation for desalination. Sep. Purif. Rev. 2020, 49, 317–356. [Google Scholar] [CrossRef]
- Yang, M.Y.; Wang, J.W.; Li, L.; Dong, B.B.; Xin, X.; Agathopoulos, S. Fabrication of low thermal conductivity yttrium silicate ceramic flat membrane for membrane distillation. J. Eur. Ceram. 2019, 39, 442–448. [Google Scholar] [CrossRef]
- Boey, M.W.; Khan, S.A.; Li, X.; Sun, J.; Farid, M.U.; An, A.K. Thermally efficient hydrophobic zirconia ceramic nanofiber membrane for enhanced membrane distillation performance. J. Chem. Eng. 2025, 512, 162582. [Google Scholar] [CrossRef]
- Guillén-Burrieza, E.; Alarcón-Padilla, D.C.; Palenzuela, P.; Zaragoza, G. Techno-economic assessment of a pilot-scale plant for solar desalination based on existing plate and frame MD technology. Desalination 2015, 374, 70–80. [Google Scholar] [CrossRef]
- Harun, Z.; Hubadillah, S.; Hasan, S.; Yunos, M.Z. Effect of thermodynamic properties on porosity of ceramic membrane prepared by phase inversion. Appl. Mech. 2014, 575, 31–35. [Google Scholar] [CrossRef]
- Lee, R.; Battahalli, N.S.; Pemberton, M.; Wazer, E.; Anacleto, B.; Schnittger, J.; Weyd, M.; Voigt, I.; Koschikowski, J.; Kumar, M.; et al. Evaluation of multi-channel ceramic tubular membranes for high-salinity water desalination by vacuum membrane distillation. Desalination 2025, 611, 118867. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, M.; Yu, D.; Wang, D. Preparation and properties of methyltrichlorosilane grafted modified ceramic membranes for membrane distillation. J. Environ. Chem. Eng. 2025, 13, 115405. [Google Scholar] [CrossRef]
- Alftessi, S.A.; Othman, M.H.D.; Adam, M.R.B.; Farag, T.M.; Tai, Z.S.; Raji, Y.O.; Rahaman, M.A.; Jaafar, J.; Ismail, A.F.; Bakar, S.A. Hydrophobic silica sand ceramic hollow fiber membrane for desalination via direct contact membrane distillation. Alex. Eng. J. 2022, 61, 9609–9621. [Google Scholar] [CrossRef]
- Chen, X.; Gao, X.; Fu, K.; Qiu, M.; Xiong, F.; Ding, D.; Cui, Z.; Wang, Z.; Fan, Y.; Drioli, E. Tubular hydrophobic ceramic membrane with asymmetric structure for water desalination via vacuum membrane distillation process. Desalination 2018, 443, 212–220. [Google Scholar] [CrossRef]
- Chen, L.H.; Chen, Y.R.; Huang, A.; Chen, C.H.; Su, D.Y.; Hsu, C.C.; Tsa, F.Y.; Tung, K.L. Nanostructure depositions on alumina hollow fiber membranes for enhanced wetting resistance during membrane distillation. J. Membr. Sci. 2018, 564, 227–236. [Google Scholar] [CrossRef]
- Abd Aziz, M.H.; Othman, M.H.D.; Alias, N.H.; Nakayama, T.; Shingaya, Y.; Hashim, N.A.; Nakayama, T.; Shingaya, Y.; Hashim, N.A.; Kurniawan, A.T.; et al. Enhanced omniphobicity of mullite hollow fiber membrane with organosilane-functionalized TiO2 micro-flowers and nanorods layer deposition for desalination using direct contact membrane distillation. J. Membr. Sci. 2020, 607, 118137. [Google Scholar] [CrossRef]
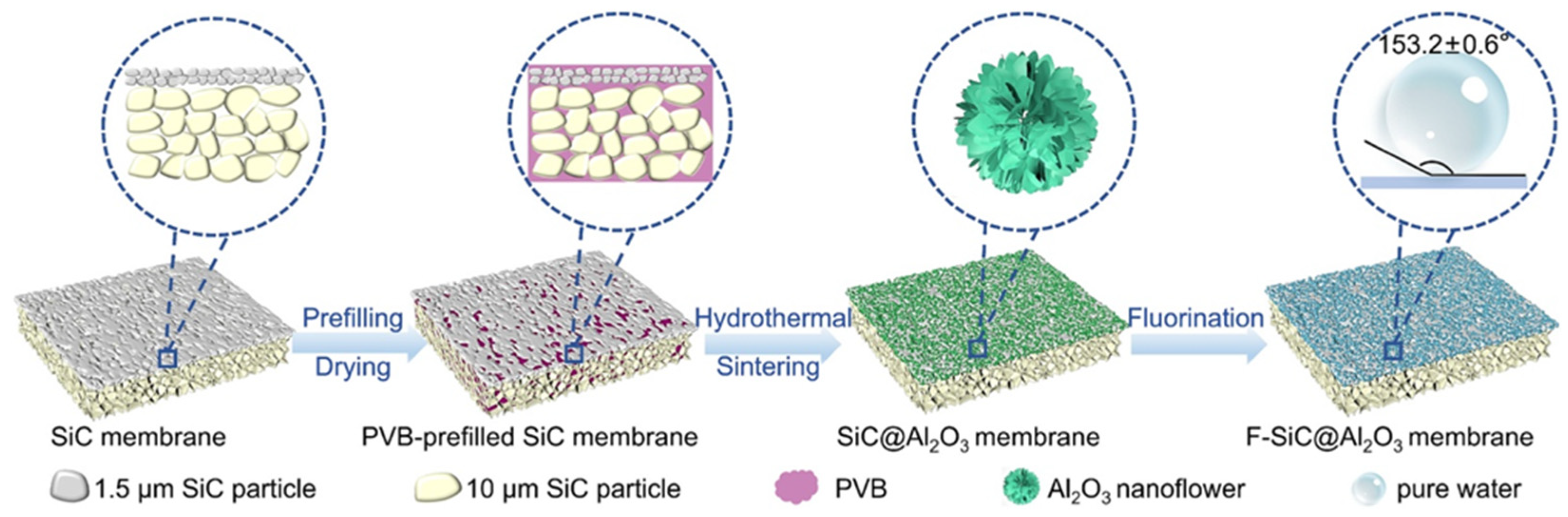
- Song, Y.; Miao, K.; Liu, J.; Kang, Y.; Zou, D.; Zhong, Z. In situ-grown Al2O3 nanoflowers and hydrophobic modification enable superhydrophobic SiC ceramic membranes for membrane distillation. Membranes 2024, 14, 117. [Google Scholar] [CrossRef]
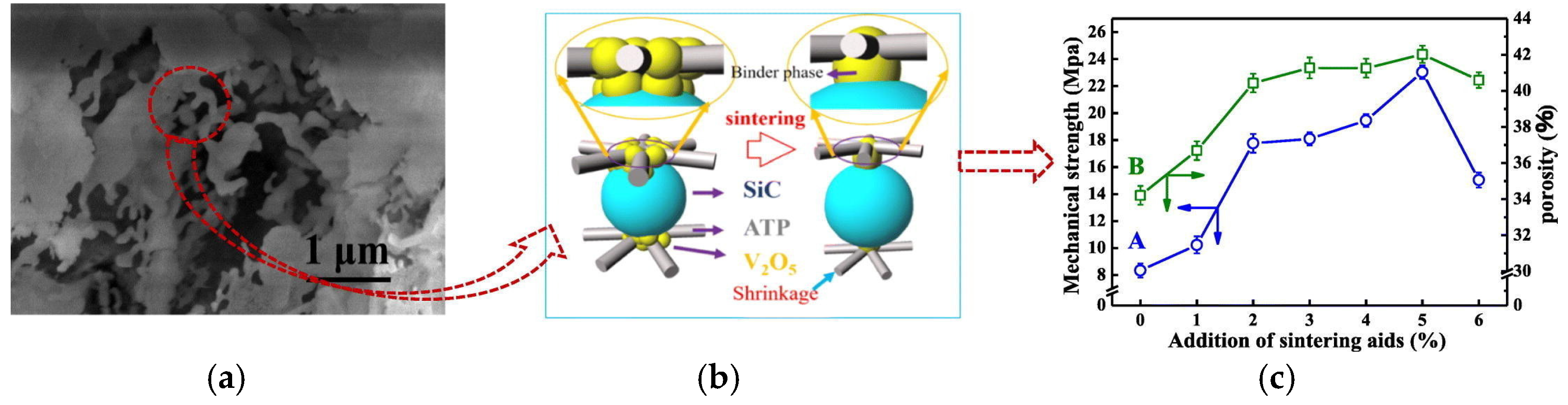
- Miao, K.; Li, H.; Xu, B.; Liu, S.; Zou, D.; Guan, K.; Wu, X.; Matsuyama, H. Prefilling polymers to regulate interfacial hierarchical structures of ceramic membranes for enhanced membrane distillation. Desalination 2025, 602, 118634. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Puteh, M.H.; Wong, K.Y.; Tan, H.; Kurniawan, T.A.; Ooi, B.S.; Nomura, M.; Iwamoto, Y. Development of superhydrophobic ceramic membrane decorated with flake-like structure by two-step synthesis for seawater desalination by membrane distillation. J. Taiwan Inst. Chem. Eng. 2024, 155, 105277. [Google Scholar] [CrossRef]
- Qian, R.; Dong, B.; Hao, S.; Wang, F.; Wang, L.; Min, Z.; Hao, L.; Xu, X.; Agathopoulos, S. Robust all-inorganic hydrophobic BN nanosheets coated β-sialon membrane for membrane distillation. J. Eur. Ceram. Soc. 2022, 42, 2672–2677. [Google Scholar] [CrossRef]
- Zahirifar, J.; Hadi, A.; Karimi-Sabet, J.; Dastbaz, A. Influence of hexagonal boron nitride nanosheets as the additives on the characteristics and performance of PVDF for air gap membrane distillation. Desalination 2019, 460, 81–91. [Google Scholar] [CrossRef]
- Jia, W.; Kharraz, J.A.; Choi, P.J.; Guo, J.; Deka, B.J.; An, A.K. Superhydrophobic membrane by hierarchically structured PDMS-POSS electrospray coating with cauliflower-shaped beads for enhanced MD performance. J. Membr. Sci. 2020, 597, 117638. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Manoj Prabhakar, M.; Saravanan, A.K.; Haiter Lenin, A.; Jerin Leno, I.; Mayandi, K.; Sethu Ramalingam, P. A short review on 3D printing methods, process parameters and materials. Mater. Today Proc. 2021, 45, 6108–6114. [Google Scholar] [CrossRef]
- Roy Barman, S.; Gavit, P.; Chowdhury, S.; Chatterjee, K.; Nain, A. 3D-Printed Materials for Wastewater Treatment. JACS Au 2023, 3, 2930–2947. [Google Scholar] [CrossRef]
- Majooni, Y.; Abioye, S.O.; Fayazbakhsh, K.; Yousefi, N. Nano-enabled 3D-Printed Structures for Water Treatment. ACS ES T Water 2024, 4, 1952–1965. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Kadir, S.H.S.A.; Jamalludin, M.R.; Harun, Z.; Abd Aziz, M.H.; Rahaman, M.A.; Jaafar, J.; Nomura, M.; Honda, S.; et al. Removal of As (III) and As (V) from water using green, silica-based ceramic hollow fibre membranes via direct contact membrane distillation. RSC Adv. 2019, 9, 3367–3376. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.R.; Kujawski, W.; Bukowska, M.; Picard, C.; Larbot, A. Application of fluoroalkylsilanes (FAS) grafted ceramic membranes in membrane distillation process of NaCl solutions. J. Membr. Sci. 2006, 281, 253–259. [Google Scholar] [CrossRef]
- Gazagnes, L.; Cerneaux, S.; Persin, M.; Prouzet, E.; Larbot, A. Desalination of sodium chloride solutions and seawater with hydrophobic ceramic membranes. Desalination 2007, 217, 260–266. [Google Scholar] [CrossRef]
- Cerneaux, S.; Strużyńska, I.; Kujawski, W.M.; Persin, M.; Larbot, A. Comparison of various membrane distillation methods for desalination using hydrophobic ceramic membranes. J. Membr. Sci. 2009, 337, 55–60. [Google Scholar] [CrossRef]
- Zhang, J.W.; Fang, H.; Wang, J.W.; Hao, L.Y.; Xu, X.; Chen, C.S. Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination. J. Membr. Sci. 2014, 450, 197–206. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Investigation of the stability of metal oxide powders and ceramic membranes grafted by perfluoroalkylsilanes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 109–117. [Google Scholar] [CrossRef]
- Das, R.; Sondhi, K.; Majumdar, S.; Sarkar, S. Development of hydrophobic clay–alumina based capillary membrane for desalination of brine by membrane distillation. J. Asian Ceram. Soc. 2016, 4, 243–251. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, X.Z.; Fan, M.; Gu, J.Q.; Hao, L.Y.; Xu, X.; Chen, C.S.; Wang, C.M.; Hao, Y.Z.; Agathopoulos, S. Porous β-Sialon planar membrane with a robust polymer-derived hydrophobic ceramic surface. J. Membr. Sci. 2017, 535, 63–69. [Google Scholar] [CrossRef]
- Ko, C.C.; Chen, C.H.; Chen, Y.R.; Wu, Y.H.; Lu, S.C.; Hu, F.C.; Li, C.L.; Tung, K.L. Increasing the performance of vacuum membrane distillation using micro-structured hydrophobic aluminum hollow fiber membranes. Appl. Sci. 2017, 7, 357. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ko, C.C.; Chen, L.H.; Huang, C.T.; Tung, K.L.; Liao, Y.C. A simple coating method to prepare superhydrophobic layers on ceramic alumina for vacuum membrane distillation. Sep. Purif. Technol. 2018, 198, 79–86. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Amin, S.Z.M. Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Purif. Technol. 2018, 205, 22–31. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Silanization of tubular ceramic membranes for application in membrane distillation. J. Membr. Sci. 2020, 601, 117911. [Google Scholar] [CrossRef]
- Dong, S.; Yun, Y.; Wang, M.; Li, C.; Fu, H.; Li, X.; Fu, H.; Li, X.; Yang, W.; Liu, G. Superhydrophobic alumina hollow ceramic membrane modified by TiO2 nanorod array for vacuum membrane distillation. J. Taiwan Inst. Chem. E. 2020, 117, 56–62. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Gani, P.; Sunar, N.M.; Tai, Z.S.; Koo, K.N.; Pauzan, M.A.B.; Ismail, N.J.; Zahari, S.S.N.S. Integrated green membrane distillation-microalgae bioremediation for arsenic removal from Pengorak River Kuantan, Malaysia. Chem. Eng. Process. Process Intensif. 2020, 153, 107996. [Google Scholar] [CrossRef]
- Schnittger, J.; McCutcheon, J.; Hoyer, T.; Weyd, M.; Fischer, G.; Puhlfürß, P.; Halisch, M.; Voigt, I.; Lerch, A. Hydrophobic ceramic membranes in MD processes–Impact of material selection and layer characteristics. J. Membr. Sci. 2021, 618, 118678. [Google Scholar] [CrossRef]
- Bandar, K.B.; Alsubei, M.D.; Aljlil, S.A.; Darwish, N.B.; Hilal, N. Membrane distillation process application using a novel ceramic membrane for Brackish water desalination. Desalination 2021, 500, 114906. [Google Scholar] [CrossRef]
- Lanjewar, T.; Satyakam, A.; Varma, M.N. Low-cost hydrophobic cenosphere ceramic membrane for the desalination application using direct contact membrane distillation. Arab. J. Sci. Eng. 2022, 47, 6445–6460. [Google Scholar] [CrossRef]
- Zewdie, T.M.; Habtu, N.G.; Dutta, A.; Van der Bruggen, B. Flat sheet metakaolin ceramic membrane for water desalination via direct contact membrane distillation. Water Reuse 2022, 12, 131–156. [Google Scholar] [CrossRef]
- Wang, J.W.; Abadikhah, H.; Wang, F.H.; Yin, L.J.; Xu, X. Robust super-hydrophobic inorganic nanoparticles modified layered structure Si2N2O membrane for membrane distillation. J. Eur. Ceram. 2022, 42, 4189–4195. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Musale, D.A.; Zhang, Y.; Asfan, R.; Galdeano, C.; Ghurye, G.L.; Wang, H. Multifunctional photocatalytic membrane distillation for treatment of hypersaline produced water using hydrophobically modified tubular ceramic membranes. J. Environ. Chem. Eng. 2023, 11, 111538. [Google Scholar] [CrossRef]
- Schnittger, J.; McCutcheon, J.R.; Hoyer, T.; Weyd, M.; Voigt, I.; Lerch, A. Modified ceramic membranes for the treatment of highly saline mixtures utilized in vacuum membrane distillation. Desalination 2023, 567, 116943. [Google Scholar] [CrossRef]
- Usman, J.; Azeem, M.A.; Obidara, T.O.; Abba, S.I.; Lawal, D.U.; Baroud, T.N. Green kaolin-tailored hydrophobic composite membrane for efficient desalination of high-salinity water. J. Eur. Ceram. Soc. 2024, 12, 114298. [Google Scholar] [CrossRef]
- Zare, J.; Abbasi, M.; Hashemifard, S.A.; Dizge, N.; Dibaj, M.; Akrami, M. Eco-Friendly Superhydrophobic Modification of Low-Cost Multi-Layer Composite Mullite Base Tubular Ceramic Membrane for Water Desalination. Water 2024, 16, 1593. [Google Scholar] [CrossRef]
- Jayakumar, S.; Trens, P.; Barboiu, M.; Cerneaux, S. Hydrophobic cordierite-based capillary membranes applied to desalination. Sep. Purif. Technol. 2025, 359, 130679. [Google Scholar] [CrossRef]
- Park, S.H.; Park, Y.G.; Lim, J.L.; Kim, S. Evaluation of ceramic membrane applications for water treatment plants with a life cycle cost analysis. Desalin. Water Treat. 2015, 54, 973–979. [Google Scholar] [CrossRef]
- Eray, E.; Candelario, V.M.; Boffa, V.; Safafar, H.; Østedgaard-Munck, D.N.; Zahrtmann, N.; Kadrispahic, H.; Jørgensen, M.K. A roadmap for the development and applications of silicon carbide membranes for liquid filtration: Recent advancements, challenges, and perspectives. Chem. Eng. J. 2021, 414, 128826. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.; Gurlo, A. Materials and applications for low-cost ceramic membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Rekik, S.B.; Bouaziz, J.; Deratani, A.; Beklouti, S. Study of ceramic membrane from naturally occurring-kaolin clays for microfiltration applications. Period. Polytech. Chem. Eng. 2017, 61, 206–215. [Google Scholar] [CrossRef]
- Rani, S.L.S.; Kumar, R.V. Insights on applications of low-cost ceramic membranes in wastewater treatment: A mini-review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Chakraborty, A.K. Phase Transformation of Kaolinite Clay; Springer India: New Delhi, India, 2014; pp. 17–19. [Google Scholar]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Ni, T.; Fontananova, E.; Tang, G.; Shon, H.; Zhao, S. Engineering antiwetting hydrophobic surfaces for membrane distillation: A review. Desalination 2023, 563, 116722. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Wang, H.; Ding, F.; Jin, G.; Li, C.; Meng, H. Superhydrophobic modification of ceramic membranes for vacuum membrane distillation. Chin. J. Chem. Eng. 2017, 25, 1395–1401. [Google Scholar] [CrossRef]
- Lu, K.J.; Chung, T.S. Membrane Distillation: Membranes, Hybrid Systems and Pilot Studies; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Purkait, M.; Singh, R. Membrane Technology in Separation Science; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Schmeda-Lopez, D.R.; Smart, S.; Meulenberg, W.A.; da Costa, J.C.D. Mixed matrix carbon stainless steel (MMCSS) hollow fibres for gas separation. Sep. Purif. Technol. 2017, 174, 150–158. [Google Scholar] [CrossRef]
- Yun, S.I.; Youm, M.R.; Nahm, S.; Park, S.W. Fabrication and properties of macro-porous SiC using Al2O3–Y2O3–SiO2 as bonding additives. Ceram. Internat. 2021, 47, 11979–11988. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, G.; Wang, Z.S.; Liu, J.W.; Luo, P.F. Improvement of microcracks resistance of porous aluminium titanate ceramic membrane support using attapulgite clay as additive. Ceram. Int. 2018, 44, 2077–2084. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, S.; Xue, A.; Li, M.; Zhang, Y.; Zhao, Y.; Xing, W. Preparation and properties of a low-cost porous ceramic support from low-grade palygorskite clay and silicon-carbide with vanadium pentoxide additives. Chin. J. Chem. Eng. 2021, 29, 417–425. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, O.Y.; Wei, C.C.; Li, K. Preparation of yttria-stabilised zirconia (YSZ) hollow fibre membranes. Desalination 2006, 199, 360–362. [Google Scholar] [CrossRef]
- Singh, R. Membrane Technology and Engineering for Water Purification: Application, Systems Design and Operation; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Drioli, E.; Alessandro, F.; Macedonio, F. Potentialities of membrane distillation and membrane crystallization. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 437–461. [Google Scholar]
- Curcio, E.; Ji, X.; Quazi, A.M.; Barghi, S.; Di Profio, G.; Fontananova, E.; Macleod, T.; Drioli, E. Hybrid nanofiltration–membrane crystallization system for the treatment of sulfate wastes. J. Membr. Sci. 2010, 360, 493–498. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Mondal, S.; Macedonio, F.; Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Membr. Sci. 2016, 505, 167–173. [Google Scholar] [CrossRef]
- Santoro, S.; Avci, A.H.; Politano, A.; Curcio, E. The advent of thermoplasmonic membrane distillation. Chem. Soc. Rev. 2022, 51, 6087–6125. [Google Scholar] [CrossRef] [PubMed]
- Jawed, A.S.; Hegab, H.M.; Kharraz, J.; Banat, F.; Al Marzooqi, F.; Hasan, S.W. Enhancement of photothermal membrane distillation efficiency with octylamine-functionalized copper oxide nanoparticles. Sep. Purif. Technol. 2025, 375, 133801. [Google Scholar] [CrossRef]
- Chen, Y.; Ju, J.; Zhang, Y.; Zhou, Y.; Wang, Y.; Kang, W. Dual-structured PTFE/PI-PI/PANI composite membranes for photothermal membrane distillation with excellent photothermal conversion and open pathways for water vapor transport. Desalination 2024, 575, 117320. [Google Scholar] [CrossRef]
- Razaqpur, A.G.; Wang, Y.; Liao, X.; Liao, Y.; Wang, R. Progress of photothermal membrane distillation for decentralized desalination: A review. Water Res. 2021, 201, 117299. [Google Scholar] [CrossRef]
- Dong, Y.; Violet, C.; Sun, C.; Li, X.; Sun, Y.; Zheng, Q.; Tang, C.; Elimelech, M. Ceramic-carbon Janus membrane for robust solar-thermal desalination. Nat. Commun. 2025, 16, 2659. [Google Scholar] [CrossRef]



| MD Configuration | Advantages | Disadvantages |
|---|---|---|
| DCMD: the permeate side consists of a condensing fluid that is directly in contact with the membrane |
|
|
| AGMD: the permeate side of the membrane consists of a condensing surface separated from the membrane by an air gap |
|
|
| SGMD: an inert sweep gas, which collects the vapor, flows at the permeate side |
|
|
| VMD: a vacuum is applied at the permeate side |
|
|
| Technique | Principle | Materials/Modifiers | Advantages | Disanvantages |
|---|---|---|---|---|
| Chemical grafting | Covalent bonding of silane agents to surface –OH groups on ceramic membranes | Fluorosilanes (e.g., PFOTES, FAS), Methyltrimethoxysilane (MTS), hexadecyltrimethoxysilane (C16), Octyltrichlorosilane (C8) |
|
|
| Physical deposition | Application of polymeric or nanoparticle coatings via physical methods | PDMS, PTFE, PVDF-HFP, ZnO, SiO2 |
|
|
| Hierarchical structuring | Creation of micro/nano-scale surface roughness for superhydrophobicity | ZnO nanorods, TiO2 microflowers, Al2O3 nanoflowers |
|
|
| Fluorine-free coatings | Use of environmentally friendly hydrophobizing agents | Methylphenyl silicone resin, boron nitride, polydimethylsiloxane (PDMS) |
|
|
| Multistep modification | Combination of surface texturing and chemical grafting for enhanced performance | CuO flakes + silane; Al2O3 nanoflowers + silane |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandro, F.; Macedonio, F. A Critical Review of Membrane Distillation Using Ceramic Membranes: Advances, Opportunities and Challenges. Materials 2025, 18, 3296. https://doi.org/10.3390/ma18143296
Alessandro F, Macedonio F. A Critical Review of Membrane Distillation Using Ceramic Membranes: Advances, Opportunities and Challenges. Materials. 2025; 18(14):3296. https://doi.org/10.3390/ma18143296
Chicago/Turabian StyleAlessandro, Francesca, and Francesca Macedonio. 2025. "A Critical Review of Membrane Distillation Using Ceramic Membranes: Advances, Opportunities and Challenges" Materials 18, no. 14: 3296. https://doi.org/10.3390/ma18143296
APA StyleAlessandro, F., & Macedonio, F. (2025). A Critical Review of Membrane Distillation Using Ceramic Membranes: Advances, Opportunities and Challenges. Materials, 18(14), 3296. https://doi.org/10.3390/ma18143296







