Biofunctional Magnetic Carbon Nanohybrid for Fast Removal of Methyl Blue from Synthetic Laboratory Effluent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation and Adsorbent’s Biosynthesis
2.3. Characterization
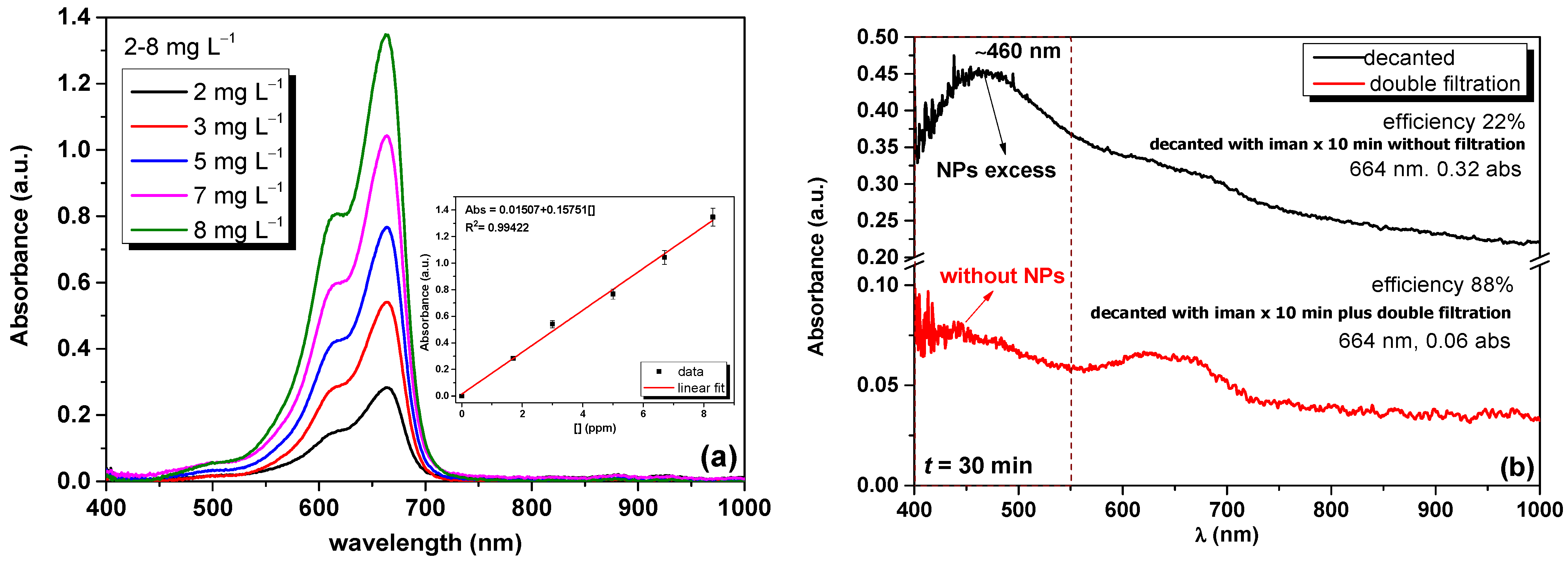
2.4. MB Adsorption Experiments
2.5. Preparation of the Synthetic MB Effluent
3. Results and Discussion
3.1. Structural, Colloidal, Surface, and Magnetic Analysis
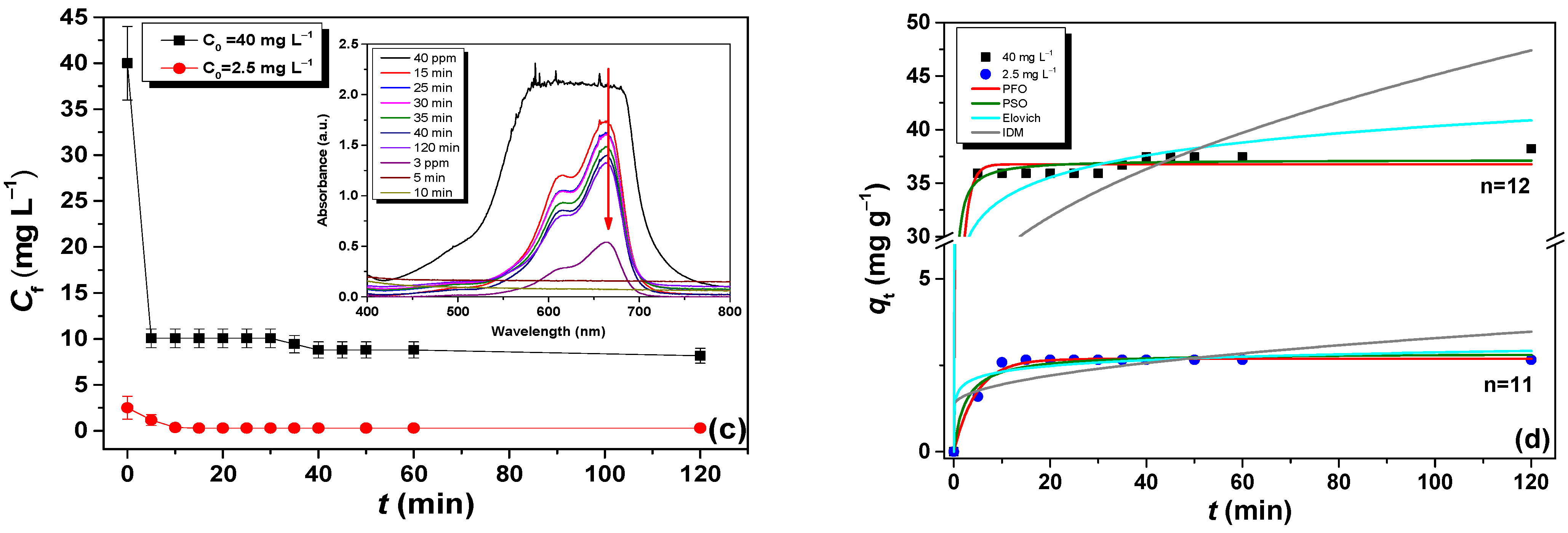
3.2. Kinetic Adsorption Results
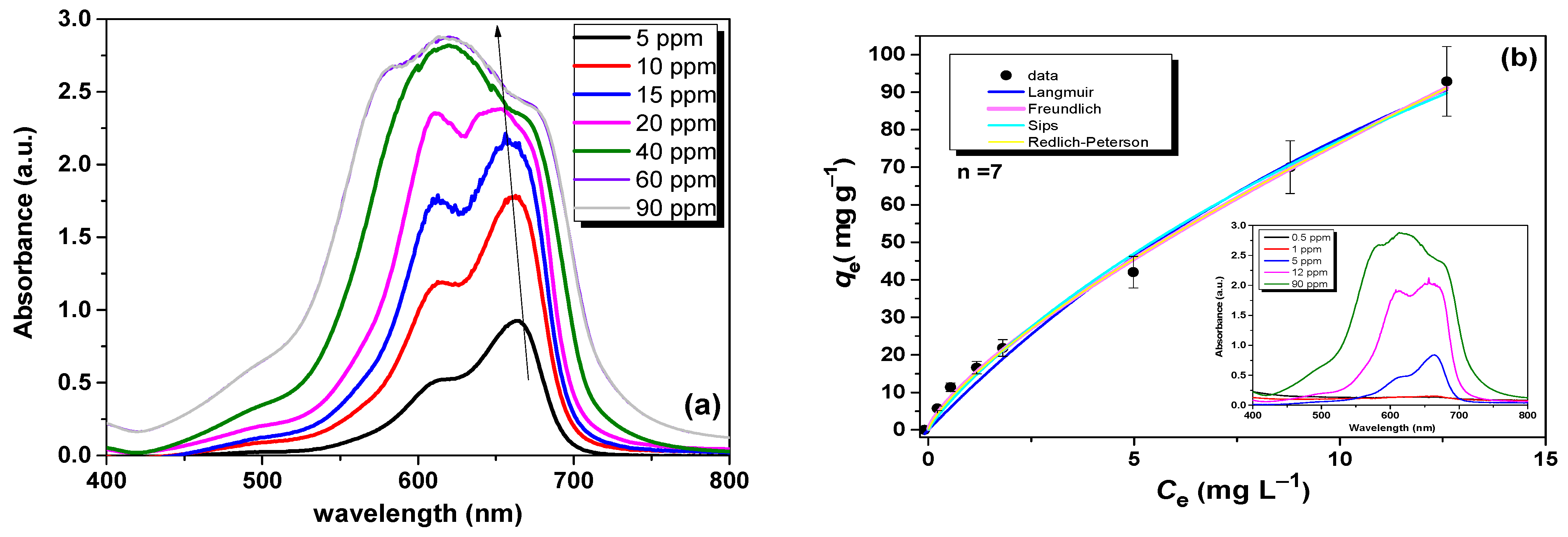
3.3. Adsorption Isotherm Analysis
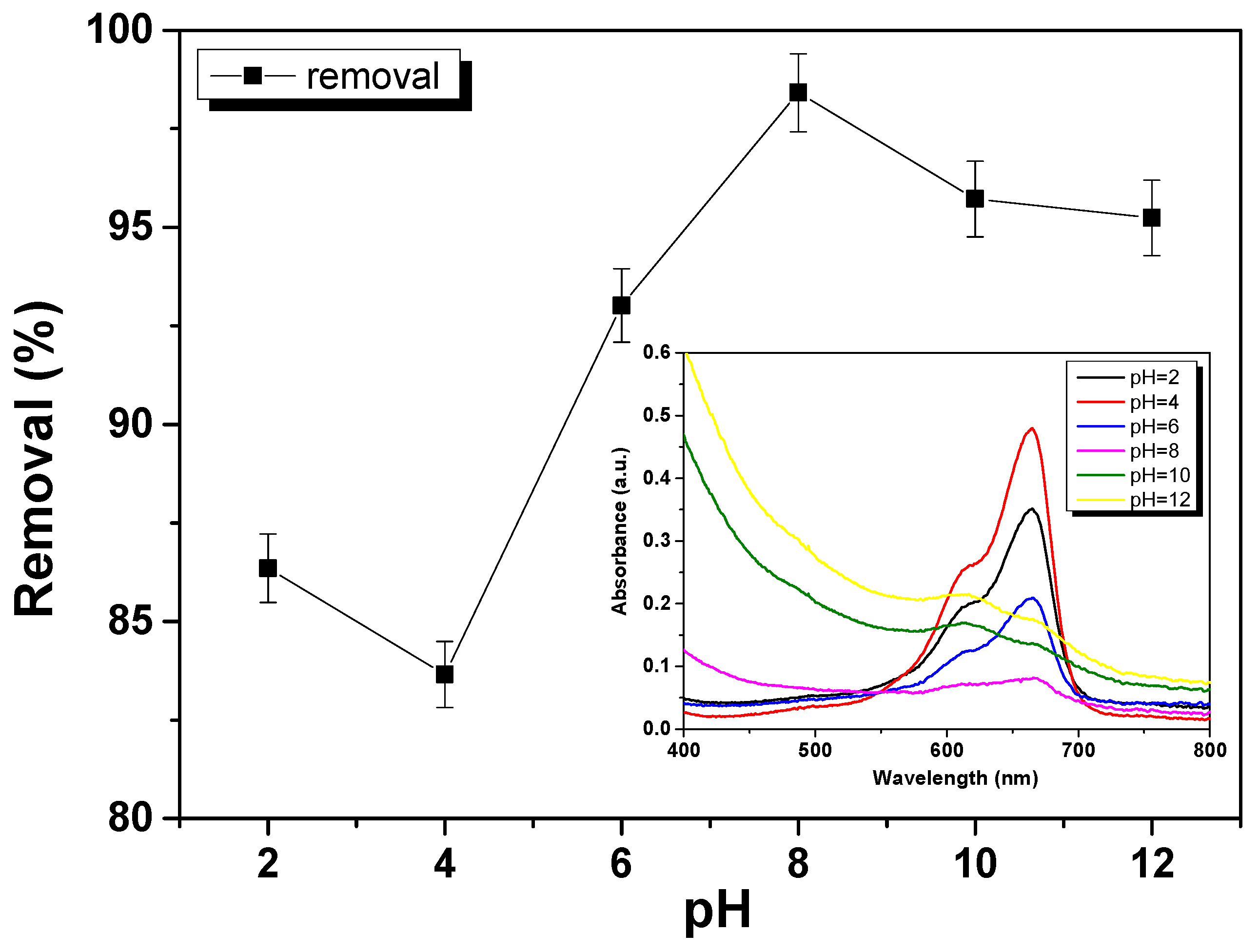
3.4. Effect of pH
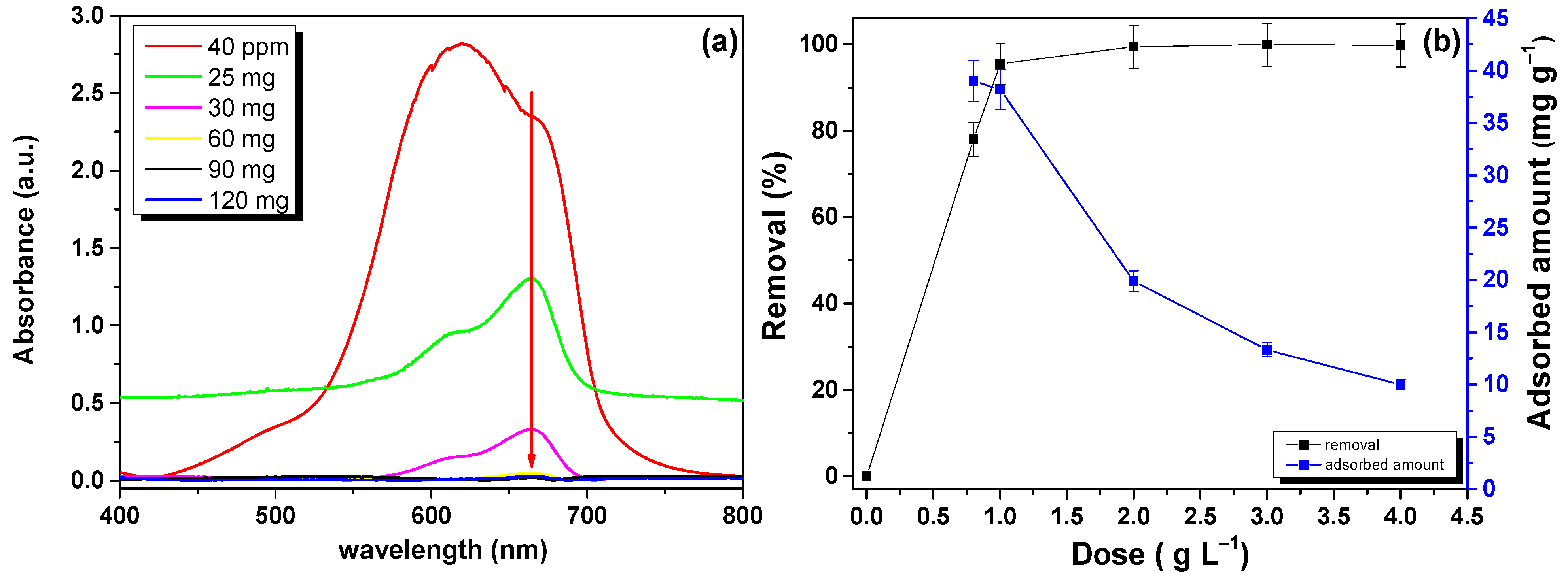
3.5. Adsorbent Dose Effect
3.6. Comparison of Adsorption Parameters with Other Adsorbents
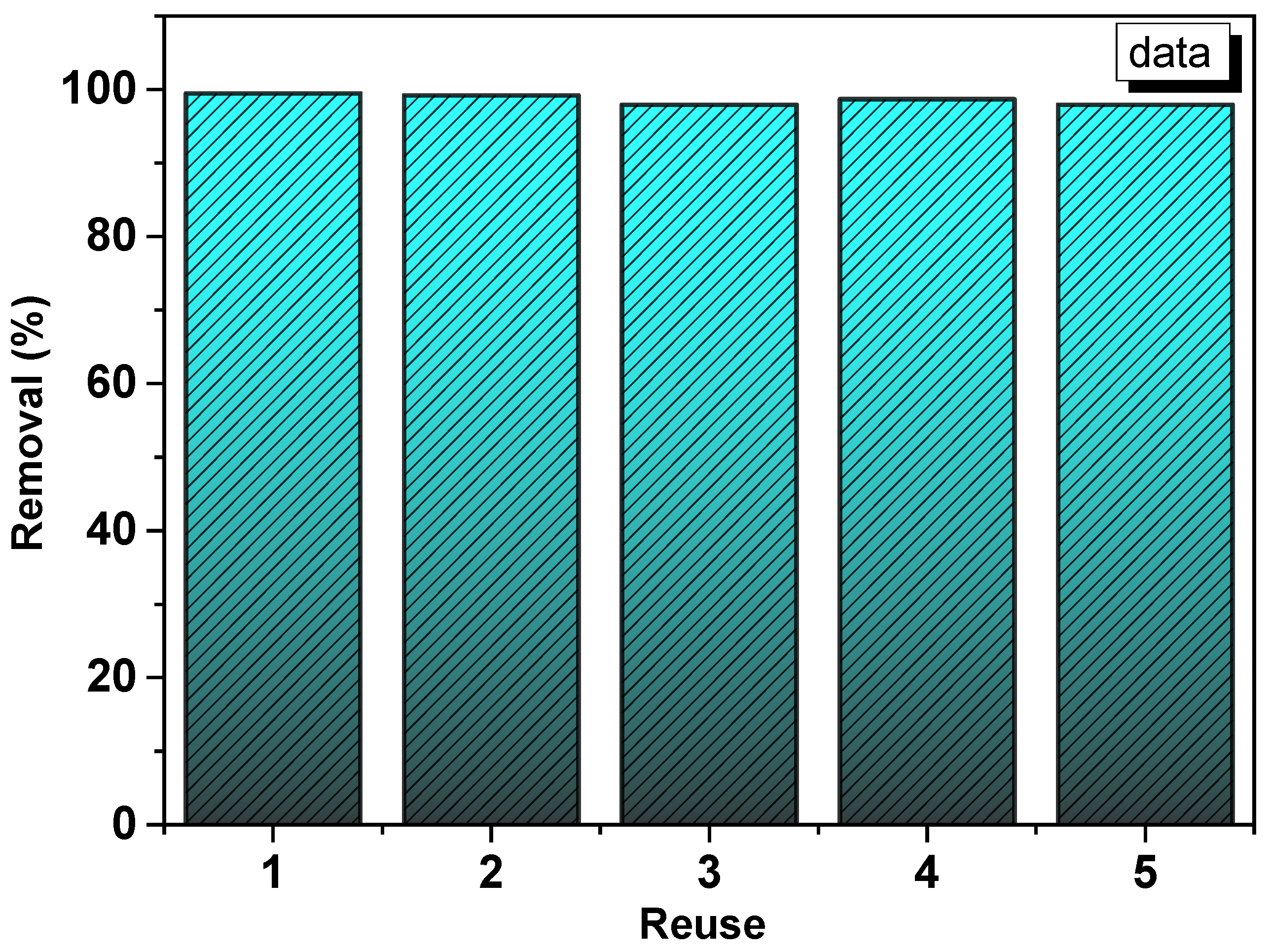
3.7. Reuse Experiments
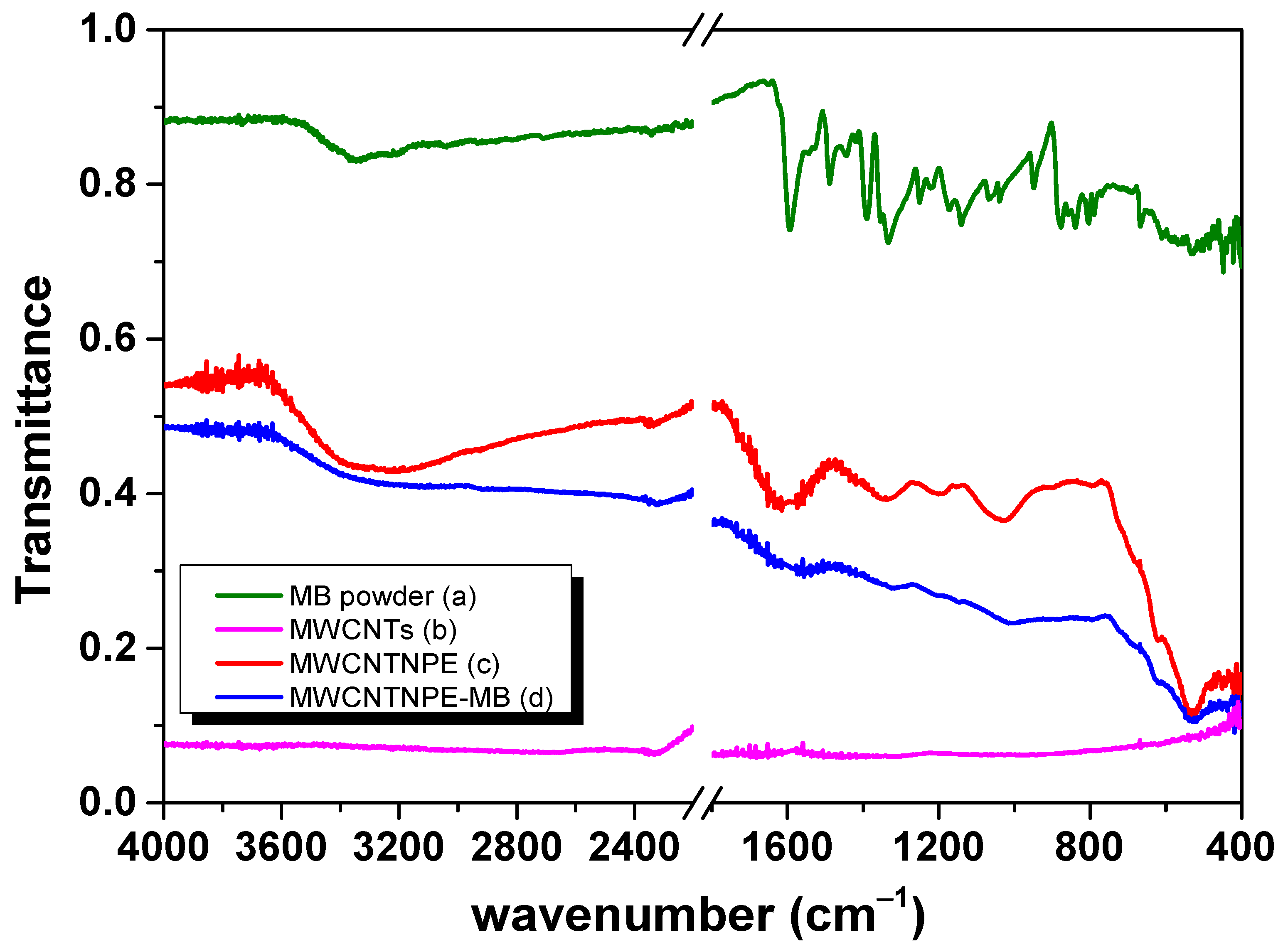
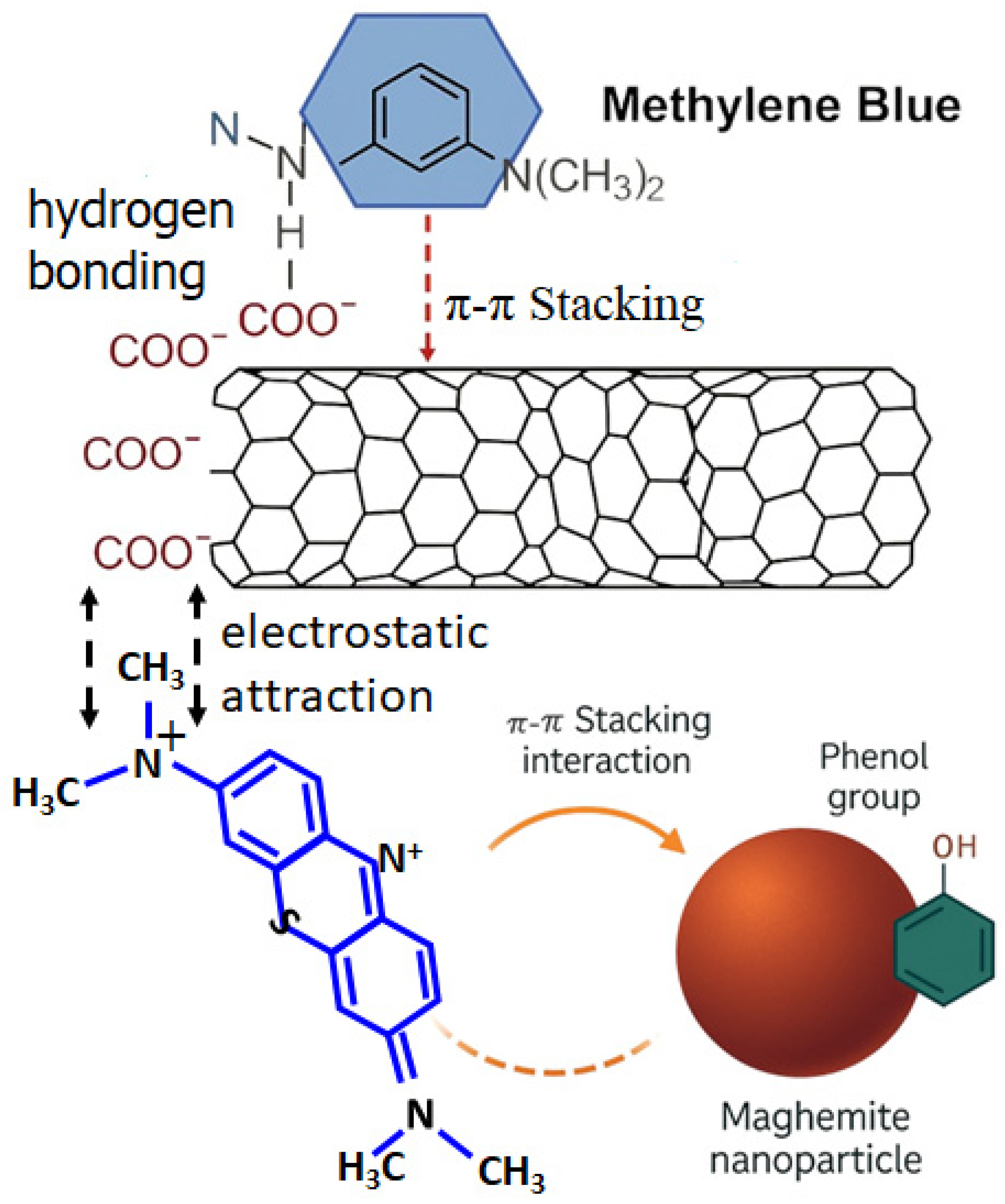
3.8. FTIR Adsorption Mechanism
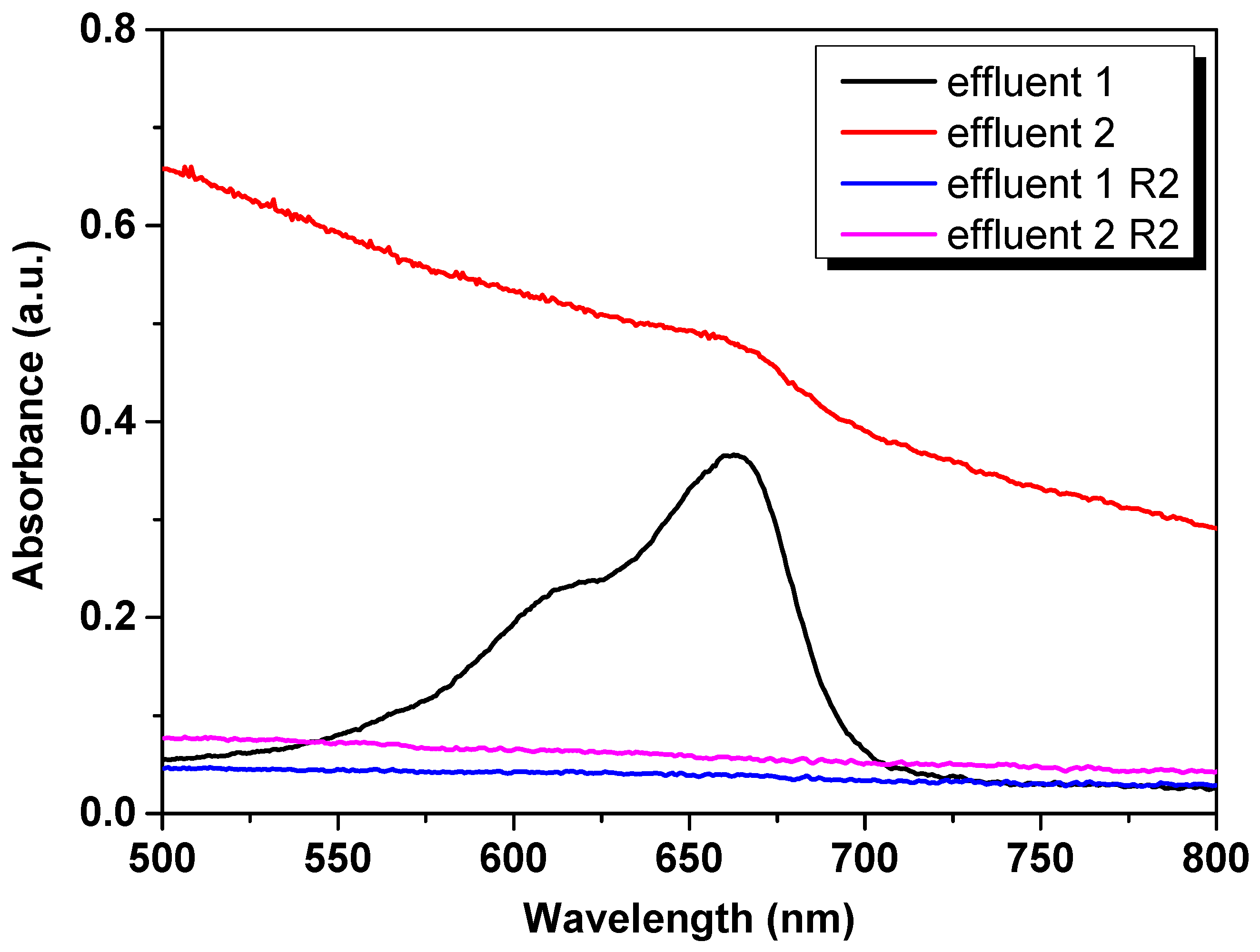
3.9. Application in a Real Effluent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Şentürk, İ.; Keklikcioğlu Çakmak, N. Use of Magnetic Nanoparticle Loaded Functionalized Multi-Walled Carbon Nanotubes for Effective Removal of Maxilon Red GRL from Aqueous Solutions. J. Iran. Chem. Soc. 2025, 22, 141–159. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Gupta, R.; Singh, R.L. Treatment and Recycling of Wastewater from Textile Industry. In Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future; Springer: Singapore, 2019; pp. 225–266. [Google Scholar]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The Utilization of Leaf-Based Adsorbents for Dyes Removal: A Review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential Candidates in Wastewater Treatment Technology—A Review on the Removal of Methylene Blue Dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Miclescu, A.A.; Wiklund, L. Methylene Blue, an Old Drug with New Indications? J. Rom. Anest. Terap. Int. 2010, 17, 35–41. [Google Scholar]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of Basic Dye Using Activated Carbon Prepared from Oil Palm Shell: Batch and Fixed Bed Studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute Toxicity of Textile Dye Methylene Blue on Growth and Metabolism of Selected Freshwater Microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on Recent Advances of Carbon Based Adsorbent for Methylene Blue Removal from Waste Water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water purification from chlorobenzenes using heteroatom-functionalized carbon nanofibers produced on self-organizing Ni-Pd catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Baratta, M.; Nezhdanov, A.V.; Mashin, A.I.; Nicoletta, F.P.; De Filpo, G. Carbon Nanotubes Buckypapers: A New Frontier in Wastewater Treatment Technology. Sci. Total Environ. 2024, 924, 171578. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Suhas Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Maghemite/Alginate/Functionalized Multiwalled Carbon Nanotubes Beads for Methylene Blue Removal: Adsorption and Desorption Studies. J. Mol. Liq. 2019, 275, 431–440. [Google Scholar] [CrossRef]
- Farghali, A.A.; Bahgat, M.; ElRouby, W.M.A.; Khedr, M.H. Decoration of Multi-Walled Carbon Nanotubes (MWCNTs) with Different Ferrite Nanoparticles and Its Use as an Adsorbent. J. Nanostructure Chem. 2013, 3, 50. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Pehlivan, E. Methylene Blue Removal Using Nano-TiO2/MWCNT/Chitosan Hydrogel Composite Beads in Aqueous Medium. Chemosphere 2024, 365, 143244. [Google Scholar] [CrossRef]
- Alca-Ramos, Y.V.; Checca-Huaman, N.-R.; Rueda-Vellasmin, R.; Passamani, E.C.; Ramos-Guivar, J.A. Biosynthesis Scale-Up Process for Magnetic Iron-Oxide Nanoparticles Using Eucalyptus globulus Extract and Their Separation Properties in Lubricant–Water Emulsions. Nanomaterials 2025, 15, 382. [Google Scholar] [CrossRef]
- de Keijser, T.H.; Langford, J.I.; Mittemeijer, E.J.; Vogels, A.B.P. Use of the Voigt Function in a Single-Line Method for the Analysis of X-Ray Diffraction Line Broadening. J. Appl. Crystallogr. 1982, 15, 308–314. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2004; Volume 16, ISBN 978-90-481-6633-6. [Google Scholar]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suárez, G. Surface Modification of Multiwall Carbon Nanotubes by Sulfonitric Treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brown, W.F. Theory of the Approach to Magnetic Saturation. Phys. Rev. 1940, 58, 736–743. [Google Scholar] [CrossRef]
- Phong, P.T.; Oanh, V.T.K.; Lam, T.D.; Phuc, N.X.; Tung, L.D.; Thanh, N.T.K.; Manh, D.H. Iron Oxide Nanoparticles: Tunable Size Synthesis and Analysis in Terms of the Core–Shell Structure and Mixed Coercive Model. J. Electron. Mater. 2017, 46, 2533–2539. [Google Scholar] [CrossRef]
- Obaidat, I.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.; Gismelseed, A. Investigating Exchange Bias and Coercivity in Fe3O4–γ-Fe2O3 Core–Shell Nanoparticles of Fixed Core Diameter and Variable Shell Thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structures, Properties, Reactions, Occurences and Uses, 2nd ed.; WILEY-VCH GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Nayek, C.; Manna, K.; Bhattacharjee, G.; Murugavel, P.; Obaidat, I. Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles. Magnetochemistry 2017, 3, 19. [Google Scholar] [CrossRef]
- Manrique-Castillo, E.V.; Marcos-Carrillo, M.d.P.; Checca-Huaman, N.-R.; Santos, B.L.D.; Macedo, W.A.A.; Barrero Meneses, C.A.; Passamani, E.C.; Greneche, J.-M.; Ramos-Guivar, J.A. Fast Kinetic Response and Efficient Removal of Methyl Blue and Methyl Green Dyes by Functionalized Multiwall Carbon Nanotubes Powered with Iron Oxide Nanoparticles and Citrus Reticulata Peel Extract. Nanomaterials 2025, 15, 603. [Google Scholar] [CrossRef]
- Mulushewa, Z.; Dinbore, W.T.; Ayele, Y. Removal of Methylene Blue from Textile Waste Water Using Kaolin and Zeolite-x Synthesized from Ethiopian Kaolin. Environ. Anal. Health Toxicol. 2021, 36, e2021007. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of Methylene Blue from Textile Industrial Wastewater Using Activated Carbon Developed from Rumex Abyssinicus Plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Marbán, G. Visible Light Spectroscopic Analysis of Methylene Blue in Water; What Comes after Dimer? ACS Omega 2020, 5, 29801–29815. [Google Scholar] [CrossRef]
- Lima, E.C.; Sher, F.; Guleria, A.; Saeb, M.R.; Anastopoulos, I.; Tran, H.N.; Hosseini-Bandegharaei, A. Is One Performing the Treatment Data of Adsorption Kinetics Correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
- Qin, N.; Tian, C.; Carter, L.; Tao, D.; Zhou, Y.; Zhang, F. Economical Magnetic Activated Carbon for Methylene Blue Removal from Water. Next Sustain. 2024, 4, 100057. [Google Scholar] [CrossRef]
- Hkiri, K.; Mohamed, H.E.A.; Abodouh, M.M.; Maaza, M. Experimental and Theoretical Insights into the Adsorption Mechanism of Methylene Blue on the (002) WO3 Surface. Sci. Rep. 2024, 14, 26991. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Asgari, E.; Danafar, H.; Salehabadi, H.; Fazli, M.M. A Comprehensive Study on Methylene Blue Removal via Polymer and Protein Nanoparticle Adsorbents. Sci. Rep. 2024, 14, 29434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Tao, Y.; Zha, L. Study on the Adsorption Characteristics of Methylene Blue by Magnesium-Modified Fly Ash. Molecules 2025, 30, 992. [Google Scholar] [CrossRef]
- Nas, M.S.; Kuyuldar, E.; Demirkan, B.; Calimli, M.H.; Demirbaş, O.; Sen, F. Magnetic Nanocomposites Decorated on Multiwalled Carbon Nanotube for Removal of Maxilon Blue 5G Using the Sono-Fenton Method. Sci. Rep. 2019, 9, 10850. [Google Scholar] [CrossRef]
- do Nascimento Moraes Calazans, T.R.; de Lima Barizão, A.C.; de Andrade Silva, T.; Campos, F.V.; Cassini, S.T.A.; Nardy Ribeiro, A.V.F.; de Godoi Pereira, M.; Cunegundes Guimarães, M.C.; de Oliveira, J.P.; Ribeiro, J.N. Methylene Blue Removal Using a Nanomagnetic Support: A Response Surface Approach. Nanoscale Adv. 2024, 6, 3887–3894. [Google Scholar] [CrossRef]
- Allou, N.B.; Tigori, M.A.; Koffi, A.A.; Halidou, M.; Eroi, N.S.; Atheba, P.; Trokourey, A. Methylene Blue Magnetic Adsorption Separation Process from Aqueous Solution Using Corn Cob. Sci. Afr. 2023, 21, e01828. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C61734&Mask=80 (accessed on 11 June 2025).
- Alshehri, A.A.; Malik, M.A. Biogenic Fabrication of ZnO Nanoparticles Using Trigonella Foenum-Graecum (Fenugreek) for Proficient Photocatalytic Degradation of Methylene Blue under UV Irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 16156–16173. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Sadrollahi, E.; Menzel, D.; Ramos Fernandes, E.G.; López, E.O.; Torres, M.M.; Arsuaga, J.M.; Arencibia, A.; Litterst, F.J. Magnetic, Structural and Surface Properties of Functionalized Maghemite Nanoparticles for Copper and Lead Adsorption. RSC Adv. 2017, 7, 28763–28779. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S.; Soga, T. Synthesis of Iron Oxide Nanoparticles by Using Eucalyptus Globulus Plant Extract. e-J. Surf. Sci. Nanotechnol. 2014, 12, 363–367. [Google Scholar] [CrossRef]
- Salgado, P.; Aedo, E.; Vidal, G. Green Synthesis of Fe2O3 Nanoparticles Using Eucalyptus Globulus Leaf Extract on Pinus Radiata Sawdust for Cationic Dye Adsorption. Nanomaterials 2024, 14, 1832. [Google Scholar] [CrossRef]
- Krestinin, A.V.; Kharitonov, A.P.; Shul’ga, Y.M.; Zhigalina, O.M.; Knerel’man, E.I.; Dubois, M.; Brzhezinskaya, M.M.; Vinogradov, A.S.; Preobrazhenskii, A.B.; Zvereva, G.I.; et al. Fabrication and characterization of fluorinated single-walled carbon nanotubes. Nanotechnol. Russ. 2009, 4, 60–78. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Shnitov, V.V.; Brzhezinskaya, M.; Baidakova, M.V.; Stolyarova, D.Y.; Ryzhkov, S.A.; Saveliev, S.D.; Shvidchenko, A.V.; Nefedov, D.Y.; Antonenko, A.O.; et al. Manifesting Epoxide and Hydroxyl Groups in XPS Spectra and Valence Band of Graphene Derivatives. Nanomaterials 2023, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Ghazal, A.; Khan, J.; Ahmad, P.; Khandaker, M.U.; Osman, H.; Alamri, S. Eucalyptus Globulus Extract-Assisted Fabrication of Copper Oxide/Zinc Oxide Nanocomposite for Photocatalytic Applications. Crystals 2022, 12, 1153. [Google Scholar] [CrossRef]
- Guel-Nájar, N.A.; Rios-Hurtado, J.C.; Muzquiz-Ramos, E.M.; Dávila-Pulido, G.I.; González-Ibarra, A.A.; Pat-Espadas, A.M. Magnetic Biochar Obtained by Chemical Coprecipitation and Pyrolysis of Corn Cob Residues: Characterization and Methylene Blue Adsorption. Materials 2023, 16, 3127. [Google Scholar] [CrossRef]
- Stegarescu, A.; Cabrera, H.; Budasheva, H.; Soran, M.-L.; Lung, I.; Limosani, F.; Korte, D.; Amati, M.; Borodi, G.; Kacso, I.; et al. Synthesis and Characterization of MWCNT-COOH/Fe3O4 and CNT-COOH/Fe3O4/NiO Nanocomposites: Assessment of Adsorption and Photocatalytic Performance. Nanomaterials 2022, 12, 3008. [Google Scholar] [CrossRef]
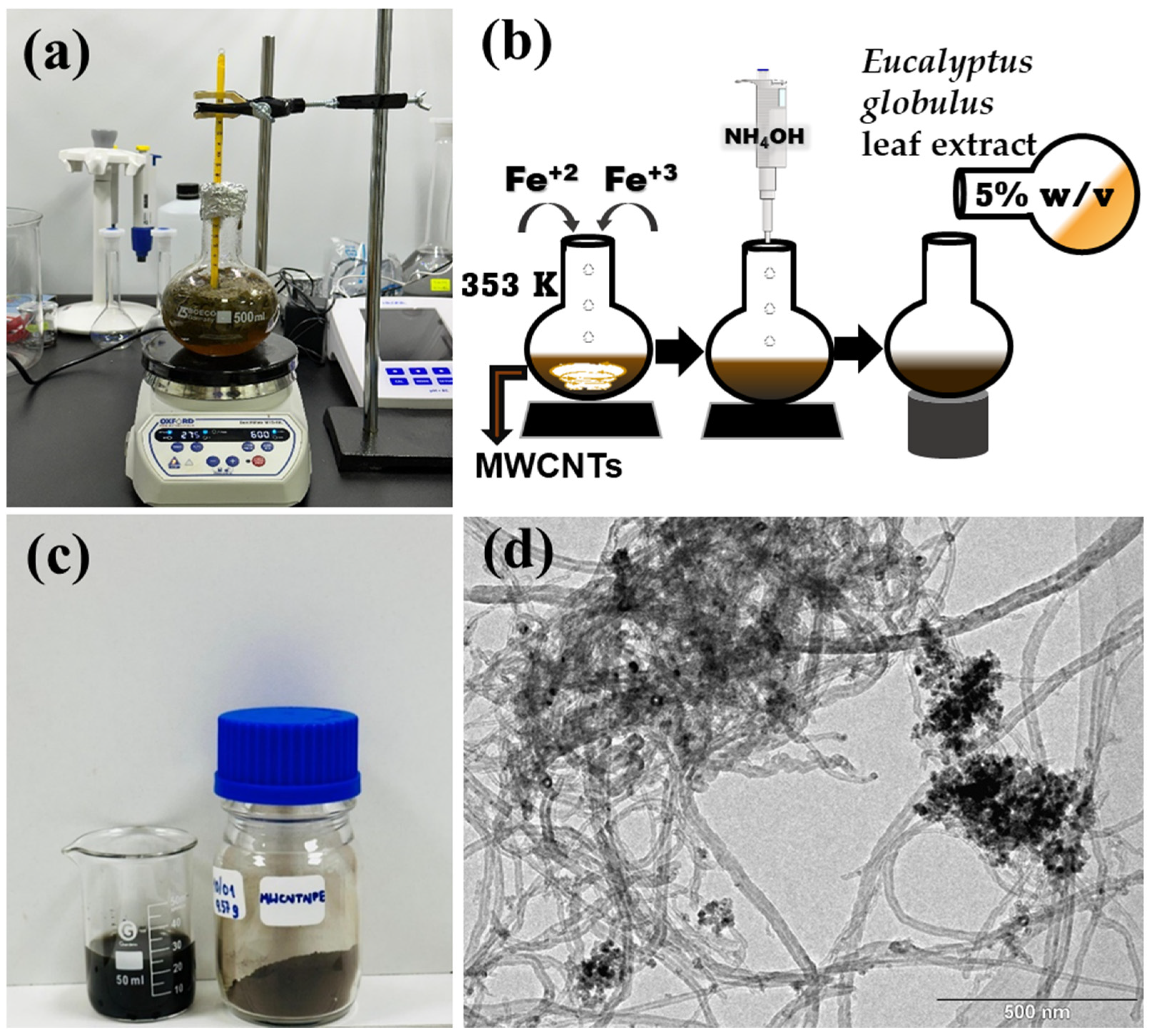
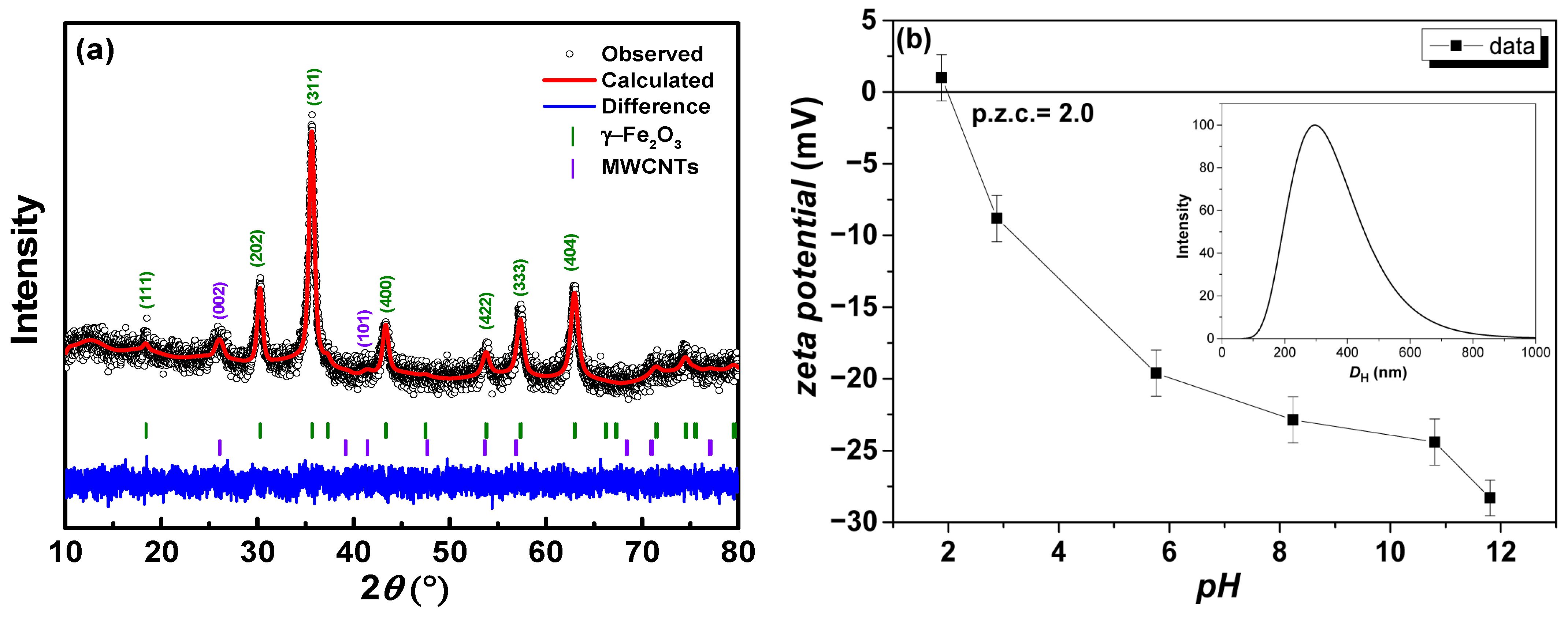
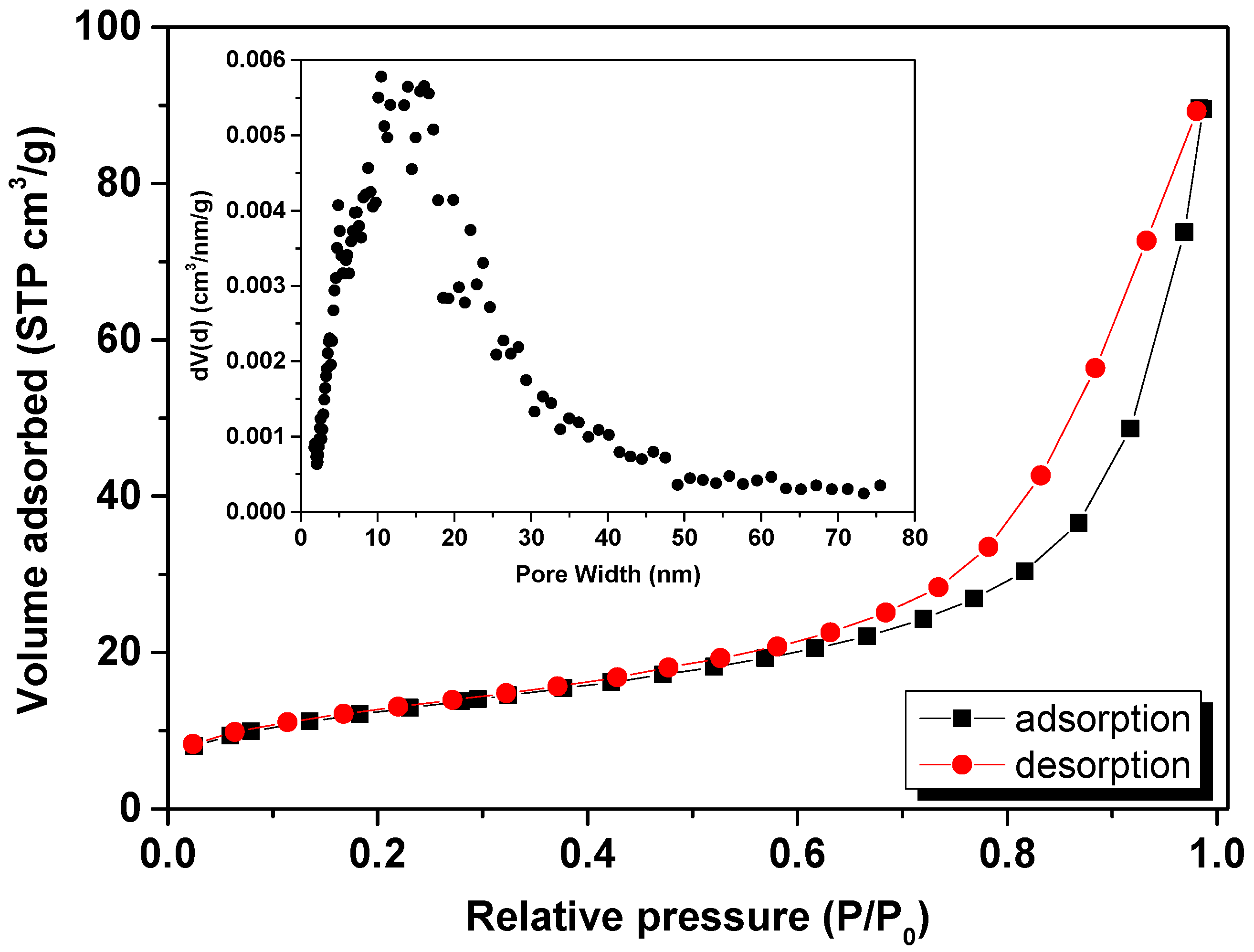
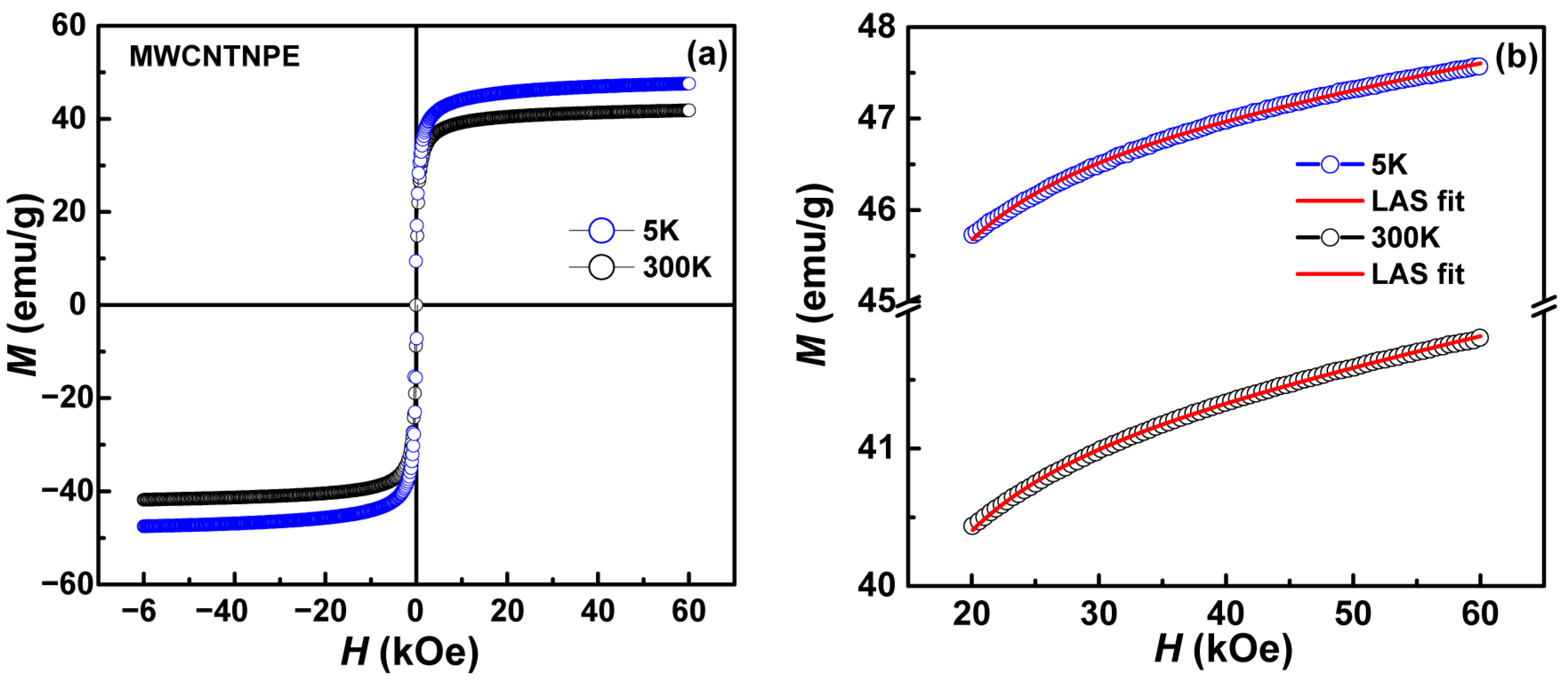
| Phase | Atoms | Atomic Positions | Occ. | Lattice Parameters (nm) | Cagliotti Coefficients | Mean Size ± 1 (nm) | Statistical Parameters | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
86% | Fe-tet | 0.1250 | 0.1250 | 0.1250 | 0.0386 | a = b = c = 0.8348 α = β = γ = 90° | U = 0.2184 V = −0.0713 W = 0.1240 | 22 | Rp = 60.5% Rwp = 28.6% Rexp = 27.4% = 1.09 |
| Fe-oct | 0.5000 | 0.5000 | 0.5000 | 0.0683 | |||||
| O | 0.2500 | 0.2500 | 0.2500 | 0.1730 | |||||
| Carbon 14% | C1 C2 | 0.0000 0.3333 | 0.0000 0.6666 | 0.0000 0.0050 | 1.0000 1.0000 | a = b = 0.2654 c = 0.6834 α = β = γ = 90° | U = 0.8011 V = −0.0891 W = 0.7974 | Not calculated | |
| Sample | T (K) | HC ±0.01 (kOe) | Mr ±0.1 (emu/g) | MS ±0.1 (emu/g) | Keff (×104 J/m3) |
|---|---|---|---|---|---|
| MWNT-NPE | 5 | 0.20 | 12.0 | 46.0 | 2.7 |
| 300 | - | - | 41.0 | 2.1 |
| PFO Model | PSO Model | |
|---|---|---|
| qe (mg g−1) | 2.68(3) × 100 | 2.86(9) × 100 |
| k1 (min−1) | 2.14(2) × 10−1 | k2 = 1.4(4) × 10−1 (g mg−1 min−1) |
| R2 | 0.986 | 0.956 |
| BIC | −19.47 | −13.72 |
| Elovich model | IDM | |
| α | (mg g−1 min−1) | kp = 2(13) × 10−1 (mg g−1 min−1/2) |
| 4.1(1) × 100 (g mg−1) | C = 1.3(4) × 100 (mg g−1) | |
| R2 | 0.910 | 0.425 |
| BIC | −11.72 | 4.179 |
| PFO Model | PSO Model | |
|---|---|---|
| qe (mg g−1) | 3.68(3) × 101 | 3.72(3) × 101 |
| k1 (min−1) | 7.5(2) × 10−1 | k2 = 9.7(4) × 10−2 (g mg−1 min−1) |
| R2 | 0.993 | 0.995 |
| BIC | 0.39 | 4.1 |
| Elovich model | IDM | |
| α | 2.3(0) × 104 (mg g−1 min−1) | kp = 2.4(9) × 100 (mg g−1 min−1/2) |
| 3.35(5) × 10−1 (g mg−1) | 2.1(5) × 101 (mg g−1) | |
| R2 | 0.969 | 0.356 |
| BIC | 37 | 62 |
| Langmuir Model | Freundlich Model | ||
|---|---|---|---|
| qmax (mg g−1) | 2.5(7) × 102 | kF | 1.4(1) × 101 |
| kL (min−1) | 4.6(2) × 10−2 | 1/n | 7.5(3) × 10−1 |
| R2 | 0.986 | R2 | 0.996 |
| BIC | 71.34 | BIC | 61.68 |
| Sips model | Redlich-Peterson | ||
| qs | 3.4(1) × 102 | A | 9.8(0) × 101 |
| Ks | 4.0(0) × 10−2 | B | 6.3(5) × 100 |
| m | 8.7(0) × 10−1 | β | 2.7(4) × 10−1 |
| R2 | 0.992 | R2 | 0.996 |
| BIC | 68.50 | BIC | 68.38 |
| Adsorbent System | Adsorption Capacity (mg g−1) | Removal (%) | pH | Equilibrium Time (min) | Dose (g L−1) | References |
|---|---|---|---|---|---|---|
| Fe3O4/Activated Carbon | 251.3 | Not specified | 6.5 | 60 | 0.1 | [33] |
| WO3 Nanoflakes (green-synthesized) | 78.14 | Not specified | Not specified | 24 h | Not specified | [34] |
| BSA Nanosorbent | 38.52 | 69% | 11 | 60 | 0.5 | [35] |
| Mg@FA (Magnesium-modified fly ash) | 14.34 | 95.61% | 7–11 | 120 | 10 | [36] |
| Fe3O4@MWCNT (sono-Fenton method) | Not specified | >90% | 9 | 120 | 0.02 | [37] |
| MNP-SDS | 19.75 | 79% | 5 | Not specified | 1 | [38] |
| Modified Magnetic Corn Cobs (MCC) | 13.23 | >80% (alkaline pH) | 7.69 | 10 | 4 | [39] |
| MWNT-NPE | 92.9 | 89% | 6 | 40 | 0.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Guivar, J.A.; Mejía-Barraza, M.-A.; Rueda-Vellasmin, R.; Passamani, E.C. Biofunctional Magnetic Carbon Nanohybrid for Fast Removal of Methyl Blue from Synthetic Laboratory Effluent. Materials 2025, 18, 3168. https://doi.org/10.3390/ma18133168
Ramos-Guivar JA, Mejía-Barraza M-A, Rueda-Vellasmin R, Passamani EC. Biofunctional Magnetic Carbon Nanohybrid for Fast Removal of Methyl Blue from Synthetic Laboratory Effluent. Materials. 2025; 18(13):3168. https://doi.org/10.3390/ma18133168
Chicago/Turabian StyleRamos-Guivar, Juan A., Melissa-Alisson Mejía-Barraza, Renzo Rueda-Vellasmin, and Edson C. Passamani. 2025. "Biofunctional Magnetic Carbon Nanohybrid for Fast Removal of Methyl Blue from Synthetic Laboratory Effluent" Materials 18, no. 13: 3168. https://doi.org/10.3390/ma18133168
APA StyleRamos-Guivar, J. A., Mejía-Barraza, M.-A., Rueda-Vellasmin, R., & Passamani, E. C. (2025). Biofunctional Magnetic Carbon Nanohybrid for Fast Removal of Methyl Blue from Synthetic Laboratory Effluent. Materials, 18(13), 3168. https://doi.org/10.3390/ma18133168








