The Effect of the Finishing Deformation Temperature on the Microstructure of CrVNb Micro-Alloyed Steel
Abstract
1. Introduction
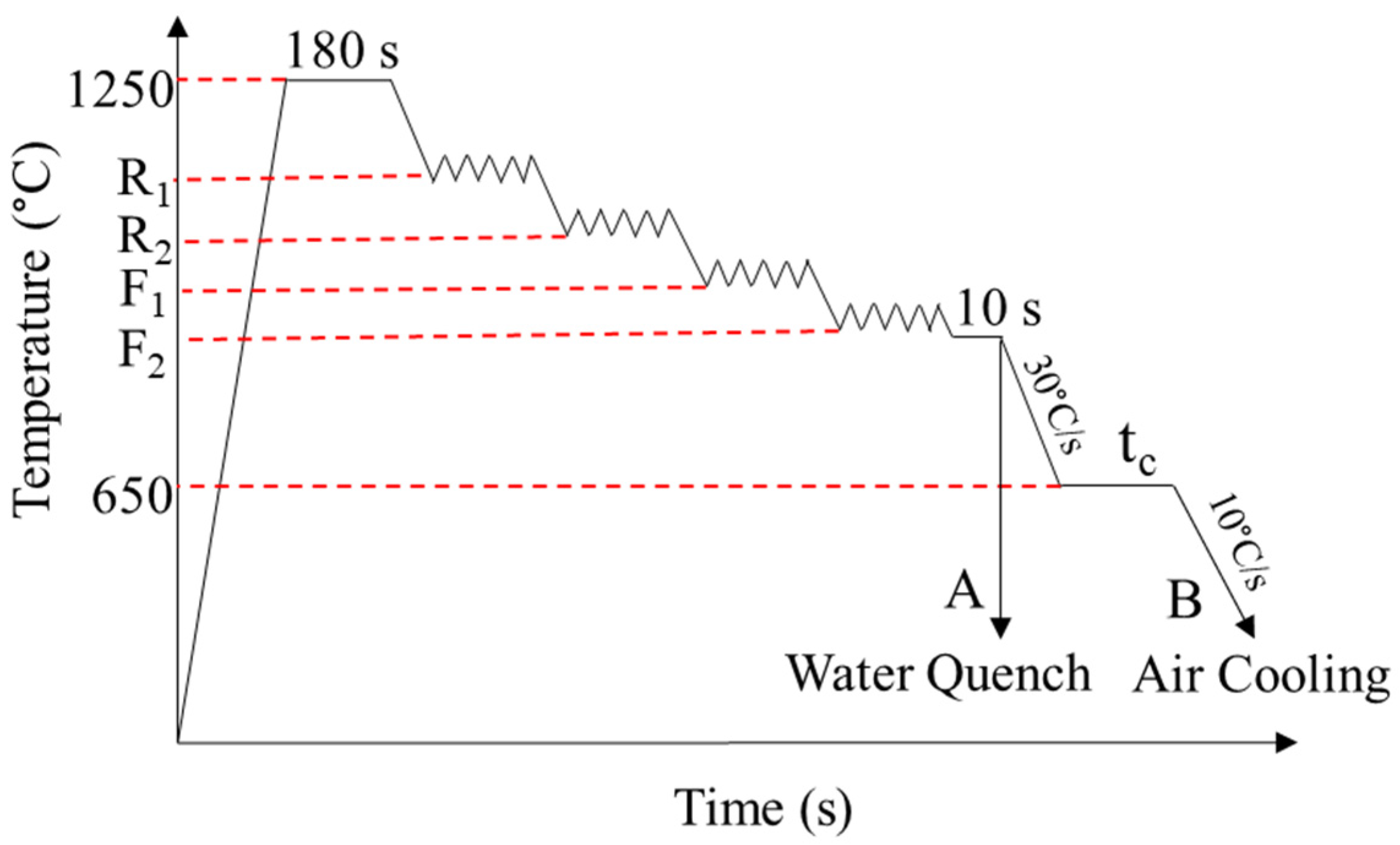
2. Materials and Methods
3. Results
3.1. OM and Scanning Electron Microscopy (SEM)
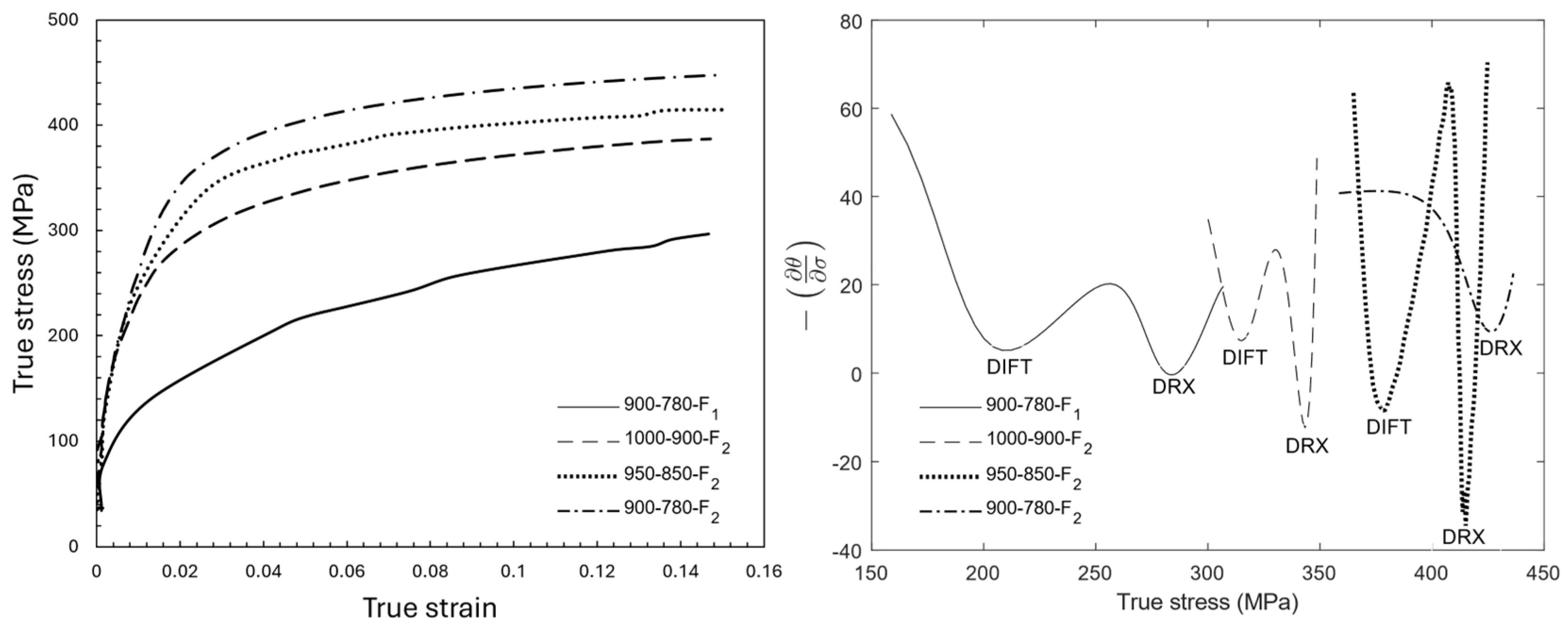
3.2. Flow Stress Investigation
3.3. EDS Analysis of Precipitates
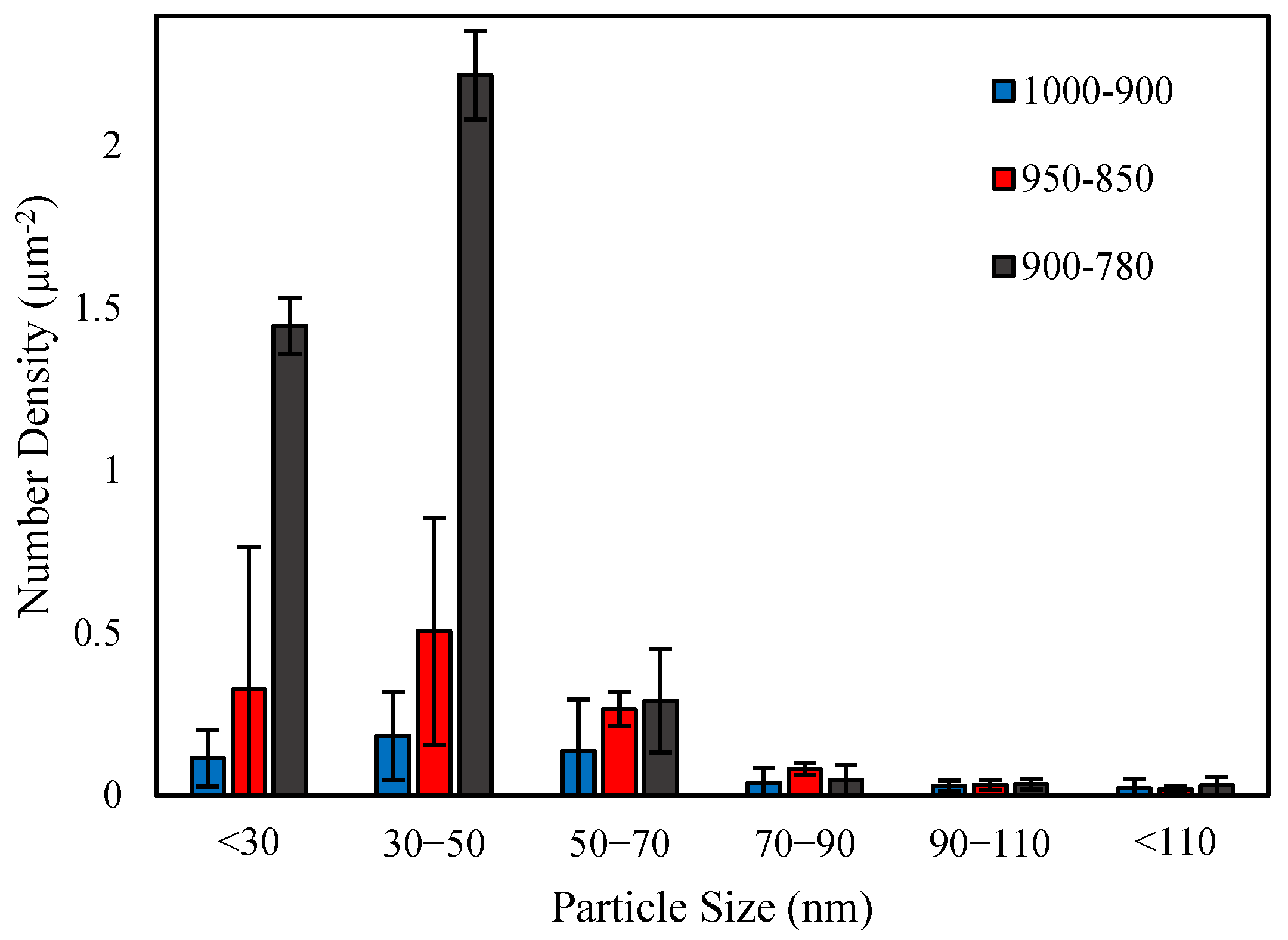
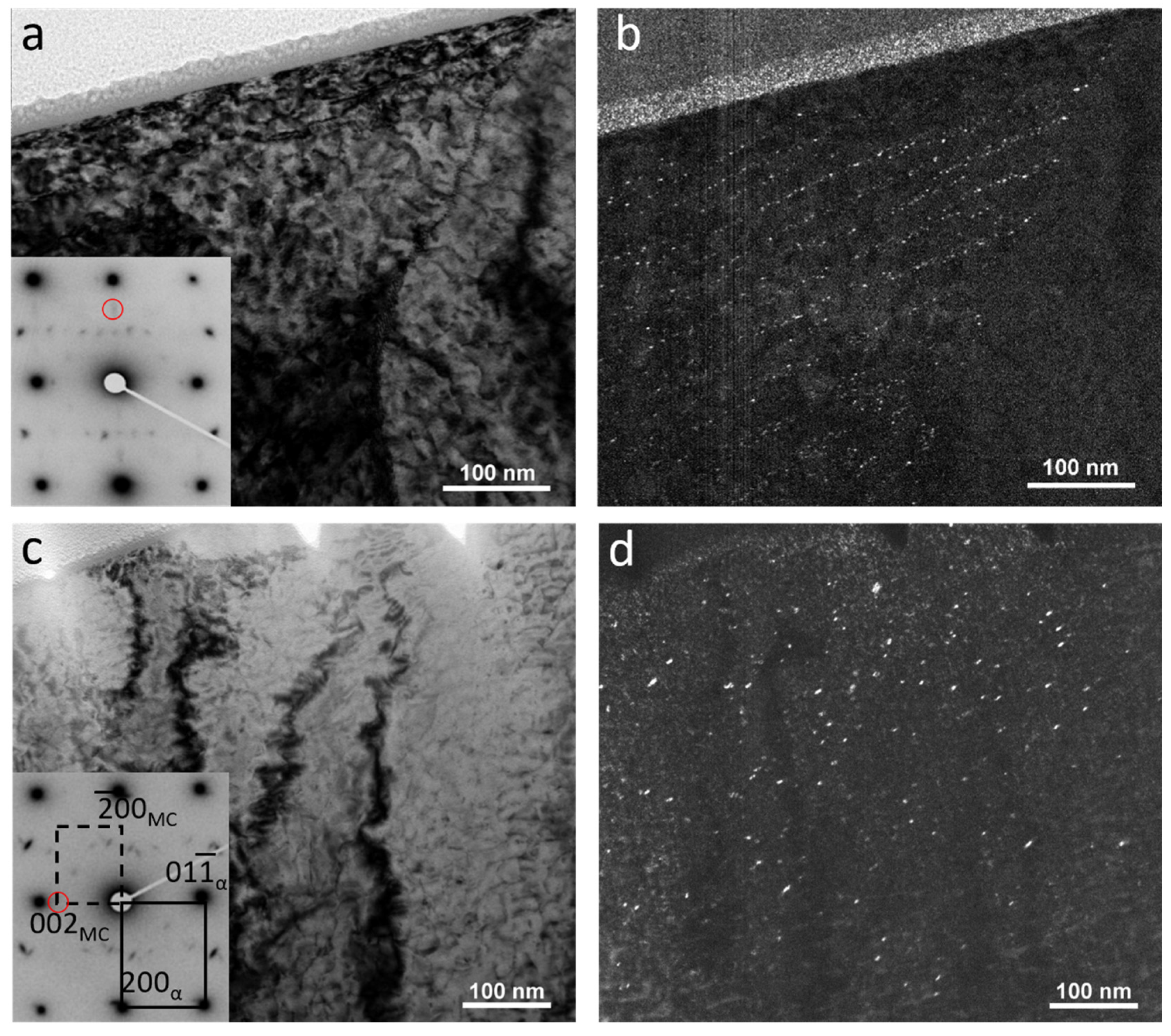
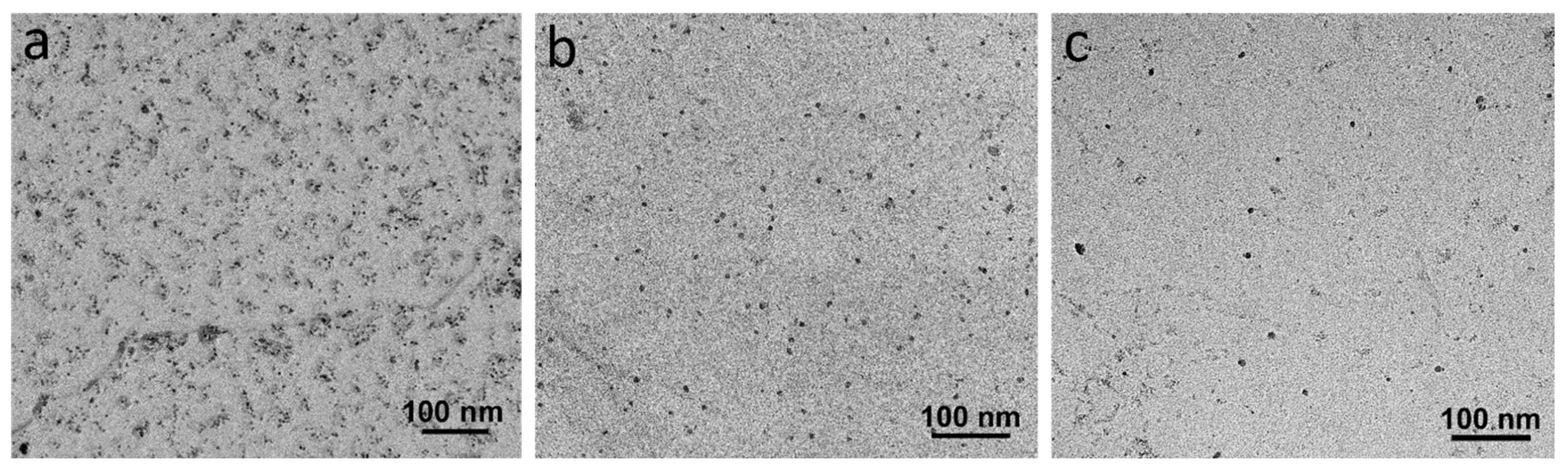
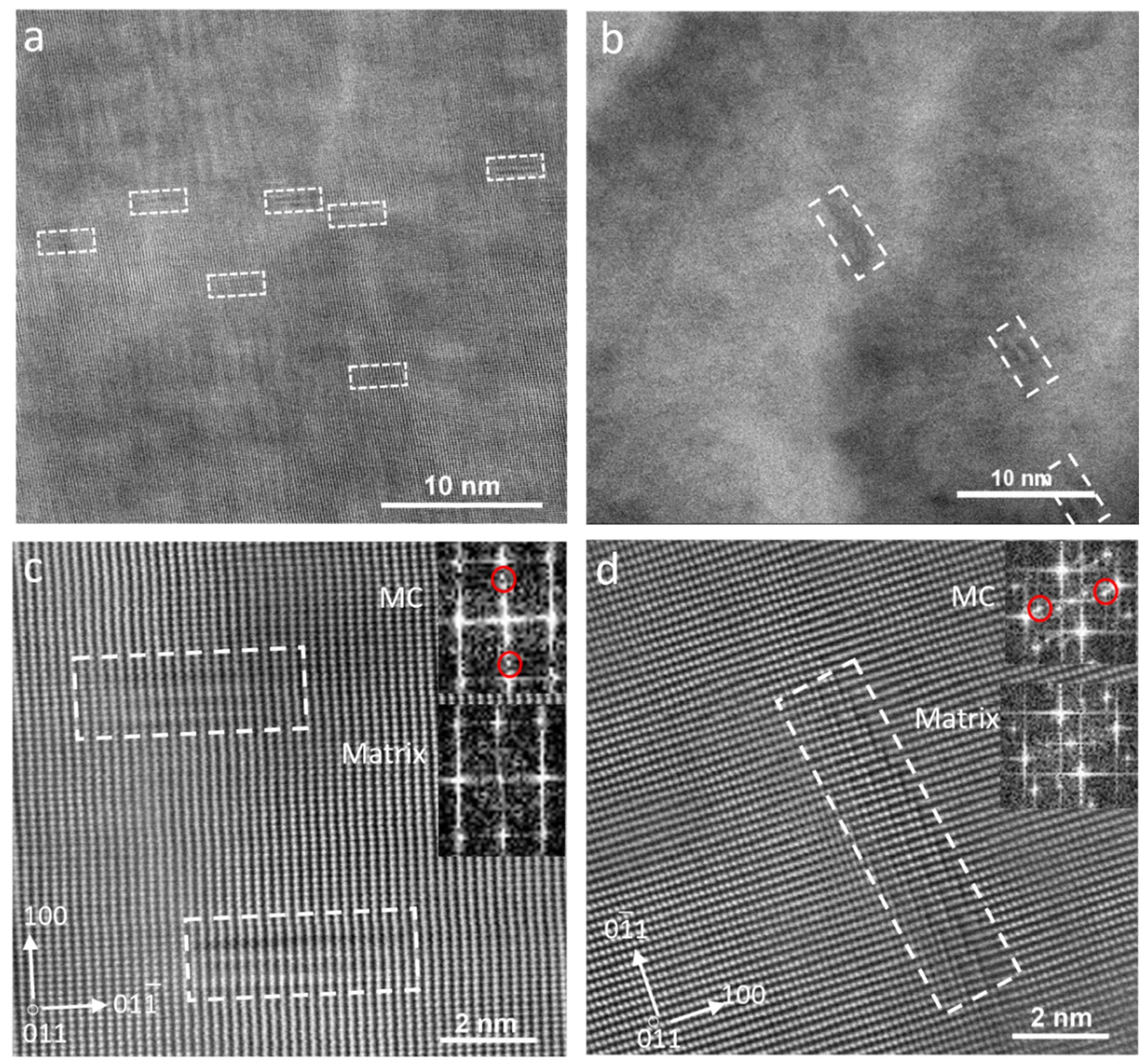
3.4. TEM Characterization
4. Discussion
5. Conclusions
- Reducing the finishing deformation temperatures to 950–850 °C refined the ferrite grains due to increased stored energy and acceleration of ferrite nucleation. At 900–780 °C, bimodal grains formed because the early DIFT grains grew fast at a high temperature, while the later-formed ferrite grew slower, and the strain-induced boundary migration during isothermal holding caused uneven grain growth.
- The DIFT and DRX softening mechanisms occurred during the last finishing deformation of the 1000–900 °C and 950–850 °C schedules, while they took place during the third deformation stage in the sample with deformation temperatures of 900–780 °C because this stage was performed below Tnr.
- The finishing deformation schedule of 950–850 °C had a higher number density of SIPs than the 1000–900 °C schedule, while the average sizes were almost equivalent. This was due to the increased work hardening creating more nucleation sites. However, a higher number of SIPs with the smaller average size formed in the sample with the lowest finishing deformation temperatures.
- The formation of large numbers of SIPs in the sample with the deformation schedule of 900–780 °C left a lower amount of dissolved alloying elements in the austenite, and subsequently, in the ferrite. This caused a reduction in the number density of random precipitates in this sample compared to the samples with higher deformation temperatures.
- The 950–850 °C finishing deformation schedule resulted in the highest hardness between among the three schedules due to a finer ferrite grain size. The sample with the lowest deformation temperatures exhibited the lowest hardness because of the lower number density of fine precipitates, bimodal ferrite grain size and lower percentage of secondary constituents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cochrane, R.C. 6—Phase transformations in microalloyed high strength low alloy (HSLA) steels. In Phase Transformations in Steel; Pereloma, E., Edmonds, D.V., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 153–212. [Google Scholar]
- Garcia, C.I. 6—High strength low alloyed (HSLA) steels. In Automotive Steels; Rana, R., Singh, S.B., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 145–167. [Google Scholar]
- Guo, A.; Misra, R.; Xu, J.; Guo, B.; Jansto, S. Ultrahigh strength and low yield ratio of niobium-microalloyed 900MPa pipeline steel with nano/ultrafine bainitic lath. Mater. Sci. Eng. A 2010, 527, 3886–3892. [Google Scholar] [CrossRef]
- Vervynckt, S.; Verbeken, K.; Lopez, B.; Jonas, J.J. Modern HSLA steels and role of non-recrystallisation temperature. Int. Mater. Rev. 2012, 57, 187–207. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel. Met. Mater. Trans. A 2001, 32, 1635–1647. [Google Scholar] [CrossRef]
- Kvackaj, T.; Bidulská, J.; Bidulský, R. Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes. Materials 2021, 14, 1988. [Google Scholar] [CrossRef]
- Das, S.K.; Chatterjee, S.; Tarafder, S. Effect of microstructures on deformation behaviour of high-strength low-alloy steel. J. Mater. Sci. 2009, 44, 1094–1100. [Google Scholar] [CrossRef]
- Shanmugam, S.; Misra, R.; Hartmann, J.; Jansto, S. Microstructure of high strength niobium-containing pipeline steel. Mater. Sci. Eng. A 2006, 441, 215–229. [Google Scholar] [CrossRef]
- Zhao, C.; Kingkam, W.; Ning, L.; Zhang, H.; Zhiming, L. Effect of Deformation Temperature on the Microstructure and Mechanical Properties of High-Strength Low-Alloy Steel During Hot Compression. J. Mater. Eng. Perform. 2018, 27, 4129–4139. [Google Scholar] [CrossRef]
- Bansal, G.; Rajinikanth, V.; Ghosh, C.; Srivastava, V.; Dutta, M.; Chowdhury, S.G. Effect of cooling rate on the evolution of microstructure and mechanical properties of nonisothermally partitioned steels. Mater. Sci. Eng. A 2020, 788, 139614. [Google Scholar] [CrossRef]
- Rodriguez-Galeano, K.F.; Nutter, J.; Azakli, Y.; Slater, C.; Rainforth, W.M. The effect of the increased strain per pass during the hot-rolling and its effect on the tensile properties of V-Mo and Cr-V-Mo microalloyed dual-phase steels. J. Mater. Sci. 2024, 59, 22228–22249. [Google Scholar] [CrossRef]
- Mandal, A.; Ghosh, A.; Chakrabarti, D.; Davis, C. Effect of coiling temperature on impact toughness of hot rolled ultra-high-strength multiphase steel strips. Mater. Sci. Eng. A 2021, 824, 141796. [Google Scholar] [CrossRef]
- Mukherjee, S.; Timokhina, I.; Zhu, C.; Ringer, S.P.; Hodgson, P.D. Clustering and precipitation processes in a ferritic titanium-molybdenum microalloyed steel. J. Alloys Compd. 2017, 690, 621–632. [Google Scholar] [CrossRef]
- Tanaka, T. Controlled rolling of steel plate and strip. Int. Met. Rev. 1981, 26, 185–212. [Google Scholar] [CrossRef]
- Fukuda, M.; Hashimoto, T.; Kunishige, K. An Investigation on Controlled Rolling of Low Carbon Killed Steel. Tetsu-to-Hagane 1972, 58, 1832–1847. [Google Scholar] [CrossRef]
- Fukuda, M.; Hashimoto, T.; Kunishige, K. Microalloying 75; Korchynsky, M., Ed.; Union Carbide Corporation: New York, NY, USA, 1977; p. 136. [Google Scholar]
- Panigrahi, B.K. Processing of low carbon steel plate and hot strip—An overview. Bull. Mater. Sci. 2001, 24, 361–371. [Google Scholar] [CrossRef]
- Inagaki, H. Role of annealing twins for grain refinement in controlled rolling of low carbon microalloyed steel. Trans. Iron Steel Inst. Jpn. 1983, 23, 1059–1067. [Google Scholar] [CrossRef]
- Sekine, H.; Maruyama, T. Retardation of Recrystallization of Austenite during Hot-rolling in Nb-containing Low-carbon Steels. Trans. Iron Steel Inst. Jpn. 1976, 16, 427–436. [Google Scholar] [CrossRef]
- Frank, F.C.; Stroh, A.N. On the Theory of Kinking. Proc. Phys. Soc. Sect. B 1952, 65, 811. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.; Kim, N.J. Transformation behavior and microstructural characteristics of acicular ferrite in linepipe steels. Mater. Sci. Eng. A 2008, 478, 361–370. [Google Scholar] [CrossRef]
- Yada, H.; Li, C.M.; Yamagata, H. Dynamic γ→α Transformation during Hot Deformation in Iron-Nickel-Carbon Alloys. ISIJ Int. 2000, 40, 200–206. [Google Scholar] [CrossRef]
- Senuma, T. Massive Type Transformation Induced by Hot Deformation in Low Carbon Steels. In Proceedings of the International Conference on Martensitic Transformations (ICOMAT-86), Nara, Japan, 26–30 August 1986; p. 6. [Google Scholar]
- Senuma, T. A New Thermomechanical Heat Treatment for Grain Refining in Low Carbon Steels. In Proceedings of the International Conference on Physical Metallurgy of Thermomechanical Processing of Steels and Other Metals, Tokyo, Japan, 6–10 June 1988; p. 8. [Google Scholar]
- Ghosh, C.; Basabe, V.V.; Jonas, J.J.; Yue, S.; Xiong, X.Y. Dynamic Transformation Behavior of a Deformed High Carbon Steel at Temperatures Above the Ae3. ISIJ Int. 2013, 53, 900–908. [Google Scholar] [CrossRef]
- Singh, N. Microstructure and Mechanical Properties of New Thermo-Mechanically Processed V-Cr (Nb-Mo) Microalloyed Steels. Ph.D. Thesis, School of Mechanical, Materials, Mechatronic and Biomedical Engineering, University of Wollongong, Wollongong, Austarlia, 2020. [Google Scholar]
- Rodriguez-Galeano, K.F.; Romano-Acosta, L.F.; Palmiere, E.J.; Rainforth, W.M. A new approach to etching low-carbon microalloyed steels to reveal prior austenite grain boundaries and the dual-phase microstructure. J. Microsc. 2023, 289, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Niessen, F. crystalAligner: A computer program to align crystal directions in a scanning electron microscope by global optimization. J. Appl. Crystallogr. 2020, 53, 282–293. [Google Scholar] [CrossRef]
- Pereloma, E.V.; Boyd, J.D. On the nature of “raised ferrite” in a low-carbon microalloyed steel. Script. Mater. 1996, 34, 703–706. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhao, X.M.; Han, H.B.; Dong, C.Y.; Yang, Y. Enhancement of pearlite transformation by Warm Rolling in 1.0C-1.5Cr Steel. Mater. Sci. Tech. 2020, 36, 1431–1439. [Google Scholar] [CrossRef]
- Aranas, C., Jr.; Rodrigues, S.F.; Grewal, R.; Jonas, J.J. Ferrite Formation above the Ae3 Temperature during the Torsion Simulation of Strip Rolling. ISIJ Int. 2015, 55, 2426–2434. [Google Scholar] [CrossRef]
- Poliak, E.I.; Jonas, J.J. A one-parameter approach to determining the critical conditions for the initiation of dynamic recrystallization. Acta Mater. 1996, 44, 127–136. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Aranas Jr, C.; Wang, T.; Jonas, J.J. Dynamic Transformation of an X70 Steel under Plate Rolling Conditions. ISIJ Int. 2017, 57, 162–169. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Siciliano, F.; Junior, C.A.; Silva, E.S.; Reis, G.S.; Jonas, J.J. High-Temperature deformation behavior of high-Nb microalloyed steel during plate rolling simulation. Tecnol. Em Metal. Mater. E Mineração 2020, 17, 105–111. [Google Scholar] [CrossRef]
- Ghosh, C.; Aranas, C., Jr.; Jonas, J.J. Dynamic transformation of deformed austenite at temperatures above the Ae3. Prog. Mater. Sci. 2016, 82, 151–233. [Google Scholar] [CrossRef]
- Ghosh, C.; Basabe, V.V.; Jonas, J.J. Determination of the Critical Strains for the Initiation of Dynamic Transformation and Dynamic Recrystallization in Four Steels of Increasing Carbon Contents. Steel Res. Int. 2013, 84, 490–494. [Google Scholar] [CrossRef]
- Ghosh, S.; Kömi, J.; Mula, S. Flow stress characteristics and design of innovative 3-steps multiphase control thermomechanical processing to produce ultrafine grained bulk steels. Mater. Des. 2020, 186, 108297. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Deng, X.; Wang, Z. The formation mechanism of complex carbides in Nb-V microalloyed steel. Mater. Let. 2022, 311, 131544. [Google Scholar] [CrossRef]
- Baker, R.; Nutting, J. The tempering of a Cr-Mo-VW and a Mo-V steel. Iron Steel Inst. Spec. Rep. 1959, 64, 1–22. [Google Scholar]
- Morales, E.V.; Gallego, J.; Kestenbach, H.-J. On coherent carbonitride precipitation in commercial microalloyed steels. Philos. Mag. Lett. 2003, 83, 79–87. [Google Scholar] [CrossRef]
- Nöhrer, M.; Zamberger, S.; Primig, S.; Leitner, H. Atom probe study of vanadium interphase precipitates and randomly distributed vanadium precipitates in ferrite. Micron 2013, 54–55, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Gouné, M.; Verdier, M.; Bréchet, Y.; Yang, J.-R. Interphase precipitation in vanadium-alloyed steels: Strengthening contribution and morphological variability with austenite to ferrite transformation. Acta Mater. 2014, 64, 78–92. [Google Scholar] [CrossRef]
- Pereloma, E.; Cortie, D.; Singh, N.; Casillas, G.; Niessen, F. Uncovering the mechanism of dislocation interaction with nanoscale (<4 nm) interphase precipitates in microalloyed ferritic steels. Mater. Res. Lett. 2020, 8, 341–347. [Google Scholar]
- Chen, J.; Li, F.; Liu, Z.Y.; Tang, S.; Wang, G.D. Influence of Deformation Temperature on γ-α Phase Transformation in Nb–Ti Microalloyed Steel during Continuous Cooling. ISIJ Int. 2013, 53, 1070–1075. [Google Scholar] [CrossRef]
- Ghosh, C.; Basabe, V.V.; Jonas, J.J.; Kim, Y.M.; Jung, I.H.; Yue, S. The dynamic transformation of deformed austenite at temperatures above the Ae3. Acta Mater. 2013, 61, 2348–2362. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, J.H.; Hong, S.G.; Lee, C.S. Effects of rolling temperature on the microstructure and mechanical properties of Ti–Mo microalloyed hot-rolled high strength steel. Mater. Sci. Eng. A 2014, 605, 244–252. [Google Scholar] [CrossRef]
- Hong, S.C.; Lim, S.H.; Lee, K.J.; Shin, D.H. Inhibition of Abnormal Grain Growth during Isothermal Holding after Heavy Deformation in Nb Steel. ISIJ Int. 2002, 42, 1461–1467. [Google Scholar] [CrossRef]
- Bodin, A.; Sietsma, J.; van der Zwaag, S. On the nature of the bimodal grain size distribution after intercritical deformation of a carbon–manganese steel. Mater. Charact. 2001, 47, 187–193. [Google Scholar] [CrossRef]
- Dutta, B.; Palmiere, E.; Sellars, C. Modelling the kinetics of strain induced precipitation in Nb microalloyed steels. Acta Mater. 2001, 49, 785–794. [Google Scholar] [CrossRef]
- Guo, B.; Fan, L.; Wang, Q.; Fu, Z.; Wang, Q.; Zhang, F. Effect of Finish Rolling Temperature on the Microstructure and Tensile Properties of Nb–Ti Microalloyed X90 Pipeline Steel. Metals 2016, 6, 323. [Google Scholar] [CrossRef]
- Kostryzhev, A.; Killmore, C.; Pereloma, E. Effect of Processing Parameters on Interphase Precipitation and Mechanical Properties in Novel CrVNb Microalloyed Steel. Metals 2021, 11, 107. [Google Scholar] [CrossRef]
- Karmakar, A.; Karani, A.; Patra, S.; Chakrabarti, D. Development of Bimodal Ferrite-Grain Structures in Low-Carbon Steel Using Rapid Intercritical Annealing. Met. Mater. Trans. A 2013, 44, 2041–2052. [Google Scholar] [CrossRef]
- Karmakar, A.; Sivaprasad, S.; Kundu, S.; Chakrabarti, D. Tensile Behavior of Ferrite-Carbide and Ferrite-Martensite Steels with Different Ferrite Grain Structures. Met. Mater. Trans. A 2014, 45, 1659–1664. [Google Scholar] [CrossRef]
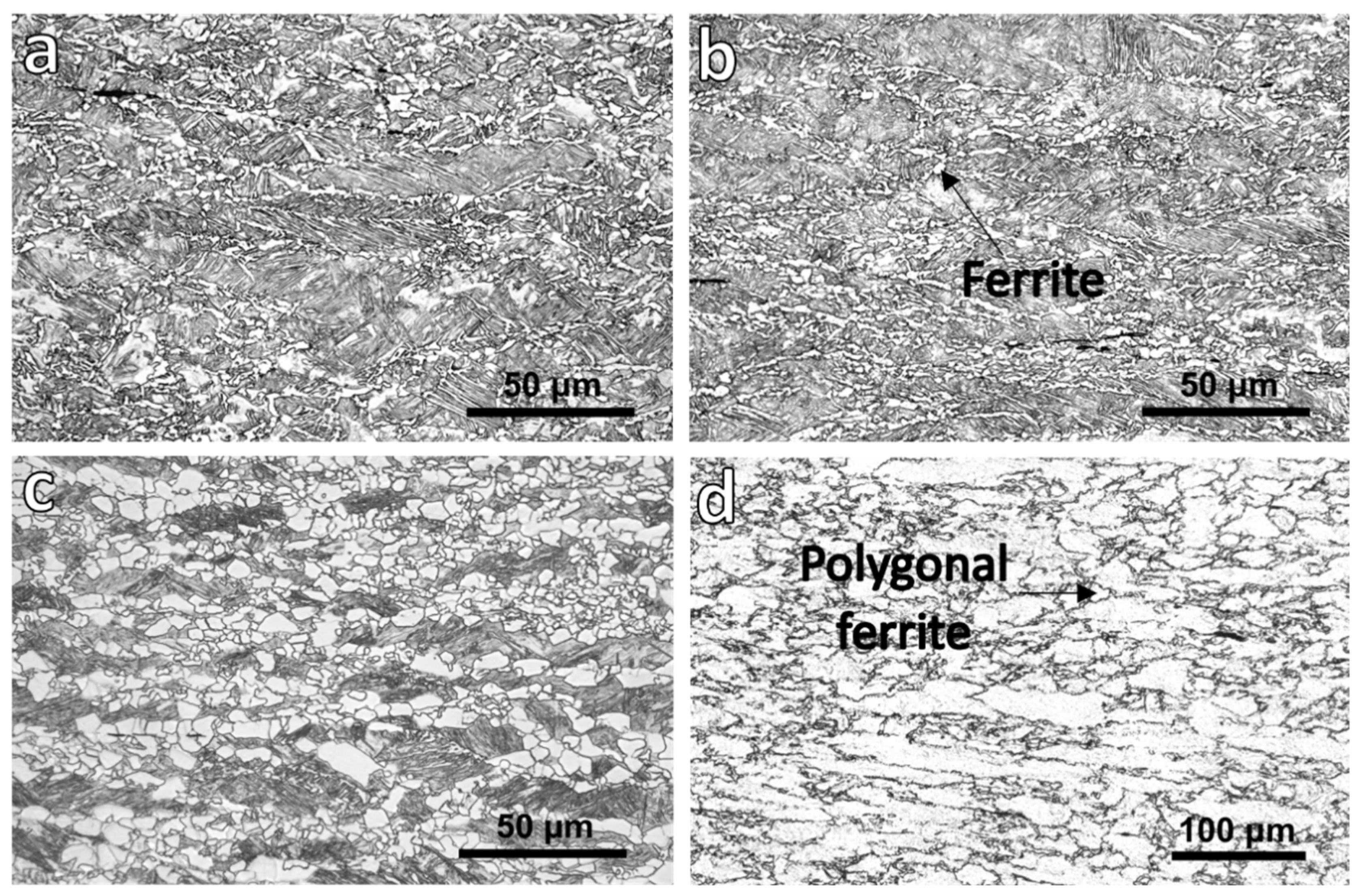
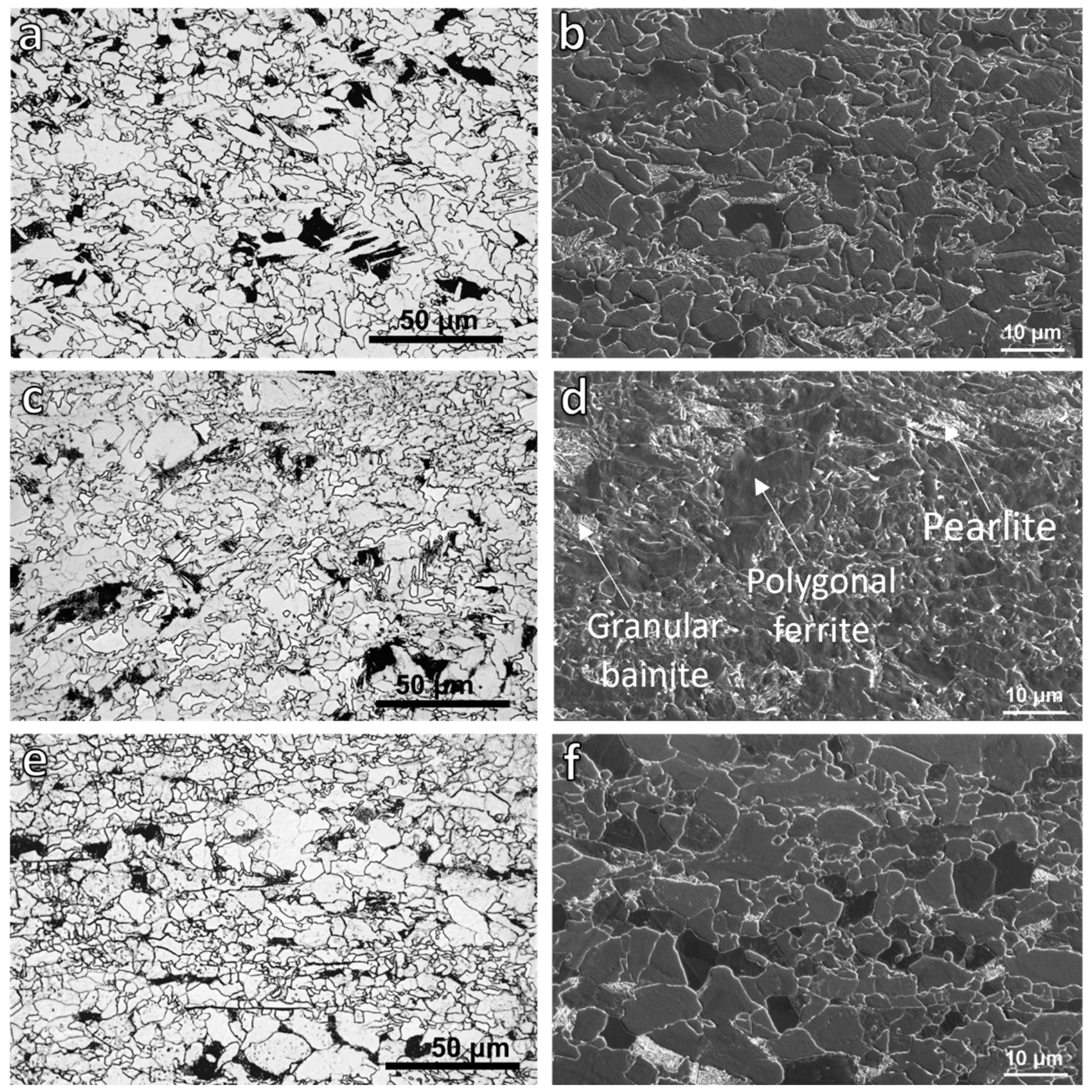

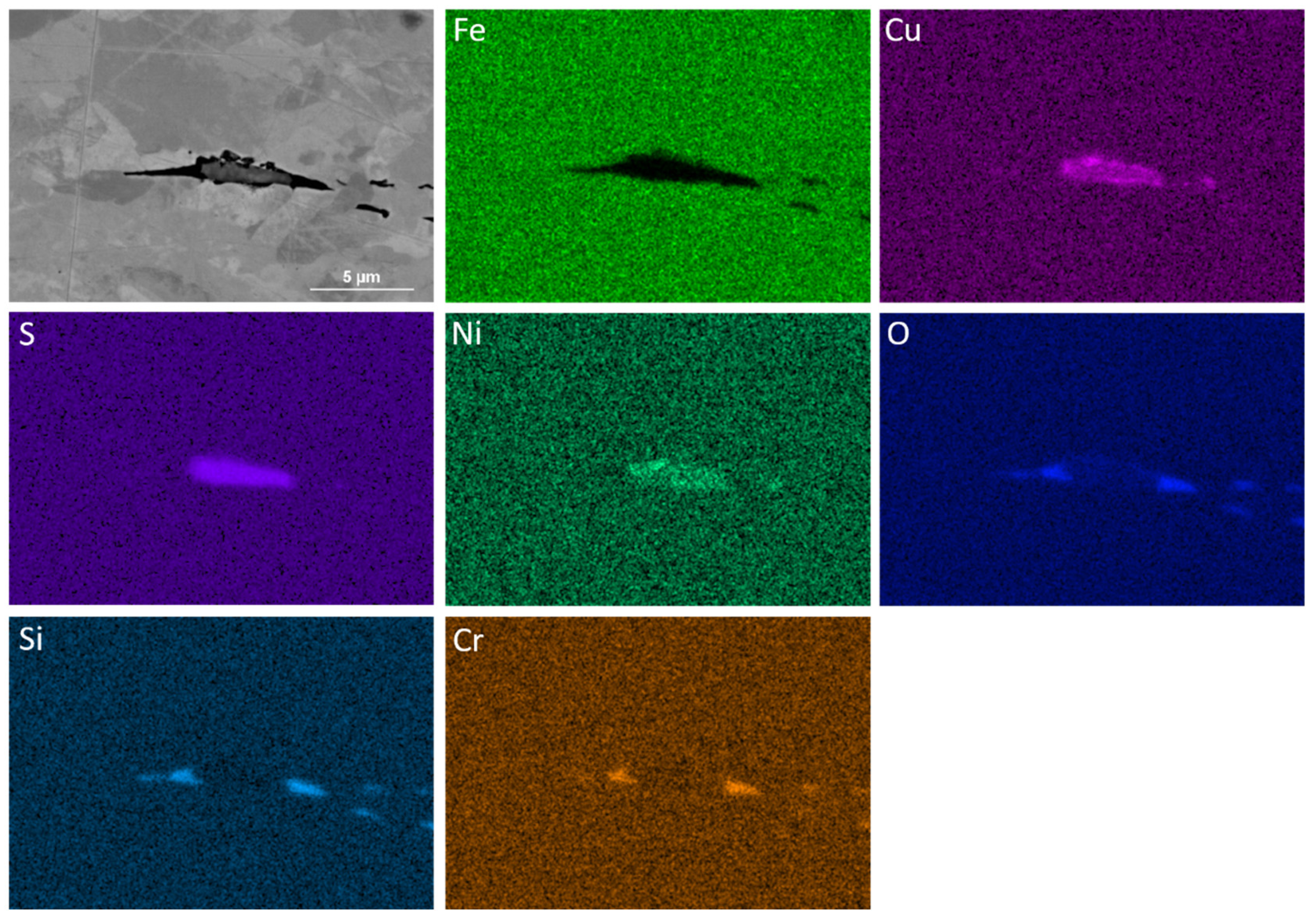
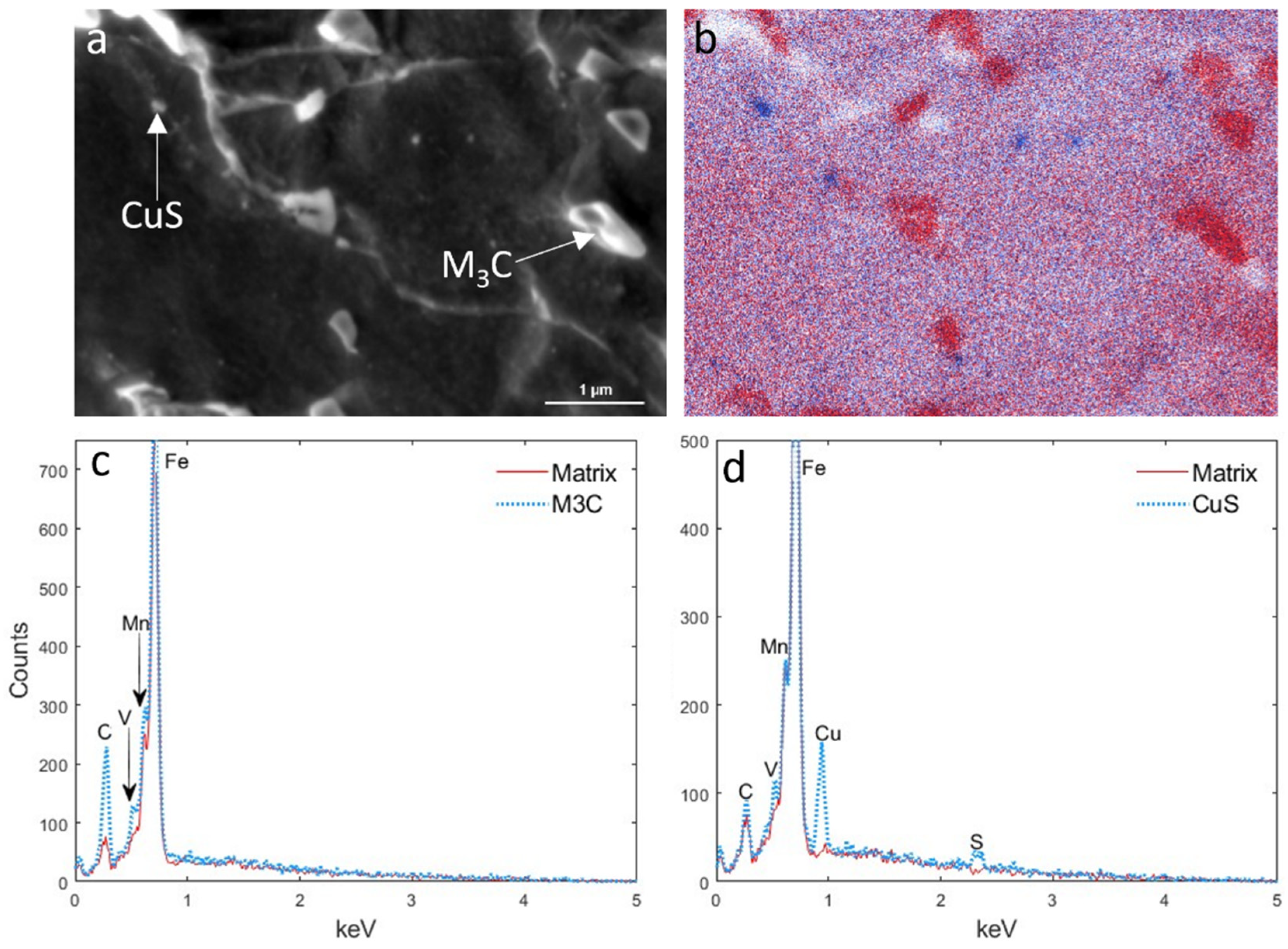
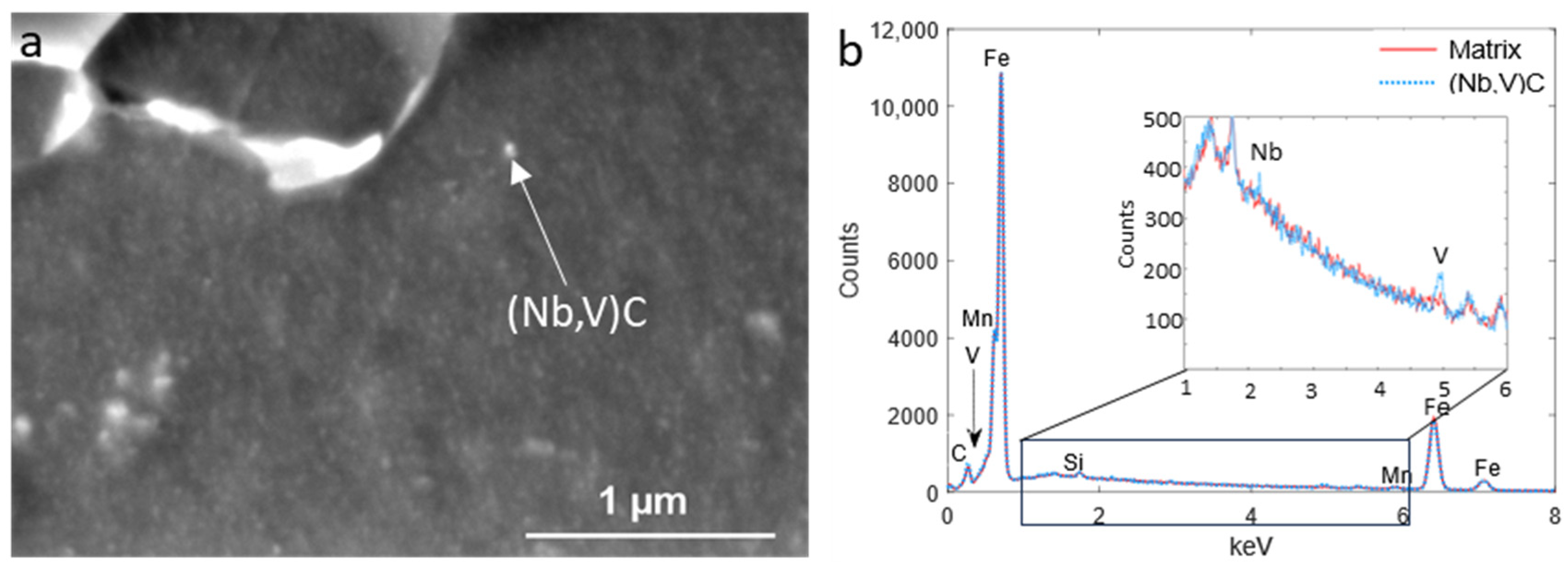



| Finishing Deformation Temperatures (°C) (F1–F2) | 1000–900 | 950–850 | 900–780 |
|---|---|---|---|
| Ferrite average grain size (µm) | 2.7 ± 1.7 | 2.4 ± 1.7 | 2.5 ± 2.1 |
| Area percentage of the second constituents (%) | 12.7 ± 0.4 | 13.1 ± 0.6 | 9.2 ± 0.4 |
| Pearlite interlamellar spacing (nm) | 179 ± 31 | 191 ± 29 | 110 ± 11 |
| Pearlite colonies average size (µm) | 4.3 ± 2.8 | 4.1 ± 2.3 | 2.4 ± 1.7 |
| Strain-induced precipitates average size (nm) | 58 ± 30 | 57 ± 29 | 38 ± 15 |
| Strain-induced precipitates number density (µm−2) | 0.5 ± 0.4 | 1.2 ± 1.1 | 4.0 ± 1.1 |
| IPs/random precipitates average size (nm) | 2.5 ± 0.8 | 3.9 ± 0.3 | 4.9 ± 0.5 |
| IPs/random precipitates number density (µm−2) | 2546 ± 952 | 1298 ± 444 | 576 ± 169 |
| Hardness (HV) | 279.9 ± 5.2 | 294.7 ± 3.8 | 257.7 ± 4.1 |
| Deformation Step | DIFT | DRX | ||
|---|---|---|---|---|
| (MPa) | (MPa) | |||
| 1000-900-F2 | 0.09 | 315 | 0.15 | 343 |
| 950-850-F2 | 0.05 | 379 | 0.11 | 408 |
| 900-780-F1 | 0.07 | 208 | 0.14 | 284 |
| 900-780-F2 | - | - | 0.08 | 425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baqeri, G.A.; Killmore, C.; Smillie, L.; Pereloma, E. The Effect of the Finishing Deformation Temperature on the Microstructure of CrVNb Micro-Alloyed Steel. Materials 2025, 18, 3234. https://doi.org/10.3390/ma18143234
Baqeri GA, Killmore C, Smillie L, Pereloma E. The Effect of the Finishing Deformation Temperature on the Microstructure of CrVNb Micro-Alloyed Steel. Materials. 2025; 18(14):3234. https://doi.org/10.3390/ma18143234
Chicago/Turabian StyleBaqeri, Gholam Ali, Chris Killmore, Lachlan Smillie, and Elena Pereloma. 2025. "The Effect of the Finishing Deformation Temperature on the Microstructure of CrVNb Micro-Alloyed Steel" Materials 18, no. 14: 3234. https://doi.org/10.3390/ma18143234
APA StyleBaqeri, G. A., Killmore, C., Smillie, L., & Pereloma, E. (2025). The Effect of the Finishing Deformation Temperature on the Microstructure of CrVNb Micro-Alloyed Steel. Materials, 18(14), 3234. https://doi.org/10.3390/ma18143234








