1. Introduction
An ongoing challenge associated with human activity is the generation and accumulation in landfills of large volumes of industrial waste and the significant technical, economic, environmental, and health issues these processes engender [
1,
2,
3].
Depending on the waste materials’ nature and characteristics, one of the main ways to achieve circularity is to use them as supplementary cementitious materials (SCMs). The examples of SCMs from the literature involve bamboo leaf ash [
4], rice husk ash [
5], plant-based husk and straw ash [
6], biomass ash powder [
7], sugarcane ash [
8], municipal solid waste incinerator [
9,
10], sisal fiber [
11], and ladle furnace slag [
12]. The cement industry has recently developed a roadmap based on the 5C approach (‘Clinker–Cement–Concrete–Construction–(re)Carbonation’) to reach climate neutrality by 2050 [
13,
14,
15]. Some of these objectives are directly related to reducing clinker, developing new eco-pozzolans, and accelerating alkaline waste material carbonation using flue gases from either the cement industry itself or other fossil fuel-intensive processes. Over the past few decades, significant progress has been made in generating scientific and technical knowledge on a wide range of new eco-pozzolans. These serve as alternatives to the materials traditionally used in commercial cement manufacture (fly ash, natural pozzolan, blast furnace slag) [
16] and offer improved performance of the blended cement matrix, mainly aimed at mechanical resistance to long curing times [
17,
18,
19] and durability (aggregate–alkali reactions, resistance to chlorides and sulfates, etc.) [
20,
21,
22,
23]. Moreover, a new scenario of great interest to the scientific and business communities is emerging as the effect of accelerated alkaline waste material carbonation and its impact on the performance of low-carbon eco-cements becomes more evident.
Pioneering researchers in this line detail the mineralization of the carbonatable phases in different alkaline wastes (slags, waste gypsum, fly ash, natural minerals, red mud, mine tailings, etc.) [
24,
25,
26,
27,
28,
29]. Thus, the mineralization process produced in alkaline wastes by accelerated carbonation is seen as a viable methodology to capture CO
2, reduce greenhouse gases, and stabilize potentially expansive phases, which, without this prior process, are not optimal for use as pozzolanic materials. Globally, a reduction of 320 Mt CO
2 through direct carbonation of alkaline waste, plus an additional 3700 Mt through the production of carbonated waste blended cements, is estimated [
26].
At the same time, the higher or lower CO
2 capture by alkaline residues depends on the conditions of the carbonation process (temperature, pressure, particle size, CO
2 gas concentration, humidity, etc.), so it is difficult to compare residues tested under different conditions. Current research focuses on optimizing carbonation reactors to accelerate the process of mineralization and CO
2 capture (static, dynamic, and supercritical) [
24,
25,
29,
30,
31] and studying various carbonated alkaline waste materials’ influence on cement matrices. Previous studies have demonstrated that the CO
2 mineralization of, among others, fly ash (5% CO
2 sequestration), red mud (7–8%), and steelmaking waste (10%) under material-specific conditions improves the performance of these carbonated waste materials, resulting in greater surface area and chemical reactivity. Furthermore, in the case of slags from the steelmaking industry, this material shows a substantial improvement in the volumetric stability after mineralization [
32].
Within this new development area, accelerated carbonation represents an ideal opportunity to obtain more reactive and stable waste materials for use in cements, especially for certain alkaline waste materials that are otherwise potentially less suitable as SCMs due to their low or non-existent pozzolanic activity and/or the presence of potentially expansive phases or elements. While construction and demolition waste (CCDW-C) is now widely used to replace cement in the production of concrete, several issues still exist, like the possible negative impact on mechanical properties and durability, and the presence of impurities [
33]. White ladle furnace slag (LFS) contains mainly CaO, SiO
2, MgO, and Al
2O
3 and, thus, can be used to partly replace Portland cement. However, its varying mineralogical composition influences its applicability in this field, with an increasing early strength but a vice versa effect after long-term curation [
34]. The implementation of biomass ash (BA) as a secondary raw material is often problematic due to its variable chemical and mineralogical composition [
35]. For this reason, in light of emerging trends in utilizing the industrially produced CO
2, the focus of this research is on these three wastes.
Related earlier studies on different alkaline waste material types—such as LFS (700,000 ton/y in Europe), fine-fraction CDW-C (in Europe, it represents between 20 and 40% of 400 mton/y), and BA (140 billion tons/year worldwide)—demonstrated their low reactivity with lime in the medium and, in some cases, the presence of potentially expansive phases such as periclase and fluorine (in LFS) and arcanite (in BA) [
36], which are stabilized by carbonation [
37]. Furthermore, it was confirmed that after carbonation, the surface area increased considerably, similar to pozzolanic reactivity due to the formation of highly reactive silica and alumina gels.
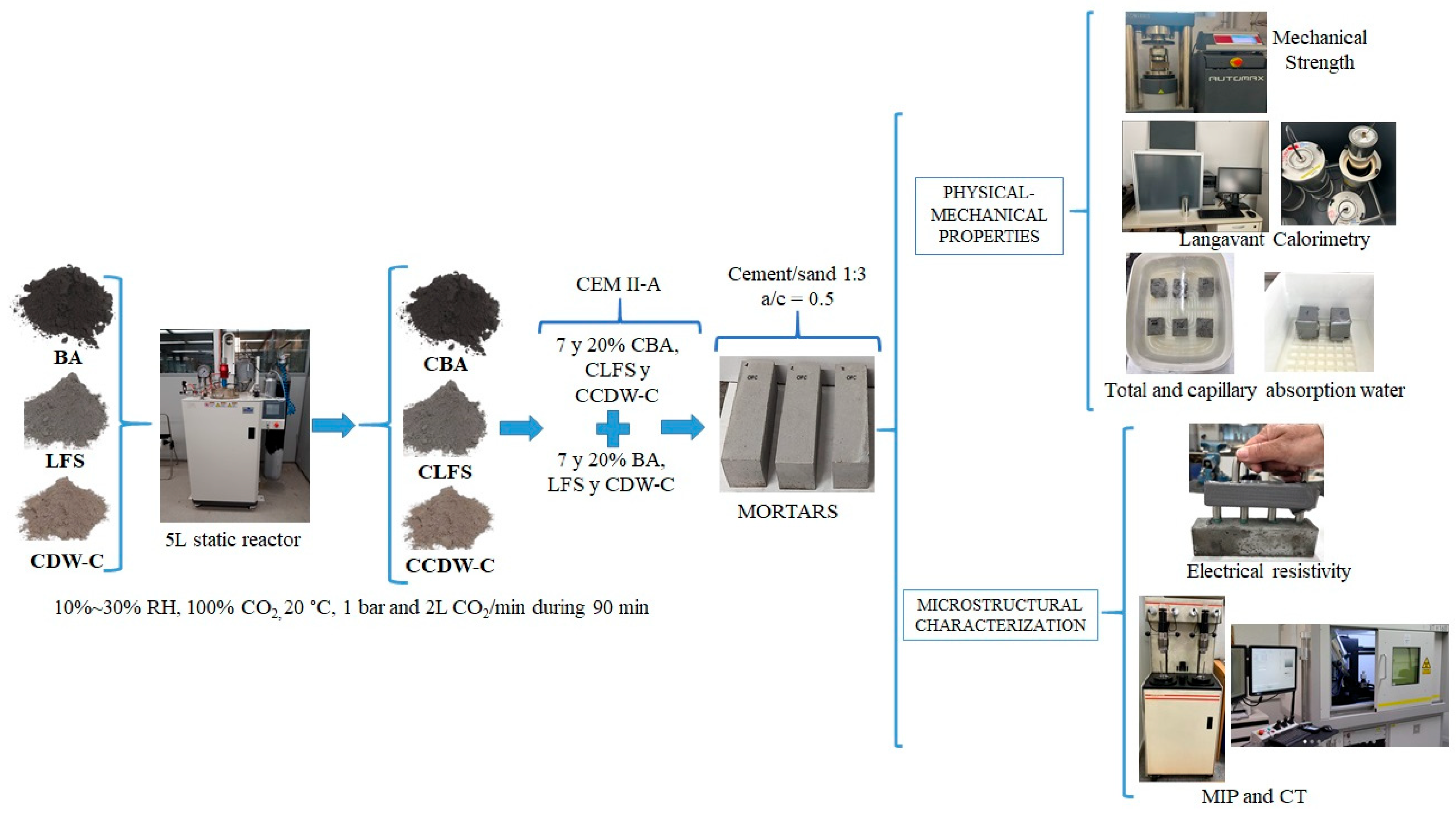
This paper generates new knowledge of three alkaline wastes (LFS, BA, and CDW-C), previously carbonated in a static reactor. The physical and mechanical behaviors and microstructure of low-carbon eco-cement-based mortars in which 7% and 20% of the clinker was replaced with carbonated alkaline waste material were investigated and compared to homologous non-carbonated mortars. To this end, total and capillary water absorption, electrical resistivity, compressive strength, and micro/macroporosity are studied in depth.
3. Results and Discussion
3.1. Calorimetric Behavior
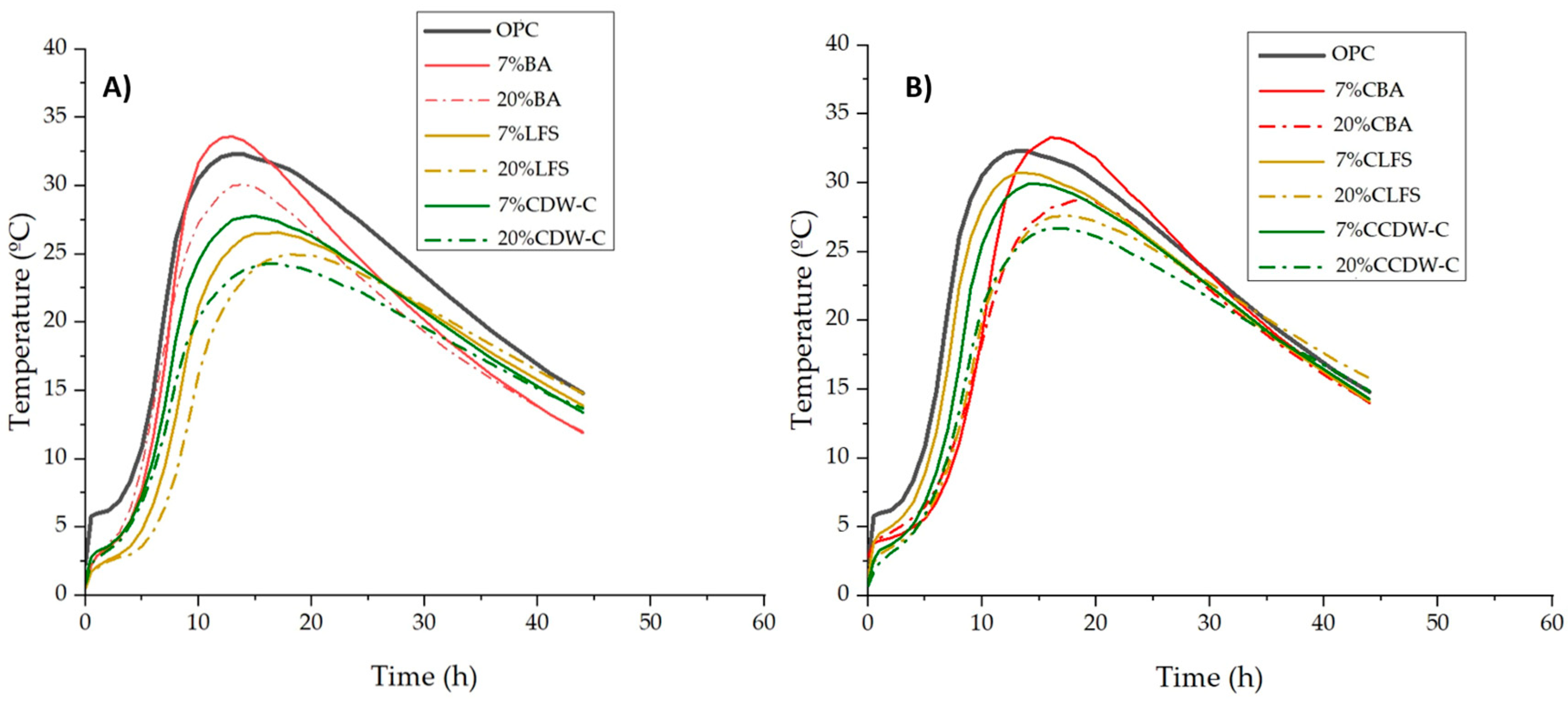
The influence of the alkaline waste materials’ nature and carbonation on the heating curve and heat of hydration is illustrated in
Figure 1 and
Figure 2. All but one of the blended mortars (
Figure 2A,B) present lower heating temperatures than the OPC mortar, with this decrease being more pronounced with the higher carbonated and non-carbonated waste material content. The exception is the 7% BA mortar, whose temperature exceeds that of the OPC mortar. The data clearly show that the maximum temperatures shift to longer reaction times—from 13 h (OPC) to 14–17 h for the blended mortars—in all cases except the 7% BA, in which reaction time is 1 h shorter (12 h). The effect of carbonation shares a certain similarity with the properties of the non-carbonated waste materials, albeit with a change in reactivity between the CLFS and the CCDW-C.
This physical property indirectly indicates different calorimetric behaviors, with the greatest reactivity presented by the BA and followed at similar levels by the LFS and CDW-C. We compared this calorimetric behavior in mortars made with carbonated waste materials to previous tests conducted on cement pastes made with non-carbonated waste materials of the same type, using R3 normalized isothermal calorimetry [
43] (where LFS is classified in group 3 while BA and CDW-C are classified in the lower reactivity group 4). Changes in reactivity are observed in the carbonated CLFS and CBA waste materials [
44]. This is consistent with the short-term behavior of low-to-medium activity pozzolans (FA, natural pozzolan, ceramics, etc.) [
45,
46,
47].
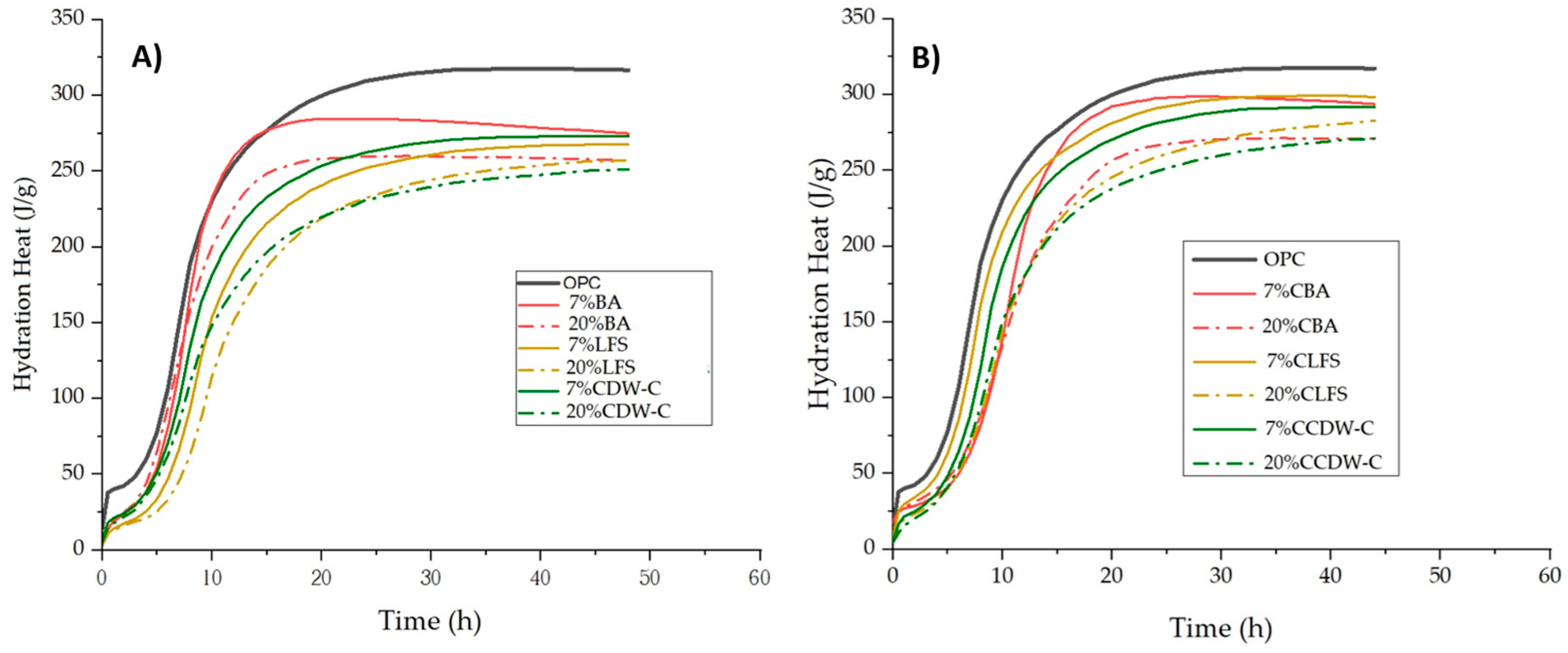
These variations in the heating curve are reflected in the heat of hydration values (
Figure 3A,B), with similar behavior observed between carbonated and non-carbonated waste materials.
Table 5 shows the heats of hydration obtained after 41 h of hydration according to the specifications in EN 197-1 [
16]. Considering the heats of hydration after this hydration age, the total heat decreases considerably for all blended mortars, with this reduction being more pronounced with the increase in the replacement percentage.
Despite these waste materials’ low pozzolanic reactivities, it is worth noting that prior carbonation generates greater heat of hydration in the mortars than recorded in their non-carbonated counterparts. This slight increase in the heat generated by the incorporation of carbonated waste to the cements is related to the increase in the surface area of both the carbonated waste and the blended cements (two–three times greater than their non-carbonated counterparts) and greater pozzolanic reactivity of this carbonated waste due to the mineralization process of some of the carbonatable phases (portlandite and hydrated phases mainly) that generate reactive silica and alumina gels, which results in increased pozzolanic reactivity [
36,
48,
49].
As per standard EN 197-1 [
16], all the mortars containing non-carbonated waste materials except the 7% BA meet the requirements for low heat-of-hydration cements (≤270 J/g). Meanwhile, among the mortars containing carbonated waste materials, only the 20% CCDW-C meets the threshold value (269.98 J/g).
3.2. Intrinsic Properties
3.2.1. Total Water Absorption (TWA)
Table 6 presents the total water absorption (TWA) obtained by Expression (6), the absorption coefficient (AR) obtained from the slopes of the regression lines of the initial segment of the curves (<2 h), and the correlation factor (R
2) obtained from obtaining AR for blended cements containing carbonated and non-carbonated alkaline waste materials, according to the Spanish standard UNE 83980 [
50].
These results show that all the non-carbonated 7% mortars experience a decrease in water absorption capacity of between 13% and 21.6% versus the OPC mortar. Meanwhile, the mortars with 20% non-carbonated waste material content present TWA values similar to those of the OPC for the BA and CDW-C samples and even higher figures for the LFS samples.
The incorporation of carbonated waste materials modifies TWA capacity, reducing the TWA and AR values in nearly all cases according to the nature of the waste material. The exceptions are the 7% CBA and 7% BA, whose rates remain unchanged. This tendency to decrease the water absorption rate is consistent with results obtained for other industrial waste materials such as ceramic CDW, as a recycled aggregate in concrete [
51], mortars made with up to 50% partial substitution by coal mining waste [
52] showed an improvement in the resistance to chloride ion ingestion and cement mortars with 39% crushed bio-mass bottom ash showed an improvement in water absorption values [
53]; however, marabou-type biomass ash with 20% substitution had the opposite effect [
54].
All the carbonated and non-carbonated mortars present TWA values well below the 10% recommended for high-quality cement-based materials [
51,
55,
56]. This beneficial behavior produced by alkaline waste materials’ prior carbonation is consistent with previous studies [
30,
49], which reported that mineralization due to carbonation produces low-solubility carbonates that clog the pore system, densify the microstructure, and improve physical properties.
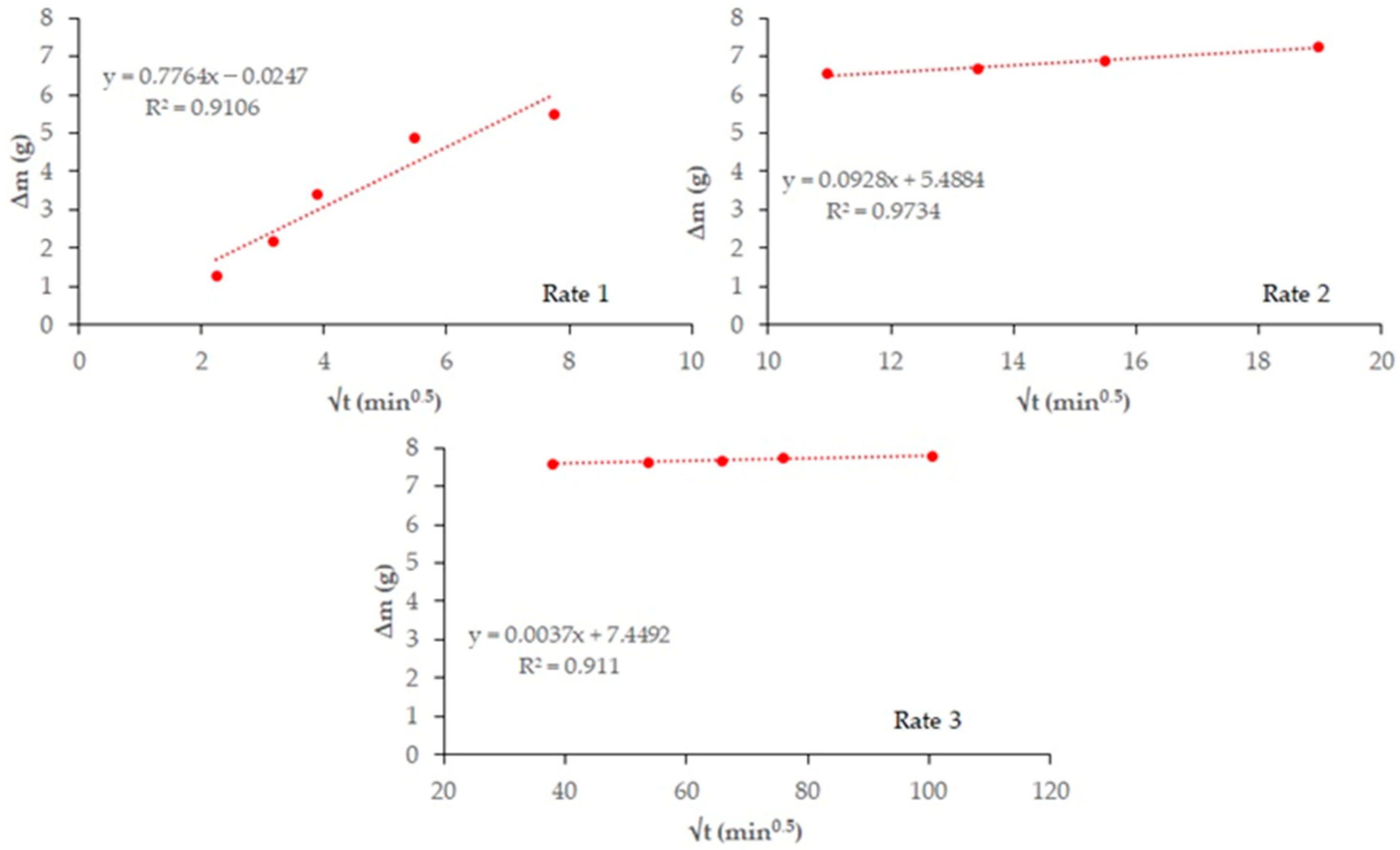
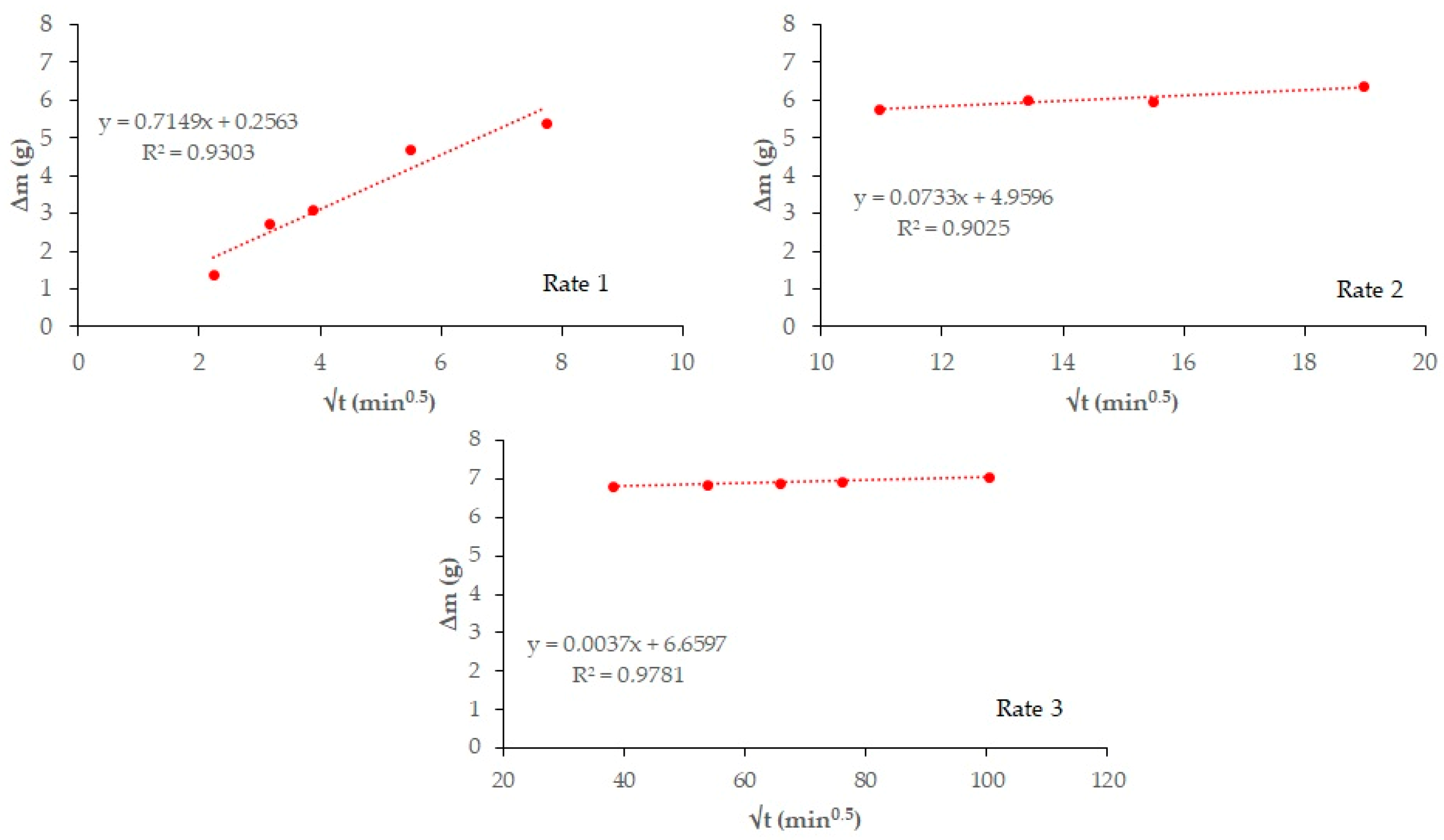
Figure 4 and
Figure 5 plot total weight gain (g) versus root mean time (min
0.5). The absorption rates (
Table 7) obtained from the regression lines of these Figures confirm the increase in this parameter in the 20% carbonated and non-carbonated mortars—by way of example,
Figure 6 and
Figure 7 show the representations of the linear regressions in each of the sections shown in
Table 6 for 20%BA and CBA—during the first and second absorption intervals (5 min–1 h) and (2–6 h), respectively. In the third interval, the rate remains constant versus the OPC mortar (>6 h). The carbonated waste materials CCDW-C (0.701 min
0.5) and CBA (0.715 min
0.5) during the first interval produce a slight decrease versus the non-carbonated materials (0.761 min
0.5 and 0.776 min
0.5, respectively), possibly due to structural densification [
57]. In the second interval, all carbonated waste materials have lower values than carbonated wastes.
3.2.2. Capillary Water Absorption
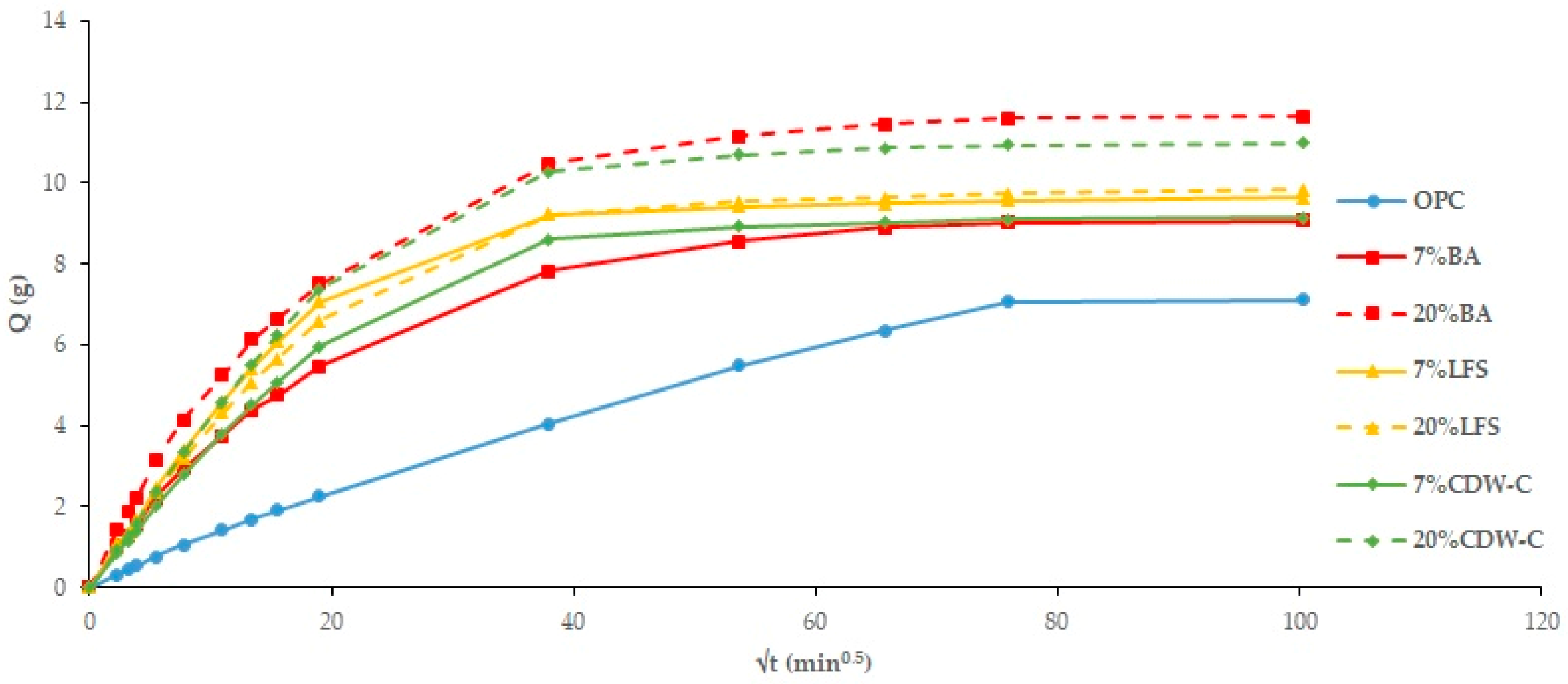
Nearly all the analyzed mortars show higher capillary absorption values (
Figure 8 and
Figure 9) than the OPC, with this increase being more pronounced the higher the waste material content [
58]. The only exception is the non-carbonated LFS mortars, which show no influence. The addition of carbonated waste materials shows a similar trend to the TWA results, with capillary absorption decreasing as a consequence of structural densification of the carbonated waste material. Two capillary behaviors are observed: the first interval (0–20 min
0.5) consists of water absorption via the capillary pore network, while the second (20–100 min
0.5) corresponds to the filling of air pores via the air diffusion and dissolution process until saturation state is reached, as described in [
59] where an experimental determination is carried out on the capillary water absorption coefficient.
By applying Equations (1)–(3) of the Fagerlund method [
39] (
Table 8), the capillary absorption coefficients (K), effective porosity (
, and resistance to water penetration by capillary absorption (m) can be determined. The blended cement mortars—both carbonated and non-carbonated—present higher K coefficients than the OPC mortar, which could indicate higher capillary porosity (0.01 μm–10 μm) and/or macropores (0.1 mm).
Regarding the m-coefficient (
Table 8), a decrease in values with respect to the OPC indicates a lower resistance to water penetration by capillarity, as well as an increase in this parameter with the percentage of addition, except in the case of CLFS. These results are in line with those obtained for other cements [
54,
60]. This parameter is not affected by prior carbonation of the waste material.
With regard to the
-coefficient (
Table 8), which provides information on the interconnected pores that allow for fluid circulation, it can be observed that all the blended cement mortars have higher values than the OPC, indicating a greater interconnection of pores in the matrix. In all cases, and considering the durability specifications set by CyTED RED DURAR [
61,
62] (where <10% indicates good concrete quality and compactness, 10–15% indicates moderate quality and >15% indicates insufficient durability), all the analyzed mortars present values < 10%, indicating that they are durable and of good quality [
63], where a detailed study of the priority durability criteria for evaluating the quality of concrete is described.
3.2.3. Resistivity of Cement Mortars
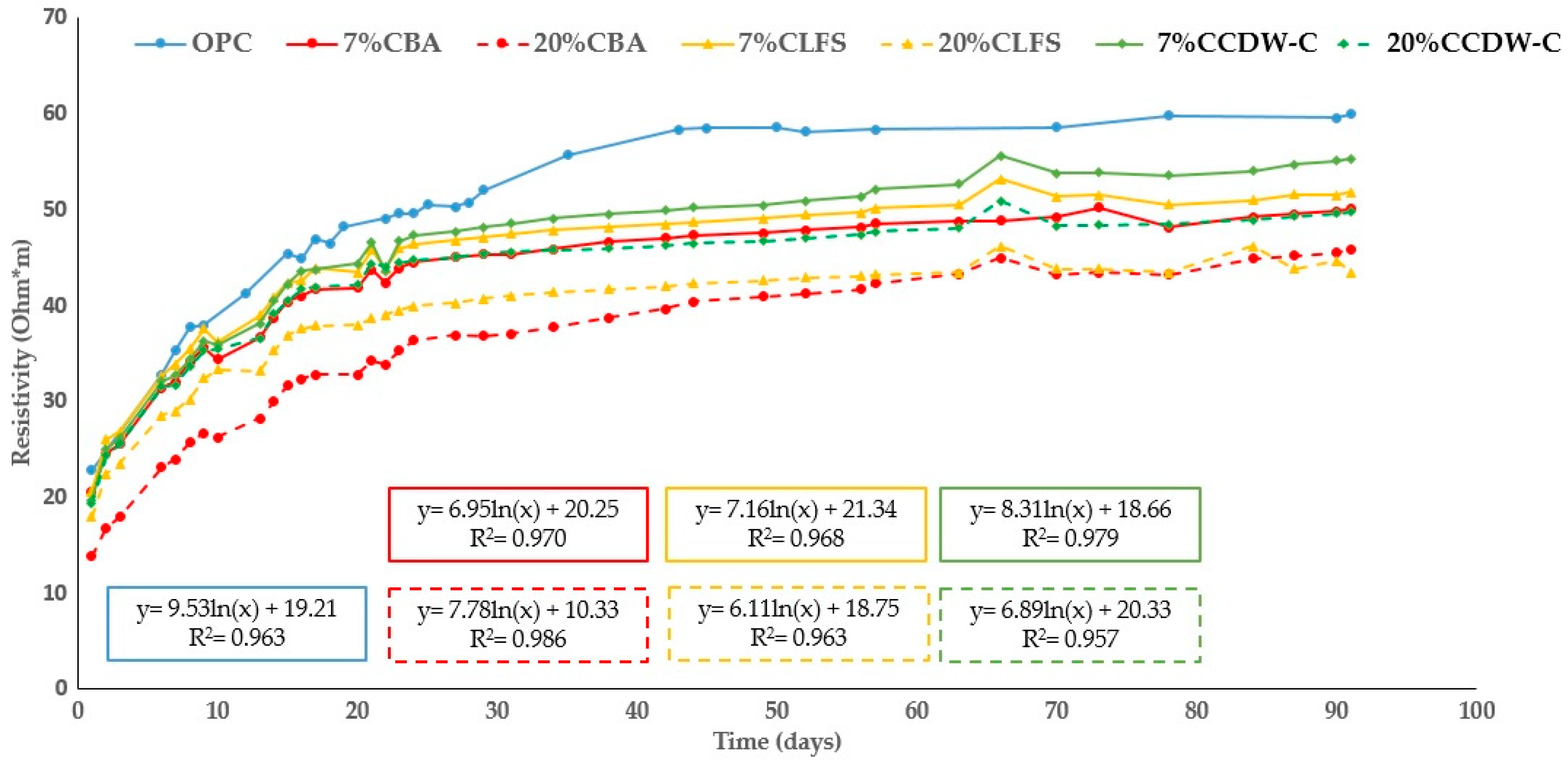
Figure 10 and
Figure 11 show the changes in the analyzed mortars’ resistivity up to 90 d of hydration. As expected, all the mortars’ resistivity increases with hydration time as a consequence of the hydrated phases produced during the hydration and pozzolanic reactions, which densify and refine the pore structure. However, the blended mortars present lower resistivity values than the OPC mortar, with this decrease being more pronounced with higher waste material content (both carbonated and non-carbonated).
The results at 90 d compared to the OPC mortar for efficiency are shown in
Table 9. This table shows a more pronounced decrease in resistivity for CCDW-C and CLFS than their non-carbonated counterparts. However, the CBA mortar performs worse than the non-carbonate mortars. All but one of the other waste materials exhibit similar behavior, with the exception being 20% CCDW-C, which shows a significant reduction of 16.8% versus 33.1% in the non-carbonated 20% CDW-C.
This fact is likely related to the addition of the various waste materials, lowering the densification of the matrix, thus offering less resistance to the electrical current [
61,
62,
63].
This negative trend among the blended mortars—both carbonated and non-carbonated—over time is clearly identified with the age factor (q), as per Equation (5) (
Section 2.3.2). The results corresponding to the age (q) and resistivity factors at time 0 (
) as per Equation (6), as well as the R
2 values of the regression, are shown in
Table 10. The q-values do not show a clear trend following either replacement percentage or the effect of prior carbonation. The greatest differences are found in the BA mortars, which range from 0.182 and 0.279 (7% and 20% BA, respectively) to 0.191 and 0.265 (7% and 20% CBA, respectively), with a considerable increase observed with the BA content. These findings are consistent with those obtained for capillary absorption (
Section 3.2.2). This q behavior in the BA mortars is consistent with that of other biomass ashes such as Marabou weed (Cuba) and Ichu grass (Peru), although in the latter case, the values are much higher (0.53 and 0.69 for Marabou weed and 10% Ichu grass ash), indicating the higher reactivity of Ichu grass versus the BA under study [
54,
64].
3.3. Mechanical Behavior
The shaking-table consistency values for all the selected mortars are shown in
Table 11. As shown in
Section 2.2, in all cases, an a/c or a/b ratio of 0.5 has been maintained in order to keep the results homogeneous and to be able to compare the different matrices. The mortars containing LFS and CDW-C behave similarly to the OPC mortar, with no clear trend being evident in relation to the percentage of replacement content. The greatest differences are detected in mortars made with BA, whose values are much lower than those of the OPC, with this difference being more pronounced with an increase in the ash content. This behavior is likely related to their greater specific surface area and lower density (
Table 4) and to the greater presence of hydroscopic phases (partially hydrated sulfates), which requires a larger amount of mixing water [
54]. Prior carbonation has a different influence on this property than in the non-carbonated waste materials, with a slight increase in consistency values being observed in the CBA mortars and a slight decrease in the LFS mortars.
Analysis of the compressive strengths of the mortars studied at different curing ages (
Figure 12) shows that adding these carbonated and non-carbonated alkaline waste materials negatively influences mortar strength. This influence becomes more pronounced as the replacement percentage increases, primarily during the first 28 d of hydration. This behavior is reported in other studies that examine the mechanical strengths of cementitious mortars with up to 20% ladle furnace slag (LFS) substitutions [
65] and 25% of recycled concrete aggregates and olive and eucalyptus biomass ashes [
66].
However, at 90 d, the mortars experience an abrupt increase in strength, even reaching values higher than those of the reference mortar, particularly the results obtained with the 7% LFS and 7% CBA. This rise is due to the medium-term pozzolanic effect of these waste materials, which exhibit behavior similar to standardized pozzolans such as fly ash and natural pozzolans.
Adding carbonated waste materials to blended cement mortars does not have a clear effect on mechanical strength versus adding non-carbonated materials, mainly due to the differences in their respective natures and their low pozzolanic activities. Only in CCDW-C mortars was a positive effect detected at 90 d. This is related to the silica and alumina gels contributed by prior carbonation of the hydrated cement adhered to the recycled siliceous aggregate in the CDW.
Conversely, a positive effect on compressive strength is evident in cement pastes [
67], a trend not observed in mortars, which could be related to the sand’s dilution effect (cement/sand = 1/3).
According to
Table 12 and as per the mechanical requirements specified in EN 197-1 [
16], the carbonated and non-carbonated blended mortars maintain their initial strength category at 2 d. However, at 28 d, the 20% BA, LFS, CDW-C, CBA, and CLFS mortars all descend in category (CEM 42.5R). The optimal replacement percentage to maintain a strength category similar to the standard cement would, therefore, be in the 7–20% range, especially as regards the LFS and CCDW-C samples.
A strong correlation coefficient is found between the compressive strength and electrical resistivity values (
Figure 13), as previously reported by other authors, in mortars with different w/c ratios [
68], particularly under standard curing conditions. This correlation enables the prediction of one parameter based on the other, preferably when R
2 ≥ 0.92. Electrical resistivity serves as an indirect indicator of pore connectivity in concrete, encompassing both total porosity and pore volume. These characteristics are governed by the degree of saturation and the physicochemical properties of the pore solution. Specifically, the electrical resistivity is influenced by the ionic conductivity and pH of pore solution, which are intrinsically linked to the composition and concentration of dissolved ions within the pore network.
3.4. Pore Network Characterization
The analysis of the microporosity of blended cements made with alkaline residues previously used as CO
2 sinks and their subsequent use as future eco-pozzolans is currently a little-known field; however, it is worth highlighting the analysis carried out in [
69,
70], where the effect of carbonated high fly ash in 20–50% blended cement pastes, among other things, on the porous structure and compressive strength, is discussed in depth. The authors point out that carbonated fly ash produces a refined porous structure and improved mechanical strength. These two parameters will be fundamental in terms of the practical applicability of these eco-cements with a lower carbon footprint in the construction sector.
Table 13 lists the analyzed mortars’ porosity (determined by mercury intrusion porosimetry) at 28 d. This parameter represents the fraction of the total volume of a material that is occupied by pores. It is a key microstructural parameter that governs fluid transport, durability, mechanical integrity, and long-term performance of porous materials like concrete. These data show that adding alkaline waste materials produced a significant increase versus the OPC mortar. The increase is most pronounced in the BA mortars, in which it rises by 70%.
The effect of carbonation on these waste materials generally produces an increase in total porosity versus the non-carbonated waste materials, except for 20% BA. This is consistent with the mechanical, electrical resistivity, and capillary absorption values mentioned previously. The CCDW mortar has the best mechanical behavior at 28 d (50.67 MPa), with a total porosity value of 15.63% and the lowest values of K (2.77 kg/m
2min
0.5) and
, which indicates that it has a microstructure with fewer interconnected pores, which implies low absorption rate (AR) coefficients and a lower absorption rate due to capillary pore filling. The observed increase in total porosity is consistent with that found in most pozzolanic additions such as fly ash, blast furnace slag, natural pozzolans, etc. [
66].
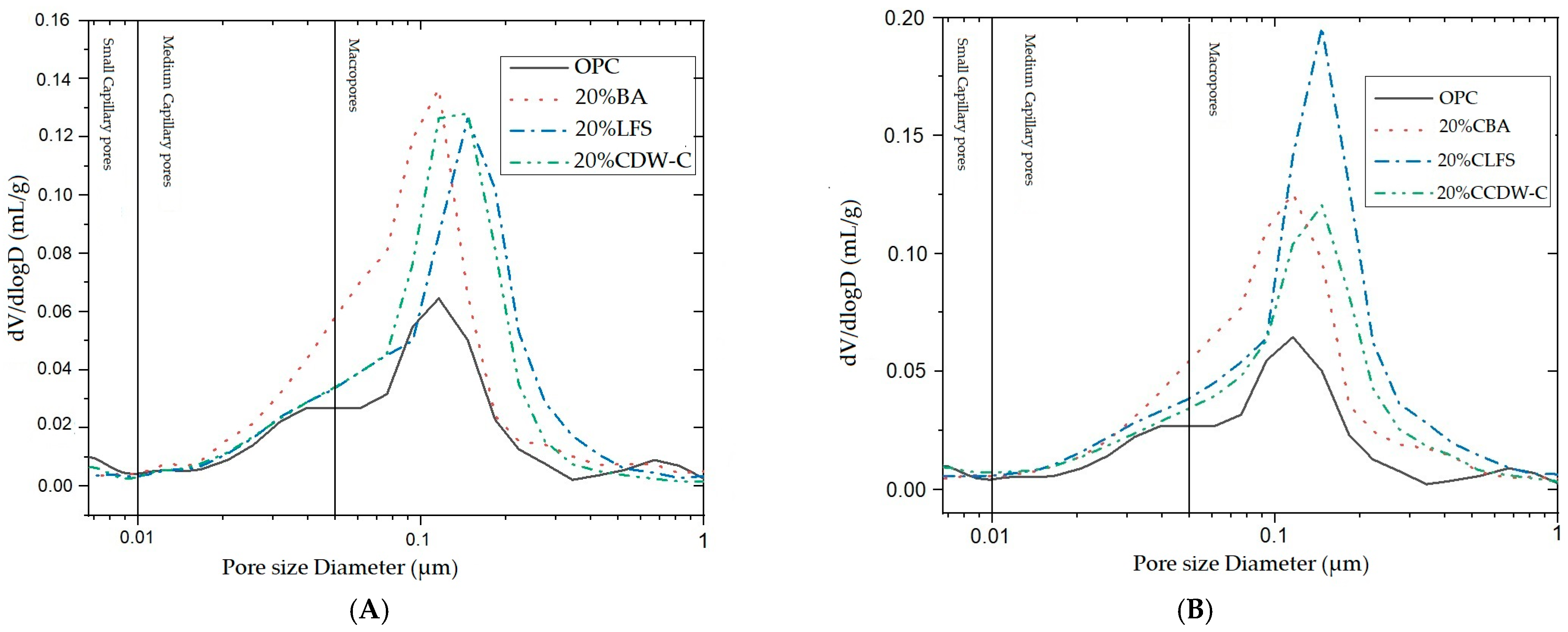
The modifications observed in the pore structure of the 20% blended mortar at 28 d (
Figure 14) have been followed by means of the pore size distribution curves. The critical pore diameter in all cases appears in the macropore area. A higher amount of macropores can be observed in the CLFS sample, which has the highest total porosity of all samples, 19.61%.
Comparing both sets of materials, OPC consistently shows the smallest critical pore diameter (~0.07–0.09 μm), indicating a tighter pore network. In contrast, blends with treated residues such as CLFS and CCDW-C display larger critical pore sizes (~0.11–0.115 μm), suggesting more open and interconnected pore structures. This implies that carbonation treatments may enhance pore connectivity and improve fluid transport potential, a factor of high relevance for durability and performance in cementitious systems.
The variations in the amount of macropores observed at 28 d in all samples with 20% substitution have been followed by computed tomography after 90 d of hydration.
Figure 15 shows the tomography-determined macroporosity values at 90 d, representing the percentage of pores per pore size interval (>0.09 mm
3, <0.09 mm
3 > 0.001 mm
3 and <0.001 mm
3).
All the analyzed blended mortars (carbonated and non-carbonated waste materials) show a reduction in the number of pores > 0.09 mm3 versus the OPC mortar. This same trend is observed in the intermediate macropores (<0.09 mm3 > 0.001 mm3), being most pronounced in the 20% CLFS. Meanwhile, smaller macropores (<0.001 mm3) increase significantly versus the OPC mortar, there being notable growth in the number of pores in the 20% CLFS mortar versus the other alkaline waste materials, a phenomenon coinciding with being the mortar with the highest porosity. The addition of carbonate waste materials to the cement produces a refinement of the pore structure with a significant reduction in the number of larger pores > 0.09 mm3 and 0.001–0.09 mm3 and, thus, a considerable increase in the number of fine macropores (<0.001 mm3) by an order of magnitude versus the non-carbonated waste materials.
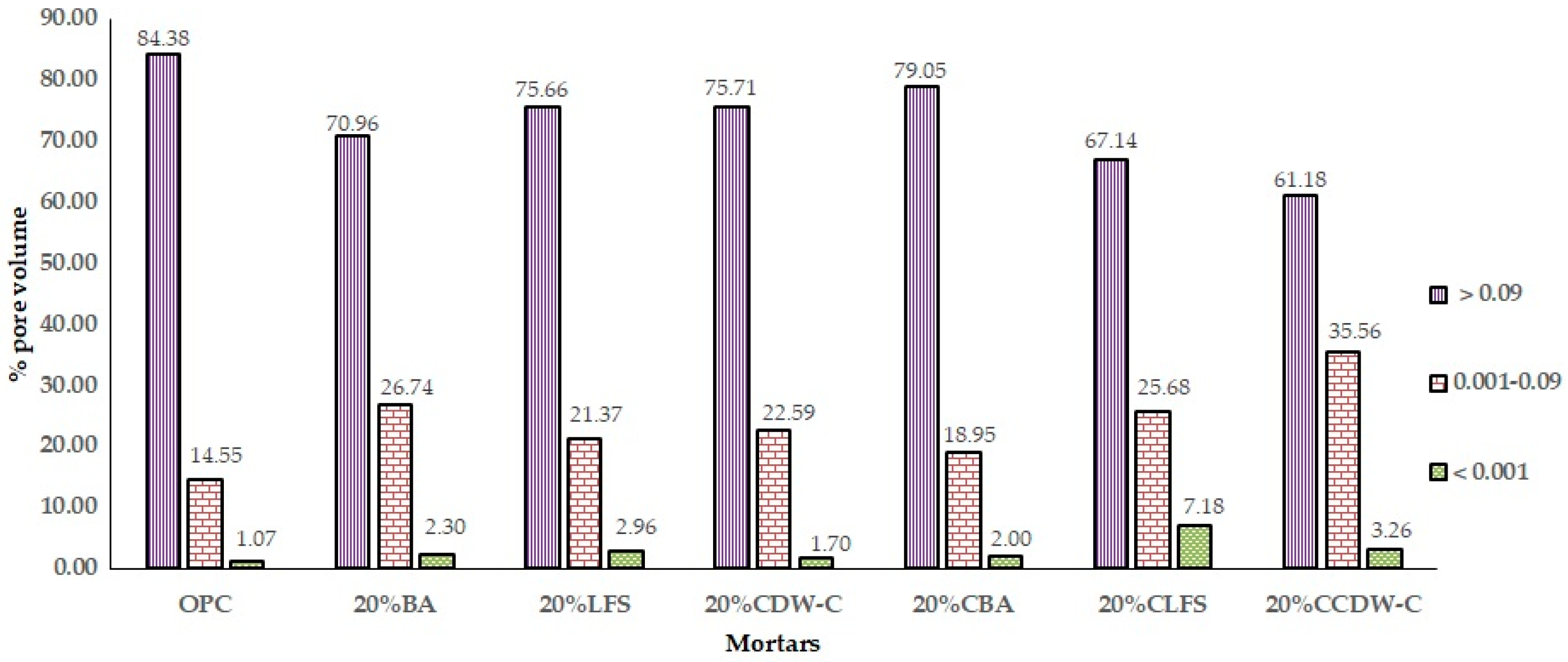
This phenomenon is reflected in the analysis of the evolution of the % pore volume (
Figure 16), where a decrease in the upper macropores (0.09 mm
3) and an increase in the intermediate ones (0.001–0.09 mm
3) and those smaller than 0.001 mm
3 are observed when compared to the OPC mortar. This results in lower TWA and AR values, as well as lower resistivity, mainly in carbonated waste materials.
The effect of adding carbonated waste materials varies depending on the nature of the waste material and the pore size interval. Thus, 20% CBA increases pore volume in the upper range and decreases it in the intermediate interval versus non-carbonated BA. In the case of 20%CLFS and 20%CCDW-C, the pores larger than 0.09 mm3 decrease, and the rest of the pores increase significantly when compared to the non-carbonated waste materials, highlighting the % of pore volume in the range 0.09–0.001 mm3 of 20%CLFS, which is the mortar with the highest total porosity.
The above is observed in
Figure 17, which shows the distribution of macropores with Ø > 0.09 mm
3 and intermediate and smaller fractions (<0.09 mm
3 > 0.001 mm
3 and <0.001 mm
3) in the cement matrix of the carbonated and non-carbonated blended mortars at 90 d. All the mortars have a lower amount of pores, Ø > 0.09 mm
3 (purple colour) than OPC, highlighting the pore content < 0.001 mm
3 (green colour) in the carbonated residues. The lower pore content of higher fractions in the case of the mortar with CCDW-C is closely related to the better mechanical performance at 90 d of this mortar. These observations indicate a refinement of the pore network of the matrices with the presence of carbonate waste materials, with the mortar with CCDW-C performing best and the CLFS mortar worst.
While mercury intrusion porosimetry revealed an increase in critical pore diameters for mixes incorporating treated residues (especially CLFS and CCDW-C), indicating enhanced pore connectivity, X-ray computed tomography confirmed that the overall pore structure remains dominated by small and medium-sized pores. This suggests that the improvement in connectivity is due to widening of pore throats rather than the formation of larger voids. The combined interpretation highlights the complementarity of both techniques for understanding structure–performance relationships.