Geopolymer CLSM with Off-Specification Fly Ash and Bottom Ash: A Sustainable Approach to Hazardous Waste Utilization
Abstract
1. Introduction
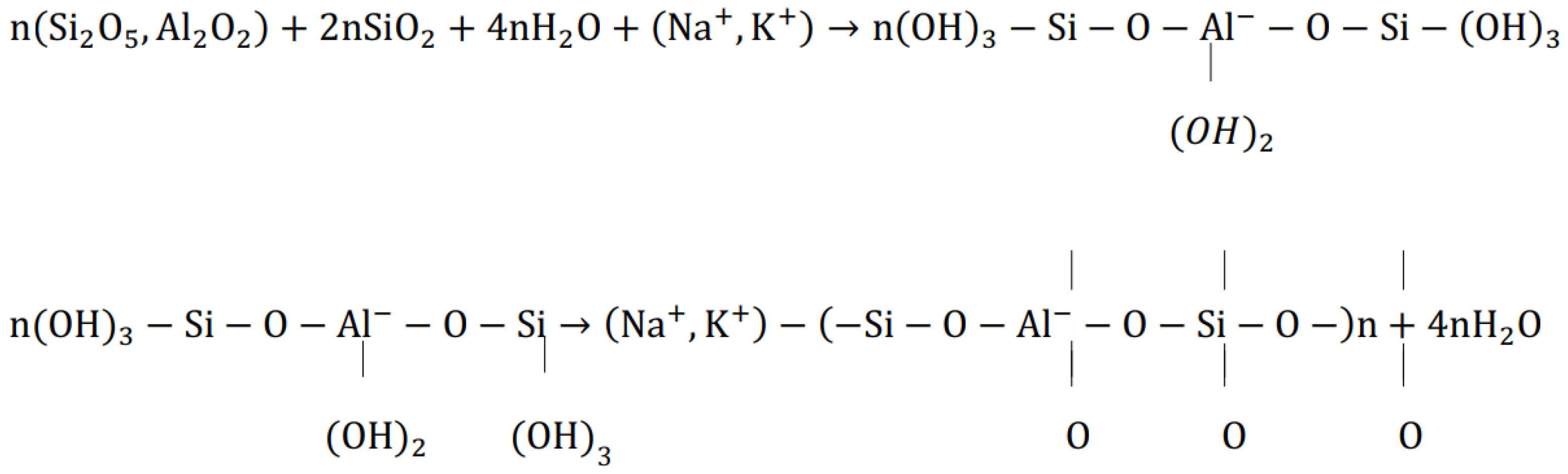
2. Material Characterization and Properties
2.1. Off-Specification Fly Ash
2.2. Bottom Ash
2.3. Activator (Sodium Hydroxide)
3. Experimental Program
3.1. Test Matrix
3.2. Flow Consistency Test
3.3. Compressive Strength Test
3.4. Cost Analysis
4. Results and Discussion
4.1. Flow Consistency
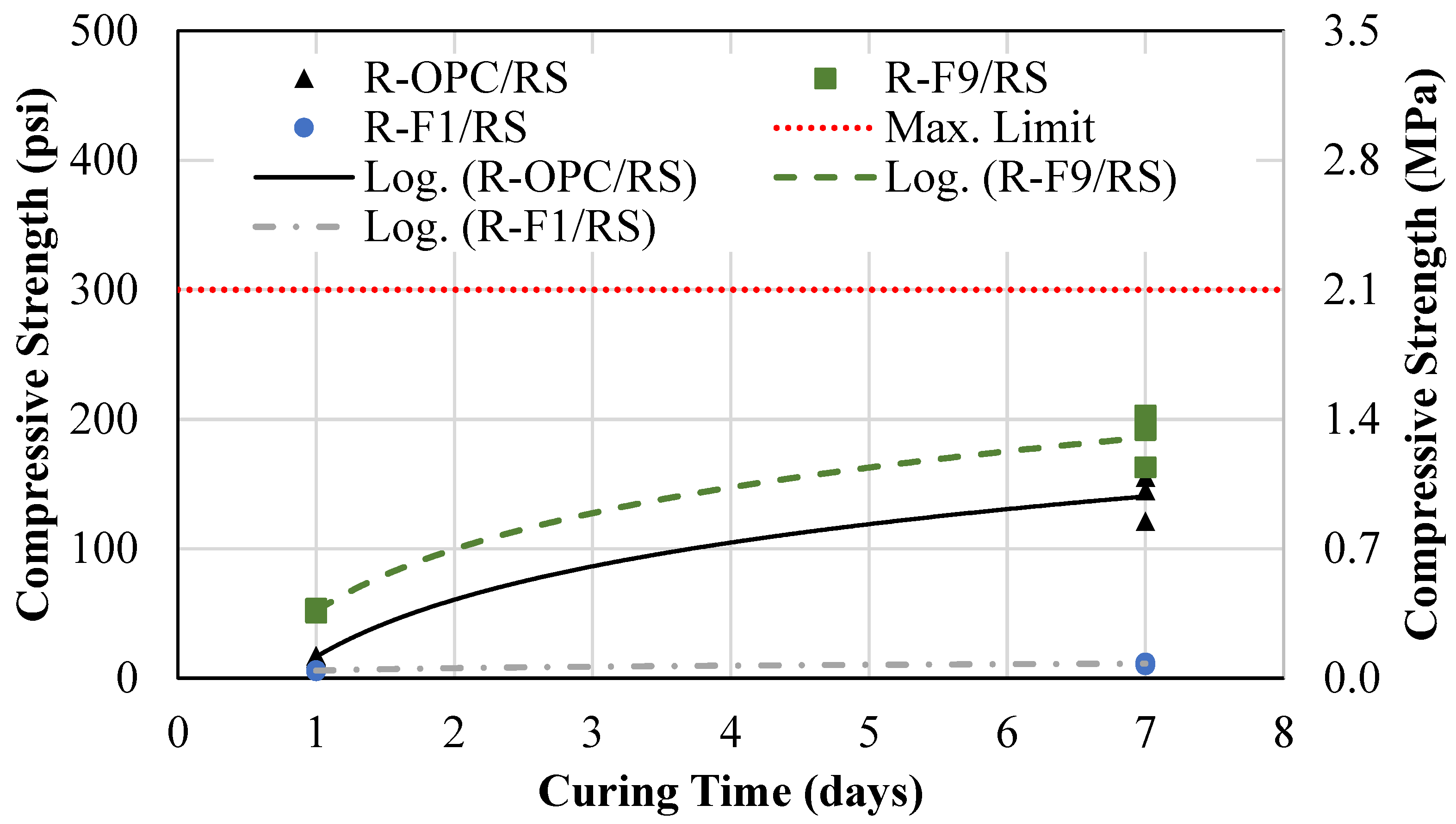
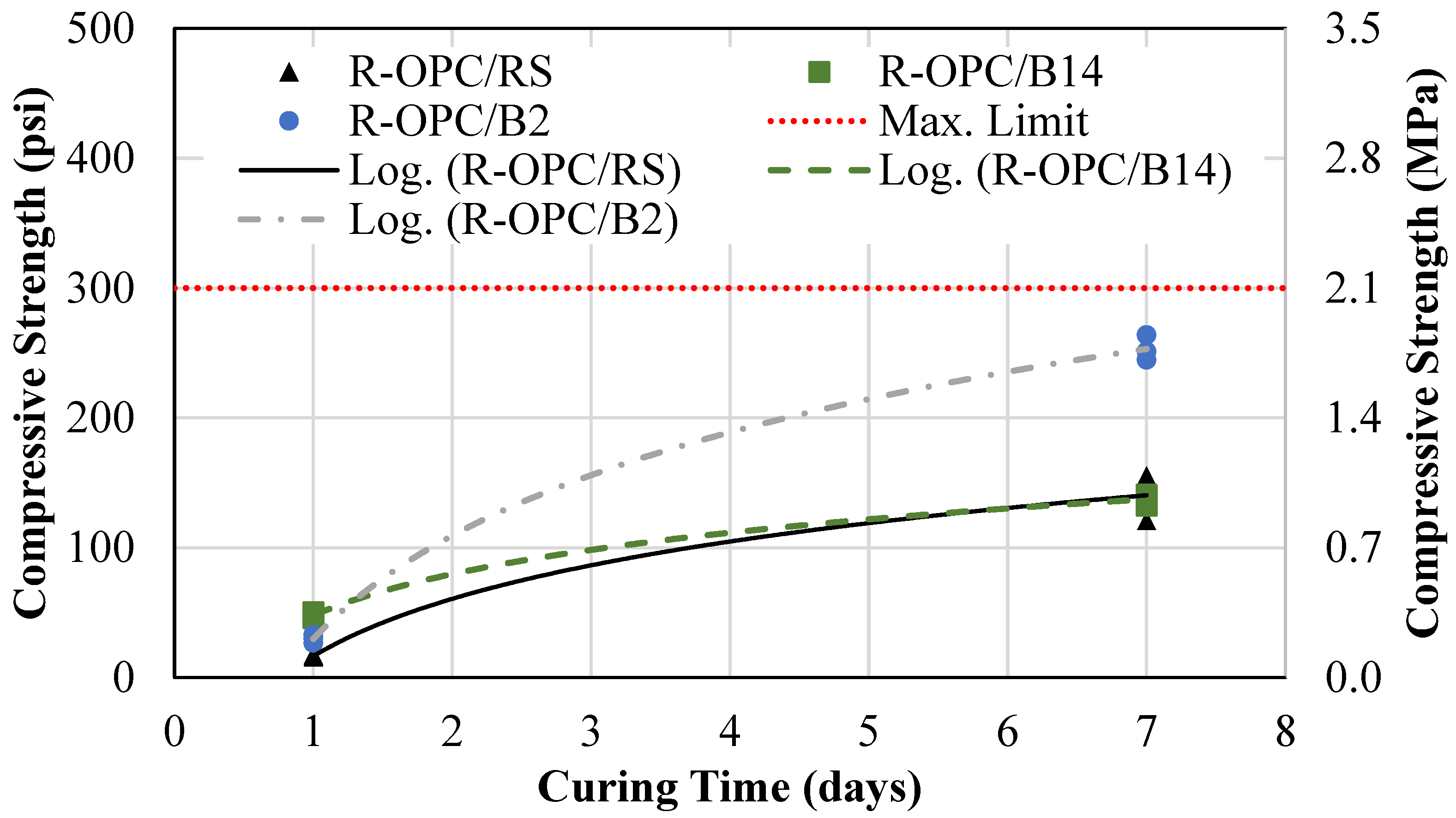
4.1.1. Reference Mixtures
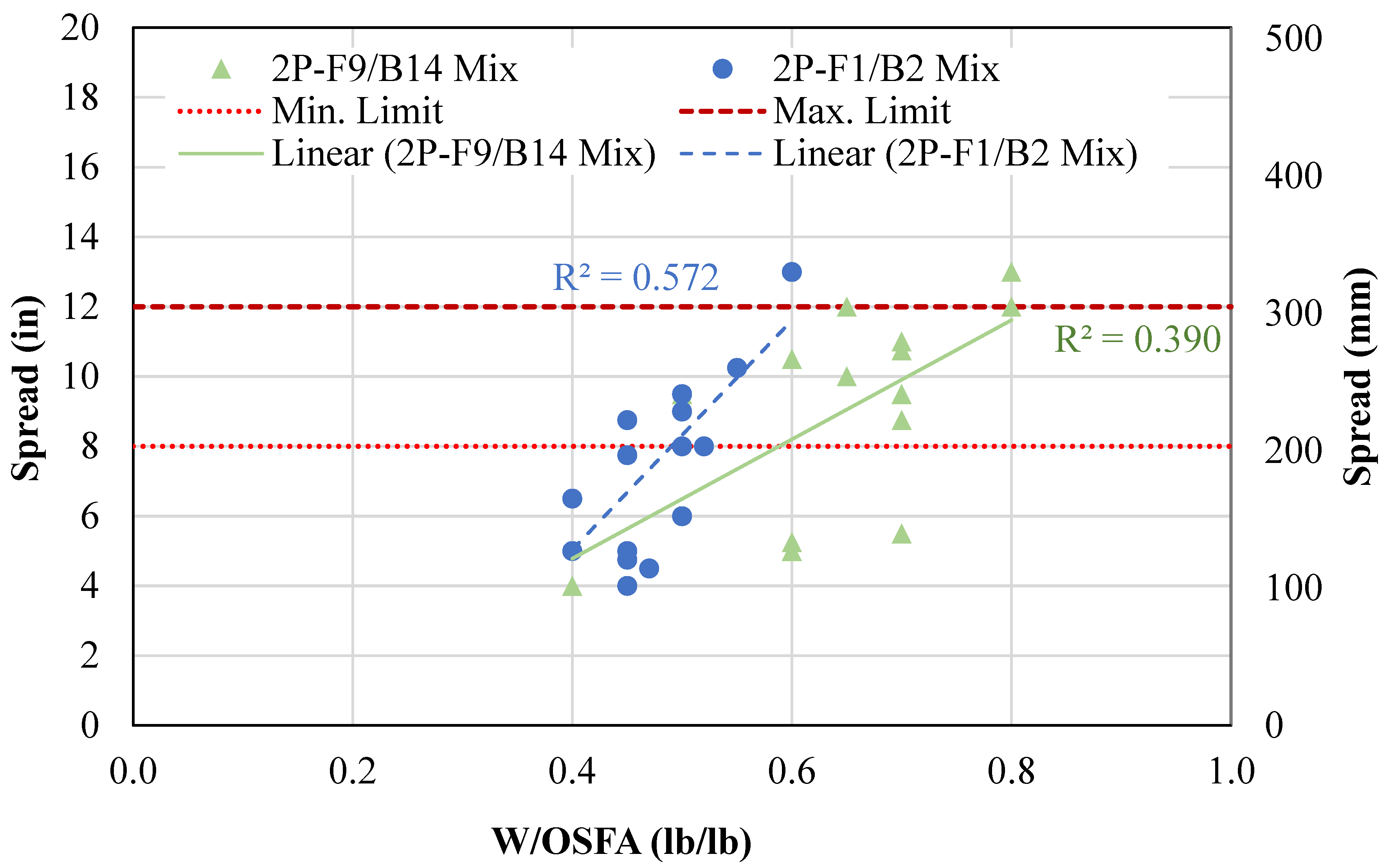
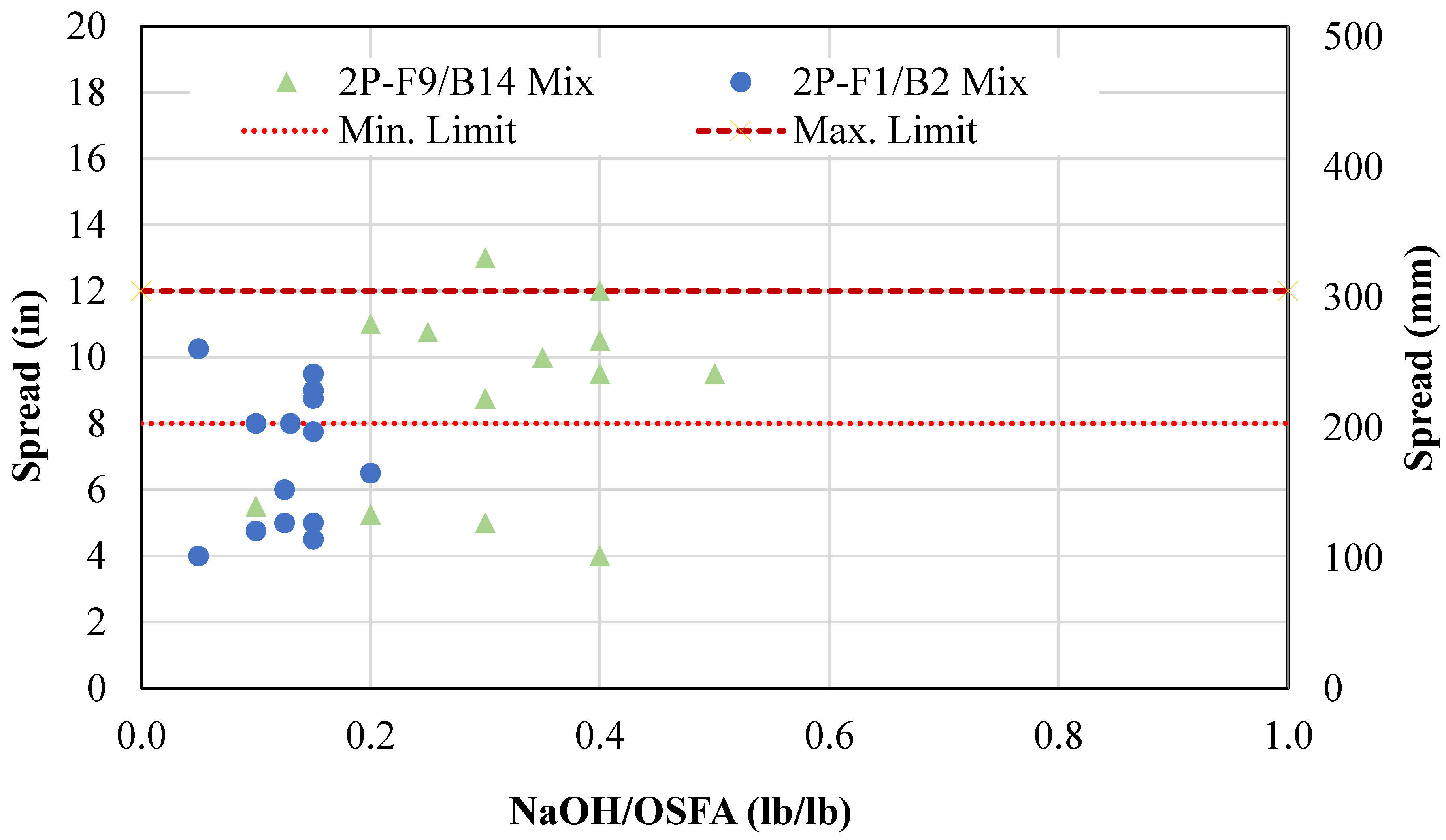
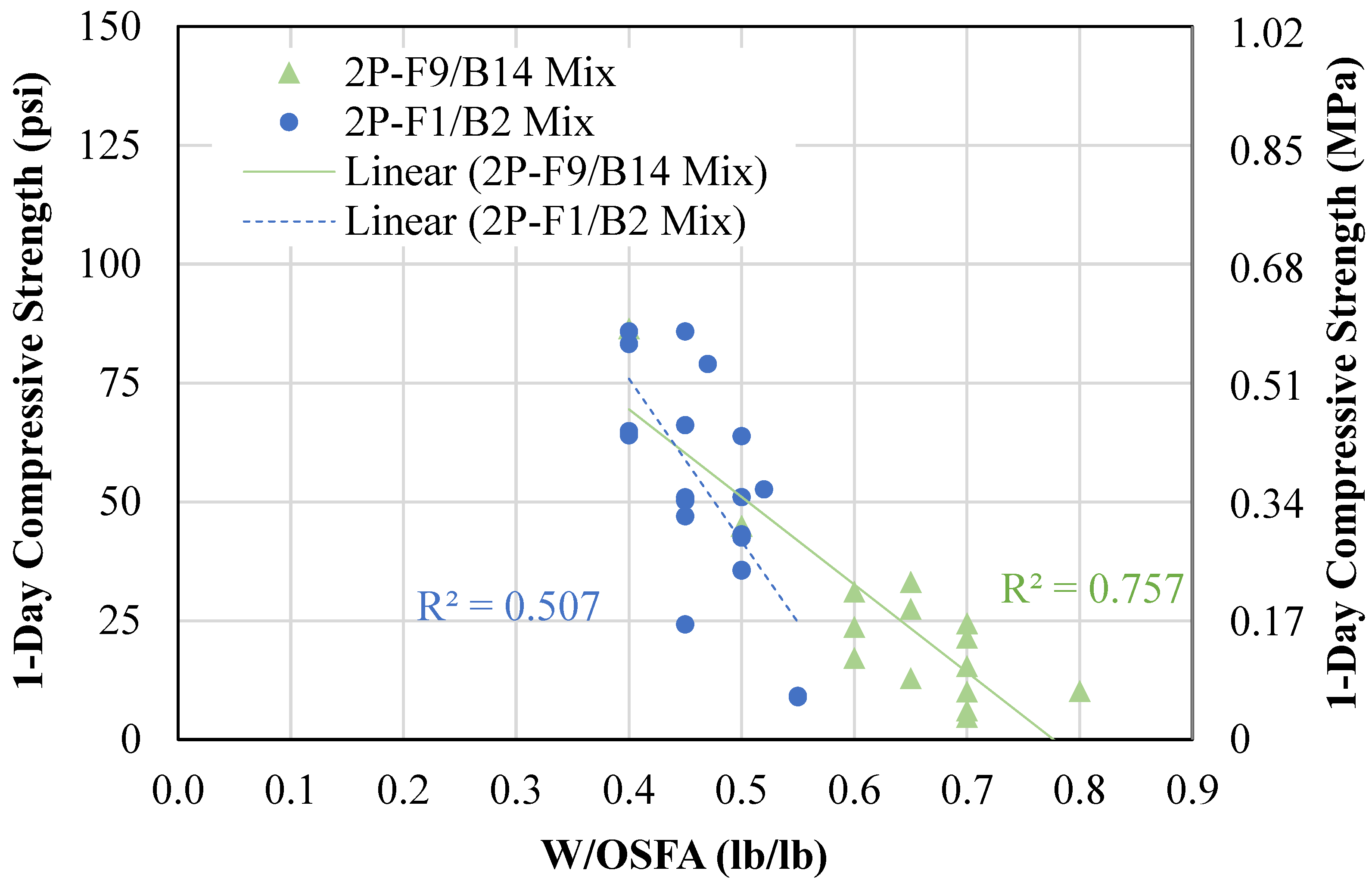
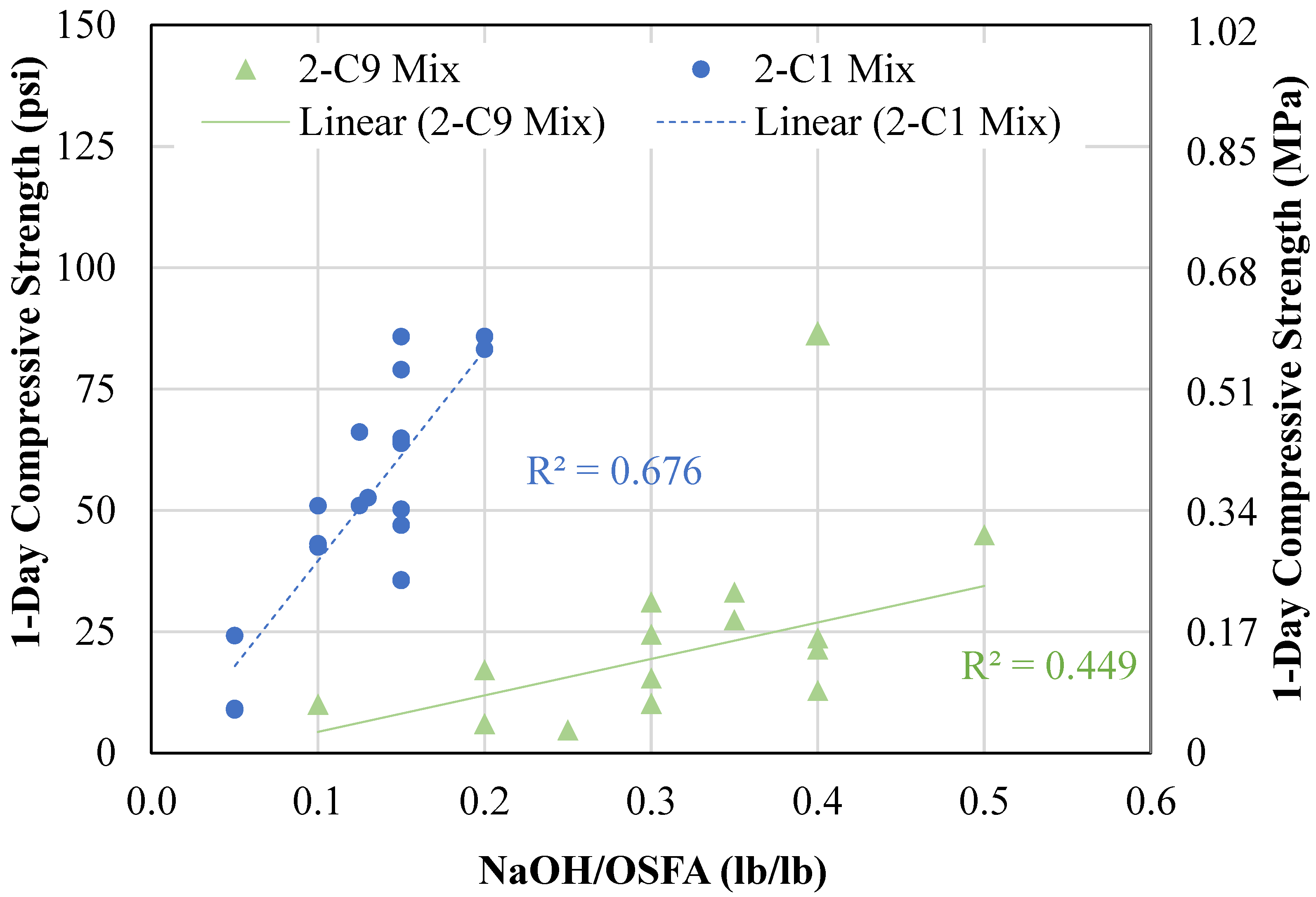
4.1.2. Two-Part Mixtures
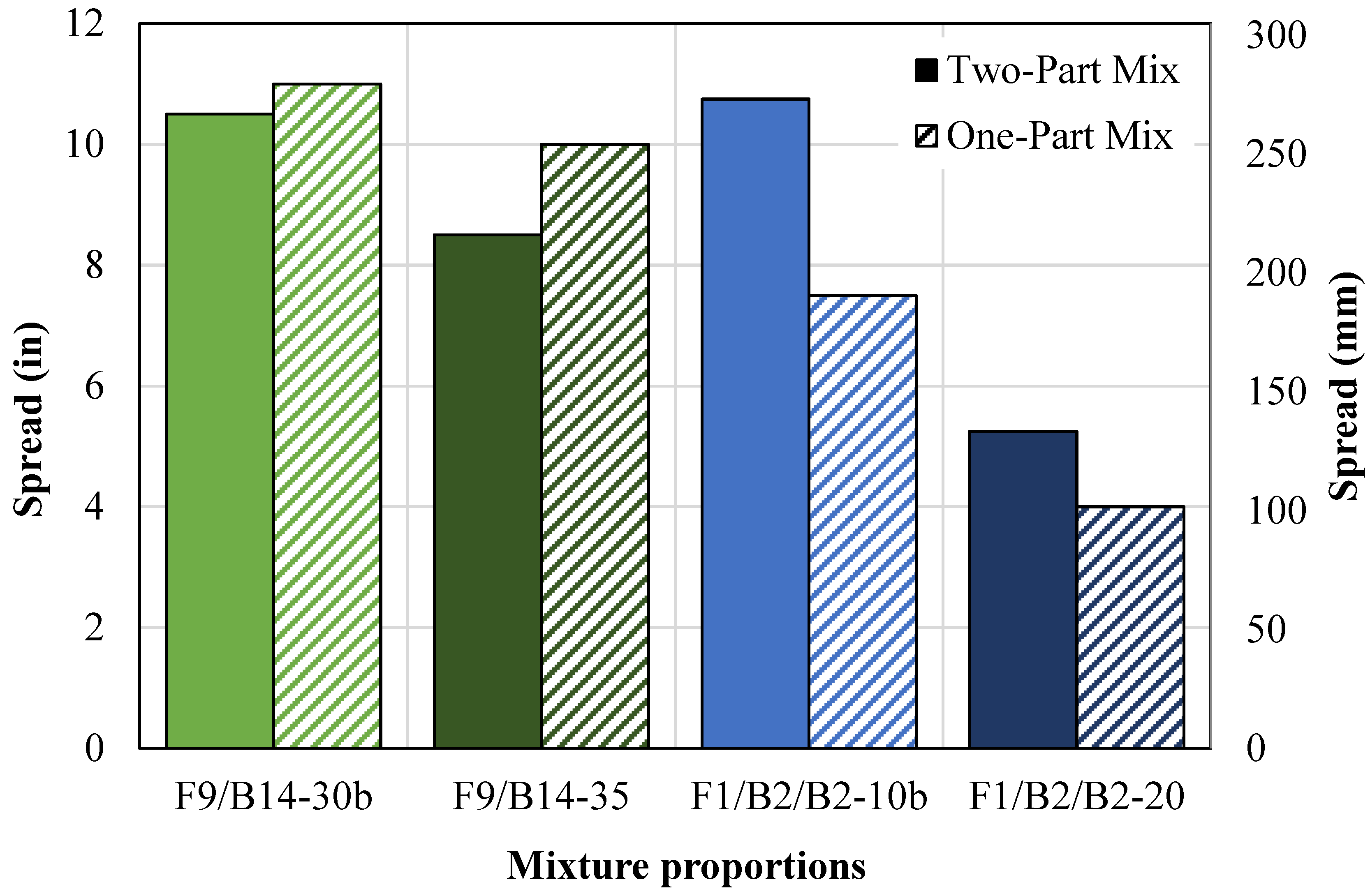
4.1.3. One-Part Mixtures
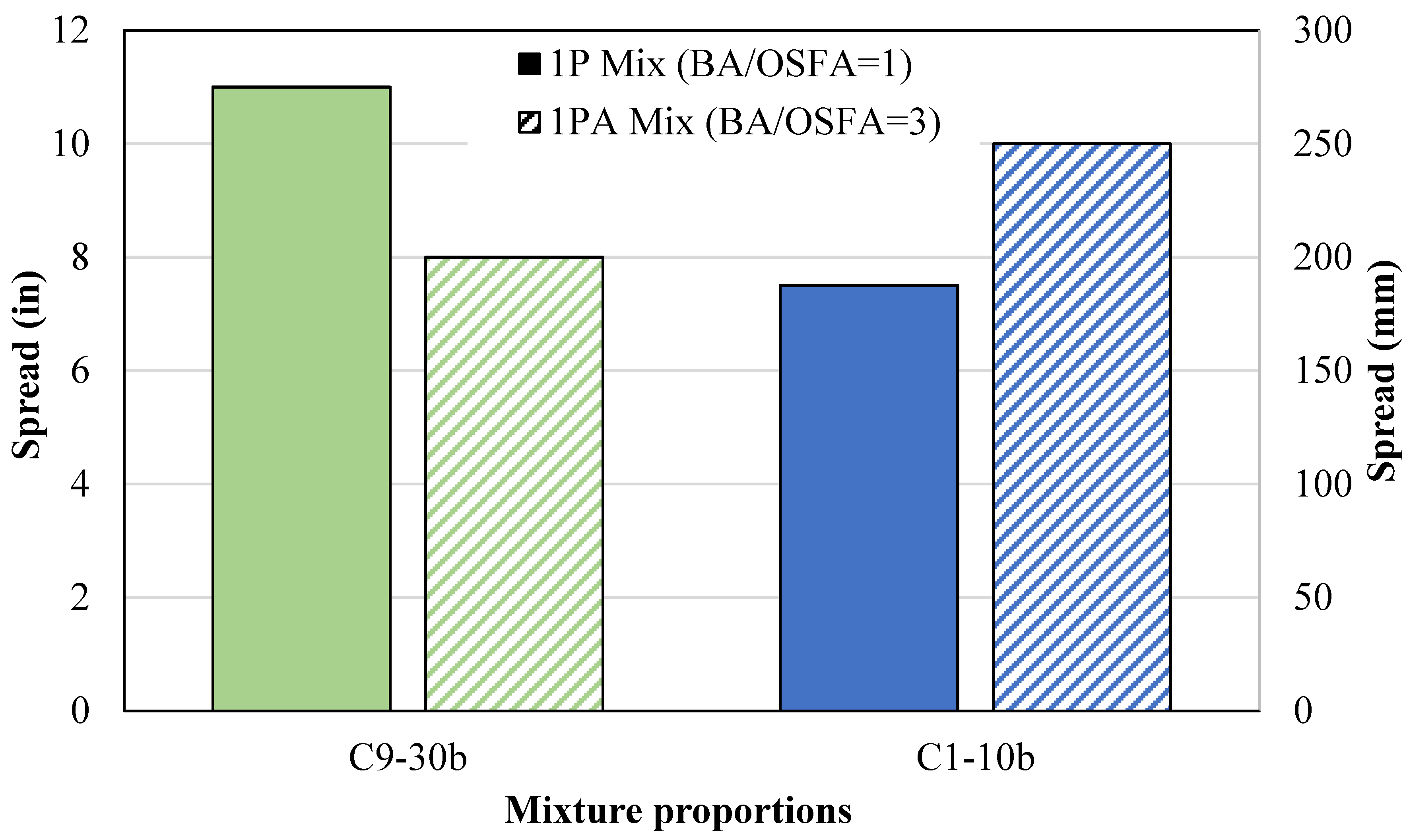
4.1.4. One-Part Ratio-Adjusted Mixtures
4.2. Compressive Strength
4.3. Cost Analysis
5. Conclusions
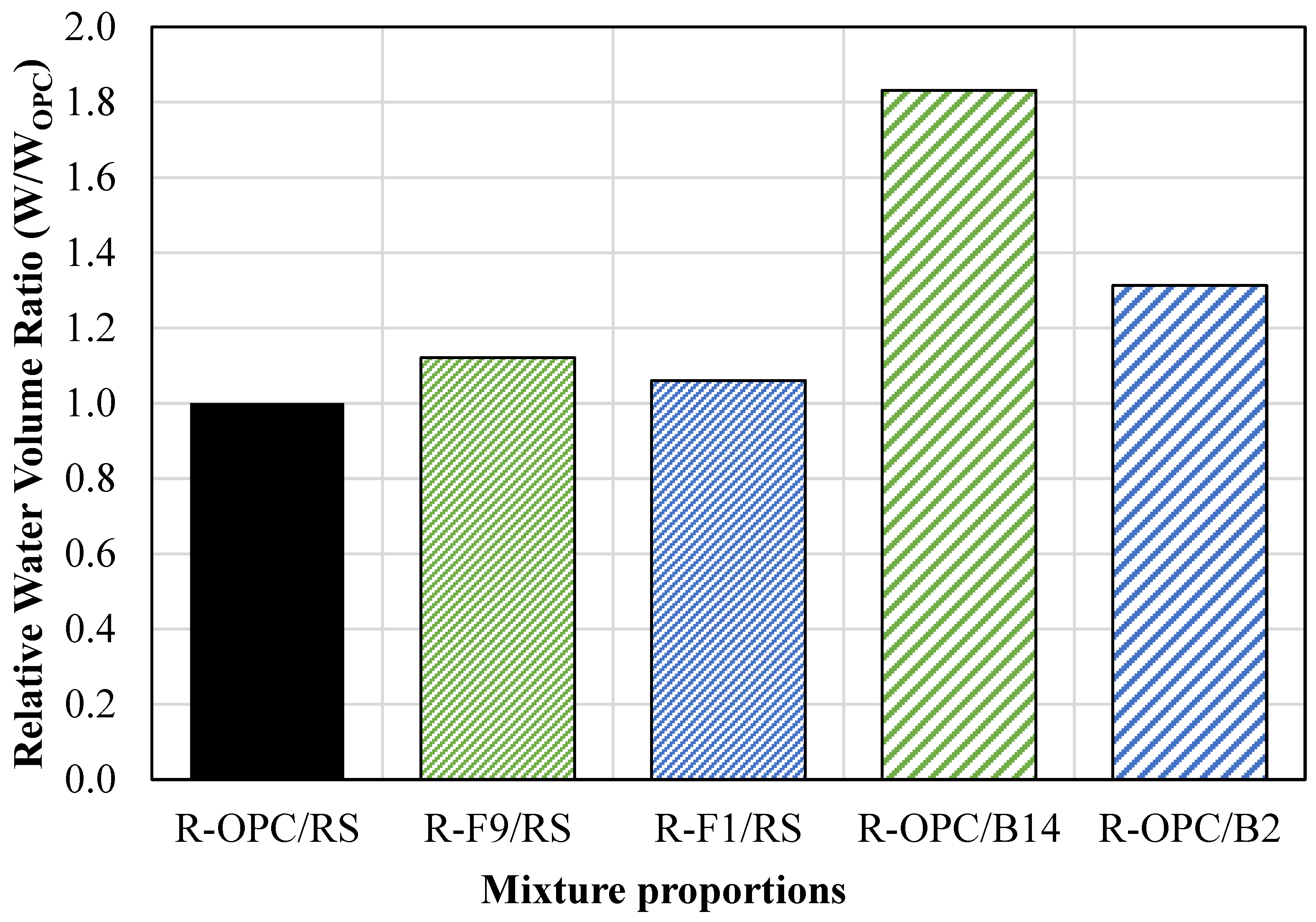
- An eco-friendly alternative CLSM was developed using off-specification fly ash (OSFA) and sodium hydroxide (NaOH) as a 100% replacement for cement, with bottom ash (BA) serving as the aggregate. This approach not only repurposes hazardous industrial by-products but also contributes to reducing the environmental impact of their disposal. The developed mixtures required 15% more water to meet flowability requirements.
- An alternative CLSM was developed by using cement and BA as a 100% sand replacement. These mixtures were subject to segregation; therefore, adjustments in the mixture proportions must be made to meet flowability requirements.
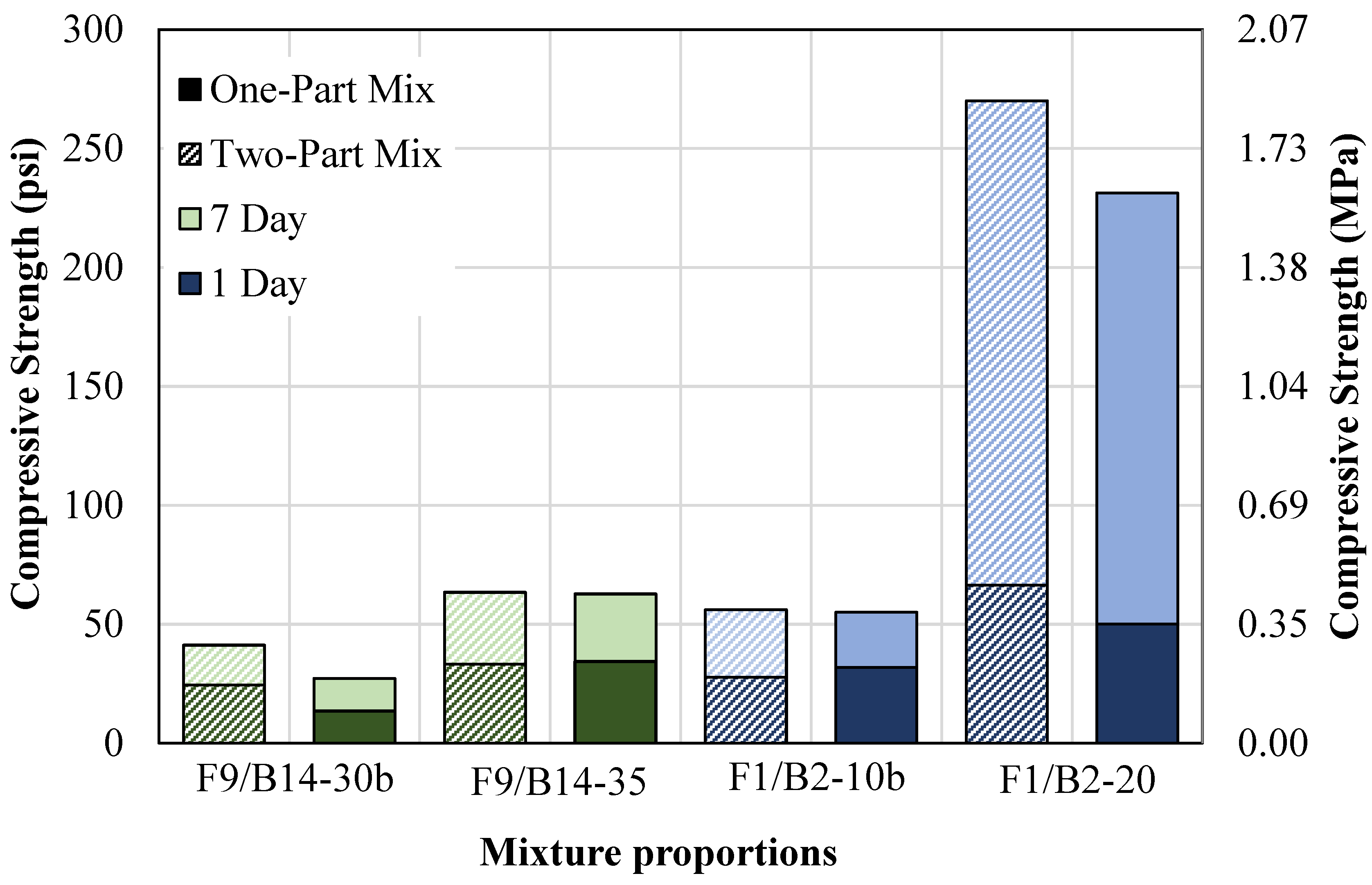
- A two-part geopolymer CLSM was developed by fully replacing both the cement and sand with OSFA and BA, respectively, and NaOH solution. With the BA-to-OSFA ratio equal to 1, the mixtures containing FA9 required a W/OSFA ratio between 0.6 and 0.8, and the mixtures containing FA1 required a W/OSFA ratio between 0.5 and 0.6 to meet flowability requirements. The mixtures meeting self-consolidation specifications had 1-day compressive strengths measuring between 5 psi (0.03 MPa) and 87 psi (0.6 MPa).
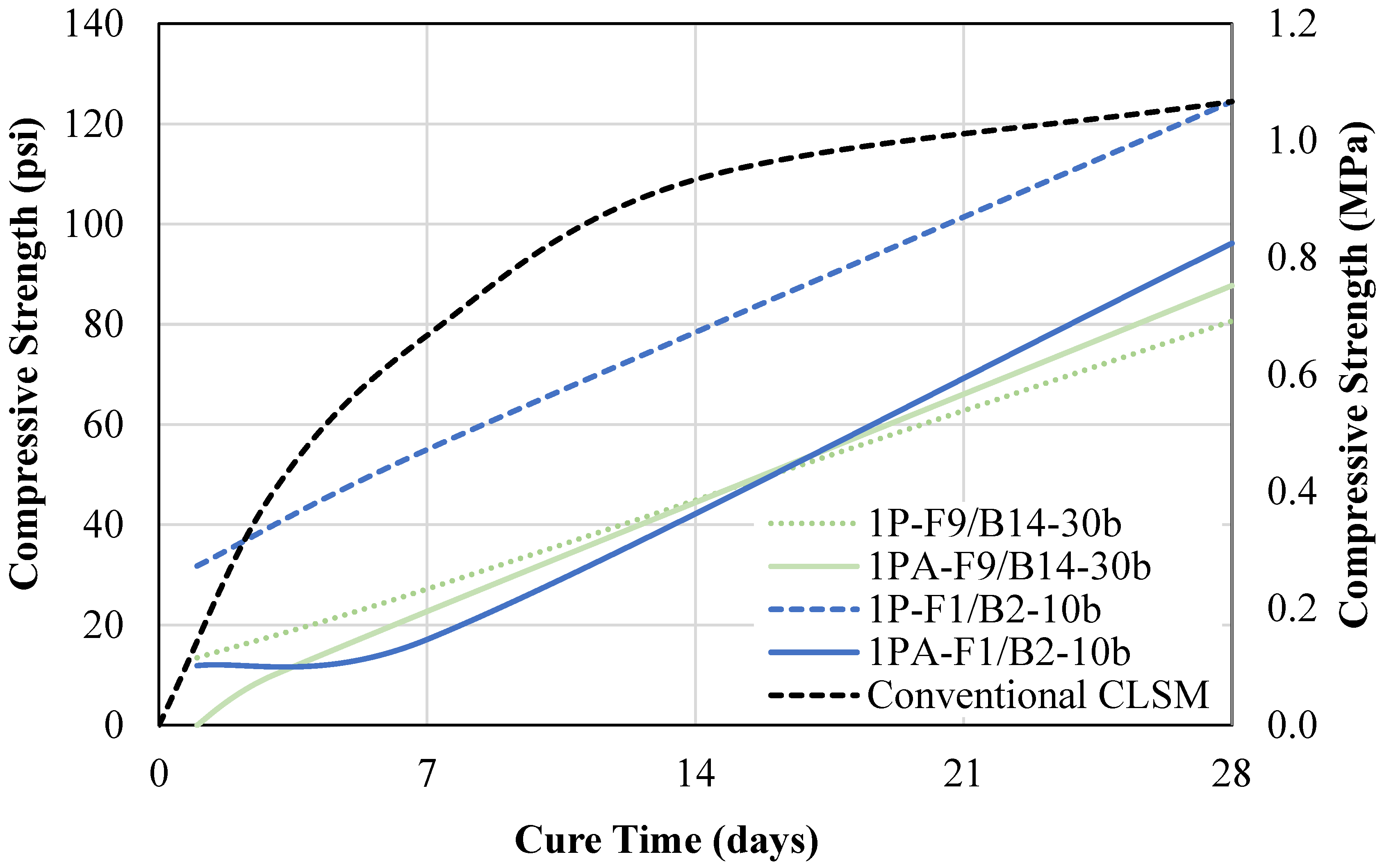
- A one-part geopolymer CLSM, using dry NaOH pellets, was developed. The mixtures containing FA9 increased in spread by at least 5% due to the excess mixing water, but the mixtures containing FA1 decreased in spread by more than 20% due to the heat caused by the exothermic reaction during mixing. The 7-day compressive strength for all one-part mixtures decreased by 1% to 34%, thereby improving the excavatability.
- An increase in the BA-to-OSFA ratio from 1:1 to 3:1 decreased the water demand due to the reduced surface area but increased the NaOH/OSFA ratio. The F9/B14 mixtures showed a decrease in spread by 28% but still met the minimum flowability requirement, and the F1/B2 mixtures showed an increase in spread by 33%. The F9/B14 mixtures increased in compressive strength after 7 days due to the increase in NaOH/OSFA but were still only 30% of the maximum capacity for excavatability by day 28.
- The one-part ratio-adjusted mixtures with F1/B2 had improved excavatability due to the inert characteristics of the fine/coarse BA particles. This mixture measured at a 28-day compressive strength of 96 psi (0.66 MPa), which was 23% lower than the one-part mix.
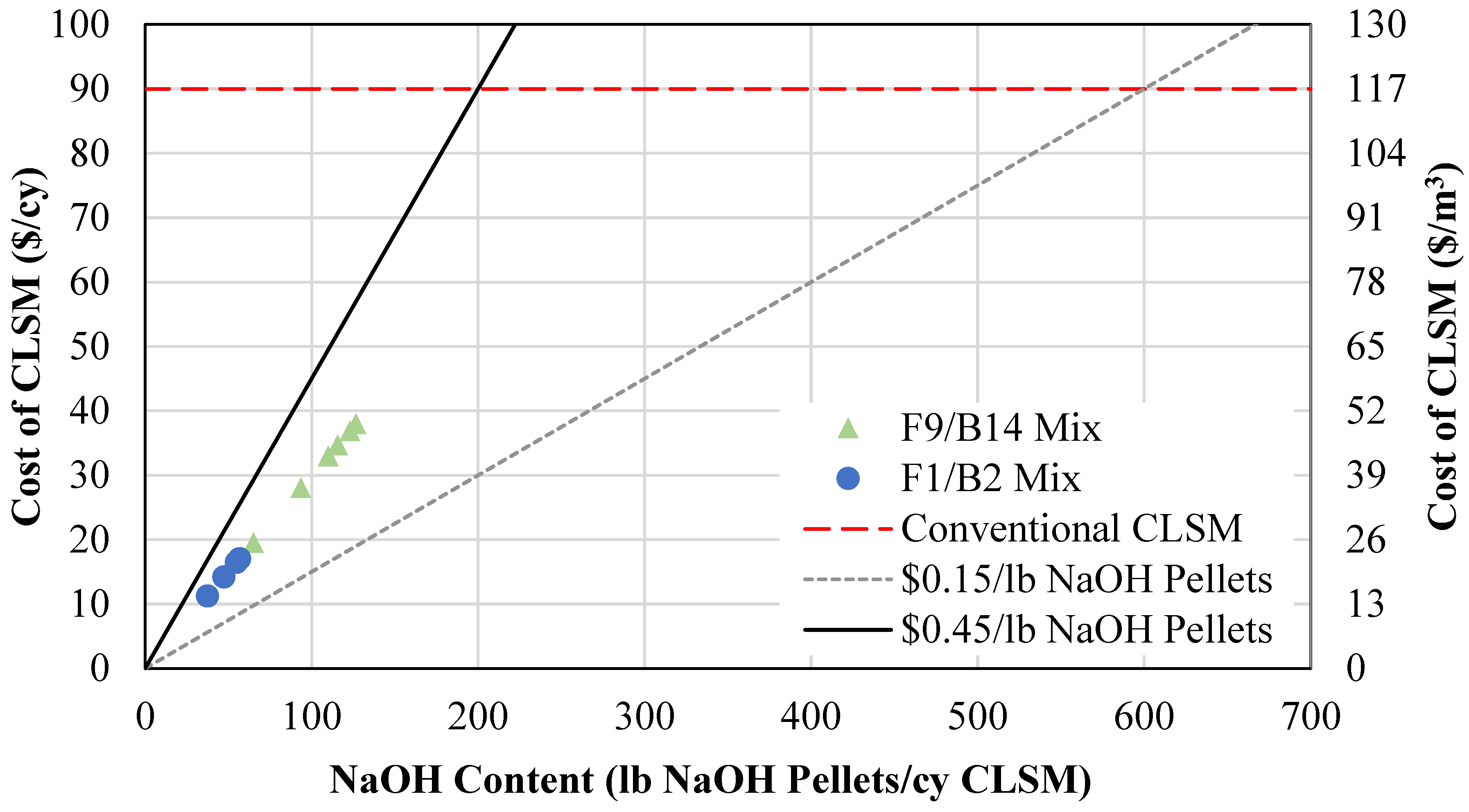
- The geopolymer CLSM mixture proportions can reduce the cost for CLSM by up to 94% with the current NaOH prices, at a price of USD 6 per cubic yard. Based on the cost analysis, one- and two-part mixtures (F1/B2-10b) with an NaOH solution of 0.10 were the most recommended option.
6. Future Work
- It is recommended to evaluate the social impact of the reduction in opportunities in the cement industry as a result of introducing no-cost byproduct as an effective replacement.
- The water absorption and the porosity of the newly proposed geopolymer CLSM need to be investigated for different types of geopolymer CLSM mixtures.
- The long-term performance and durability of the newly proposed geopolymer CLSM need to be investigated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACI Committee 229. Report on Controlled Low-Strength Materials; ACI Committee: Farmington Hills, MI, USA, 2013. [Google Scholar]
- National Ready Mixed Concrete Association. Guide Specification for Controlled Low Strength Materials (CLSM); Specification Guide; National Ready Mixed Concrete Association: Alexandria, VA, USA, 2006. [Google Scholar]
- Rajendran, N. Controlled Low Strength Materials (CLSM), Reported by ACI Committee 229; Westinghouse Savannah River Co.: Aiken, SC, USA, 1997. [Google Scholar]
- Smith, A. Controlled low-strength material. Aberdeen’s Concr. Constr. 1991, 36, 389. [Google Scholar]
- ASTM C494/C494M-17; Standard Specification for Chemical Admixtures for Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- Chittoori, B.; Puppala, A.J.; Pedarla, A.; Vanga, D.P.R. Durability studies on native soil-based controlled low strength materials. In Ground Improvement and Geosynthetics; American Society of Civil Engineers: Reston, VA, USA, 2014; pp. 249–257. [Google Scholar]
- ASTM D6103/D6103M-17; Standard Test Method for Flow Consistency of Controlled Low Strength Material (CLSM). ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4832-16; Standard Test Method for Preparation and Testing of Controlled Low Strength Material (CLSM) Test Cylinders. ASTM International: West Conshohocken, PA, USA, 2019.
- Gomaa, E.; Gheni, A.A.; Kashosi, C.; ElGawady, M.A. Bond strength of eco-friendly class C fly ash-based thermally cured alkali-activated concrete to portland cement concrete. J. Clean. Prod. 2019, 235, 404–416. [Google Scholar] [CrossRef]
- Garside, M. Major Countries in Worldwide Cement Production in 2022. Statista. 2022. Available online: https://www.statista.com/statistics/267364/world-cement-production-bycountry (accessed on 15 July 2022).
- Hanle, L.J.; Jayaraman, K.R.; Smith, J.S. CO2 Emissions Profile of the US Cement Industry; U.S. Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Gomaa, E.; Sargon, S.; Kashosi, C.; Gheni, A.; ElGawady, M.A. Mechanical properties of high early strength class C fly ash-based alkali activated concrete. Transp. Res. Rec. 2020, 2674, 430–443. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2017; U.S. Geological Survey: Reston, VA, USA, 2017; 202p. [Google Scholar]
- Torres, A.; Liu, J.; Brandt, J.; Lear, K. The World Is Facing a Global Sand Crisis. The Conversation. 2017. Available online: https://scholarworks.boisestate.edu/cgi/viewcontent.cgi?article=1018&context=hes_facpubs (accessed on 26 June 2025).
- World Coal Association. Coal’s Role in Electricity Generation Worldwide; Coal & Electricity; World Coal Association: London, UK, 2018. [Google Scholar]
- American Coal Ash Association. 2016 Coal Combustion Product (CCP) Production & Use Survey Report; American Coal Ash Association: Aurora, CO, USA, 2016. [Google Scholar]
- American Coal Ash Association. 2017 Coal Combustion Product (CCP) Production & Use Survey Report; American Coal Ash Association: Aurora, CO, USA, 2017. [Google Scholar]
- United States Environmental Protection Agency. Frequent Questions About the 2015 Coal Ash Disposal Rule; United States Environmental Protection Agency: Washington, DC, USA, 2018. [Google Scholar]
- Sargon, S.; Gomaa, E.; Gheni, A.A.; ElGawady, M.A. Optimization of Curing Parameters of Class C Fly-Ash-Based Alkali-Activated Mortar. ACI Mater. J. 2022, 119, 53–66. [Google Scholar]
- Physicians for Social Responsibility. Coal Ash: Hazardous to Human Health; Physicians for Social Responsibility: Washington, DC, USA, 2018. [Google Scholar]
- Burt, E.; Orris, P.; Buchanan, S. Scientific Evidence of Health Effects from Coal Use in Energy Generation; School of Public Health, University of Illinois and Health Care Without Harm: Chicago, IL, USA; Washington, DC, USA, 2013. [Google Scholar]
- Kuźnia, M. A Review of Coal Fly Ash Utilization: Environmental, Energy, and Material Assessment. Energies 2024, 18, 52. [Google Scholar] [CrossRef]
- Raghavendra, T.; Udayashankar, B. Flow and strength characteristics of CLSM using ground granulated blast furnace slag. J. Mater. Civ. Eng. 2013, 26, 04014050. [Google Scholar] [CrossRef]
- Pierce, C.E.; Tripathi, H.; Brown, T.W. Cement kiln dust in controlled low-strength materials. ACI Mater. J. 2003, 100, 455–462. [Google Scholar]
- Do, T.-M.; Kim, Y.-S.; Ryu, B.-C. Improvement of engineering properties of pond ash based CLSM with cementless binder and artificial aggregates made of bauxite residue. Int. J. Geo-Eng. 2015, 6, 8. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Do, T.M.; Kim, H.-K.; Kang, G. Utilization of excavated soil in coal ash-based controlled low strength material (CLSM). Constr. Build. Mater. 2016, 124, 598–605. [Google Scholar] [CrossRef]
- Raghavendra, T.; Udayashankar, B. Engineering properties of controlled low strength materials using flyash and waste gypsum wall boards. Constr. Build. Mater. 2015, 101, 548–557. [Google Scholar] [CrossRef]
- Gemperline, C.S.; Durham, S. Beneficial use of recycled materials in controlled low strength materials. In ICPTT 2012: Better Pipeline Infrastructure for a Better Life; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 1305–1316. [Google Scholar]
- Le, D.-H.; Nguyen, K.-H. An Assessment of Eco-Friendly Controlled Low-Strength Material. Procedia Eng. 2016, 142, 260–267. [Google Scholar] [CrossRef][Green Version]
- Batt, A.; Garg, A. Partial Replacement of Wood Ash with Ordinary Portland Cement and Foundry Sand as Fine Aggregate. J. Civ. Env. Eng. 2017, 7, 2. [Google Scholar] [CrossRef]
- Naik, T.R.; Kraus, R.N.; Siddique, R. Demonstration of Manufacturing Technology for Concrete and CLSM Utilizing Wood Ash from Wisconsin; Report No REP-485; UMW Center for By-Products Utilization: Milwaukee, WI, USA, 2002. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Compiegne, France, 2008. [Google Scholar]
- Nguyen, H.T.; Pham, T.K.; Promentilla, M.A. Development of Geopolymer-Based Materials from Coal Bottom Ash and Rice Husk Ash with Sodium Silicate Solutions. In Proceedings of the Congrès International de Géotechnique–Ouvrages–Structures, Ho Chi Minh City, Vietnam, 26–27 October 2017; pp. 402–410. [Google Scholar]
- Gunasekara, M.; Law, D.; Setunge, S. Effect of composition of fly ash on compressive strength of fly ash based geopolymer mortar. In Proceedings of the 23rd Australasian Conference on the Mechanics of Structures and Materials (ACMSM23), Byron Bay, Australia, 9–12 December 2014. [Google Scholar]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. Mater. J. 2004, 101, 467–472. [Google Scholar]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Fernández-Pereira, C. Coal fly ash-slag-based geopolymers: Microstructure and metal leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef]
- Han, T.; Gomaa, E.; Gheni, A.; Huang, J.; ElGawady, M.; Kumar, A.J. Machine learning enabled closed-form models to predict strength of alkali-activated systems. J. Am. Ceram. Soc. 2022, 105, 4414–4425. [Google Scholar] [CrossRef]
- Zarębska, K.; Szczurowski, J.; Muszyńska, J.; Baran, P. Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management. Materials 2024, 17, 3515. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Cong, T.D.; Phuong, T.; Vu, M.T.; Nguyen, T.H. Effect of calcium hydroxide on compressive strength and microstructure of geopolymer containing admixture of kaolin, fly ash, and red mud. Appl. Sci. 2023, 13, 5034. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Jamali, S.; Naganathan, S. Performance Assessment of Cementless Controlled Low-Strength Material (CLSM) Utilizing Coal Ashes. Jordan J. Civ. Eng. 2015, 9, 102–116. [Google Scholar]
- Lee, N.K.; Kim, H.K.; Park, I.; Lee, H.-K. Alkali-activated, cementless, controlled low-strength materials (CLSM) utilizing industrial by-products. Constr. Build. Mater. 2013, 49, 738–746. [Google Scholar] [CrossRef]
- Naganathan, S.; Razak, H.A.; Hamid, S.N.A. Corrosivity and leaching behavior of controlled low-strength material (CLSM) made using bottom ash and quarry dust. J. Environ. Manag. 2013, 128, 637–641. [Google Scholar] [CrossRef]
- Lee, C.; Shin, H.; Lee, J.-S. Behavior of sand–rubber particle mixtures: Experimental observations and numerical simulations. Int. J. Numer. Anal. Methods Geomech. 2014, 38, 1651–1663. [Google Scholar] [CrossRef]
- Naganathan, S.; Razak, H.A.; Hamid, S.N.A. Properties of controlled low-strength material made using industrial waste incineration bottom ash and quarry dust. Mater. Des. 2012, 33, 56–63. [Google Scholar] [CrossRef]
- Razak, H.A.; Naganathan, S.; Hamid, S.N.A. Performance appraisal of industrial waste incineration bottom ash as controlled low-strength material. J. Hazard. Mater. 2009, 172, 862–867. [Google Scholar] [CrossRef]
- Nataraja, M.; Rao, N.V. Controlled Low Strength Material with Fly Ash and Cinder Aggregates: An Effective Replacement for the Compacted Backfill. Indian J. Adv. Chem. Sci. 2016, S1, 289–293. [Google Scholar]
- Ohlheiser, T.R. Utilization of recycled glass as aggregate in controlled low-strength material (CLSM). In The Design and Application of Controlled Low-Strength Materials (Flowable Fill); ASTM International: West Conshohocken, PA, USA, 1998. [Google Scholar]
- Horiguchi, T.; Fujita, R.; Shimura, K. Applicability of controlled low-strength materials with incinerated sewage sludge ash and crushed-stone powder. J. Mater. Civ. Eng. 2010, 23, 767–771. [Google Scholar] [CrossRef]
- Hanson, J.; Stone, G.; Yesiller, N. Use of Post-Consumer Corrugated Fiberboard as Fine Aggregate Replacement in Controlled Low-Strength Materials. In Testing and Specification of Recycled Materials for Sustainable Geotechnical Construction; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Cheung, T.; Jansen, D.C.; Hanson, J.L. Engineering controlled low strength materials using scrap tire rubber. In GeoCongress 2008: Characterization, Monitoring, and Modeling of GeoSystems; American Society of Civil Engineers: Reston, VA, USA, 2008; pp. 622–629. [Google Scholar]
- Pierce, C.; Blackwell, M. Potential of scrap tire rubber as lightweight aggregate in flowable fill. Waste Manag. 2003, 23, 197–208. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chen, B.-T.; Wu, Y.-W. A study of the fresh properties of controlled low-strength rubber lightweight aggregate concrete (CLSRLC). Constr. Build. Mater. 2013, 41, 526–531. [Google Scholar] [CrossRef]
- Gheni, A.A.; ElGawady, M.A.; Myers, J.J. Mechanical Characterization of Concrete Masonry Units Manufactured with Crumb Rubber Aggregate. ACI Mater. J. 2017, 114, 65–76. [Google Scholar] [CrossRef]
- Bhat, S.T.; Lovell, C. Flowable fill using waste foundry sand: A substitute for compacted or stabilized soil. In Testing Soil Mixed with Waste or Recycled Materials; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Tikalsky, P.; Gaffney, M.; Regan, R. Properties of controlled low-strength material containing foundry sand. ACI Struct. J. 2000, 97, 698–702. [Google Scholar]
- Finney, A.J.; Shorey, E.F.; Anderson, J. Use of native soil in place of aggregate in controlled low strength material (CLSM). In Pipelines 2008: Pipeline Asset Management: Maximizing Performance of our Pipeline Infrastructure; American Society of Civil Engineers: Reston, VA, USA, 2008; pp. 1–13. [Google Scholar]
- Mneina, A.; Soliman, A.; Ahmed, A.; El Naggar, M. Engineering properties of Controlled Low-Strength Materials containing Treated Oil Sand Waste. Constr. Build. Mater. 2018, 159, 277–285. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Wang, H.-Y.; Shu, C.-Y.; Su, D.-S. Engineering properties of controlled low-strength materials containing waste oyster shells. Constr. Build. Mater. 2013, 46, 128–133. [Google Scholar] [CrossRef]
- Kong, X.; Wang, G.; Rong, S.; Liang, Y.; Liu, M.; Zhang, Y. Utilization of fly ash and red mud in soil-based controlled low strength materials. Coatings 2023, 13, 893. [Google Scholar] [CrossRef]
- Han, J.; Jo, Y.; Kim, Y.; Kim, B. Development of high-performance fly-ash-based controlled low-strength materials for backfilling in metropolitan cities. Appl. Sci. 2023, 13, 9377. [Google Scholar] [CrossRef]
- ASTM D7348-13; Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D4326-13; Standard Test Method for Major and Minor Elements in Coal and Coke Ash by X-Ray Fluorescence. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM C136/C136M-14; Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. ASTM International: West Conschohocken, PA, USA, 2014.
- ASTM C33/C33M-16e1; Standard Specification for Concrete Aggregates. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C128-15; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C117-17; Standard Test Method for Materials Finer than 75-m (No. 200) Sieve in Mineral Aggregates by Washing. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D7428-15; Standard Test Method for Resistance of Fine Aggregate to Degradation by Abrasion in the Micro-Deval Apparatus. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C1252-17; Standard Test Methods for Uncompacted Void Content of Fine Aggregate (as Influenced by Particle Shape, Surface Texture, and Grading). ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C40/C40M-16; Standard Test Method for Organic Impurities in Fine Aggregates for Concrete. ASTM International: West Conshohocken, PA, USA, 2016.
- InfoMine USA, Inc. 2018 Mine & Mill Equipment Costs Complete Guide; InfoMine USA, Inc.: Spokane Valley, WA, USA, 2018. [Google Scholar]
- Rolla Ready Mix. 2018. Available online: https://bmcenterprises.com/locations/rrm/ (accessed on 26 June 2025).


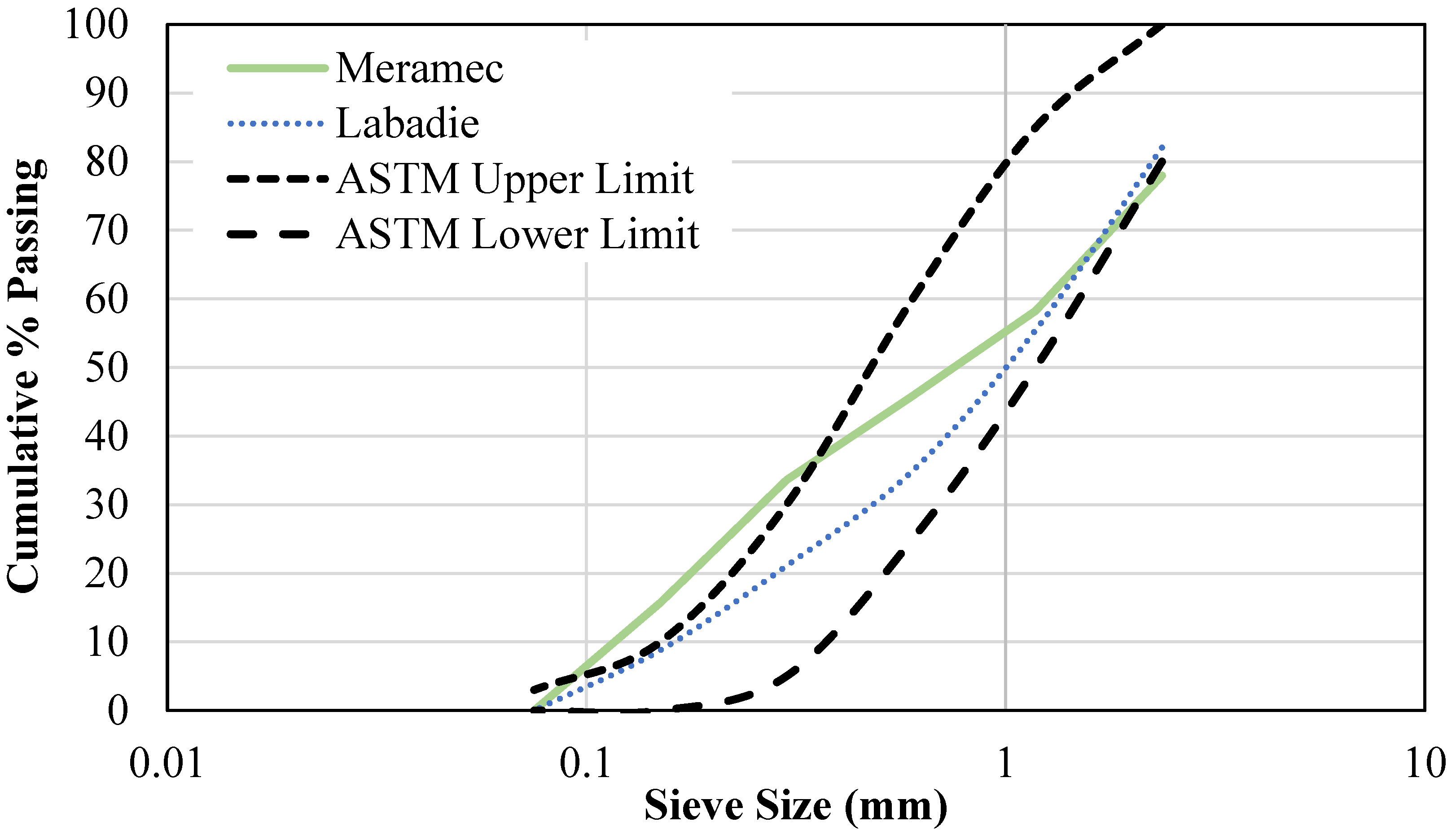
| Meramec | Labadie | |||||
|---|---|---|---|---|---|---|
| Fly Ash (F9) | Bottom Ash (B14) | Fly Ash (F1) | Bottom Ash (B2) | |||
| Powder | Powder | Coarse | Powder | Powder | Coarse | |
| Na2O | 1.29 | 0.39 | 0.51 | 1.43 | 0.61 | 0.69 |
| MgO | 4.99 | 3.99 | 3.68 | 5.36 | 4.42 | 3.88 |
| Al2O3 | 20.1 | 17.6 | 18.1 | 19.7 | 18.1 | 18.3 |
| SiO2 | 38.3 | 41.6 | 45.9 | 36.0 | 39.9 | 44.7 |
| P2O5 | 0.22 | 0.20 | 0.22 | 1.97 | 1.11 | 1.38 |
| K2O | 0.49 | 0.49 | 0.52 | 0.52 | 0.44 | 0.42 |
| CaO | 25.0 | 25.0 | 21.9 | 26.9 | 26.3 | 23.0 |
| TiO2 | 1.44 | 1.57 | 1.48 | 1.49 | 1.56 | 1.44 |
| Fe2O3 | 6.54 | 8.09 | 8.66 | 6.65 | 7.46 | 6.73 |
| SiO2 + Al2O3 + Fe2O3 | 64.9 | 67.3 | 70.9 | 62.4 | 65.5 | 69.7 |
| Loss on Ignition | 8.54 | 14.4 | 0.93 | 1.70 | ||
| Standard | Property | B14 | B2 |
|---|---|---|---|
| ASTM C136 [68] | Powder particle composition, % | 21.8 | 9.20 |
| Fine particle composition, % | 66.7 | 78.3 | |
| Coarse particle composition, % | 11.4 | 12.4 | |
| ASTM C128 [70] | Specific gravity, oven dry | 2.53 | 2.20 |
| Specific gravity, saturated surface dry | 2.58 | 2.29 | |
| Specific gravity, apparent | 2.67 | 2.41 | |
| Water absorption, % | 2.20 | 4.00 | |
| ASTM C117 [71] | Materials finer than #200 sieve, % | 2.60 | 1.17 |
| ASTM D7428 [72] | Micro-Deval, % material lost | 12.6 | 8.48 |
| ASTM C1252 [73] | Uncompacted voids, % | 75.2 | 56.9 |
| ASTM C40 [74] | Organic impurities | 0 | 2 |
| Test | Mix Design | Cement (C) | NaOH Solution (NL) | NaOH Pellets (NP) | Class C Fly Ash (FA) | Off-Specification Fly Ash (OSFA) | River Sand (RS) | Bottom Ash (BA) | Water (W) |
|---|---|---|---|---|---|---|---|---|---|
| Reference | R-OPC/RS | 0.25 | 0.75 | 6.75 | 0.95 | ||||
| R-F9/RS | 0.09 | 1.00 | 7.06 | 1.11 * | |||||
| R-F1/RS | 0.03 | 1.00 | 7.06 | 1.05 * | |||||
| R-OPC/B14 | 0.25 | 0.75 | 2.75 | 1.74 * | |||||
| R-OPC/B2 | 0.25 | 0.75 | 5.48 | 1.25 * | |||||
| 2-Part | 2P-F9/B14-10 | 0.10 | 1.00 | 1.00 | 0.70 | ||||
| 2P-F9/B14-20a | 0.20 | 1.00 | 1.00 | 0.60 | |||||
| 2P-F9/B14-20b | 0.20 | 1.00 | 1.00 | 0.70 | |||||
| 2P-F9/B14-25 | 0.25 | 1.00 | 1.00 | 0.70 | |||||
| 2P-F9/B14-30a | 0.30 | 1.00 | 1.00 | 0.60 | |||||
| 2P-F9/B14-30b | 0.30 | 1.00 | 1.00 | 0.70 | |||||
| 2P-F9/B14-30c | 0.30 | 1.00 | 1.00 | 0.80 | |||||
| 2P-F9/B14-35 | 0.35 | 1.00 | 1.00 | 0.65 | |||||
| 2P-F9/B14-40a | 0.40 | 1.00 | 1.00 | 0.40 | |||||
| 2P-F9/B14-40b | 0.40 | 1.00 | 1.00 | 0.60 | |||||
| 2P-F9/B14-40c | 0.40 | 1.00 | 1.00 | 0.65 | |||||
| 2P-F9/B14-40d | 0.40 | 1.00 | 1.00 | 0.70 | |||||
| 2P-F9/B14-50 | 0.50 | 1.00 | 1.00 | 0.50 | |||||
| 2P-F1/B2-05a | 0.05 | 1.00 | 1.00 | 0.45 | |||||
| 2P-F1/B2-05b | 0.05 | 1.00 | 1.00 | 0.55 | |||||
| 2P-F1/B2-10a | 0.10 | 1.00 | 1.00 | 0.45 | |||||
| 2P-F1/B2-10b | 0.10 | 1.00 | 1.00 | 0.55 | |||||
| 2P-F1/B2-13a | 0.13 | 1.00 | 1.00 | 0.45 | |||||
| 2P-F1/B2-13b | 0.13 | 1.00 | 1.00 | 0.50 | |||||
| 2P-F1/B2-13c | 0.13 | 1.00 | 1.00 | 0.52 | |||||
| 2P-F1/B2-15a | 0.15 | 1.00 | 1.00 | 0.40 | |||||
| 2P-F1/B2-15b | 0.15 | 1.00 | 1.00 | 0.45 | |||||
| 2P-F1/B2-15c | 0.15 | 1.00 | 1.00 | 0.47 | |||||
| 2P-F1/B2-15d | 0.15 | 1.00 | 1.00 | 0.50 | |||||
| 2P-F1/B2-20 | 0.20 | 1.00 | 1.00 | 0.40 | |||||
| 1-Part | 1P-F9/B14-30b | 0.30 | 1.00 | 1.00 | 0.71 | ||||
| 1P-F9/B14-30b | 0.09 | 1.00 | 1.00 | 0.90 | |||||
| 1P-F9/B14-35 | 0.35 | 1.00 | 1.00 | 0.71 | |||||
| 1P-F9/B14-35 | 0.10 | 1.00 | 1.00 | 0.91 | |||||
| 1P-F1/B2-10b | 0.10 | 1.00 | 1.00 | 0.50 | |||||
| 1P-F1/B2-10b | 0.03 | 1.00 | 1.00 | 0.57 | |||||
| 1P-F1/B2-20 | 0.20 | 1.00 | 1.00 | 0.70 | |||||
| 1P-F1/B2-20 | 0.06 | 1.00 | 1.00 | 0.91 | |||||
| Ratio | 1P-F9/B14-30b | 0.09 | 1.00 | 1.00 | 0.82 | ||||
| 1PA-F9/B14-30b | 0.18 | 1.00 | 1.00 | 0.82 | |||||
| 1PA-F1/B2-10b | 0.03 | 1.00 | 1.00 | 0.57 | |||||
| 1PA-F1/B2-10b | 0.06 | 1.00 | 3.00 | 1.14 |
| Mix Design | Cost, min (USD/cy) | Cost, max (USD/cy) | Cost, ave (USD/cy) |
|---|---|---|---|
| F9/B14-20a | 10 | 29 | 20 |
| F9/B14-30b | 14 | 42 | 28 |
| F9/B14-35 | 16 | 49 | 33 |
| F9/B14-40b | 19 | 57 | 38 |
| F9/B14-40c | 18 | 55 | 37 |
| F9/B14-40d | 17 | 52 | 35 |
| F1/B2-10b | 6 | 17 | 11 |
| F1/B2-13c | 7 | 21 | 14 |
| F1/B2-15b | 9 | 26 | 17 |
| F1/B2-15d | 8 | 25 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanDomelen, A.K.; Gheni, A.A.; Gomaa, E.; ElGawady, M.A. Geopolymer CLSM with Off-Specification Fly Ash and Bottom Ash: A Sustainable Approach to Hazardous Waste Utilization. Materials 2025, 18, 3105. https://doi.org/10.3390/ma18133105
VanDomelen AK, Gheni AA, Gomaa E, ElGawady MA. Geopolymer CLSM with Off-Specification Fly Ash and Bottom Ash: A Sustainable Approach to Hazardous Waste Utilization. Materials. 2025; 18(13):3105. https://doi.org/10.3390/ma18133105
Chicago/Turabian StyleVanDomelen, Alexis K., Ahmed A. Gheni, Eslam Gomaa, and Mohamed A. ElGawady. 2025. "Geopolymer CLSM with Off-Specification Fly Ash and Bottom Ash: A Sustainable Approach to Hazardous Waste Utilization" Materials 18, no. 13: 3105. https://doi.org/10.3390/ma18133105
APA StyleVanDomelen, A. K., Gheni, A. A., Gomaa, E., & ElGawady, M. A. (2025). Geopolymer CLSM with Off-Specification Fly Ash and Bottom Ash: A Sustainable Approach to Hazardous Waste Utilization. Materials, 18(13), 3105. https://doi.org/10.3390/ma18133105






