Effect of 3 wt% Cu on the Microstructure and Hardness of a Ti-10Ta-1.6Zr Alloy
Abstract
1. Introduction
2. Modelling and Experimental Procedures
2.1. Computational Modelling of Alloys
2.2. Alloy Preparation
2.3. Sample Preparation
2.4. XRD and Microscopy Analysis
2.5. Image Analysis
2.6. Hardness Measurements
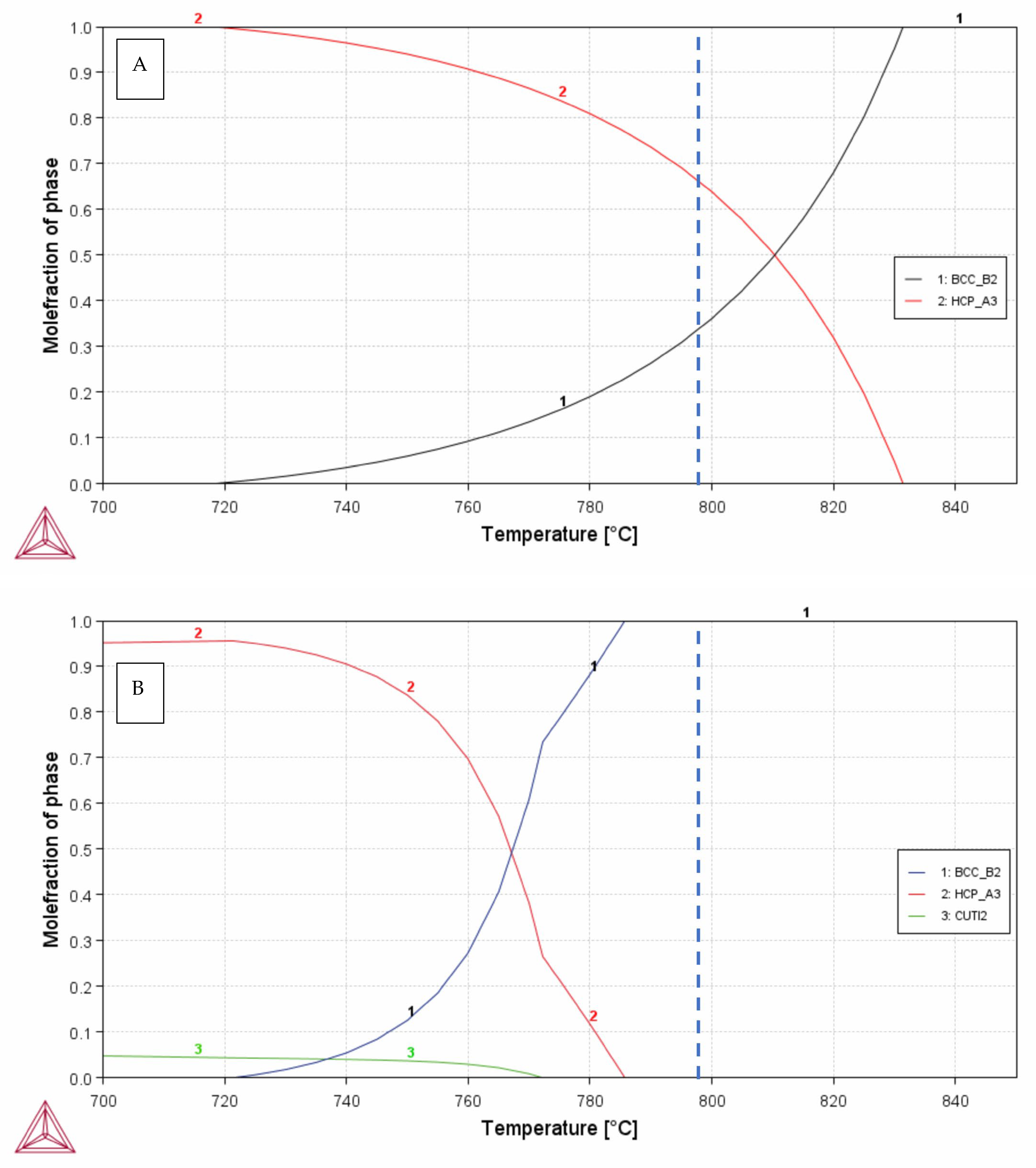
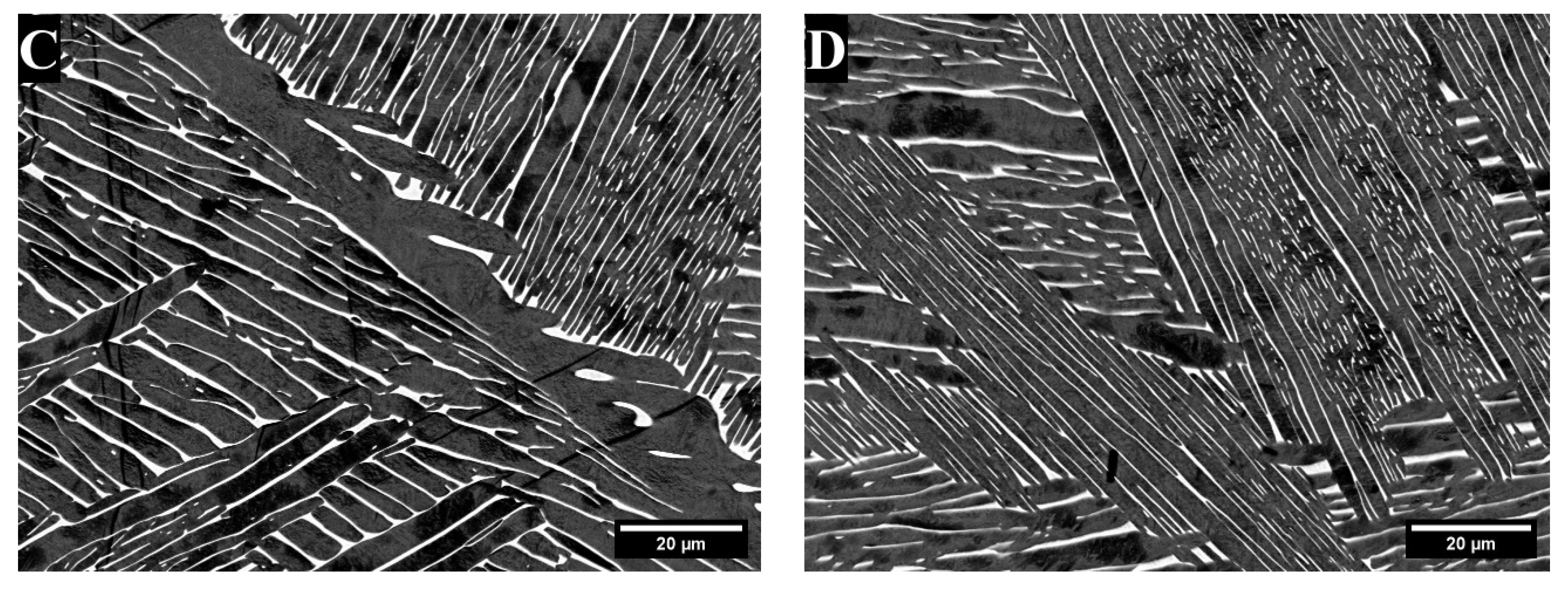
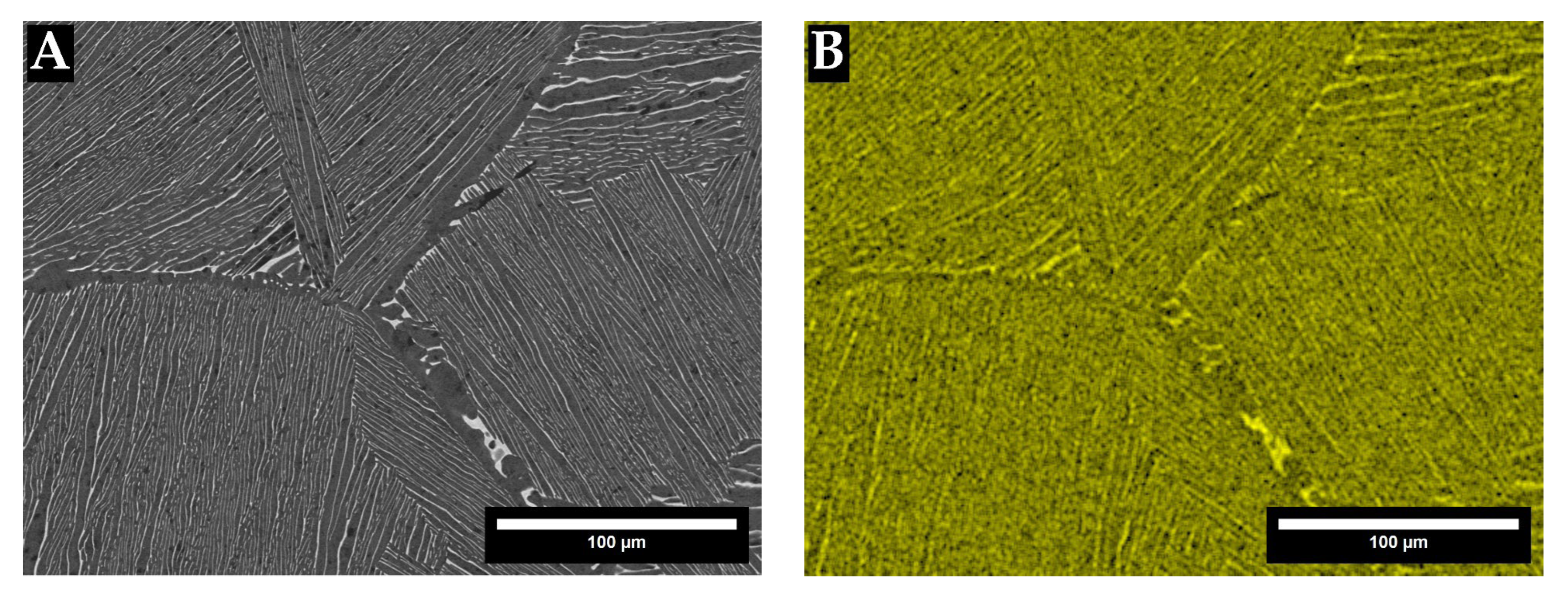
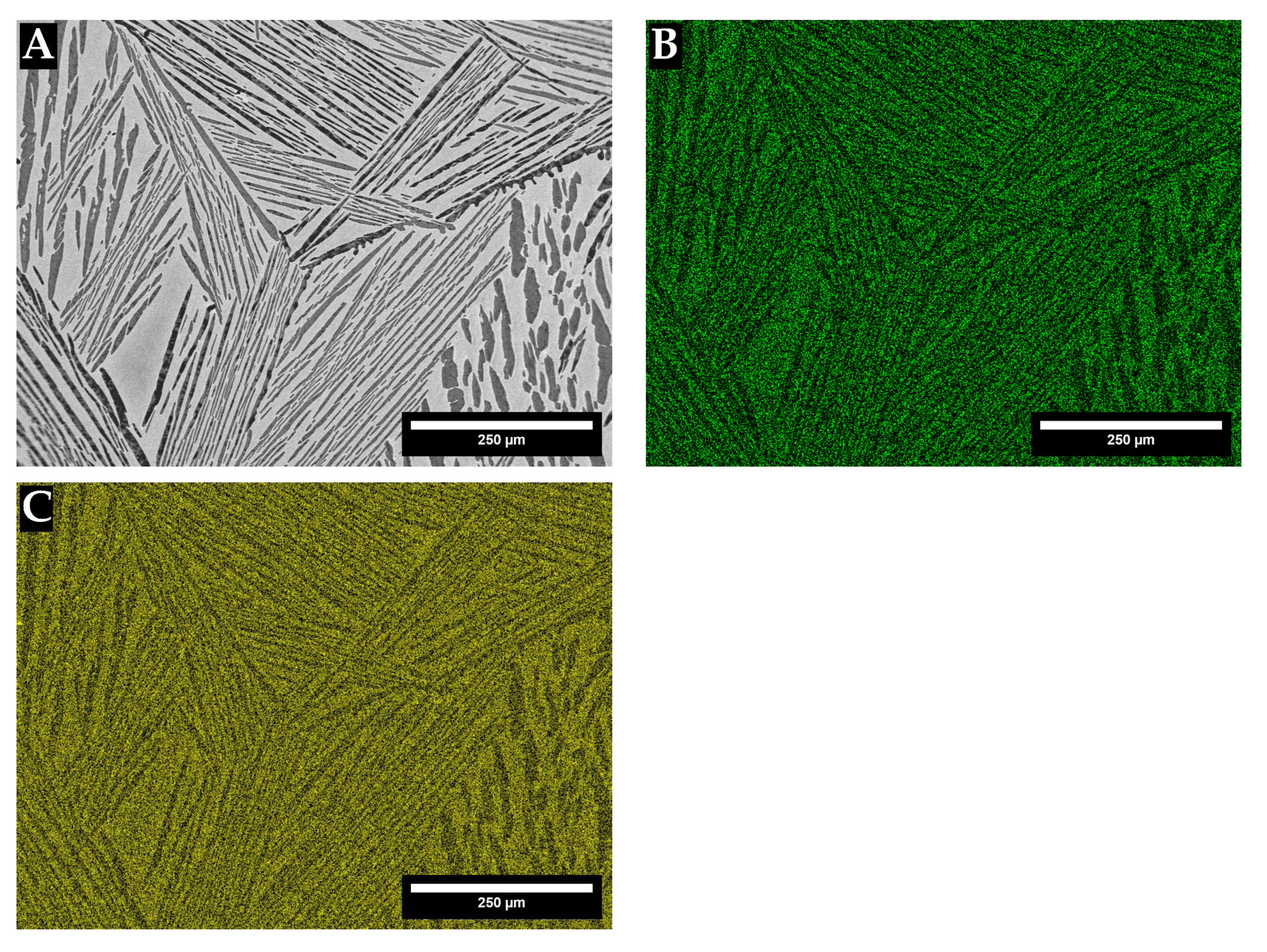
3. Results
3.1. X-Ray Diffraction
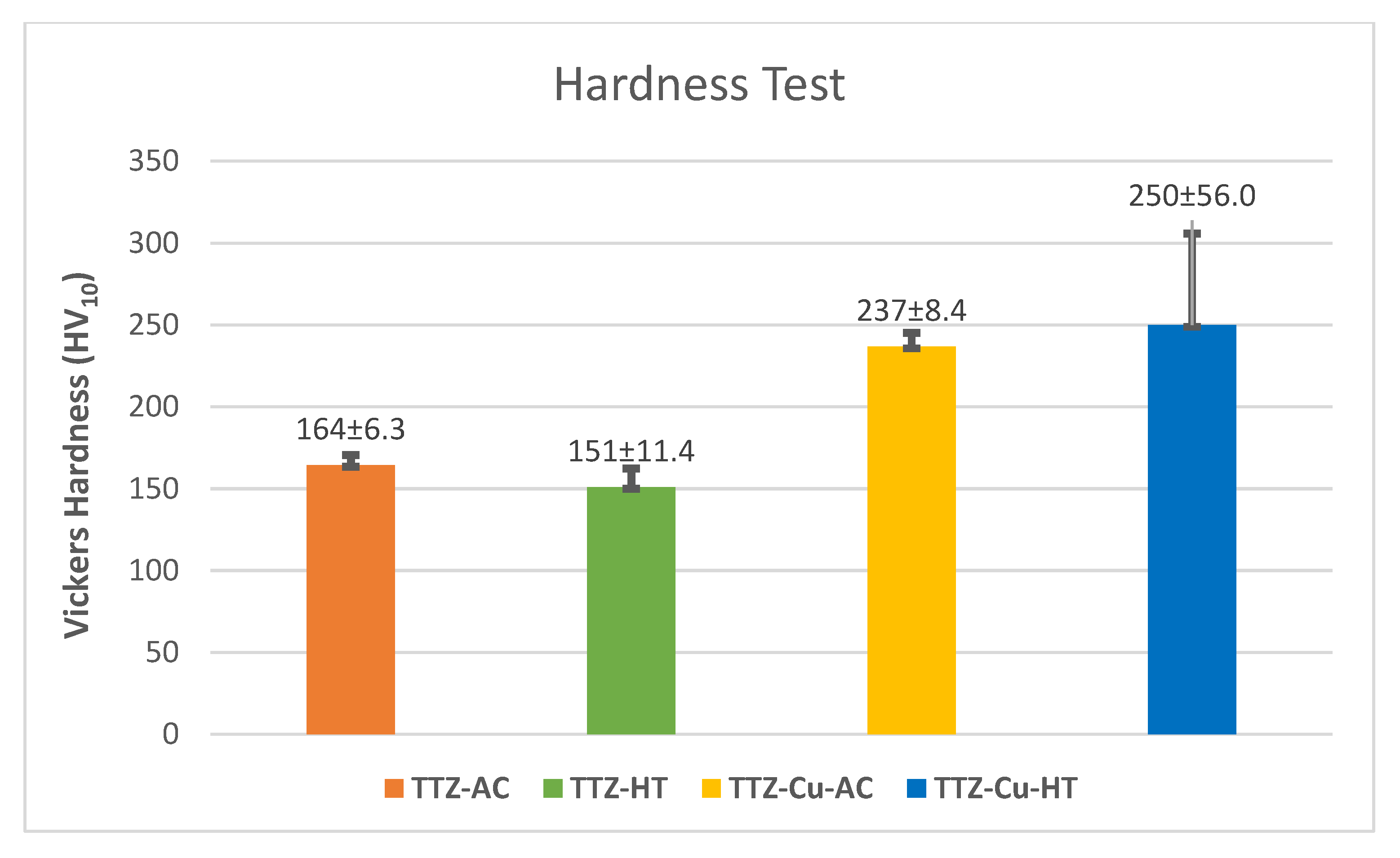
3.2. Hardness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Patel, N.R.; Gohil, P.P. A Review on Biomaterials: Scope, Applications and Human Anatomy Significance. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 99–101. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Ogawa, A.; Akatsuka, R.; Tamauchi, H.; Hio, K.; Kanematsu, H. Influence of the nickel-titanium alloy components on biological functions. BMC Proc. 2011, 5 (Suppl. S8), 79. [Google Scholar] [CrossRef]
- Grosgogeat, E.Z.B.; Vaicelyte, A.; Gauthier, R.; Janssen, C.; Le Borgne, M. Toxicological Risks of the Cobalt–Chromium Alloys in Dentistry: A Systematic Review. Materials 2022, 15, 5801. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based alloys and composites for orthopedic implants Applications: A comprehensive review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Ahmed, M.M.S.; Chowdhury, M.A.; Mobarak, M.H.; Rahman, M.M.; Hossain, M.H. Advances and significances of titanium in dental implant applications. Results Chem. 2024, 7, 101394. [Google Scholar] [CrossRef]
- Shao, L.; Du, Y.; Dai, K.; Wu, H.; Wang, Q.; Liu, J.; Tang, Y.; Wang, L. Ti Alloys for Orthopedic and Dental Applications: A Review of Progress on Improvement of Properties through Surface Modification. Coatings 2021, 11, 1446. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Langley, A.; Dameron, C.T. Modern metal implant toxicity and anaesthesia. In Australasian Anaesthesia 2015: Invited Papers and Selected Continuing Education Lectures; Riley, R., Ed.; Australian and New Zealand College of Anaesthetists: Melbourne, VIC, Australia, 2015; pp. 57–65. [Google Scholar]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Digole, S.; Desai, J.; Christopher, C.; Bohara, S.; Witharamage, C.S.; Kothapalli, C.; Gupta, R.K.; Borkar, T. Improved Tribological Performance of Nitride-Reinforced Biocompatible Titanium–Niobium–Zirconium–Tantalum (TNZT) Alloys for Advanced Orthopedic Applications. Metals 2024, 14, 122. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, T. Phase transformation and mechanical properties of the Ti50Zr30Nb10Ta10 alloy with low modulus and biocompatible. J. Alloys Compd. 2005, 392, 291–294. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, X.; Dong, Z. Effect of Nb on microstructure and mechanical properties of Ti-xNb-4Zr–8Sn alloys. Mater. Sci. Eng. A 2021, 825, 141741. [Google Scholar] [CrossRef]
- Dercz, G.; Matuła, I.; Prusik, K.; Zając, J.; Szklarska, M.; Kazek-Kęsik, A.; Simka, W. Effect of Nb and Zr alloying additives on structure and properties of Ti-Ta-Nb-Zr alloys for medical applications. Arch. Metall. Mater. 2023, 68, 1137–1142. [Google Scholar] [CrossRef]
- Dobri, G.; Banu, A.; Donath, C.; Marcu, M. The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy. Metals 2023, 13, 1294. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Gogia, A.K.; Asokamani, R. Effect of thermomechanical processing on evolution of various phases in Ti–Nb–Zr alloys. J. Alloys Compd. 2004, 384, 131–144. [Google Scholar] [CrossRef]
- Elias, L.M.; Schneider, S.G.; Schneider, S.G.; Silva, H.M.; Malvisi, F. Microstructural and mechanical characterization of biomedical Ti–Nb–Zr(–Ta) alloys. Mater. Sci. Eng. A. 2006, 432, 108–112. [Google Scholar] [CrossRef]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti–Ta–Nb–Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E., Jr.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1334. [Google Scholar] [CrossRef] [PubMed]
- Fowler, L.; van Vuuren, A.J.; Goosen, W.; Engqvist, H.; Öhman-Mägi, C.; Norgren, S. Investigation of copper alloying in a TNTZ-Cux alloy. Materials 2019, 12, 3691. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Rdzawski, Z. Antibacterial properties of copper and its alloys. Arch. Mater. Sci. Eng. 2012, 56, 53–60. [Google Scholar]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium–copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C. 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Zhang, E.; Ren, J.; Li, S.; Yang, L.; Qin, G. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of as-cast Ti-Cu alloys. Biomed. Mater. 2016, 11, 65001. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ma, Z.; Li, M.; Zhang, Y.; Liu, W.; Liao, Z.; Yang, K. Antibacterial Properties of Ti–6Al–4V–xCu Alloys. J. Mater. Sci. Technol. 2014, 30, 699–705. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, L.; Zhang, S.; Wei, X.; Yang, K.; Dai, K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021, 119, 472–484. [Google Scholar] [CrossRef]
- Wang, R.; Wang, R.; Chen, D.; Qin, G.; Zhang, E. Novel CoCrWNi alloys with Cu addition: Microstructure, mechanical properties, corrosion properties and biocompatibility. J. Alloys Compd. 2020, 824, 153924. [Google Scholar] [CrossRef]
- Lu, Y.; Ren, L.; Xu, X.; Yang, Y.; Wu, S.; Luo, J.; Yang, M.; Liu, L.; Zhuang, D.; Yang, K.; et al. Effect of Cu on microstructure, mechanical properties, corrosion resistance and cytotoxicity of CoCrW alloy fabricated by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018, 81, 130–141. [Google Scholar] [CrossRef]
- Duan, J.; Yang, Y.; Zhang, E.; Wang, H. Co-Cr-Mo-Cu alloys for clinical implants with osteogenic effect by increasing bone induction, formation and development in a rabbit model. Burn. Trauma 2020, 8, tkaa036. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Shahzad, M.B.; Xua, D.; Sun, Z.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Ukabhai, K.D.; Curle, U.A.; Masia, N.D.E.; Smit, M.; Mwamba, I.A.; Norgren, S.; Öhman-Mägi, C.; Hashe, N.G.; Cornish, L.A. Formation of Ti2Cu in Ti-Cu Alloys. J. Phase Equilibria Diffus. 2022, 43, 332–344. [Google Scholar] [CrossRef]
- Chong, Y.; Bhattacharjee, T.; Tsuji, N. Bi-lamellar microstructure in Ti–6Al–4V: Microstructure evolution and mechanical properties. Mater. Sci. Eng. A. 2019, 762, 138077. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C. Titanium; Springer: Berlin/Heidelberg, Germany, 2013; Available online: https://books.google.se/books?id=wBXsCAAAQBAJ (accessed on 21 January 2023).
- Thermo-Calc Software, Thermo-Calc Documentation Set, Version 2022a, Sci. Gr. Thermodata Eur. Solut. Database Version 5, SSOL5. Available online: https://thermocalc.com/support/documentation (accessed on 21 January 2023).
- Lin, I.T.; Chiou, T.C.; Da Lee, C.; Gierlotka, W. Ab-initio and molecular dynamics supported thermodynamic modeling of binary Cu—Ta system. Mater. Chem. Phys. 2019, 232, 422–427. [Google Scholar] [CrossRef]
- Kumar, K.C.H.; Ansara, I.; Wollants, P.; Delaey, L. Thermodynamic Optimisation of the Cu-Ti System. Int. J. Mater. Res. 1996, 87, 666–672. [Google Scholar] [CrossRef]
- Canale, P.; Servant, C. Thermodynamic assessment of the Cu–Ti system taking into account the new stable phase CuTi3. Int. J. Mater. Res. 2002, 93, 273–276. [Google Scholar] [CrossRef]
- ASTM E92-17; Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials. ASTM volume 03.01. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Maykuth, D.J.; Ogden, H.R.; Jaffee, R.I. Titanium-tungsten and titanium-tantalum systems. Trans. Am. Inst. Min. Metall. Eng. 1953, 197, 231. [Google Scholar]
- Powder Diffraction File (JCPDS International Center for Diffraction Data, Swarthmore PA) Inorganic 21–1276. International Centre for Diffraction Data: Newton Square, PA, USA. Available online: https://www.icdd.com/ (accessed on 1 January 2023).
- Takahashi, M.; Sato, K.; Togawa, G.; Takada, Y. Mechanical Properties of Ti-Nb-Cu Alloys for Dental Machining Applications. J. Funct. Biomater. 2022, 13, 263. [Google Scholar] [CrossRef]
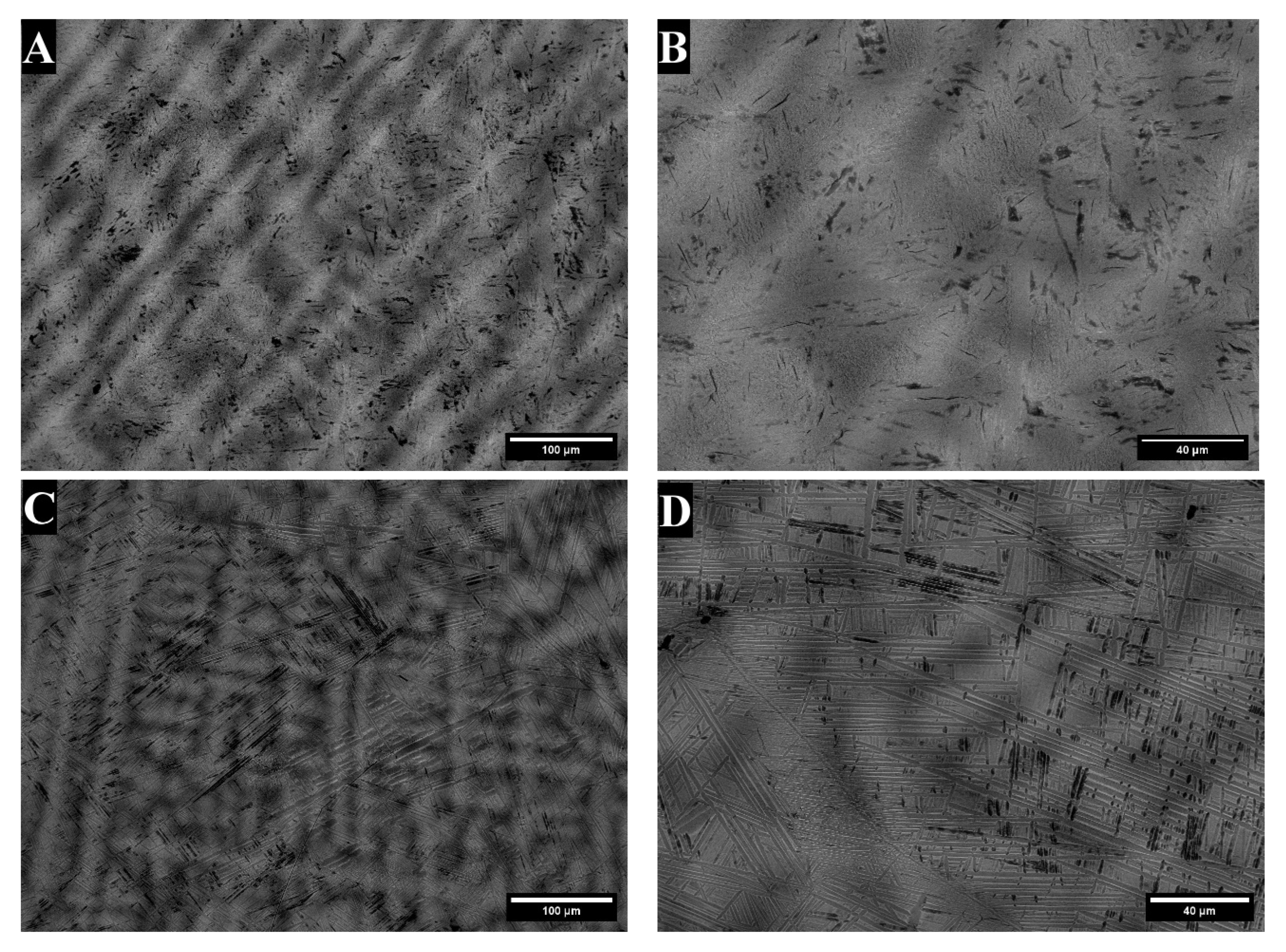
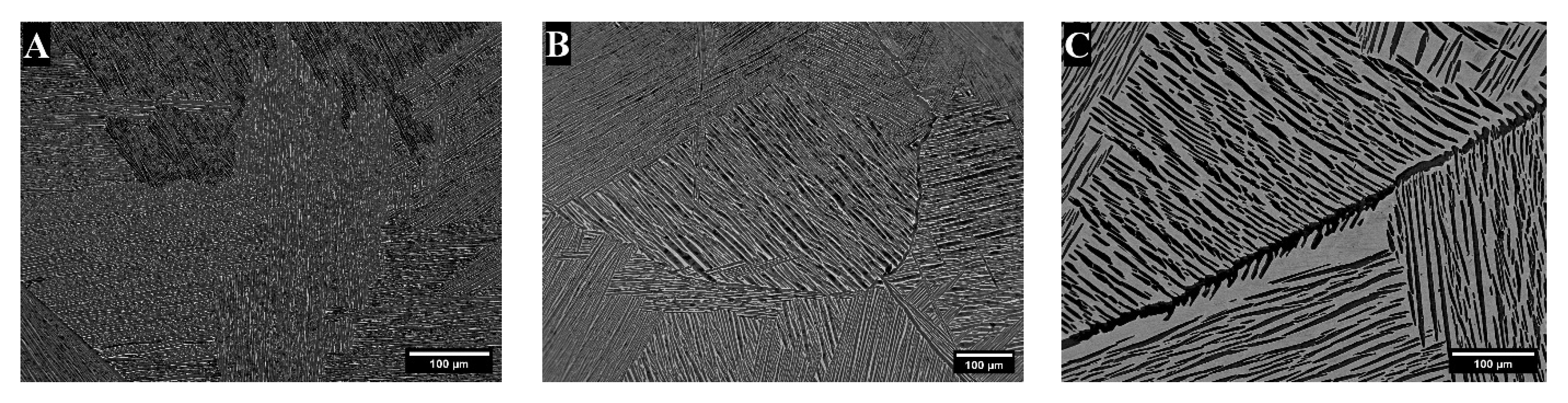

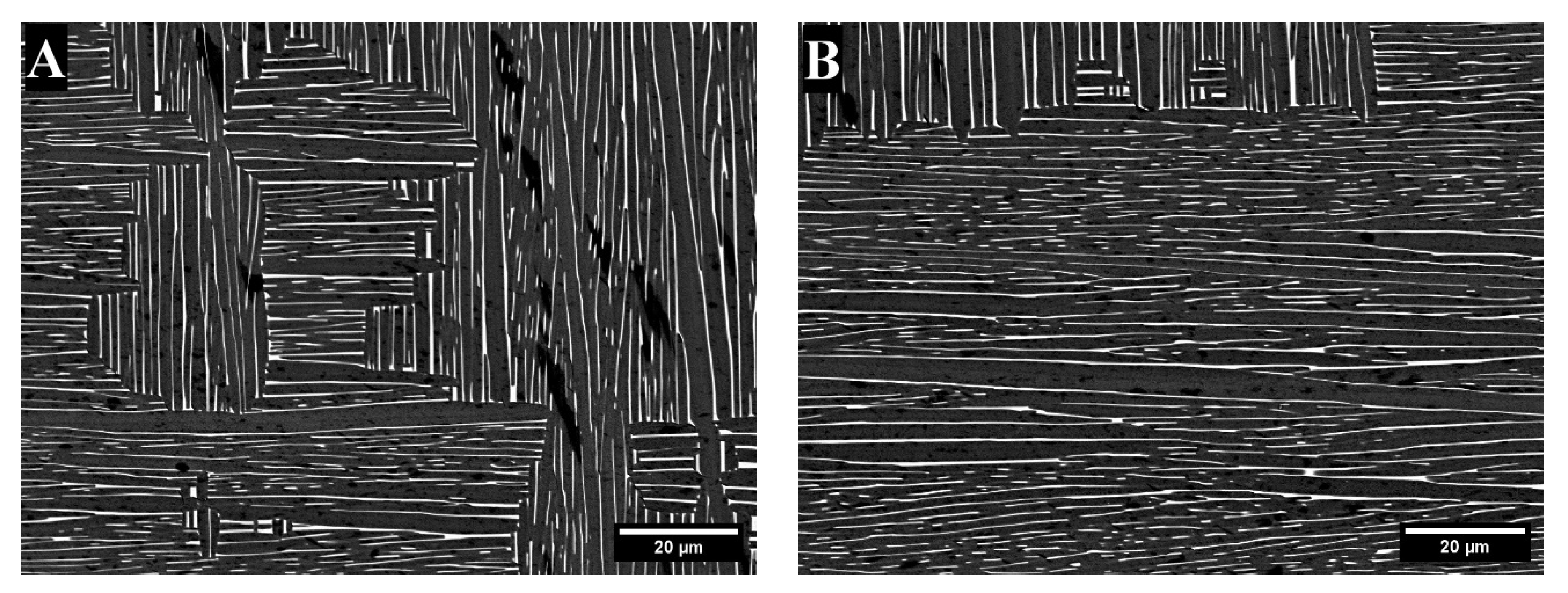



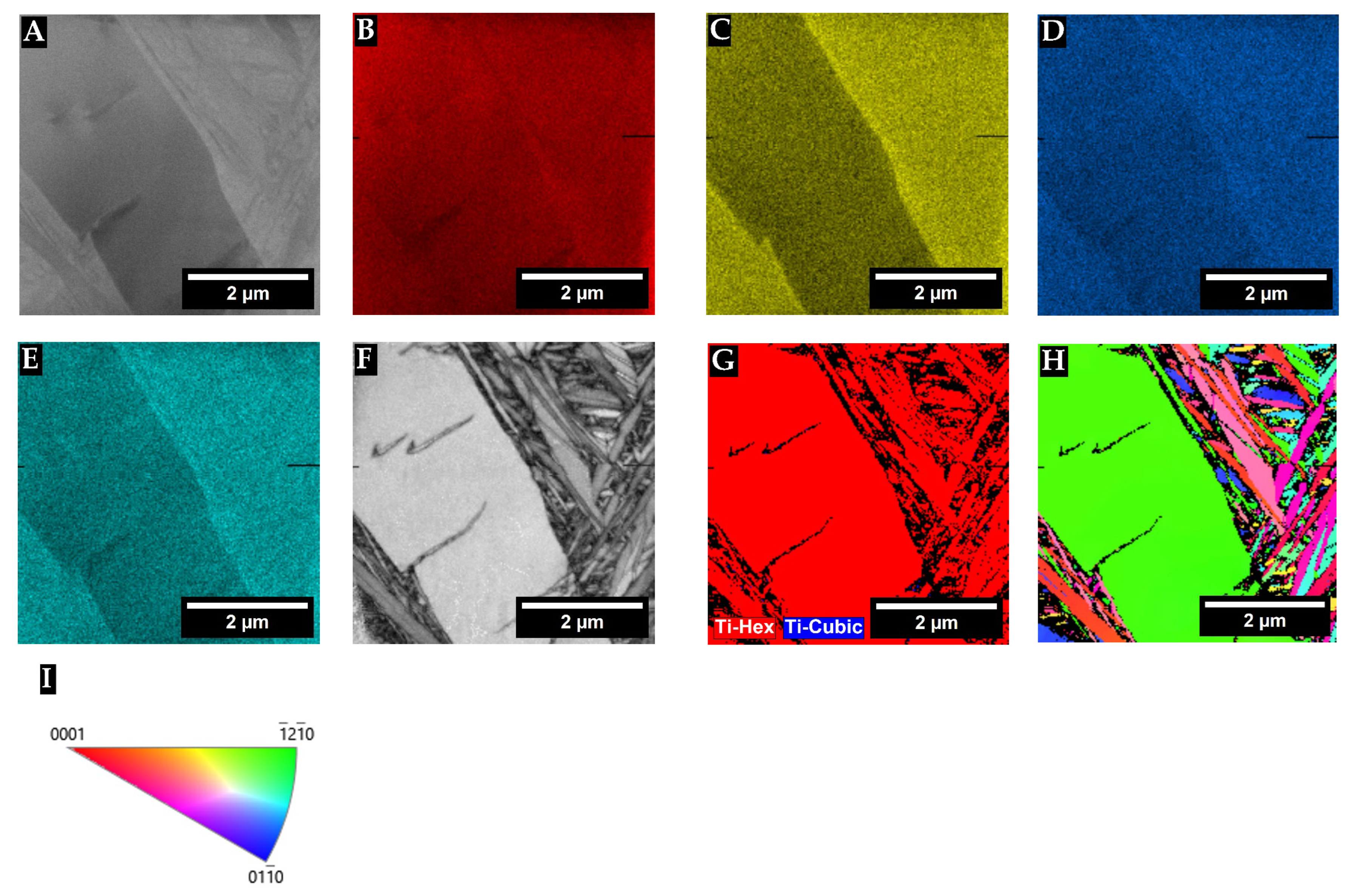

| Alloy | Area * | Bright- Contrast Phase (%) | Dark-Contrast Phase (%) |
| TTZ-HT | Dark contrast (N = 10) ** | 17.2 ± 2.5 | 82.8 ± 2.5 |
| TTZ-Cu-HT | Dark contrast (N = 4) | 21.3 ± 3.9 | 78.7 ± 3.9 |
| Bright contrast (N = 9) | 58.2 ± 1.7 | 41.8 ± 1.7 |
| Element | Dark-Contrast Phase | Medium-Contrast Phase | Bright-Contrast Phase |
| Ti | |||
| Cu | |||
| Zr | |||
| Ta | |||
| W |
| Element | Medium-Contrast Phase | Dark-Contrast Phase | Bright-Contrast Phase |
| Ti | |||
| Zr | |||
| Ta | |||
| W |
| Element | Medium-Contrast Phase | Dark-Contrast Phase | Bright-Contrast Phase |
| Ti | |||
| Zr | |||
| Ta | |||
| W |
| Element | Medium-Contrast Phase | Dark-Contrast Phase | Bright-Contrast Phase |
| Ti | |||
| Cu | |||
| Zr | |||
| Ta | |||
| W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashe, N.G.; Fowler, L.; Norgren, S.; Cornish, L.A.; Chown, L.H.; Goosen, W.E.; Westraadt, J.E.; Hadebe, N.D.E.; Öhman-Mägi, C. Effect of 3 wt% Cu on the Microstructure and Hardness of a Ti-10Ta-1.6Zr Alloy. Materials 2025, 18, 3163. https://doi.org/10.3390/ma18133163
Hashe NG, Fowler L, Norgren S, Cornish LA, Chown LH, Goosen WE, Westraadt JE, Hadebe NDE, Öhman-Mägi C. Effect of 3 wt% Cu on the Microstructure and Hardness of a Ti-10Ta-1.6Zr Alloy. Materials. 2025; 18(13):3163. https://doi.org/10.3390/ma18133163
Chicago/Turabian StyleHashe, Nobom G., Lee Fowler, Susanne Norgren, Lesley A. Cornish, Lesley H. Chown, William E. Goosen, Johan E. Westraadt, Nomsombuluko D. E. Hadebe, and Caroline Öhman-Mägi. 2025. "Effect of 3 wt% Cu on the Microstructure and Hardness of a Ti-10Ta-1.6Zr Alloy" Materials 18, no. 13: 3163. https://doi.org/10.3390/ma18133163
APA StyleHashe, N. G., Fowler, L., Norgren, S., Cornish, L. A., Chown, L. H., Goosen, W. E., Westraadt, J. E., Hadebe, N. D. E., & Öhman-Mägi, C. (2025). Effect of 3 wt% Cu on the Microstructure and Hardness of a Ti-10Ta-1.6Zr Alloy. Materials, 18(13), 3163. https://doi.org/10.3390/ma18133163







