Influence of Annealing Atmosphere on the Phosphatability of Ultra-High-Strength Automotive Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
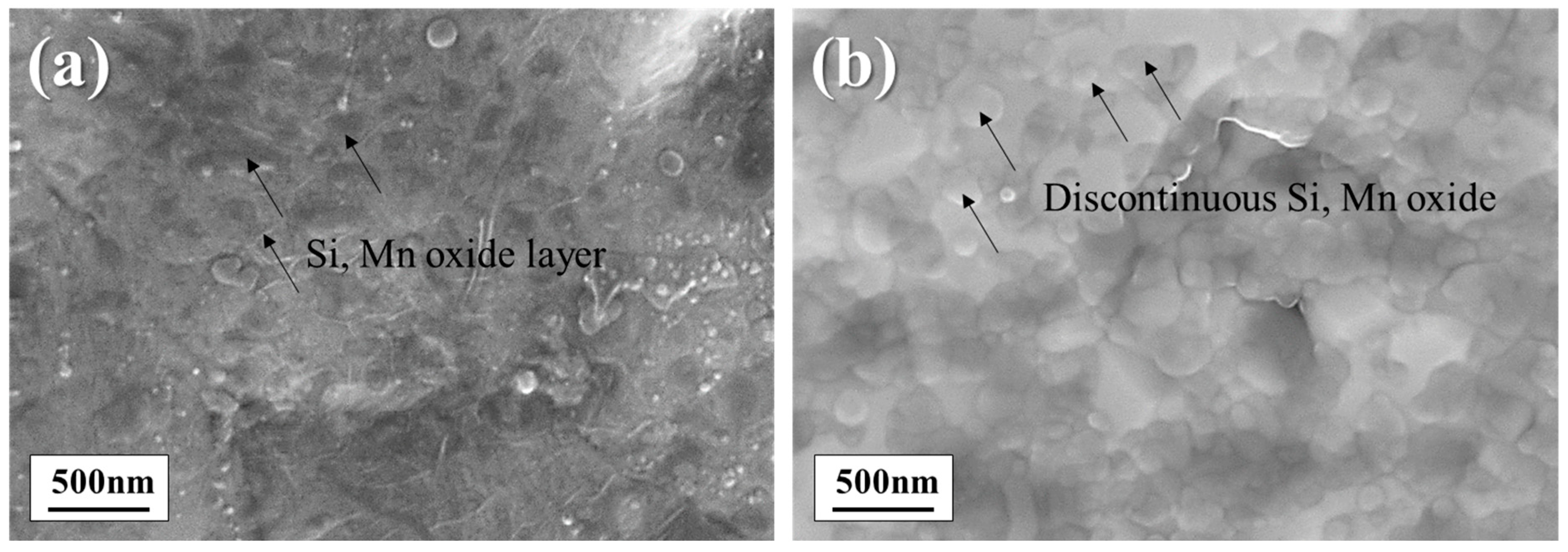
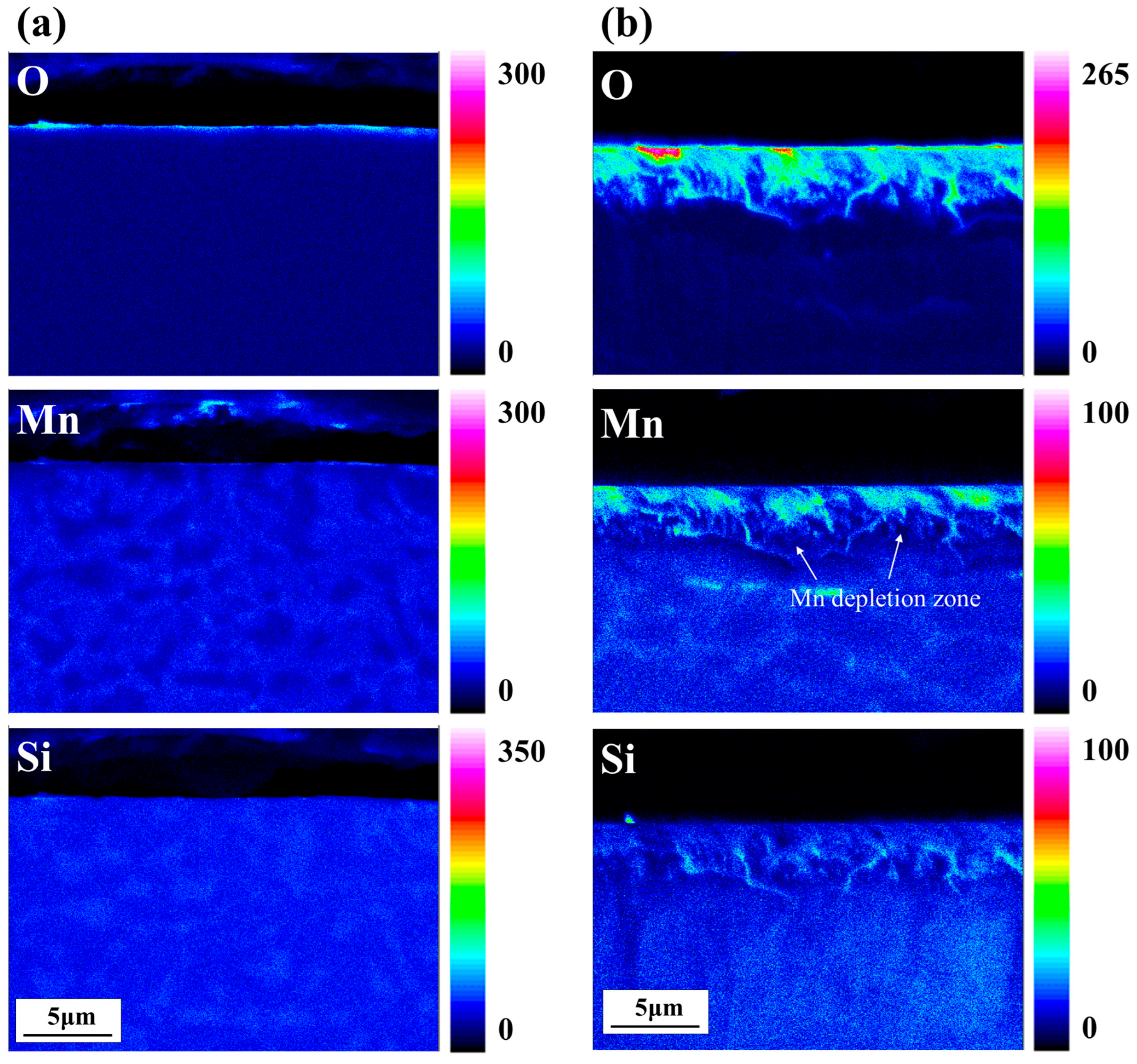
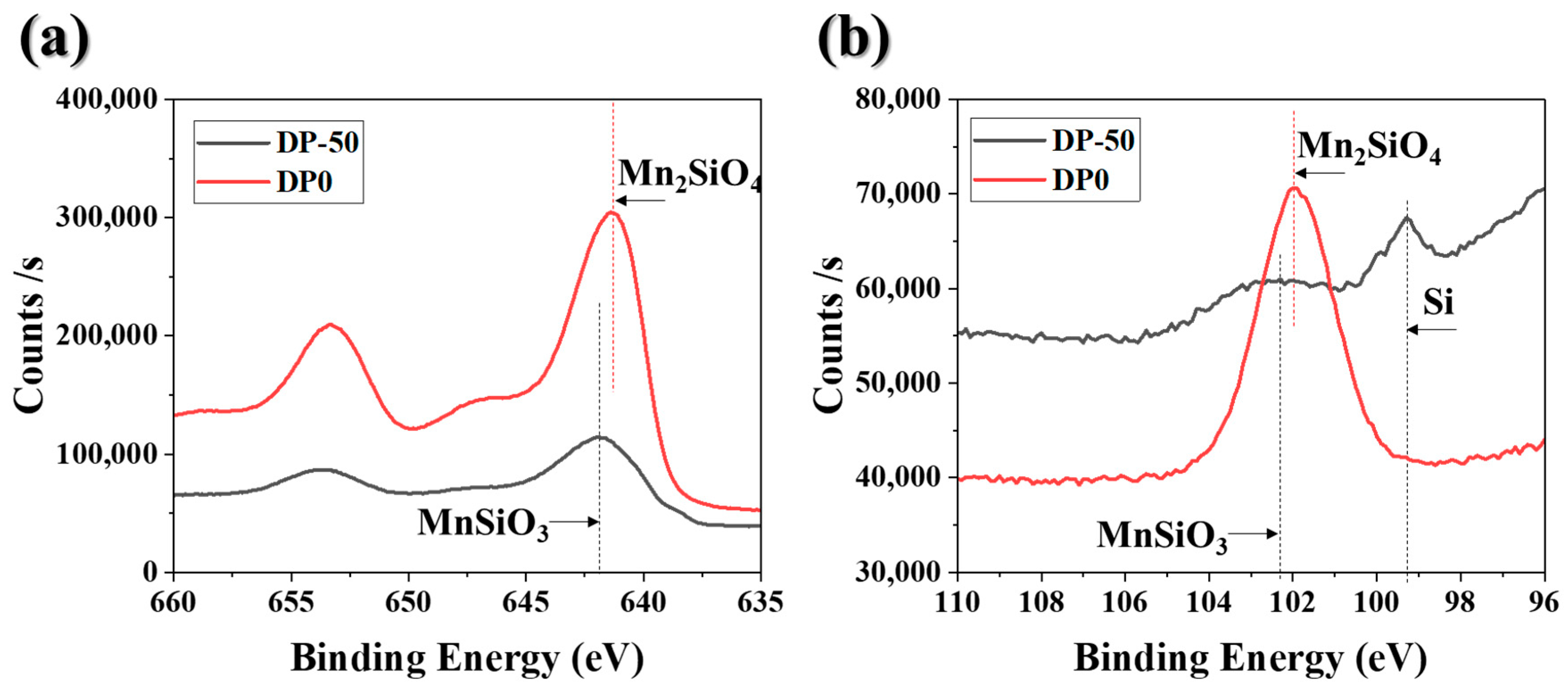
3.1. Surface Structure and Composition
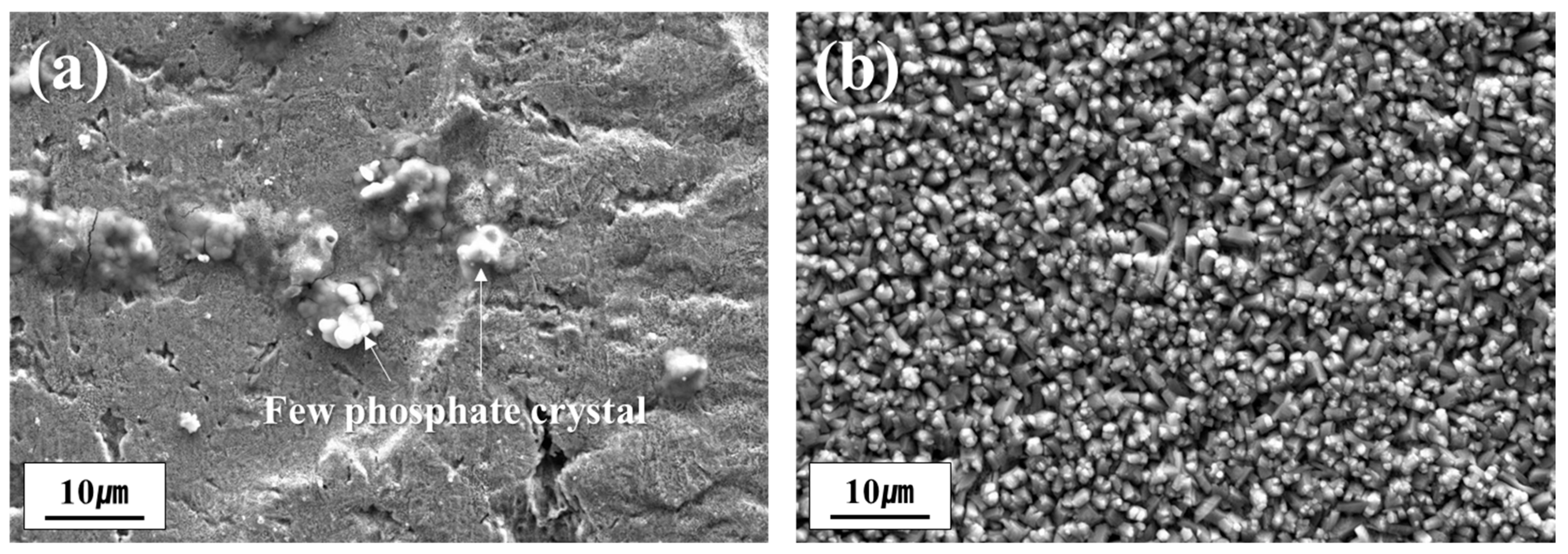
3.2. Influence of Dew Point on the Formation of Phosphate Coating
3.3. Electrochemical Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmitt, J.H.; Iung, T. New developments of advanced high-strength steels for automotive applications. Comptes Rendus Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced lightweight materials for Automobiles: A review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Umehara, S.; Moriya, Y.; Matsushima, Y. Phosphatability of Cold Rolled Steel Sheet for Automotive Bodies. Tetsu-to-Hagané 1982, 68, 720–731. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N. Surface pretreatment by phosphate conversion coatings—A review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Suzuki, Y.; Yamashita, T.; Sugimoto, Y.; Fujita, S.; Yamaguchi, S. Thermodynamic analysis of selective oxidation behavior of Si and Mn-added steel during recrystallization annealing. ISIJ Int. 2009, 49, 564–573. [Google Scholar] [CrossRef]
- Gong, Y.F.; Kim, H.S.; Cooman, B.C.D. Internal oxidation during intercritical annealing of CMnSi TRIP steel. ISIJ Int. 2009, 49, 557–563. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Park, J.; Jeon, S.H. Effect of Si content on wettability of dual phase high strength steels by liquid Zn-0.23 wt.% Al. Met. Mater. Int. 2011, 17, 607–611. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, K.S.; Jeon, S.H.; Chin, K.G.; Lee, J. The influence of the dew point on the wettability of twinning-induced-plasticity steels by liquid Zn–0.23-wt% Al. Corros. Sci. 2014, 85, 364–371. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Shin, K.S.; Jeon, S.H.; Chin, K.G. Effect of dew point on the formation of surface oxides of twinning-induced plasticity steel. Mater. Charact. 2014, 89, 138–145. [Google Scholar] [CrossRef]
- Story, M.E.; Webler, B.A. Effect of surface microstructure on oxidation of a CMnSi advanced high strength steel. ISIJ Int. 2017, 57, 1468–1475. [Google Scholar] [CrossRef]
- Pourmajidian, M.; Mcdermid, J.R. Effect of annealing temperature on the selective oxidation and reactive wetting of a 0.1 C-6Mn-2Si advanced high strength steel during continuous galvanizing heat treatments. ISIJ Int. 2018, 58, 1635–1643. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J. Effect of water pressure and soaking time on the selective oxidation of DP980 advanced high strength steel. Appl. Surf. Sci. 2018, 453, 252–262. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S. Influence of various factors on phosphatability—An overview. Met. Finish. 1996, 94, 86. [Google Scholar] [CrossRef]
- Maeda, S.; Asai, T.; Arai, S.; Suzuki, K. Phosphatability and surface characteristics of silicon-manganese dual phase steel. Tetsu-to-Hagané 1982, 68, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Hashimoto, I.; Kozuma, S.; Kamura, M.; Omiya, Y. Effects of surface oxides on the phosphatability of the high strength cold rolled steels. Tetsu-to-Hagané 2006, 92, 378–384. [Google Scholar] [CrossRef]
- Masuoka, H.; Yamamoto, S.; Taira, S.; Ando, S.; Yoshimi, N. Effects of the Surface State on Phosphatability for High Strength Cold-Rolled Steel Sheets. J. Surf. Finish. Soc. Jpn. 2015, 66, 372–376. [Google Scholar] [CrossRef]
- Cho, S.; Ko, S.J.; Yoo, J.S.; Park, J.C.; Yoo, Y.H.; Kim, J.G. Optimization of pickling solution for improving the phosphatability of advanced high-strength steels. Materials 2021, 14, 233. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, Y.; Dai, Y.; Li, J.; Shi, J.; Gao, X.; Xu, H.; Yu, K.; Luo, W. The influence of silicon on the formation of phosphate coatings for low-carbon IF steels. Surf. Coat. Technol. 2022, 441, 128599. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.; Joe, J.D.; Jung, Y.; Song, Y.; Lee, J.S.; Heo, Y.U. Enhanced phosphatability by decorating ferrite layer on the surface of a multi-phase steel. Mater. Charact. 2022, 194, 112373. [Google Scholar] [CrossRef]
- Furuya, S.; Chiba, T.; Mizuno, D. Effect of B on Surface Oxidation Behavior and Phosphatability of Si–Mn-added Cold-Rolled Steel Sheets. Tetsu-to-Hagané 2025, 111, 275–287. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Heo, Y.U.; Lee, J. Effect of dew point during annealing on phosphatability of ultra-high-strength steel. J. Mater. Res. Technol. 2024, 33, 8564–8572. [Google Scholar] [CrossRef]
- Sankar, M.; Baligidad, R.G.; Satyanarayana, D.V.V.; Gokhale, A.A. Effect of internal oxidation on the microstructure and mechanical properties of C-103 alloy. Mater. Sci. Eng. A 2013, 574, 104–112. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Bellhouse, E.M.; Korinek, A.; Bugnet, M.; McDermid, J.R. XPS and EELS characterization of Mn2SiO4, MnSiO3 and MnAl2O4. Appl. Surf. Sci. 2016, 379, 242–248. [Google Scholar] [CrossRef]
- Swaminathan, S.; Spiegel, M. Thermodynamic and kinetic aspects on the selective surface oxidation of binary, ternary and quarternary model alloys. Appl. Surf. Sci. 2007, 253, 4607–4619. [Google Scholar] [CrossRef]
- Tegehall, P.E.; Vannerberg, N.G. Nucleation and formation of zinc phosphate conversion coating on cold-rolled steel. Corros. Sci. 1991, 32, 635–652. [Google Scholar] [CrossRef]
- Huin, D.; Flauder, P.; Leblond, J.-B. Numerical simulation of internal oxidation of steels during annealing treatments. Oxid. Met. 2005, 64, 131–167. [Google Scholar] [CrossRef]
- Ghali, E.I.; Potvin, R.J.A. The mechanism of phosphating of steel. Corros. Sci. 1972, 12, 583–594. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Z.; Pan, H.; Cheng, X. The initiation and formation of a double-layer phosphate conversion coating on steel. Electrochem. Commun. 2020, 114, 106676. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Zheng, W.; Wang, H.; Zhang, Y.; Li, L. Effect of hot-dip galvanizing process on selective oxidation and galvanizability of medium manganese steel for automotive application. Coatings 2020, 10, 1265. [Google Scholar] [CrossRef]
- Gaderbauer, W.; Arndt, M.; Truglas, T.; Steck, T.; Klingner, N.; Stifter, D.; Faderl, J.; Groiss, H. Effects of alloying elements on surface oxides of hot–dip galvanized press hardened steel. Surf. Coat. Technol. 2020, 404, 126466. [Google Scholar] [CrossRef]
- Minenkov, A.; Mörtlbauer, T.; Arndt, M.; Hesser, G.; Angeli, G.; Groiss, H. Towards a dependable TEM characterization of hot-dip galvanized steels with low and high Si content. Mater. Des. 2023, 227, 111684. [Google Scholar] [CrossRef]


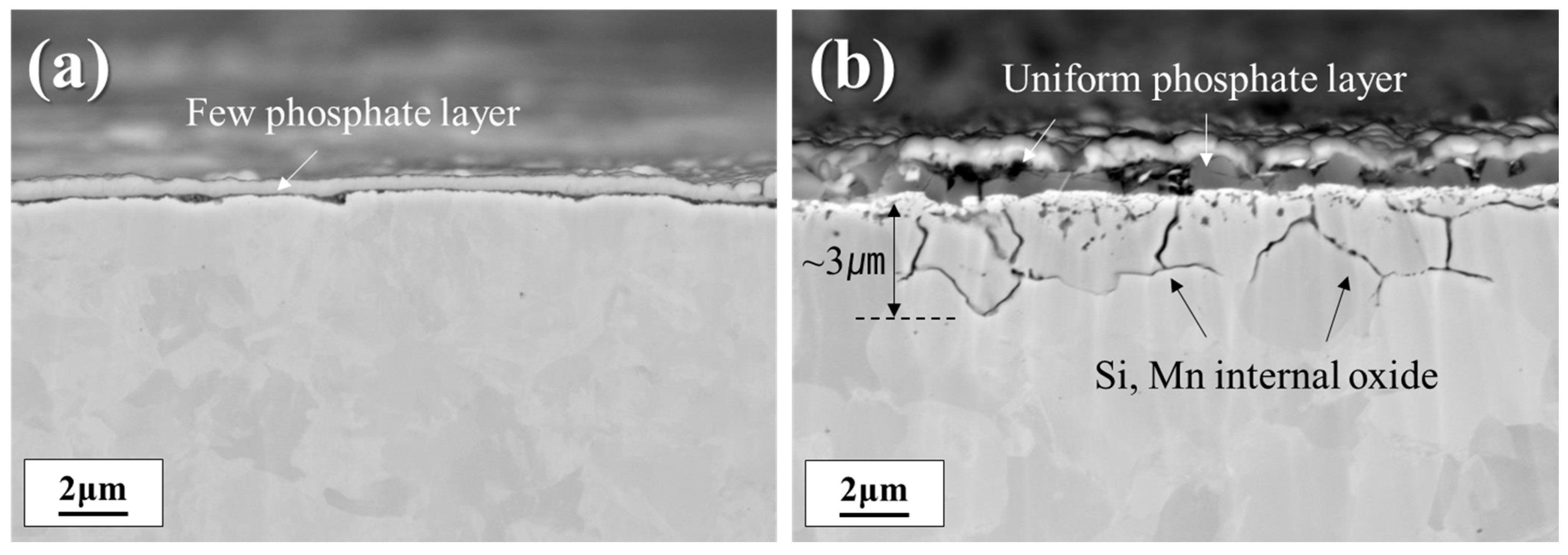

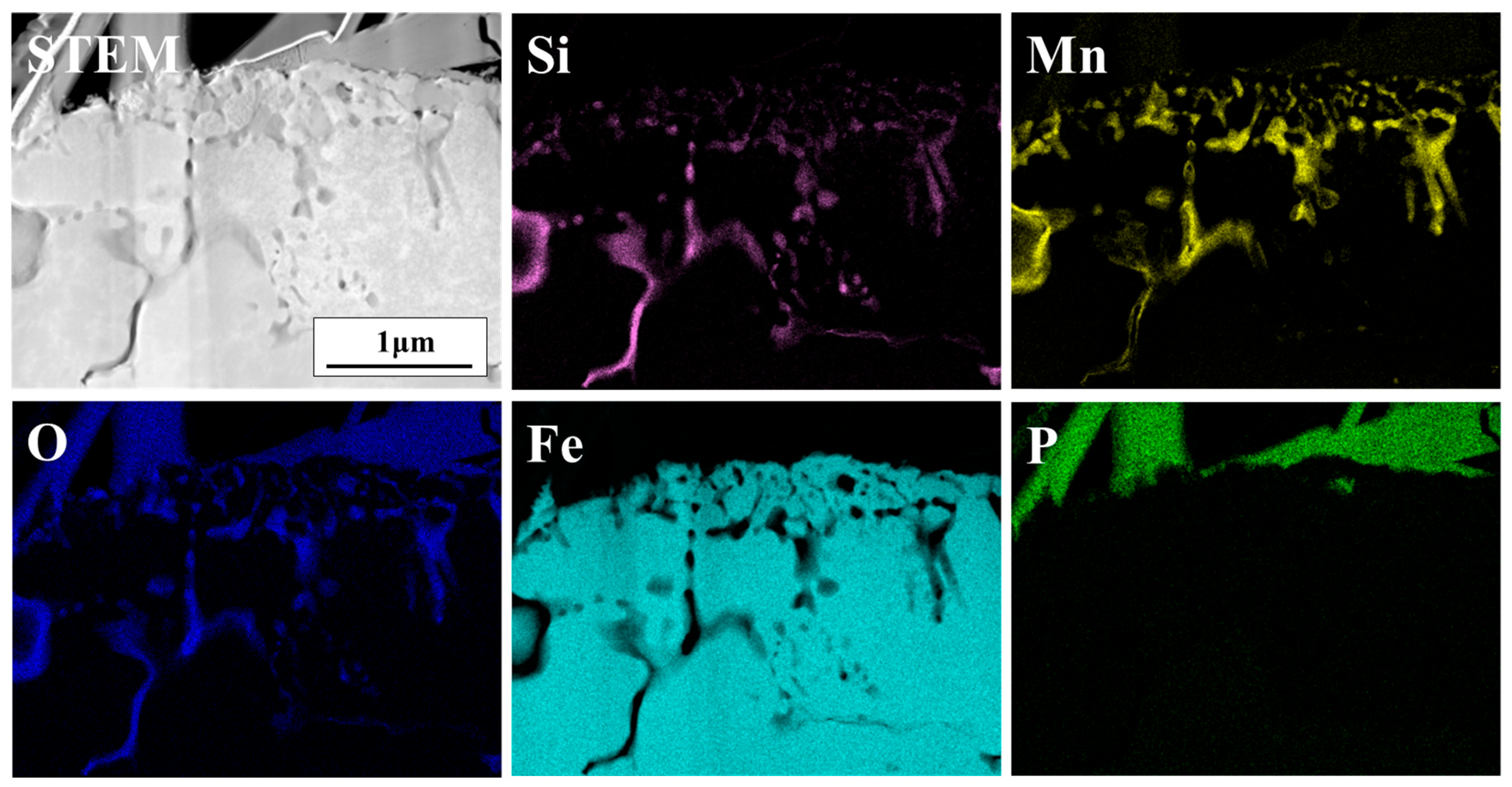
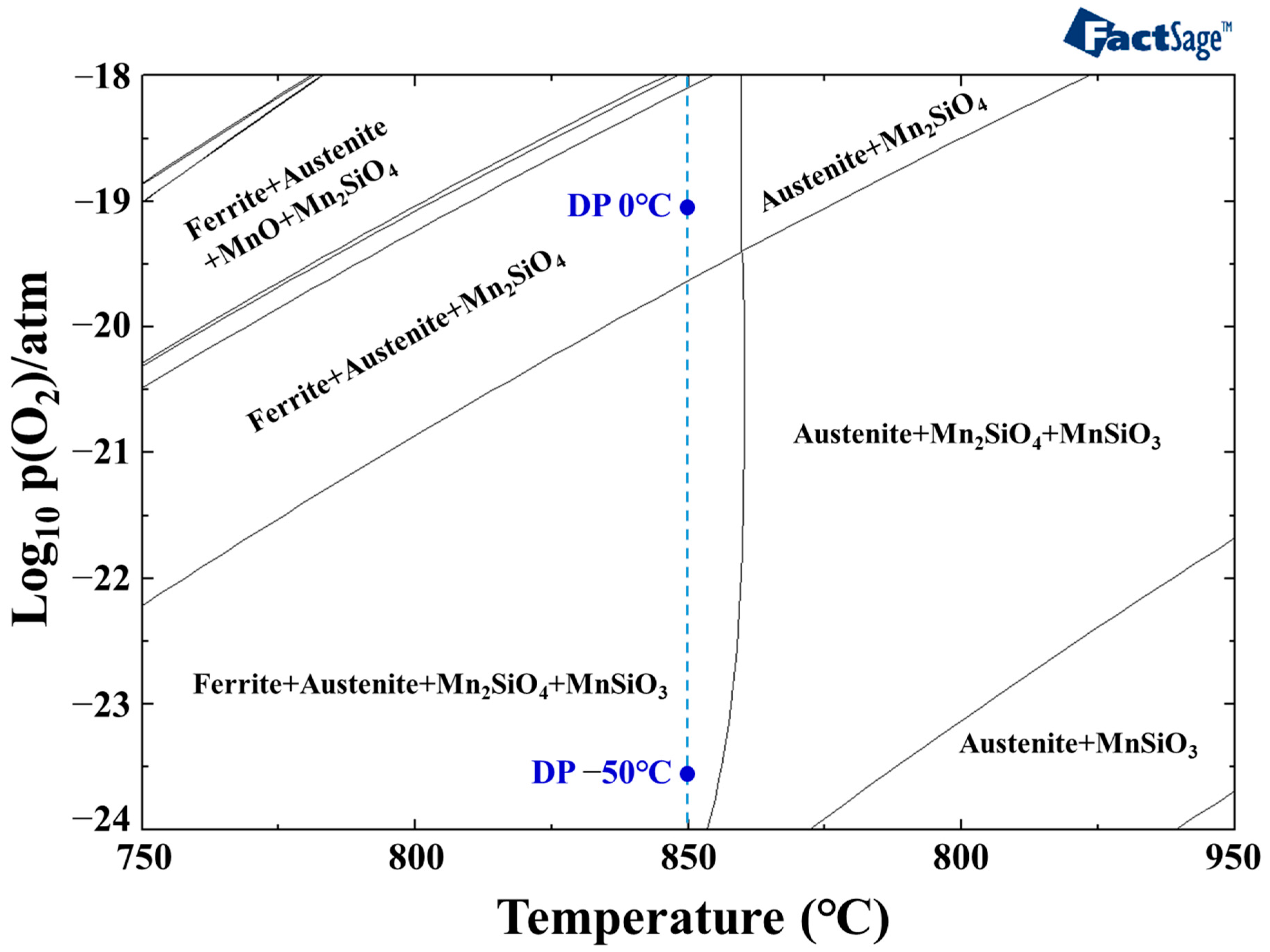


| Sample | Chemical Analysis Results (wt pct) | Annealing Condition | ||||
|---|---|---|---|---|---|---|
| C | Si | Mn | P | Al | ||
| DP-50 | 0.12 | 1.0 | 2.35 | 0.01 | 0.03 | Dew point −50 °C |
| DP0 | 0.12 | 1.0 | 2.35 | 0.01 | 0.03 | Dew point 0 °C |
| DP 0 °C | DP −50 °C | |
|---|---|---|
| (atm) | 6.026 × 10−3 | 3.895 × 10−5 |
| (atm) | 7.181 × 10−20 | 3.001 × 10−24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, J. Influence of Annealing Atmosphere on the Phosphatability of Ultra-High-Strength Automotive Steels. Materials 2025, 18, 3170. https://doi.org/10.3390/ma18133170
Park J, Lee J. Influence of Annealing Atmosphere on the Phosphatability of Ultra-High-Strength Automotive Steels. Materials. 2025; 18(13):3170. https://doi.org/10.3390/ma18133170
Chicago/Turabian StylePark, Joongchul, and Joonho Lee. 2025. "Influence of Annealing Atmosphere on the Phosphatability of Ultra-High-Strength Automotive Steels" Materials 18, no. 13: 3170. https://doi.org/10.3390/ma18133170
APA StylePark, J., & Lee, J. (2025). Influence of Annealing Atmosphere on the Phosphatability of Ultra-High-Strength Automotive Steels. Materials, 18(13), 3170. https://doi.org/10.3390/ma18133170








