Hybrid Binders Through Alkaline Activation of Fine Construction and Demolition Waste
Abstract
1. Introduction
2. Materials and Methodology
2.1. Characterization of Materials
2.2. Techniques
2.2.1. Pastes
2.2.2. Mortars
2.3. Experimental Methodology
3. Results and Discussion
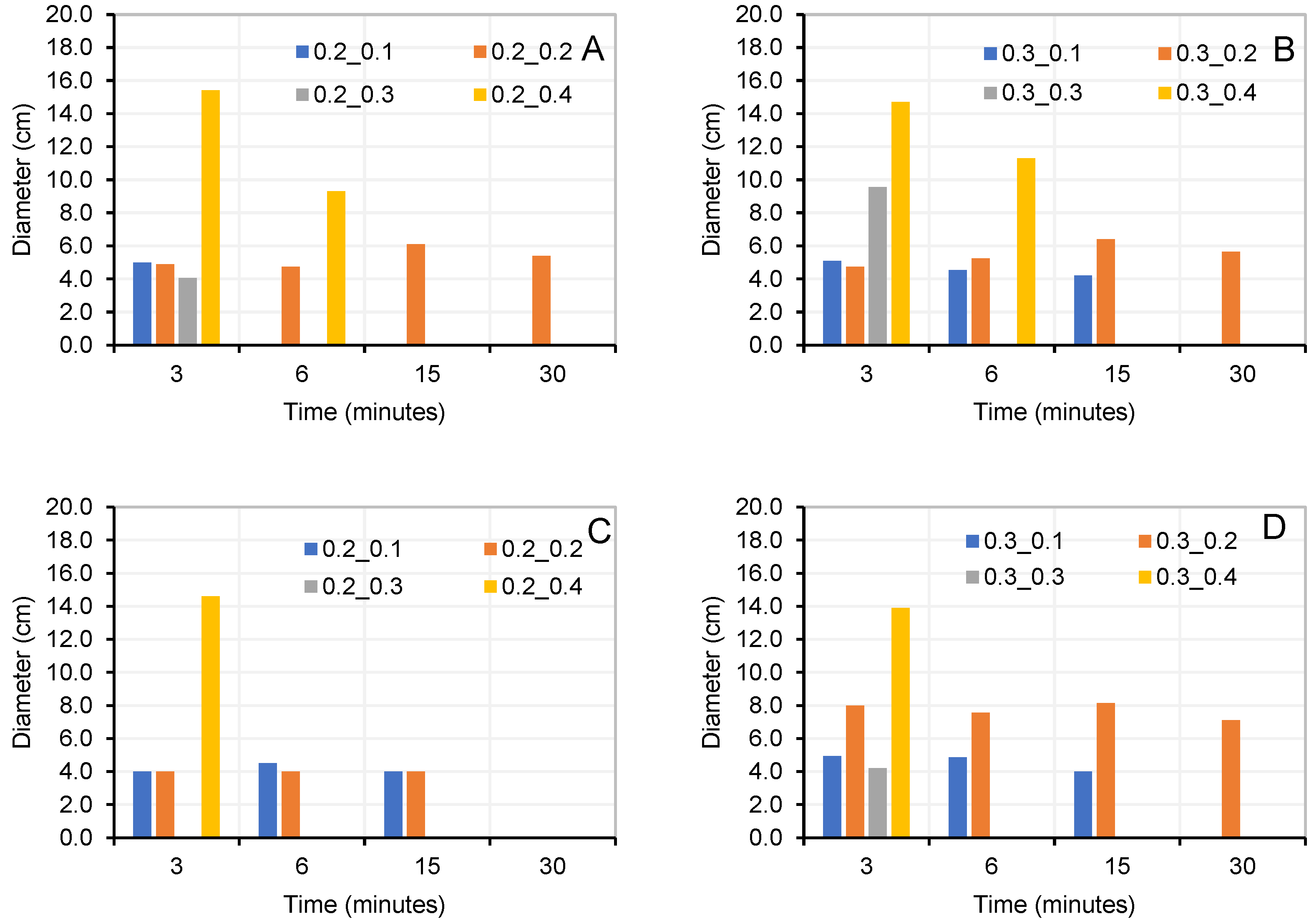
3.1. Initial Dosages to Determine Optimum NaOH/Na2SiO3 Ratio
3.2. Dosages to Determine Optimum Alkaline Activator/Precursor Ratio (AA/0.9P + 0.1OPC)
3.3. Optimised Dosages for Pastes and Mortars
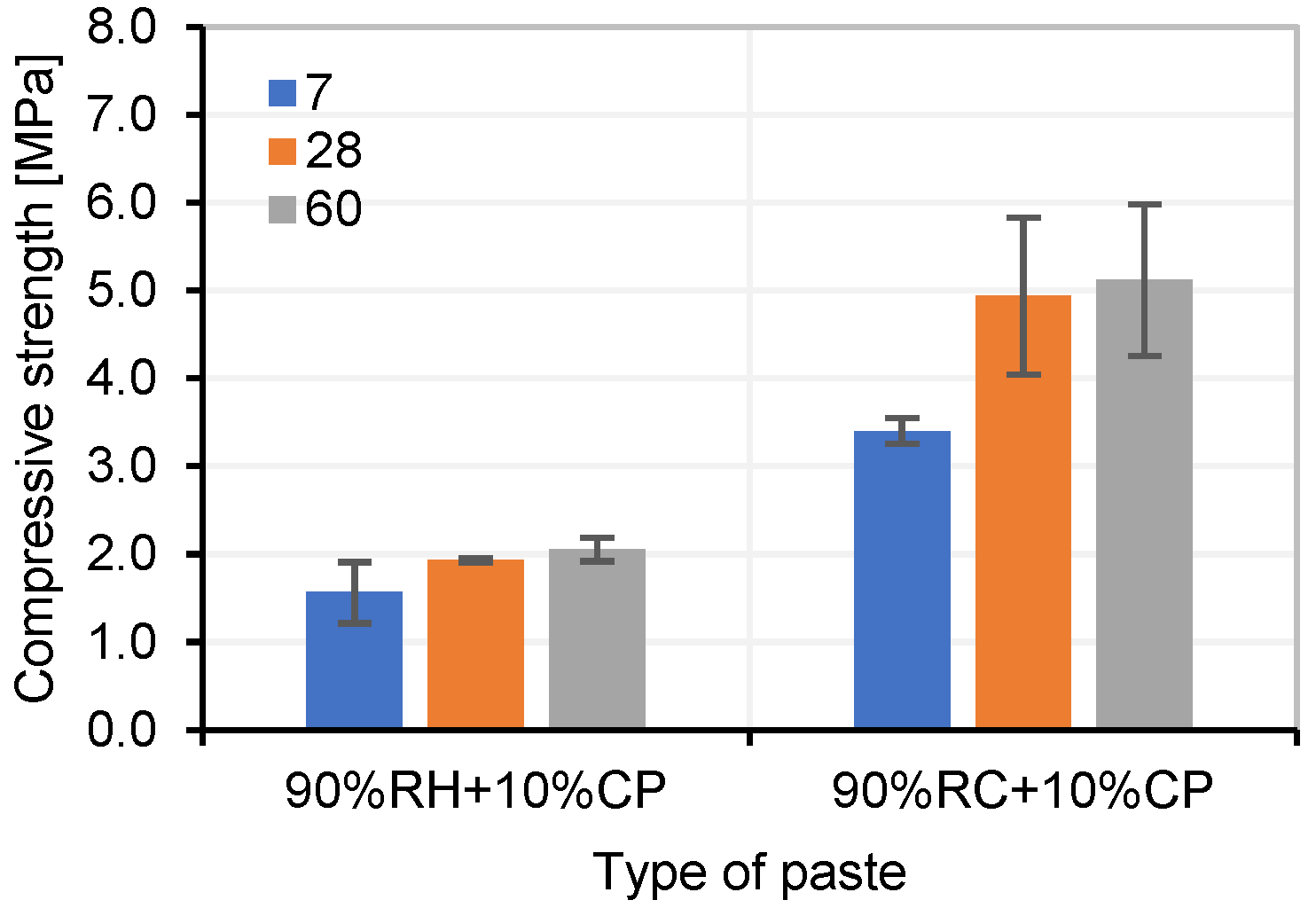
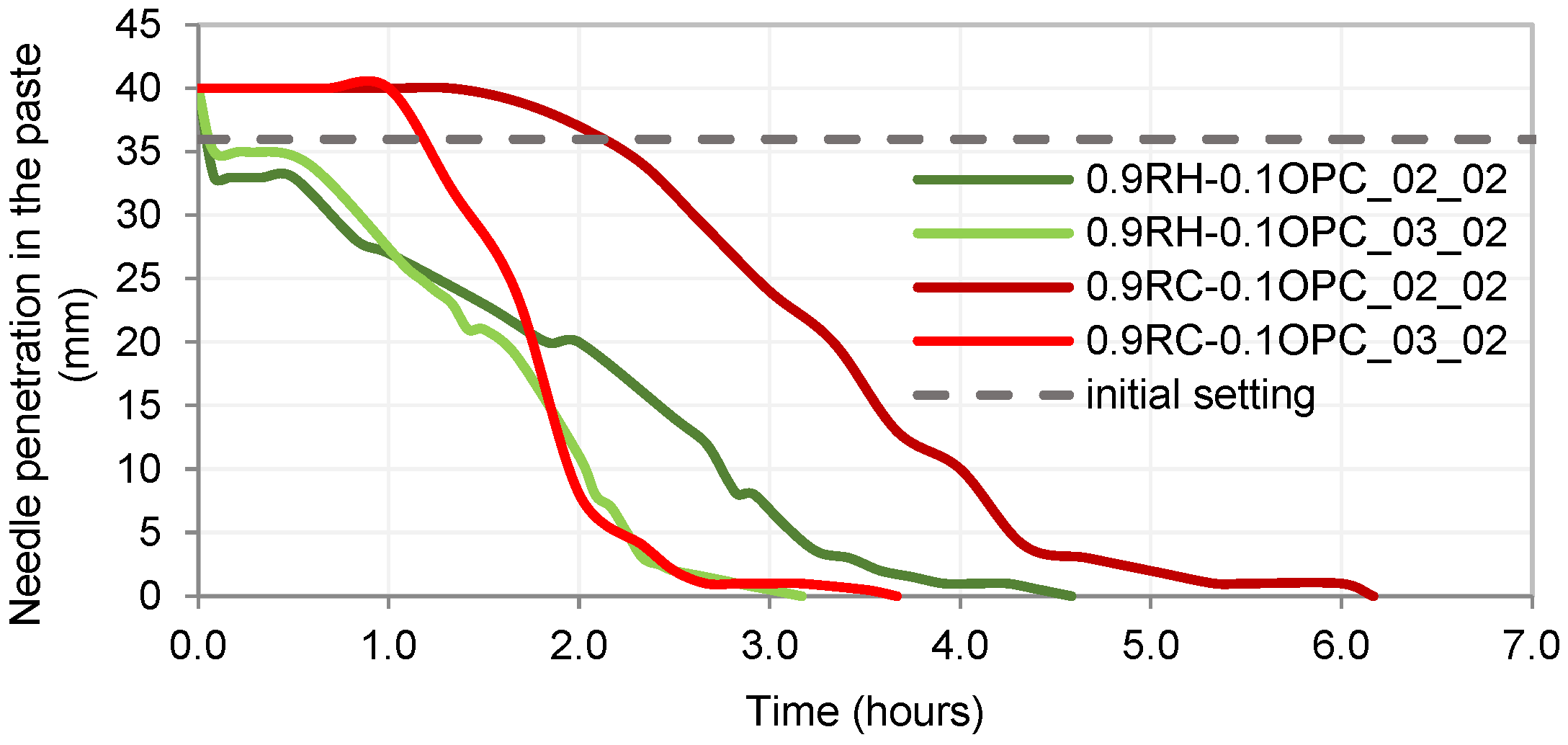
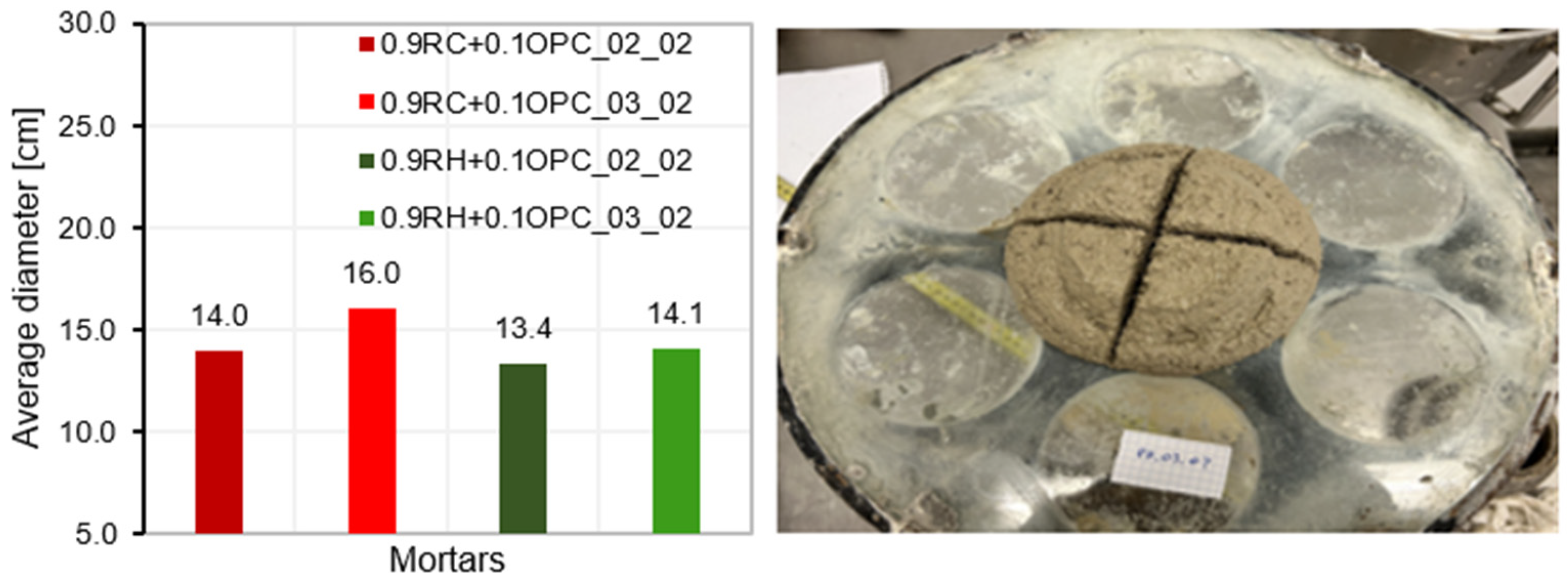
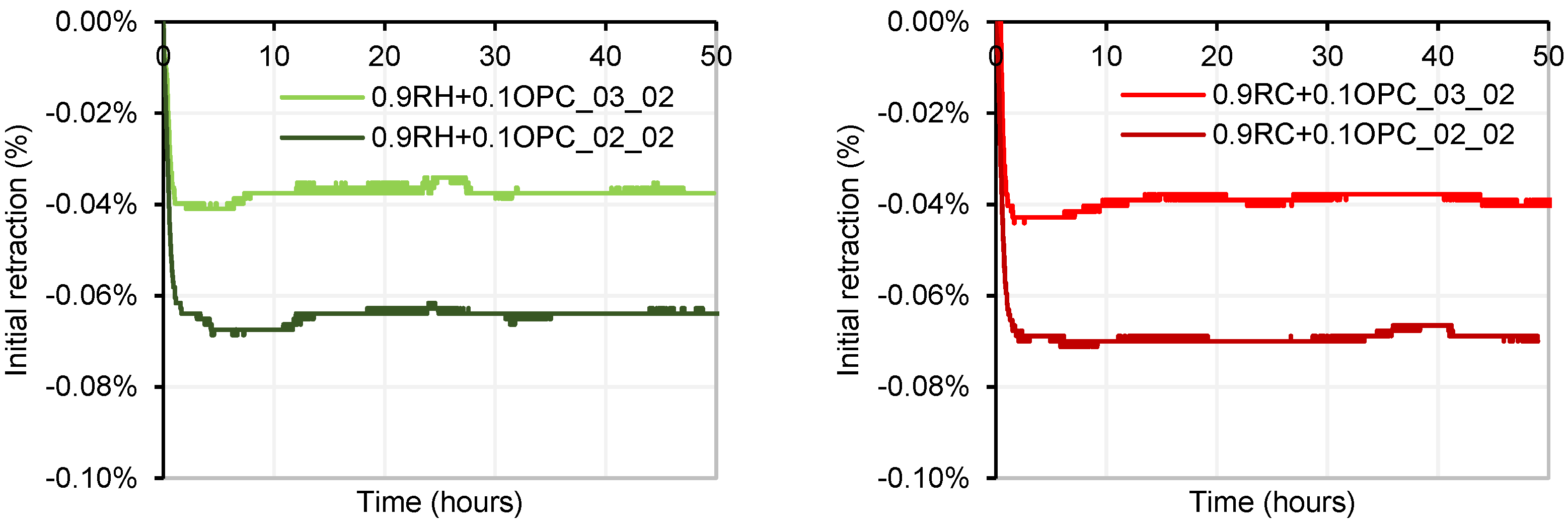
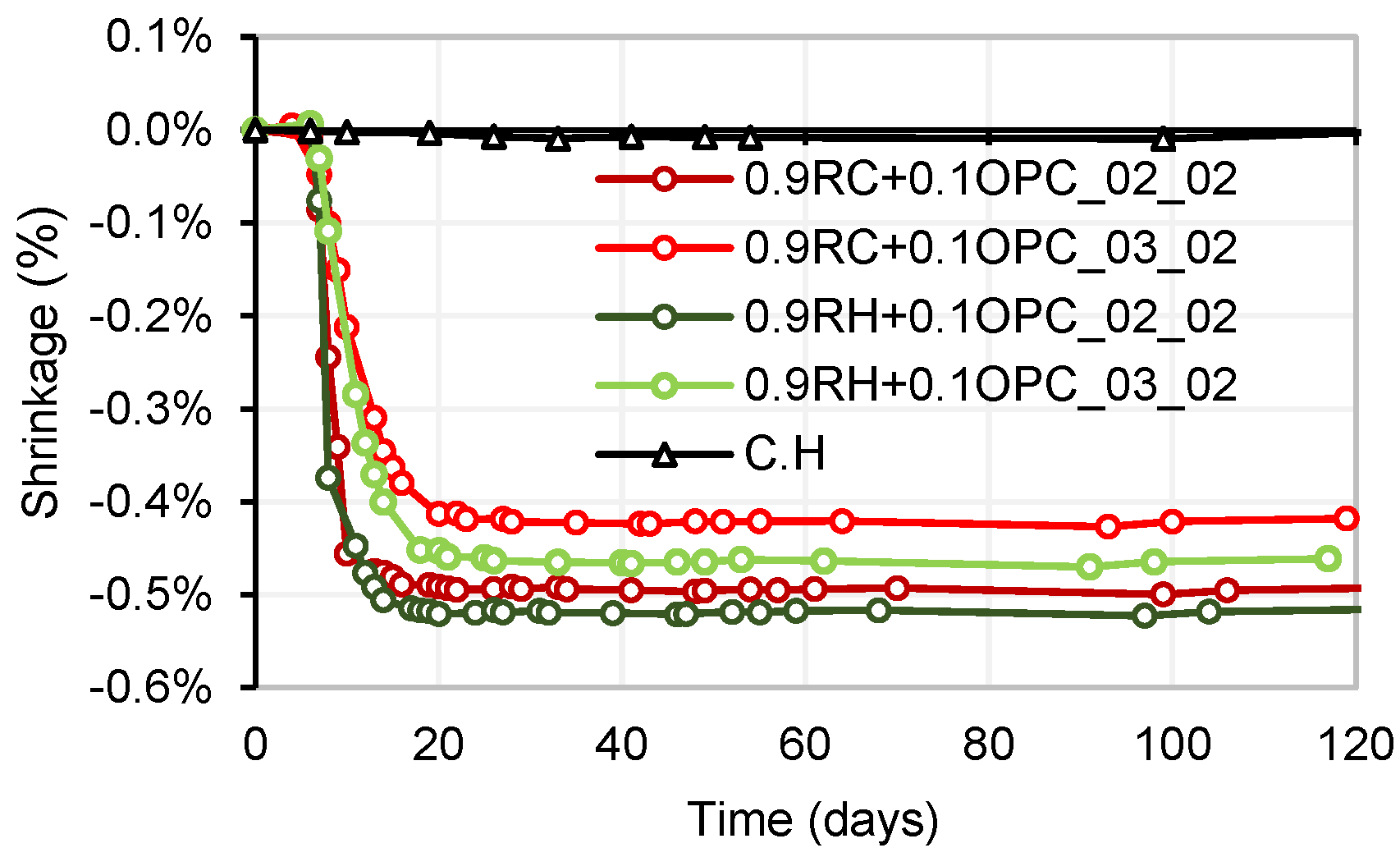
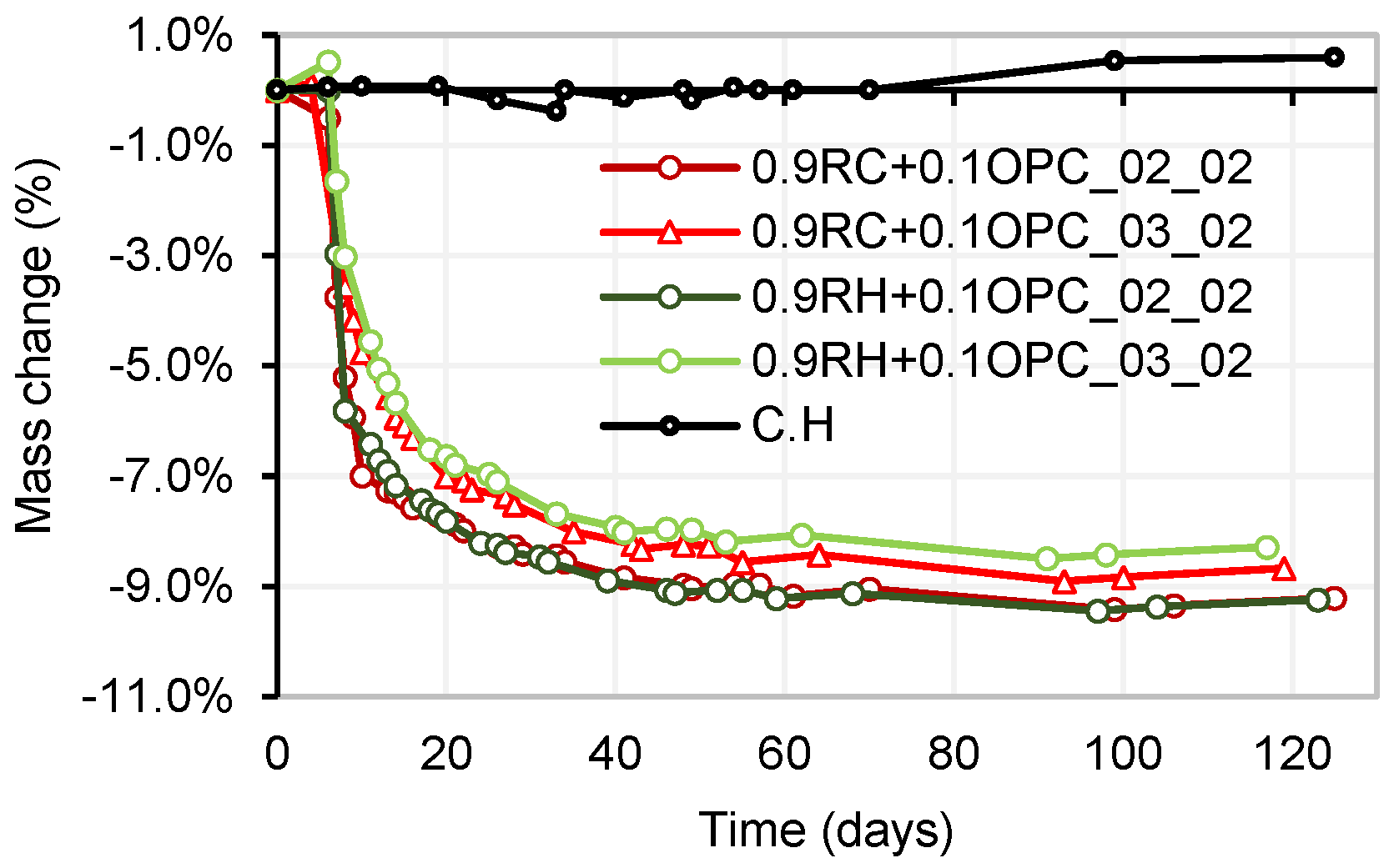
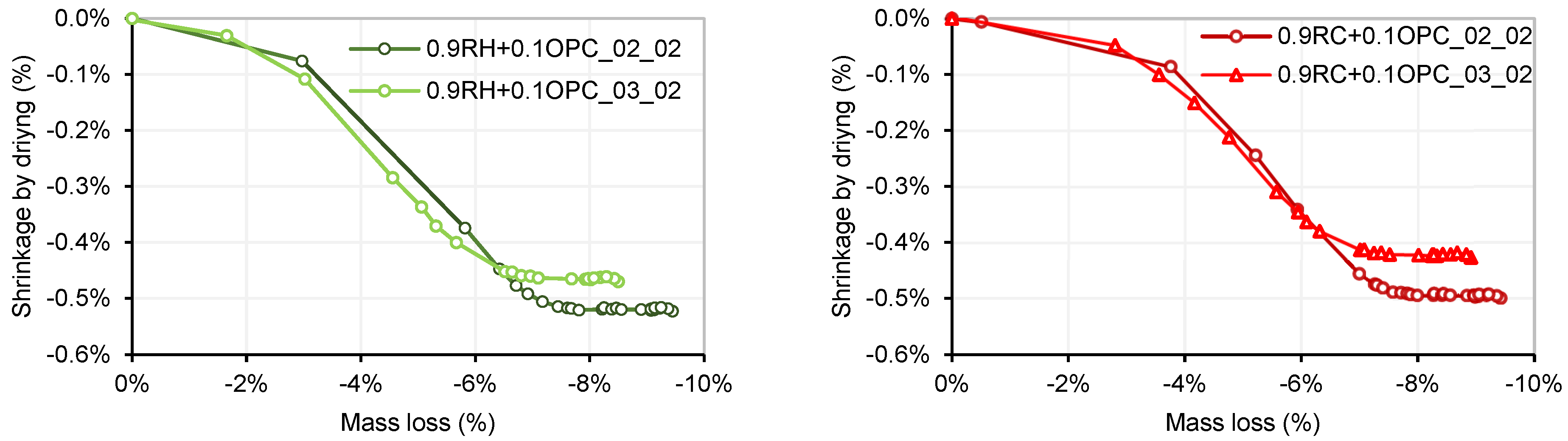
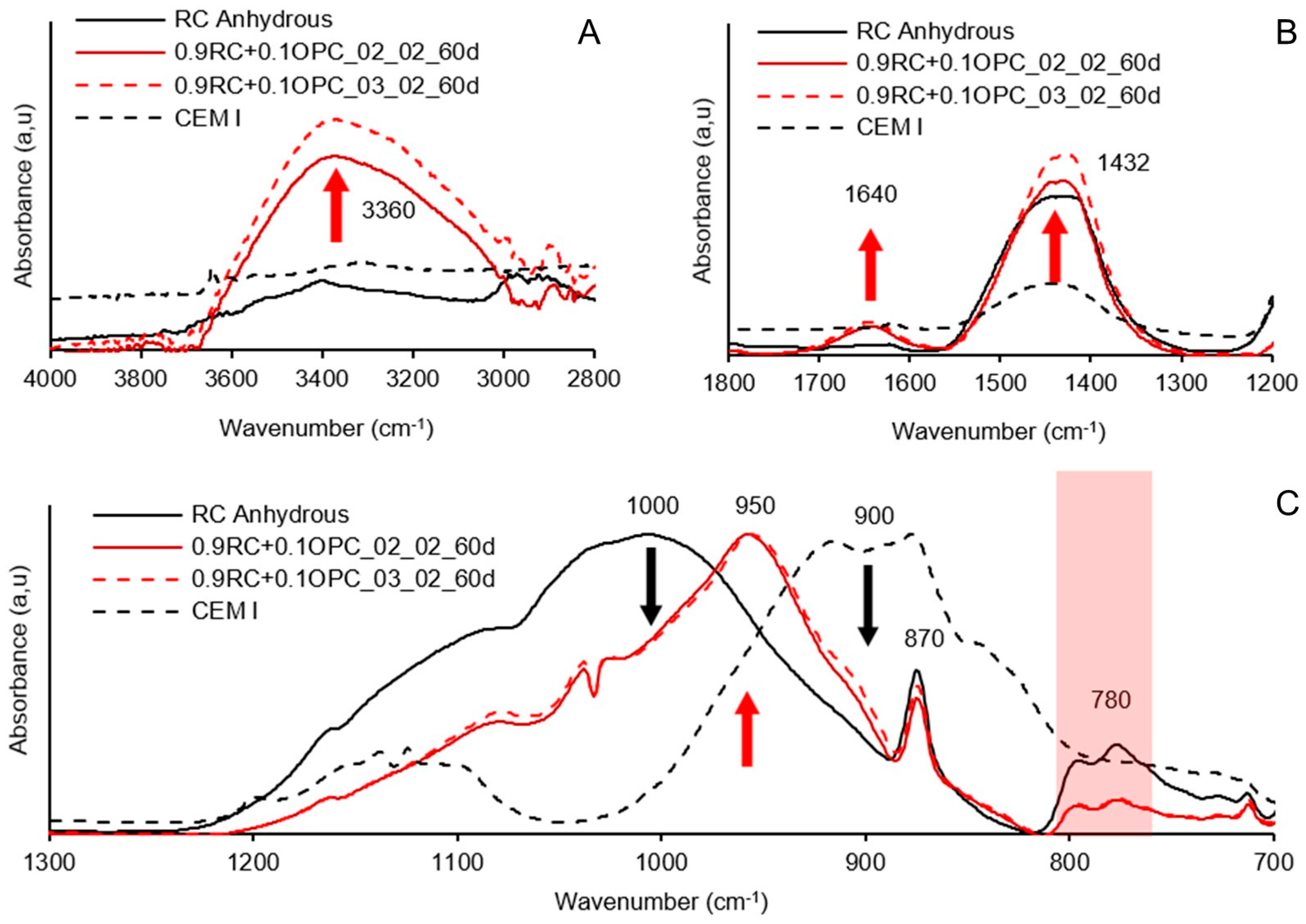
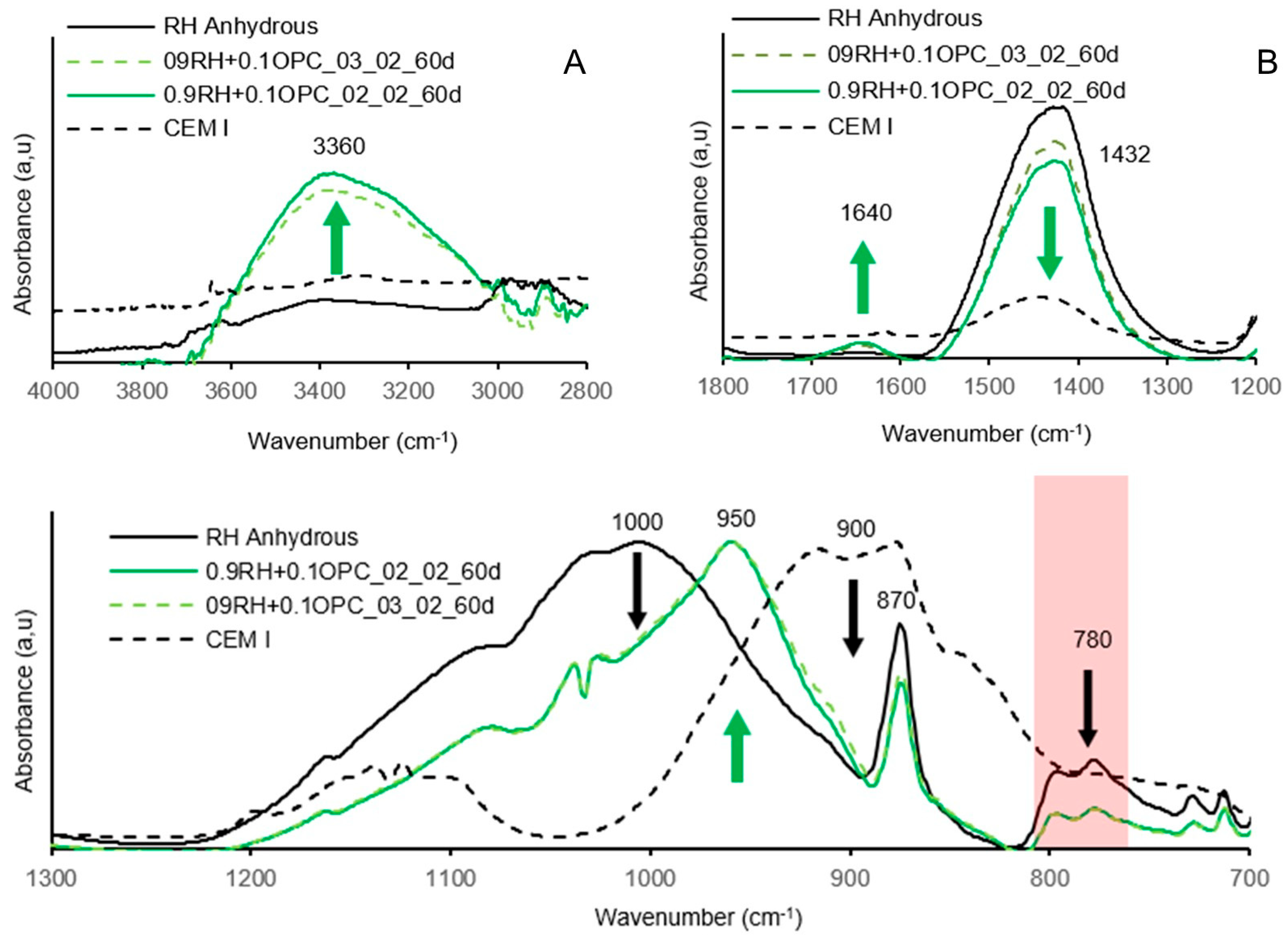
3.4. Testing of Pastes and Mortars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simpson, M.J. Global climate change impacts in the United States. J. Environ. Qual. 2011, 40, 279. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. /Mater. Constr. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Asharfi, A.; Erfanifard, Y.; Amiraslani, F.; Boloorani, A.D.; Mousivand, A. Quantitative assessment of forest ecosystem stress caused by cement plant pollution using in situ measurements and Sentinel-2 satellite data in a part of the UNESCO World Heritage site. Eur. J. Environ. Sci. 2020, 10, 22–31. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Galvez-Martos, J.L.; Schoenberger, H. An analysis of the use of life cycle assessment for waste co-incineration in cement kilns. Resour. Conserv. Recycl. 2014, 86, 118–131. [Google Scholar] [CrossRef]
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Brito, J.; Saikia, N. Recycled aggregate in concrete: Use of industrial, construction and demolition waste. In Green Energy and Technology; Springer Science & Business Media: Berlin, Germany, 2013; Volume 54. [Google Scholar] [CrossRef]
- Ledesma, E.F.; Jiménez, J.R.; Ayuso, J.; Fernández, J.M.; De Brito, J. Maximum feasible use of recycled sand from construction and demolition waste for eco-mortar production—Part I: Ceramic masonry waste. J. Clean. Prod. 2015, 87, 692–706. [Google Scholar] [CrossRef]
- Pereira, P.; Evangelista, L.; De Brito, J. The effect of superplasticisers on the workability and compressive strength of concrete made with fine recycled concrete aggregates. Constr. Build. Mater. 2012, 28, 722–729. [Google Scholar] [CrossRef]
- Evangelista, L.; de Brito, J. Durability performance of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2010, 32, 9–14. [Google Scholar] [CrossRef]
- Corinaldesi, V. Environmentally-friendly bedding mortars for repair of historical buildings. Constr. Build. Mater. 2012, 35, 778–784. [Google Scholar] [CrossRef]
- Duan, Z.; Hou, S.; Xiao, J.; Li, B. Study on the essential properties of recycled powders from construction and demolition waste. J. Clean. Prod. 2020, 253, 119865. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Mejía-Arcila, J.; Mejía de Gutiérrez, R.; Martínez, E. Life cycle assessment (LCA) of an alkali-activated binary concrete based on natural volcanic pozzolan: A comparative analysis to OPC concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M. Synthesis of ceramic materials from ecofriendly geopolymer precursors. Mater. Lett. 2018, 230, 300–304. [Google Scholar] [CrossRef]
- Bagriacik, B. Utilization of alkali-activated construction demolition waste for sandy soil improvement with large-scale laboratory experiments. Constr. Build. Mater. 2021, 302, 124173. [Google Scholar] [CrossRef]
- Su, L.; Wu, S.; Fu, G.; Zhu, W.; Zhang, X.; Liang, B. Creep characterisation and microstructural analysis of municipal solid waste incineration fly ash geopolymer backfill. Sci. Rep. 2024, 14, 29828. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Milestones in the analysis of alkali-activated binders. J. Sustain. Cem. Based Mater. 2014, 4, 74–84. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Lei, Z.; Qi, W.; Zhang, L.; Yang, J.; Zhuorui, Z. Preparation and comprehensive performance optimization of green insulation building materials based on blast furnace slag. J. Build. Eng. 2025, 106, 112591. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Lach, M.; Mikula, J. Alkali activation of waste clay bricks: Influence of the silica modulus, SiO2/Na2O, H2O/Na2O molar ratio, and liquid/solid ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef]
- García-Díaz, A.; Delgado-Plana, P.; Bueno-Rodríguez, S.; Eliche-Quesada, D. Investigation of waste clay brick (chamotte) addition and activator modulus in the properties of alkaline activation cements based on construction and demolition waste. J. Build. Eng. 2024, 84, 108568. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Valencia-Saavedra, W.; Mejía de Gutiérrez, R. Reuse of powders and recycled aggregates from mixed construction and demolition waste in alkali-activated materials and precast concrete units. Sustainability 2022, 14, 9685. [Google Scholar] [CrossRef]
- Borrachero, M.V.; Payá, J.; Brito, S.; Segura, Y.P.; Soriano, L.; Tashima, M.M.; Monzó, J.M. Reusing construction and demolition waste to prepare alkali-activated cement. Materials 2022, 15, 3437. [Google Scholar] [CrossRef] [PubMed]
- Robayo-Salazar, R.; Valencia-Saavedra, W.; Mejía de Gutiérrez, R. Recycling of concrete, ceramic, and masonry waste via alkaline activation: Obtaining and characterization of hybrid cements. J. Build. Eng. 2022, 46, 103698. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Sui, T.; Akbarnezhad, A.; Duan, Z. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Jiang, J.; Yu, C.; Yuan, B.; Lin, Q.; Wu, Q. Comparative study on different methods of activation of recycled powder grouts. Mater. Sci. Pol. 2024, 42, 21–33. [Google Scholar] [CrossRef]
- Bassani, M.; Tefa, L.; Coppola, B.; Palmero, P. Alkali-activation of aggregate fines from construction and demolition waste: Valorisation in view of road pavement subbase applications. J. Clean. Prod. 2019, 234, 71–84. [Google Scholar] [CrossRef]
- Tan, J.; Cizer, Ö.; De Vlieger, J.; Dan, H.; Li, J. Impacts of milling duration on construction and demolition waste (CDW) based precursor and resulting geopolymer: Reactivity, geopolymerization and sustainability. Resour. Conserv. Recycl. 2022, 184, 106433. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. Strength development and acid resistance of geopolymer based on waste clay brick powder and phosphorous slag. Struct. Concr. 2019, 20, 1596–1606. [Google Scholar] [CrossRef]
- UNE-EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. AENOR: Madrid, Spain, 2011.
- Han, T.; Aponte, D.; Valls, S.; Bizinotto, M.B. Impact of Recycled Concrete and Ceramic Fillers on the Performance of Cementitious Systems: Microstructural, Mechanical, and Durability Aspects. Recycling 2025, 10, 108. [Google Scholar] [CrossRef]
- UNE 80103; Test Methods of Cements. Physical Analysis. Actual Density Determination. AENOR: Madrid, Spain, 2013.
- UNE-EN 1097-6; Test for Mechanical and Physical Properties of Aggregate—Part 6: Determination of Particle Density and Water Absorption. AENOR: Madrid, Spain, 2014.
- Kuzielová, E.; Slaný, M.; Žemlička, M.; Másilko, J.; Palou, M.T. Phase composition of silica fume—Portland cement systems formed under hydrothermal curing evaluated by FTIR, XRD, and TGA. Materials 2021, 14, 2786. [Google Scholar] [CrossRef] [PubMed]
- Snellings, R.; Chwast, J.; Cizer, Ö.; de Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. Report of TC 238-SCM: Hydration stoppage methods for phase assemblage studies of blended cements—Results of a round robin test. Mater. Struct./Mater. Constr. 2018, 51, 111. [Google Scholar] [CrossRef]
- UNE-EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. AENOR: Madrid, Spain, 2018.
- UNE-EN 445; Grout for Prestressing Tendons—Test Methods. AENOR: Madrid, Spain, 2009.
- UNE-EN 196-3; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. AENOR: Madrid, Spain, 2016.
- UNE-EN 1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). AENOR: Madrid, Spain, 2000.
- ASTM C642-13; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM C490-04; Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete. ASTM International: West Conshohocken, PA, USA, 2004.
- Deir, E.; Gebregziabiher, B.S.; Peethamparan, S. Influence of starting material on the early age hydration kinetics, microstructure and composition of binding gel in alkali activated binder systems. Cem. Concr. Compos. 2014, 48, 108–117. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R. Hydration properties of alkali-activated quick cooled copper slag and slow cooled copper slag. J. Therm. Anal. Calorim. 2020, 139, 3383–3394. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. RILEM State-of-the-Art Report, RILEM TC 224-AAM.10.1007/978-94-007-7672-2. Available online: http://www.springer.com/series/8780 (accessed on 23 October 2024).
- Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R. Comparison of different activators for alkaline activation of construction and demolition wastes. Constr. Build. Mater. 2021, 281, 122599. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
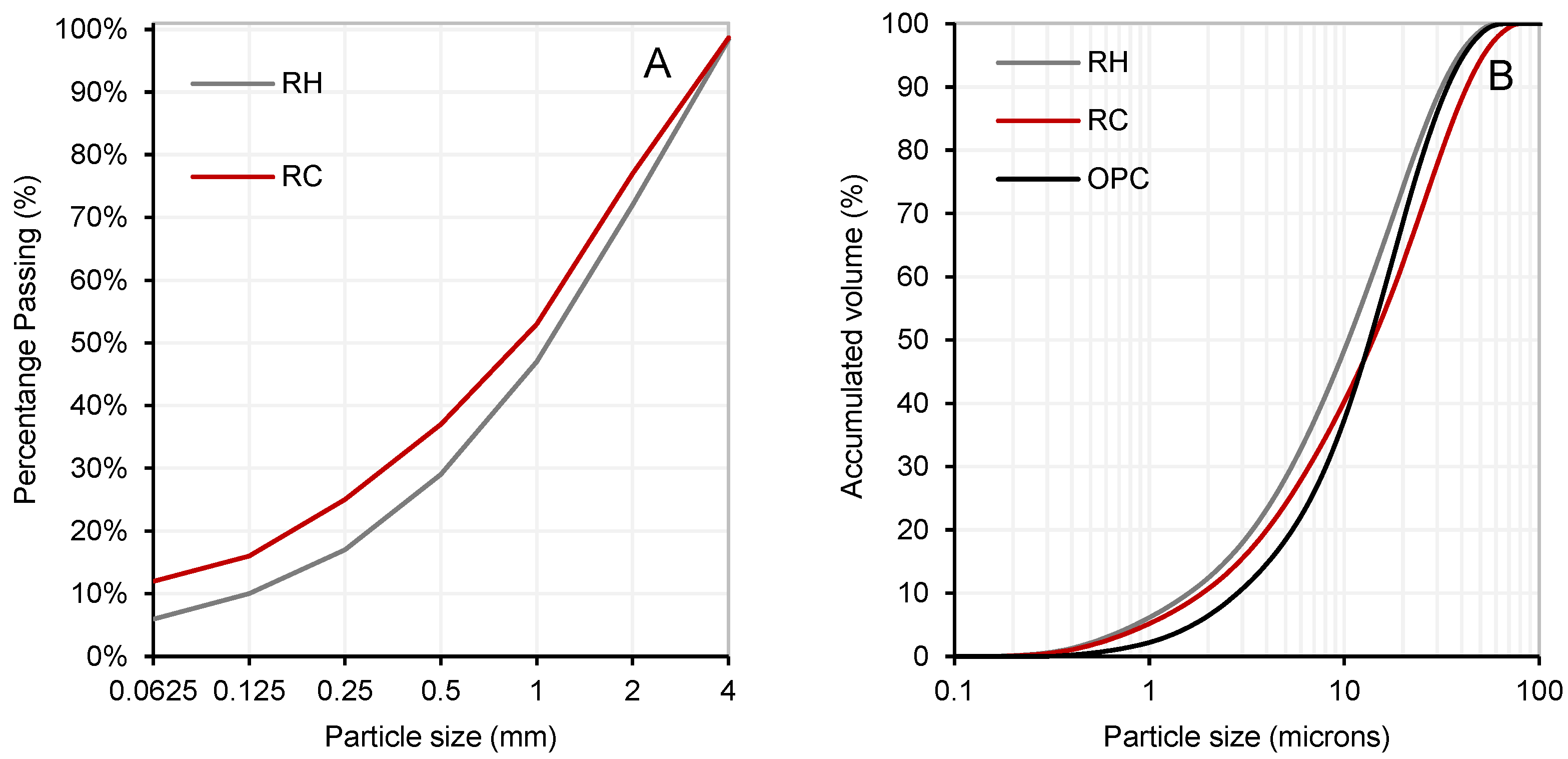

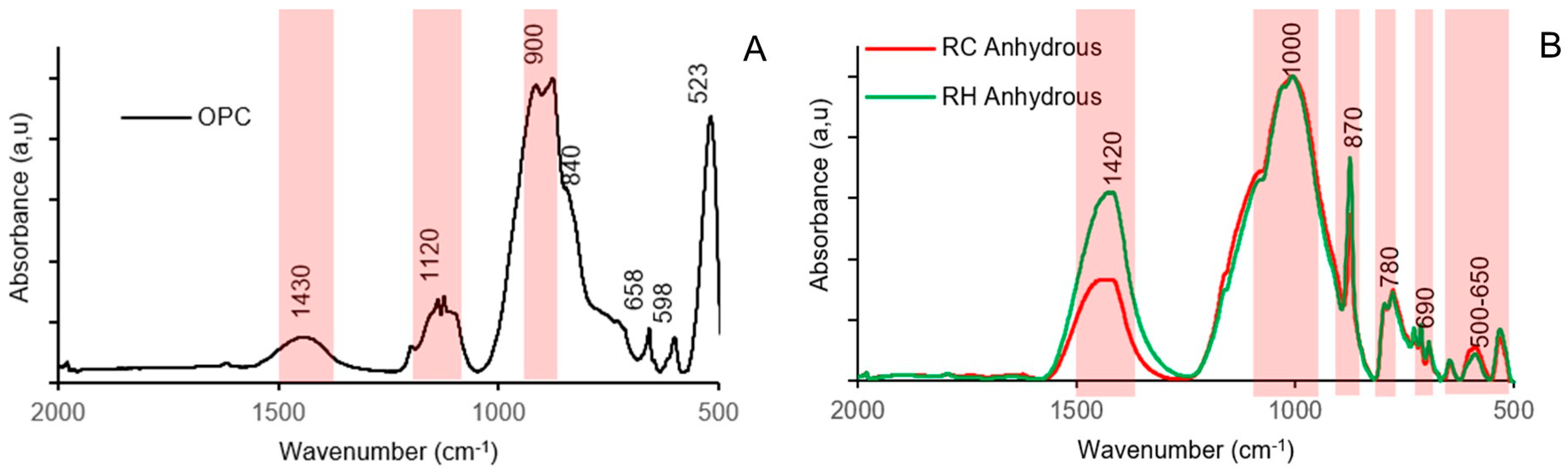
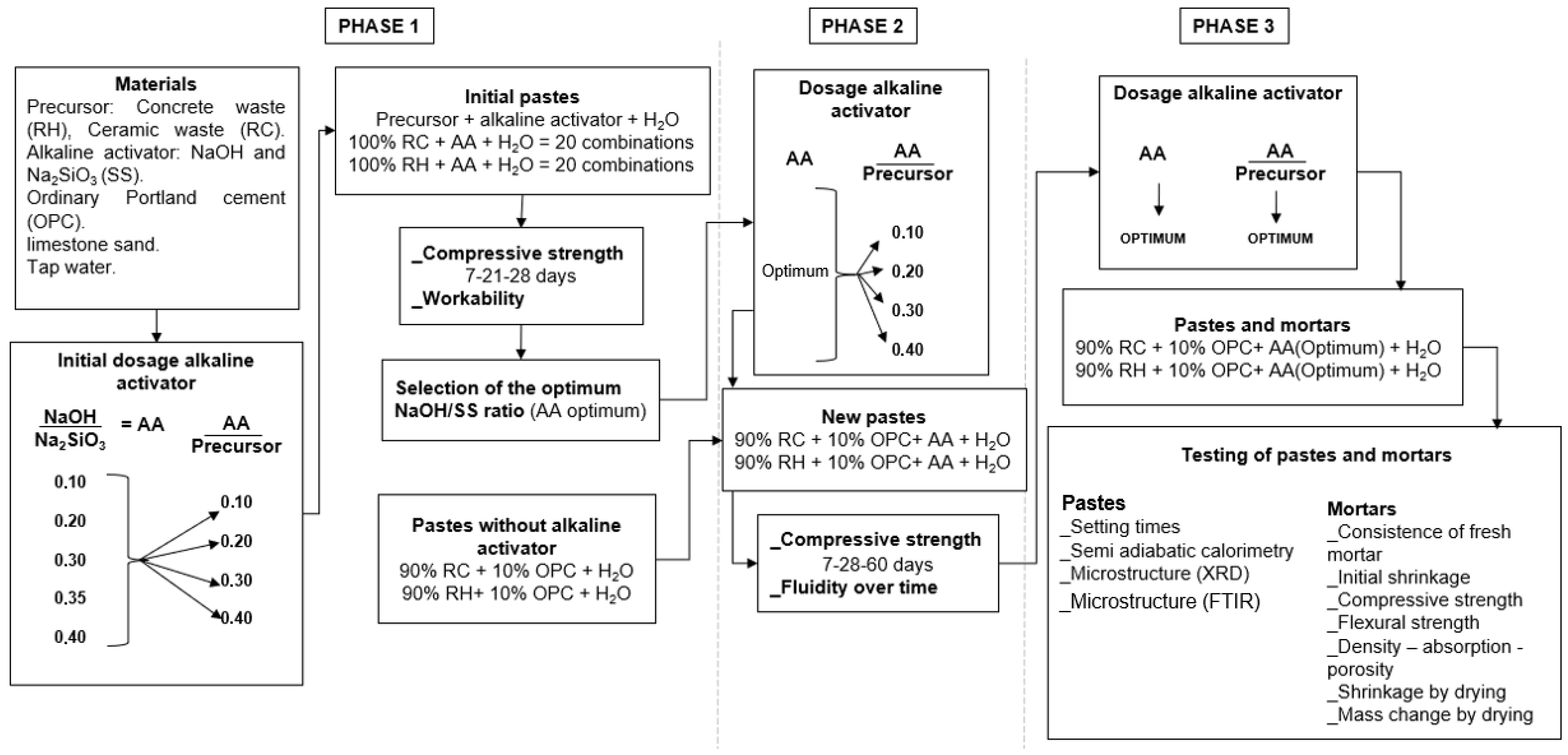









| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | MnO | SO3 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| RH | 47.93 | 6.25 | 2.31 | 18.52 | 2.82 | 2.06 | 0.04 | 0.50 | 1.22 | 18.35 |
| RC | 58.13 | 8.96 | 3.20 | 13.04 | 1.10 | 2.53 | 0.05 | 1.17 | 1.68 | 10.14 |
| OPC | 19.96 | 4.68 | 3.32 | 63.27 | 1.52 | 0.81 | 0.03 | 3.11 | 0.67 | 2.63 |
| Type of Paste | NaOH/ Na2SiO3 | AA/P | L/S |
|---|---|---|---|
| P_01_01 | 0.1 | 0.1 | 0.4 |
| P_01_02 | 0.2 | ||
| P_01_03 | 0.3 | ||
| P_01_04 | 0.4 | ||
| P_02_01 | 0.2 | 0.1 | |
| P_02_02 | 0.2 | ||
| P_02_03 | 0.3 | ||
| P_02_04 | 0.4 | ||
| P_03_01 | 0.3 | 0.1 | |
| P_03_02 | 0.2 | ||
| P_03_03 | 0.3 | ||
| P_03_04 | 0.4 | ||
| P_035_01 | 0.1 | ||
| P_035_02 | 0.35 | 0.2 | |
| P_035_03 | 0.3 | ||
| P_035_04 | 0.4 | ||
| P_04_01 | 0.4 | 0.1 | |
| P_04_02 | 0.2 | ||
| P_04_03 | 0.3 | ||
| P_04_04 | 0.4 |
| Type of Paste | NaOH/ Na2SiO3 | AA/(0.9P + 0.1OP) | L/S |
|---|---|---|---|
| 0.9P + 0.1OPC_02_01 | 0.2 | 0.1 | 0.4 |
| 0.9P + 0.1OPC_02_02 | 0.2 | ||
| 0.9P + 0.1OPC_02_03 | 0.3 | ||
| 0.9P + 0.1OPC_02_04 | 0.4 | ||
| 0.9P + 0.1OPC_03_01 | 0.3 | 0.1 | |
| 0.9P + 0.1OPC_03_02 | 0.2 | ||
| 0.9P + 0.1OPC_03_03 | 0.3 | ||
| 0.9P + 0.1OPC_03_04 | 0.4 |
| Type of Paste | NaOH/ Na2SiO3 | AA/(0.9P + 0.1OP) | L/S |
|---|---|---|---|
| 0.9P + 0.1OPC_02_02 | 0.2 | 0.2 | 0.4 |
| 0.9P + 0.1OPC_03_02 | 0.3 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retamal-Rojas, M.; Aponte, D.; Valencia-Saavedra, W.; Robayo-Salazar, R.; Barra-Bizinotto, M. Hybrid Binders Through Alkaline Activation of Fine Construction and Demolition Waste. Materials 2025, 18, 3227. https://doi.org/10.3390/ma18143227
Retamal-Rojas M, Aponte D, Valencia-Saavedra W, Robayo-Salazar R, Barra-Bizinotto M. Hybrid Binders Through Alkaline Activation of Fine Construction and Demolition Waste. Materials. 2025; 18(14):3227. https://doi.org/10.3390/ma18143227
Chicago/Turabian StyleRetamal-Rojas, Manuel, Diego Aponte, William Valencia-Saavedra, Rafael Robayo-Salazar, and Marilda Barra-Bizinotto. 2025. "Hybrid Binders Through Alkaline Activation of Fine Construction and Demolition Waste" Materials 18, no. 14: 3227. https://doi.org/10.3390/ma18143227
APA StyleRetamal-Rojas, M., Aponte, D., Valencia-Saavedra, W., Robayo-Salazar, R., & Barra-Bizinotto, M. (2025). Hybrid Binders Through Alkaline Activation of Fine Construction and Demolition Waste. Materials, 18(14), 3227. https://doi.org/10.3390/ma18143227








