Effect of Silica Sol on the Preparation and Oxidation Resistance of MoSi2@SiO2
Abstract
1. Introduction
2. Materials and Methods
3. Results
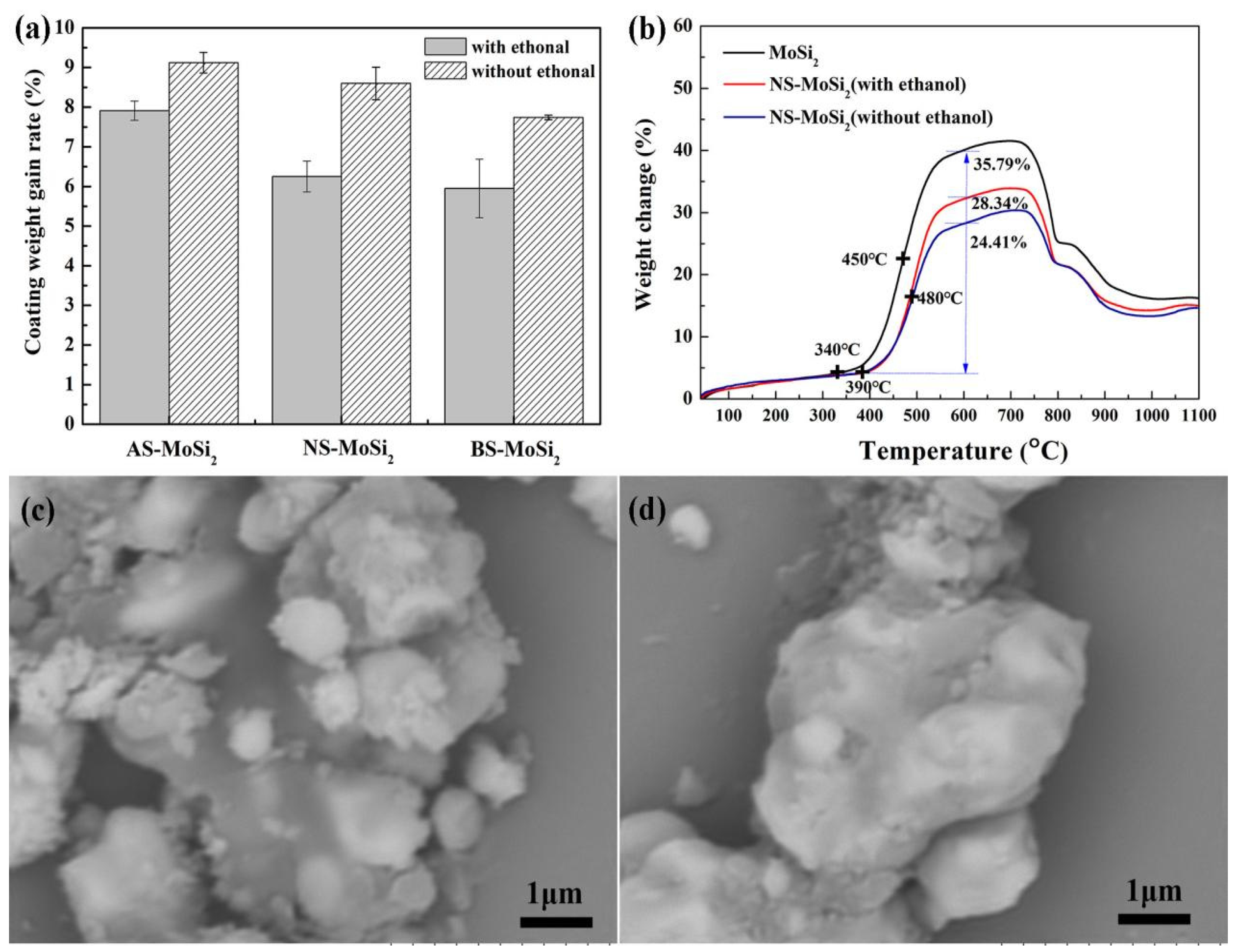
3.1. Effects of Ethanol-Mediated Process on Coating Efficiency and Oxidation Resistance
3.2. Effect of Silica Sol on the Oxidation Resistance of Gel Coating MoSi2
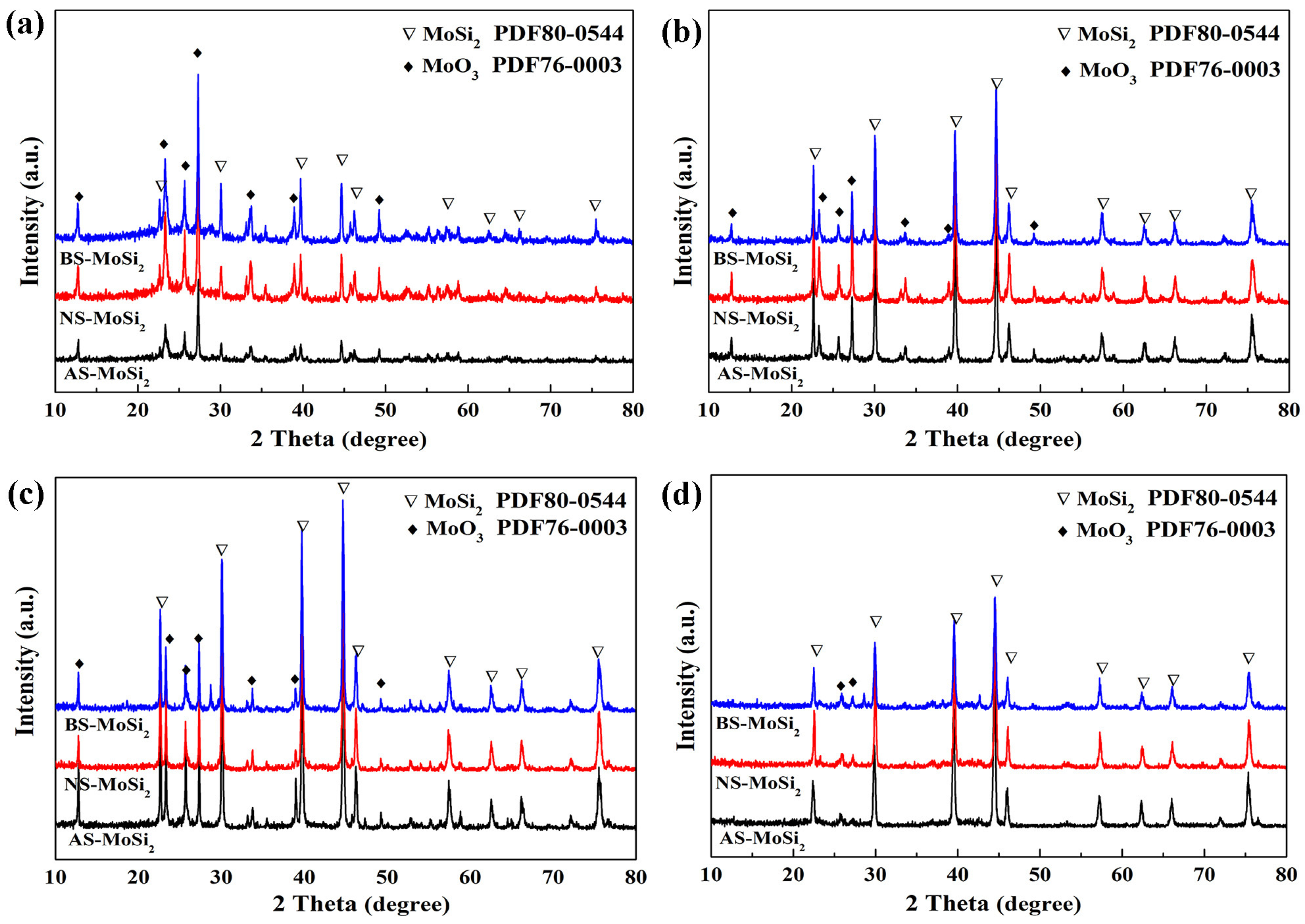
3.2.1. Effect of Silica Sol on Coating Formation
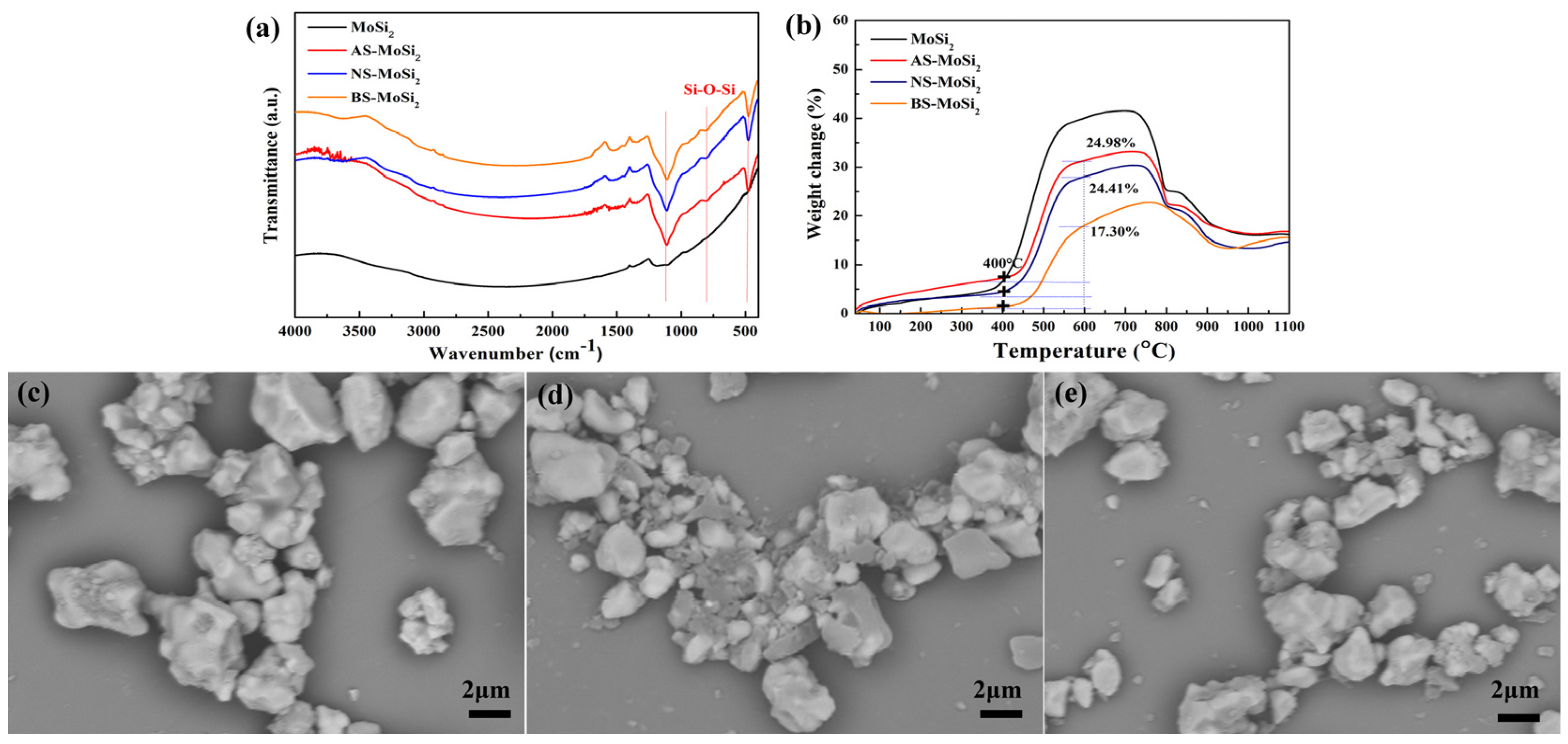
3.2.2. Surface Characteristics and Thermogravimetric Analysis
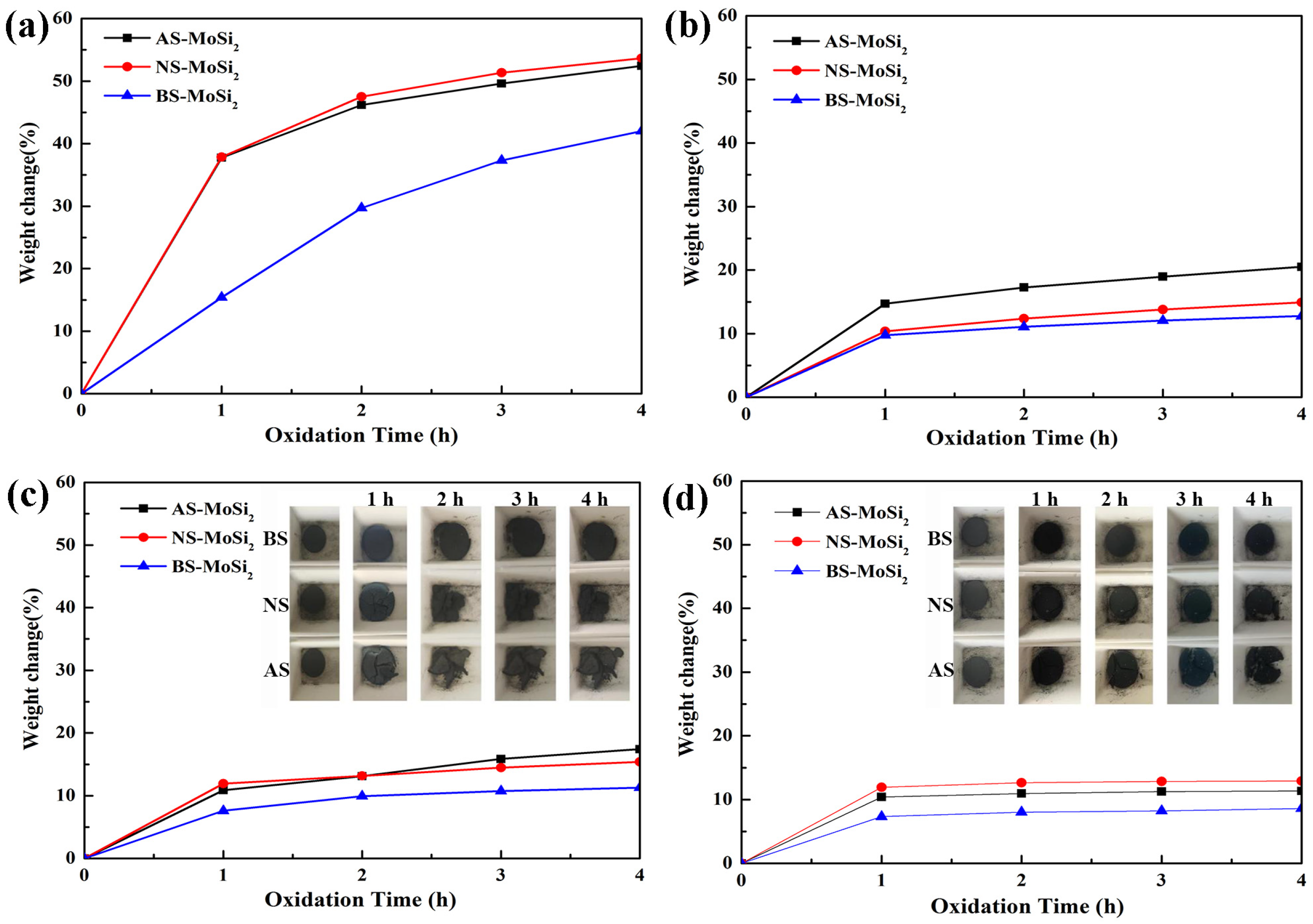
3.2.3. Oxidation Behavior of the Silica-Coated MoSi2
3.3. Mechanism Analysis of Coating Encapsulation of MoSi2
4. Conclusions
- (1)
- AS-MoSi2, NS-MoSi2, and BS-MoSi2 specimens exhibited significantly reduced coating efficiency values (maximum 27%) when fabricated via ethanol-mediated dispersion compared with direct mixing. All xerogel powder exhibited reduced mass gain compared to pristine MoSi2 in TG analysis, with ethanol-free preparations showing 10–20% lower weight gains between 340 °C and 600 °C than their ethanol-mediated dispersed counterparts. The NS-MoSi2 demonstrated delayed oxidation kinetics: oxidation initiation shifted from 340 °C (MoSi2) to 390 °C, with peak reaction rates occurring at 480 °C (vs. 450 °C for MoSi2).
- (2)
- There is a clear correlation between the coating weight gain rate and the type of silica sol used. The AS-MoSi2 sample exhibits the highest coating weight gain rate at 9.12 ± 0.26%, followed by NS-MoSi2 with 8.60 ± 0.41%, while BS-MoSi2 demonstrates the lowest value at 7.74 ± 0.06%. While the weight gains between 340 °C and 600 °C due to oxidation were quantified as 31.58%, 28.34%, and 18.45% for AS-MoSi2, NS-MoSi2, and BS-MoSi2, respectively, indicate that BS-MoSi2 exhibits superior oxidant resistance.
- (3)
- The oxidation resistance of BS-MoSi2 arises from the coating quality, which is determined by optimal gelation kinetics under alkaline conditions (slow, homogeneous growth); enhanced surface coverage via reduced agglomeration in high-pH media; and suppressed premature nucleation through controlled charge interactions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huimin, X.; Fuzhi, D.; Yanchun, Z. Secrets of high thermal emission of transition metal disilicides TMSi2 (TM = Ta, Mo). J. Mater. Sci. Technol. 2021, 89, 114–121. [Google Scholar] [CrossRef]
- Tao, X.; Xu, X.; Guo, L.; Hong, W.; Guo, A.; Hou, F.; Liu, J. MoSi2-borosilicate glass coating on fibrous ceramics prepared by in-situ reaction method for infrared radiation. Mater. Des. 2016, 103, 144–151. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Li, J.; Liu, J.; Wang, M.; Tao, X. High Emissivity MoSi2-SiC-Al2O3 Coating on Rigid Insulation Tiles with Enhanced Thermal Protection Performance. Materials 2024, 17, 220. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, D.; Chen, X.; Huang, Y.; Peng, Y.; Xu, C.; Huang, S.; Pu, R.; Liu, S.; Zhao, X.; et al. A novel ultra-high-temperature oxidation protective MoSi2-TaSi2 ceramic coating for tantalum substrate. J. Eur. Ceram. Soc. 2019, 39, 2277–2286. [Google Scholar] [CrossRef]
- Xuanru, R.; Junshuai, L.; Wei, L.; Yuwen, H.; Ke, S.; Can, M.; Hong’ao, C.; Weiguang, W.; Leihua, X.; Ziyu, L.; et al. Influence of MoSi2 on oxidation protective ability of TaB2-SiC coating in oxygen-containing environments within a broad temperature range. J. Adv. Ceram 2020, 9, 703–715. [Google Scholar] [CrossRef]
- Ying, C.; Xun, Z.; Zwaag, S.; Willem, G.; Sloof, P.X. Damage evolution in a self-healing air plasma sprayed thermal barrier coating containing self-shielding MoSi2 particles. J. Am. Ceram. Soc. 2019, 102, 4899–4910. [Google Scholar] [CrossRef]
- Denise, K.; Denial, E.; Robert, V. Degradation and lifetime of self-healing thermal barrier coatings containing MoSi2 as self-healing particles in thermo-cycling testing. Surf. Coat. Technol. 2022, 437, 128353. [Google Scholar] [CrossRef]
- Chou, T.; Nieh, T. Mechanism of MoSi2 pest during low temperature oxidation. J. Mater. Res. 1993, 8, 214–226. [Google Scholar] [CrossRef]
- Hansson, K.; Halvarsson, M.; Tang, J.; Pompe, R.; Sundberg, M.; Svensson, J. Oxidation behaviour of a MoSi2-based composite in different atmospheres in the low temperature range (400–550 °C). J. Eur. Ceram. Soc. 2004, 24, 3559–3573. [Google Scholar] [CrossRef]
- Zenk, C.; Gibson, K.; Maier-Kiener, V.; Neumeier, S.; Korte-Kerzel, S. Low temperature deformation of MoSi2 and the effect of Ta, Nb and Al as alloying elements. Acta Mater. 2019, 181, 385–398. [Google Scholar] [CrossRef]
- Niannian, L.; Jun, G.; Wanxia, W.; Sheng-Chi, C.; Kunlun, W.; Yong, W.; Chao-Kuang, W.; Hui, S. Oxidation resistance of Cr-modified MoSi2 composites at high temperature. Int. J. Refract. Met. Hard Mater. 2024, 119, 106497. [Google Scholar] [CrossRef]
- Sedighi, A.; Adeli, M.; Soltanieh, M. Investigation of the effect of SiC additions on the high-temperature oxidation behavior of combustion-synthesized MoSi2. J. Mater. Res. Technol. 2024, 30, 187–196. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Y.; Yao, L.; Zhang, Y.; Ren, X.; Wang, P.; Korneev, P.; Levashov, E.; Shi, J.; Kang, X.; et al. Enhanced oxidation resistance of ZrB2-MoSi2 coating through MoSi2-TaSi2 double-silicide alloying modifying. Corro. Sci. 2024, 233, 112070. [Google Scholar] [CrossRef]
- Bundschuh, K.; Schüze, M.; Müller, C.; Greil, P.; Heider, W. Selection of materials for use at temperatures above 1500 °C in oxidizing atmospheres. J. Eur. Ceram. Soc. 1998, 18, 2389–2391. [Google Scholar] [CrossRef]
- Feng, P.; Wang, X.; He, Y.; Qiang, Y. Effect of high-temperature preoxidation treatment on the low-temperature oxidation behavior of a MoSi2-based composite at 500 °C. J. Alloys Compd. 2009, 473, 185–189. [Google Scholar] [CrossRef]
- Yakaboylu, G.; Yumak, T.; Sabolsky, K.; Sabolsky, E. Effect of high temperature preoxidation treatment on the oxidation behavior of MoSi2- and WSi2-Al2O3 composites. J. Alloys Compd. 2020, 816, 152499. [Google Scholar] [CrossRef]
- Guo, L.; Liu, B.; Zhang, J.; Zhang, X.; Miao, C.; Wu, Y.; Du, H. Low-temperature oxidation resistance of core-shell structure MoSi2@SiO2. J. Chin. Ceram. Soc. 2022, 50, 270–276. [Google Scholar] [CrossRef]
- Guerrero, M.; Pérez, J.; Liz, M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef]
- Guo, L.; Hu, X.; Tao, X.; Du, H.; Guo, A.; Liu, J. Low-temperature oxidation resistance of the silica-coated MoSi2 powders prepared by sol-gel preoxidation method. Ceram. Int. 2020, 46, 23471–23478. [Google Scholar] [CrossRef]
- Matsunaga, H.; Iwata, M.; Shinji, K.; Kenji, Y.; Kazuhiro, H.; Yoshitsugu, K.; Takamitsu, I.; Masakazu, H.; Takamitsu, F. High Emittance Glass Coating Material, High Emittance Glass Coating, and Method of Produce High Emittance Glass Coating. U.S. Patent NO. 6391383, 21 May 2002. [Google Scholar]
- Chen, Y.; Zhu, S.; Ji, Y.; Ma, Z.; Wei, H. Oxidation resistance and infrared emissivity of MoSi2@SiO2 particles prepared via TEOS hydrolysis self-assembly method. J. Alloys Compd. 2019, 810, 151745. [Google Scholar] [CrossRef]
- Lei, X.; Sun, H.; Yuan, X.; Zhang, W.; Jiang, P.; Zhang, L. Exploration of silica sol-gel coating for the preparation of core-shell structure VO2(M)@SiO2. Inorg. Chem. Ind. 2024, 56, 46–54. [Google Scholar]
- Tao, X.; Li, J.; Liu, J.; Cai, G.; Zhang, J.; Wang, M. Improved oxidation resistance and infrared emissivity of tantalum disilicide particles with sol-gel derived SiO2 coatings. J. Alloys Compd. 2024, 971, 172765. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Wang, Z.; Qi, C.; Zhuo, J.; Wan, Y.; Zhang, Y.; Liu, H.; Xiao, B.; Wang, M. Preparation of porous mullite ceramics composed entirely of overlapping and interlocking mullite whiskers through whisker in-situ growth. J. Adv. Ceram. 2025, 14, 9221055. [Google Scholar] [CrossRef]
- Yoko, F.; Patrick, S. Relative colloidal stability in ethanol of powders with Si-O surface species. J. Ame. Ceram. Soc. 2004, 85, 2945–2948. [Google Scholar] [CrossRef]
- Yoko, F.; Patrick, S. The role of Si-O species in the colloidal stability of silicon-containing ceramic powders. J. Eur. Ceram. Soc. 2004, 24, 17–23. [Google Scholar] [CrossRef]
- Sandlin, M.; Butt, D.; Taylor, T.; Petrovic, J. Aqueous zeta potentials of molybdenum disilicide. J. Mater. Sci. Let. 1997, 16, 1336–1338. [Google Scholar] [CrossRef]
- Tamar, Y.; Sasson, Y. Examination of the regime controlling sol-gel based colloidal silica aggregation. J. Non-Cryst. Solids 2013, 380, 35–41. [Google Scholar] [CrossRef]
- Dyah, A.; Budi, H.; Neny, K.; Nazopatul, P.; Noviyan, D.; Irzaman, I. Functional groups, band gap energy, and morphology properties of annealed silicon dioxide (SiO2). Egypt. J. Chem. 2023, 66, 529–535. [Google Scholar] [CrossRef]
- Liu, J.; Wan, Y.; Xiao, B.; Li, J.; Hu, Z.; Zhang, R.; Hu, X.; Liu, J.; Cai, G.; Liu, H.; et al. The preparation and performance analysis of zirconium-modified aluminum phosphate-based high-temperature (RT-1500 °C) resistant adhesive for joining alumina in extreme environment. J. Adv. Ceram. 2024, 13, 911–932. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Yang, N.; Hou, Y.; Chen, D.; Liu, J.; Cai, G.; Wang, M. The effect of different diluents and curing agents on the performance of epoxy resin-based intumescent flame-retardant coatings. Materials 2024, 17, 348. [Google Scholar] [CrossRef]
- Cui, A.; Wang, T.; Jin, Y.; Sun, M. Thermodynamic research on the coating process of silica nano film on titanate particles surface. Chem. J. Chin. Univ. 2001, 22, 1543–1545. [Google Scholar] [CrossRef]
- Cui, A.; Wang, T.; Jin, Y.; Xiao, S.; Ge, X. Mechanism and structure analysis of TiO2 surface coated with SiO2 and Al2O3. Chem. J. Chem. J. Chin. Univ. 1998, 19, 1727–1729. [Google Scholar] [CrossRef]
- Wenfu, Y.; Ruren, X. Chemical reactions in aqueous solutions with condensed liquid state. Prog. Chem. 2022, 34, 1454–1491. [Google Scholar]
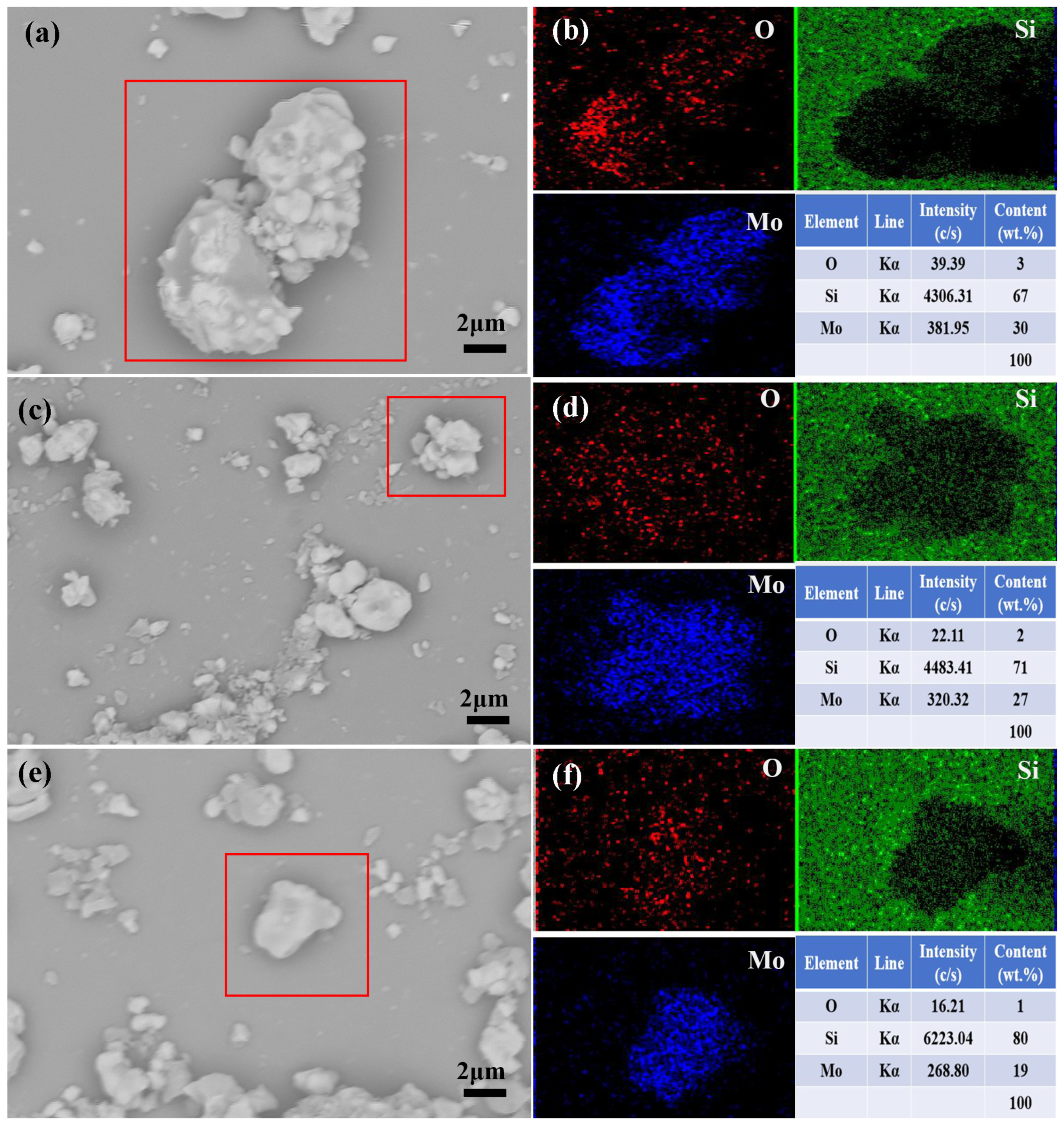
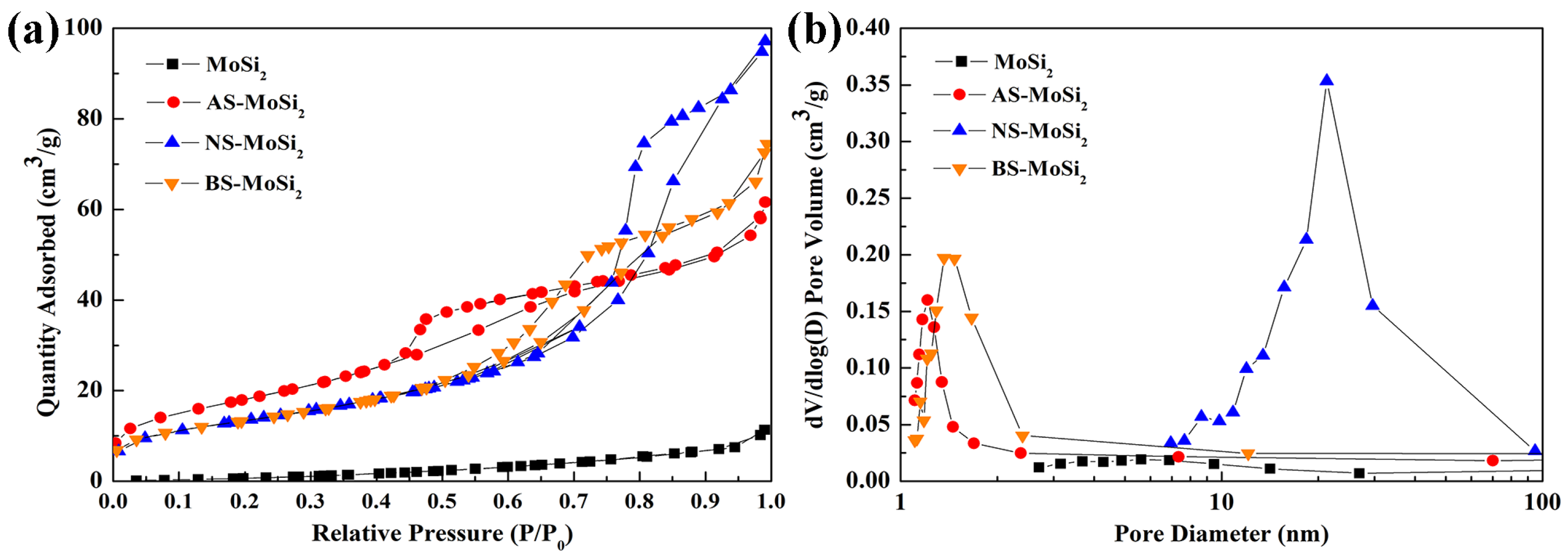
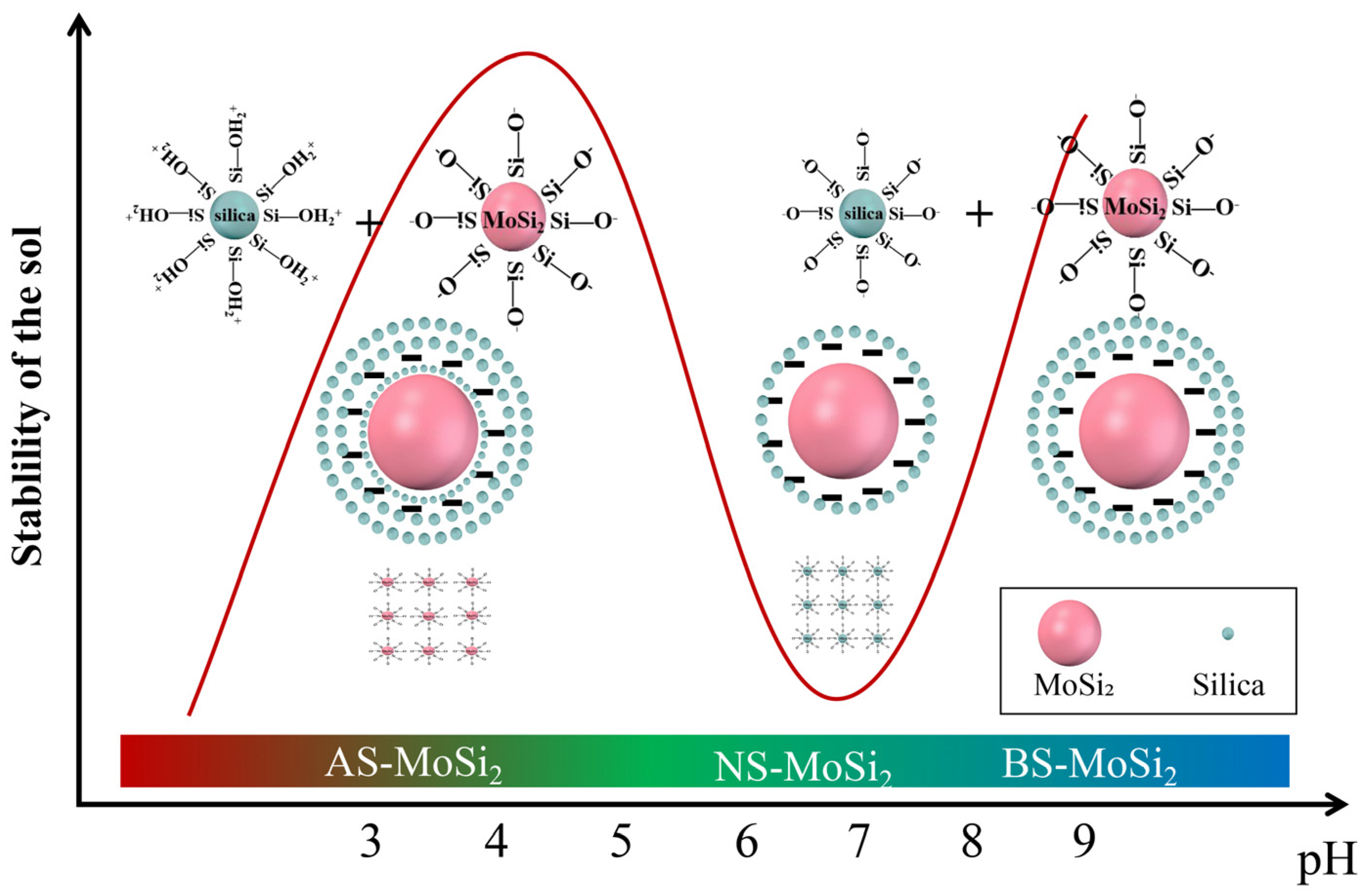
| Type | pH | SiO2 Content (%) | Na2O Content (%) | Density (20 °C, g·cm−3) | Viscosity (20 °C, mPas) |
|---|---|---|---|---|---|
| Acid silica sol | 2.92 ± 0.01 | 30 ± 0.1 | ≤0.06 | 1.17 ± 0.01 | ≤7 |
| Neutral silica sol | 7.31 ± 0.01 | 32 ± 0.1 | ≤0.1 | 1.18 ± 0.01 | ≤7 |
| Alkaline silica sol | 9.49 ± 0.01 | 32 ± 0.1 | ≤0.3 | 1.20 ± 0.01 | ≤10 |
| Type | MoSi2 | AS-MoSi2 | NS-MoSi2 | BS-MoSi2 |
|---|---|---|---|---|
| BET surface area (m2/g) | 6.15 | 66.61 | 49.17 | 48.75 |
| Total pore volume of adsorption (cm3/g) | 0.021 | 0.102 | 0.160 | 0.126 |
| Pore size (nm) | 5.72 | 2.86 | 6.11 | 4.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Zhang, J.; Miao, C.; Feng, S.; Fan, X.; Du, H.; Liu, J.; Wang, M. Effect of Silica Sol on the Preparation and Oxidation Resistance of MoSi2@SiO2. Materials 2025, 18, 3203. https://doi.org/10.3390/ma18133203
Guo L, Zhang J, Miao C, Feng S, Fan X, Du H, Liu J, Wang M. Effect of Silica Sol on the Preparation and Oxidation Resistance of MoSi2@SiO2. Materials. 2025; 18(13):3203. https://doi.org/10.3390/ma18133203
Chicago/Turabian StyleGuo, Linlin, Jinjun Zhang, Chengpeng Miao, Shuang Feng, Xiaozhen Fan, Haiyan Du, Jiachen Liu, and Mingchao Wang. 2025. "Effect of Silica Sol on the Preparation and Oxidation Resistance of MoSi2@SiO2" Materials 18, no. 13: 3203. https://doi.org/10.3390/ma18133203
APA StyleGuo, L., Zhang, J., Miao, C., Feng, S., Fan, X., Du, H., Liu, J., & Wang, M. (2025). Effect of Silica Sol on the Preparation and Oxidation Resistance of MoSi2@SiO2. Materials, 18(13), 3203. https://doi.org/10.3390/ma18133203







