Recrystallization and Second-Phase Precipitation in Nb-V Microalloyed Steels: A Thermal Simulation Study
Abstract
1. Introduction
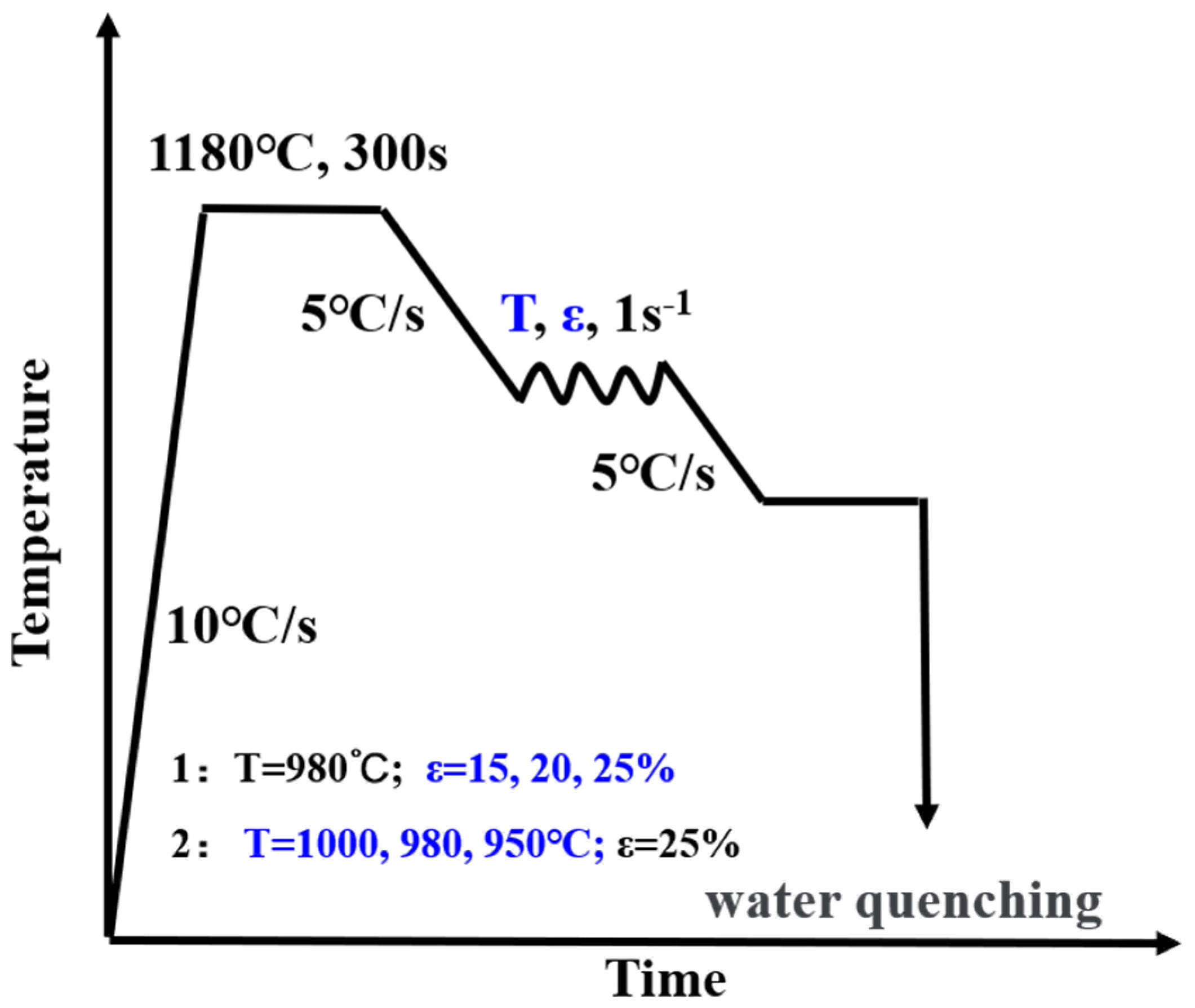
2. Materials and Methods
3. Results
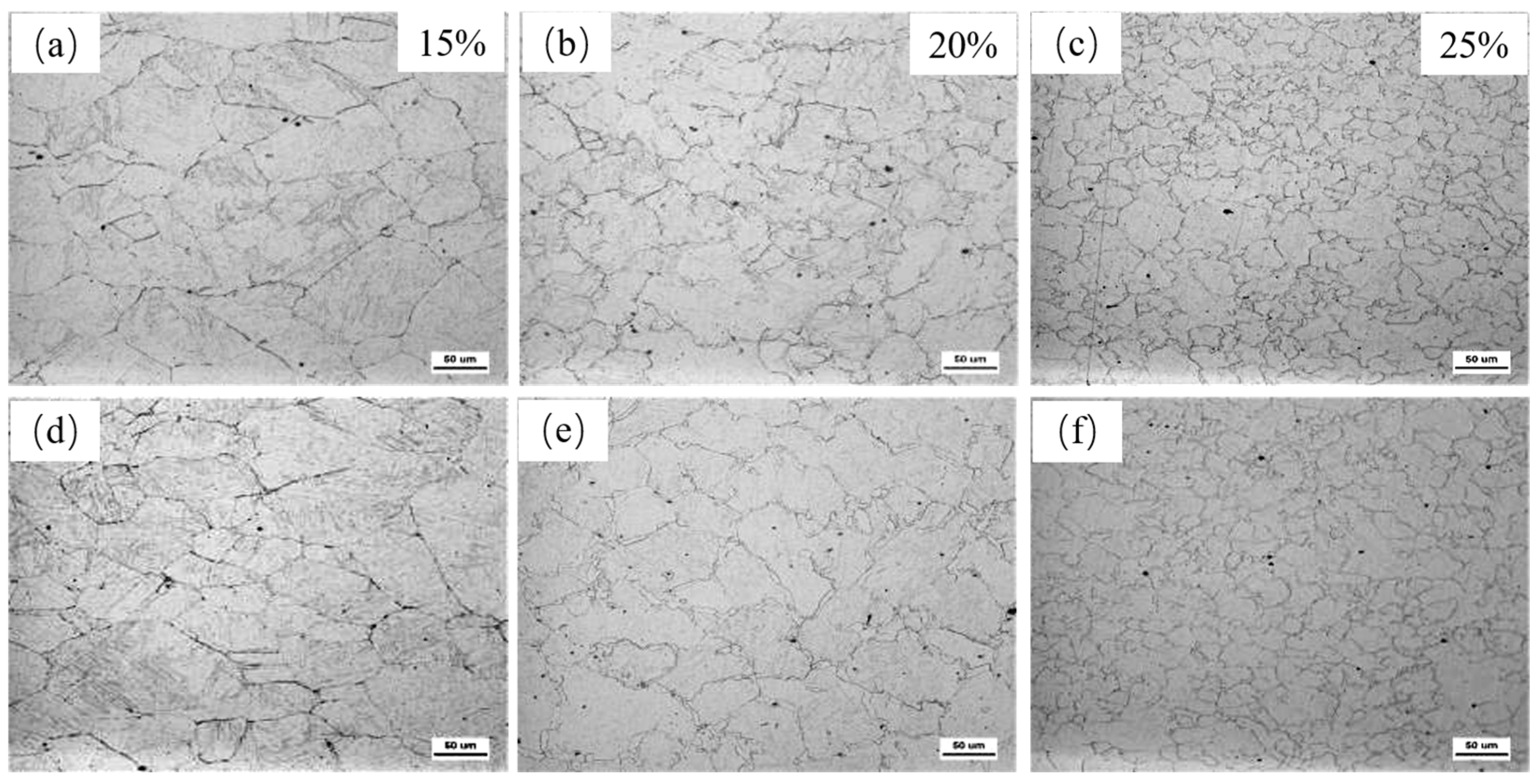
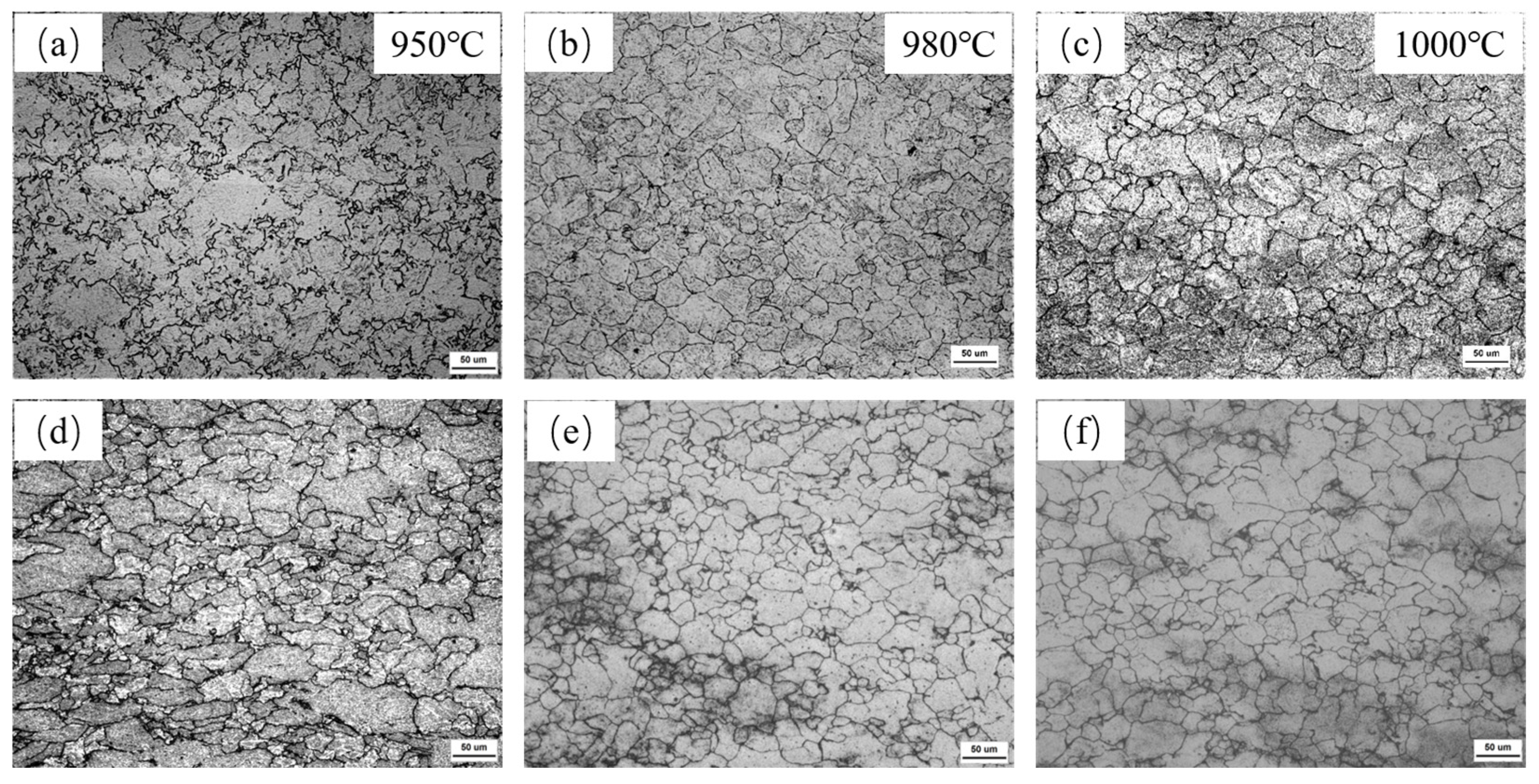
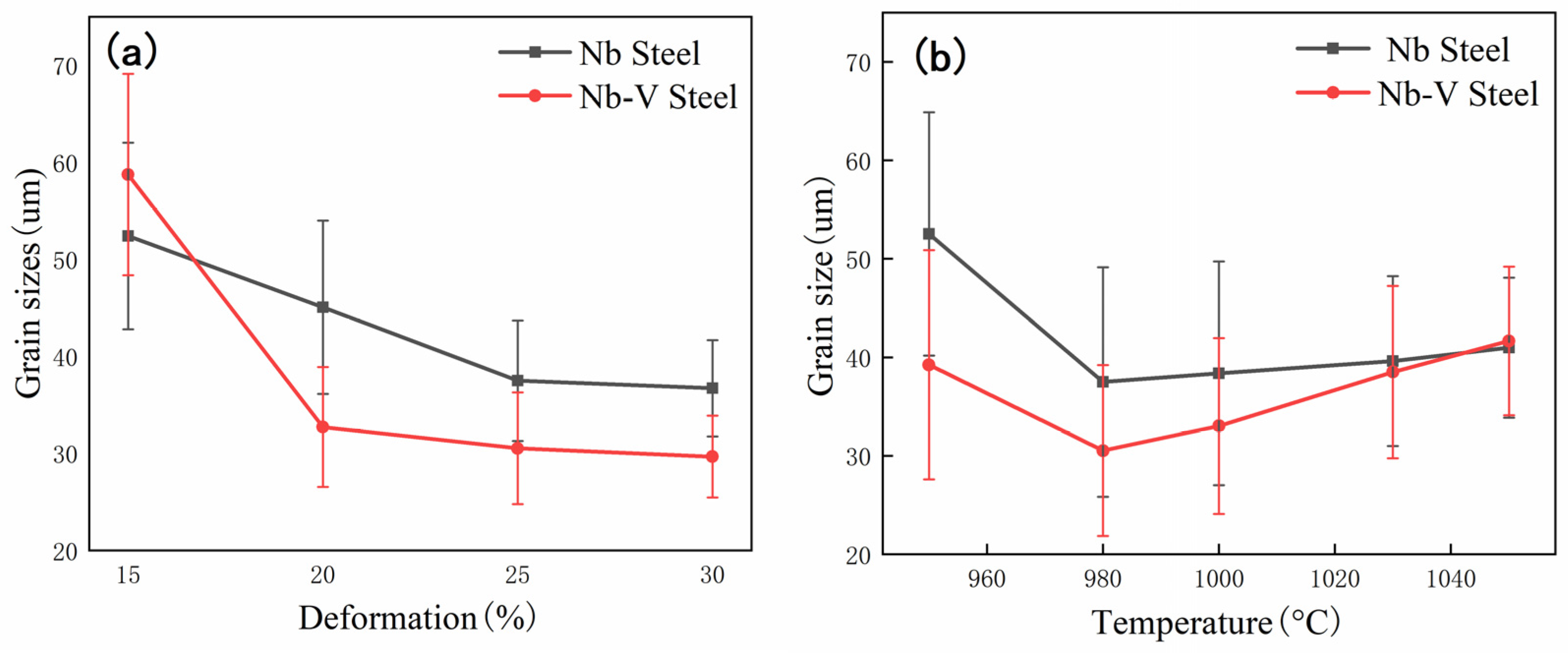
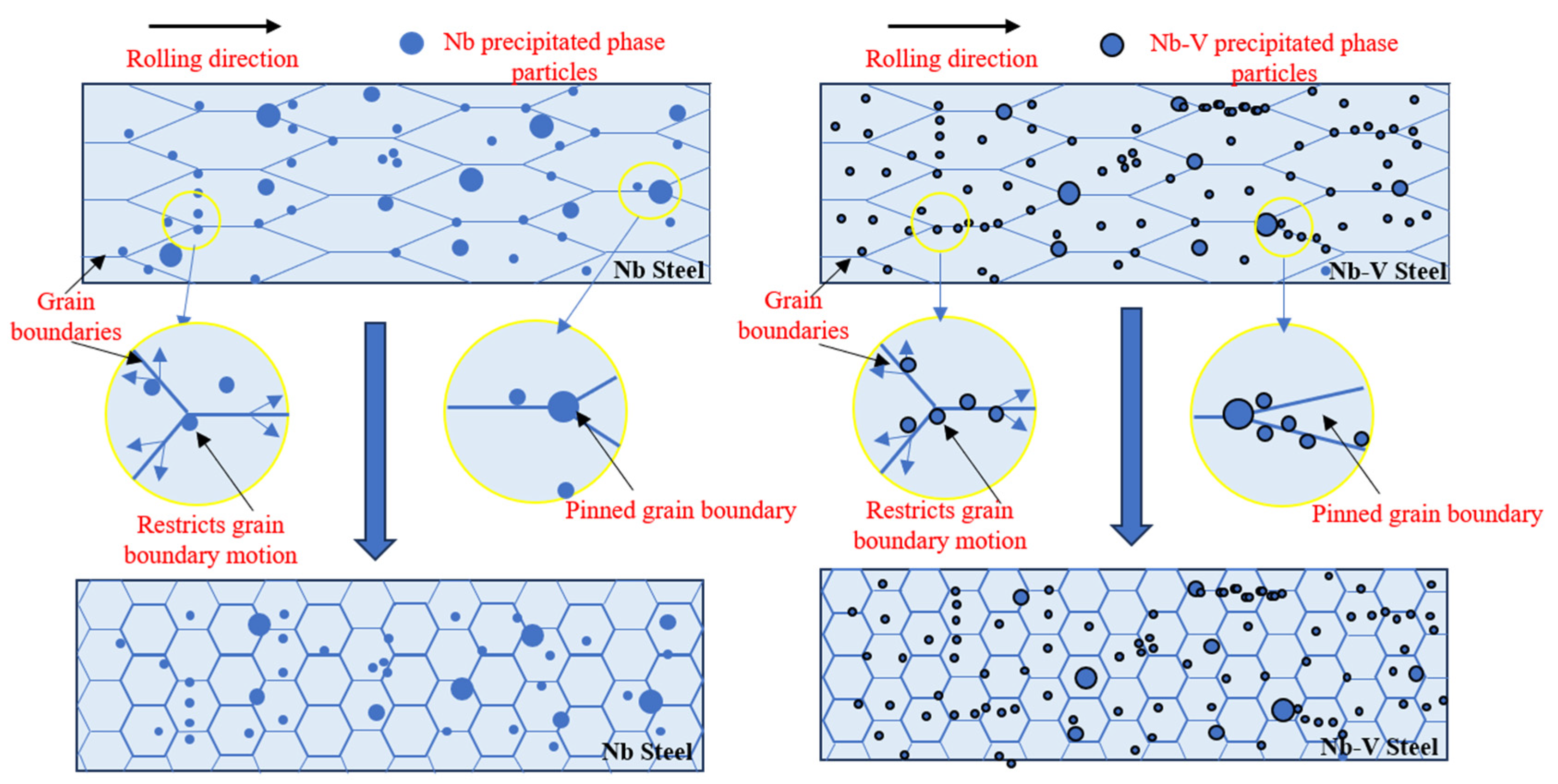
3.1. Effect of Nb-V Composite on Recrystallized Grain Size
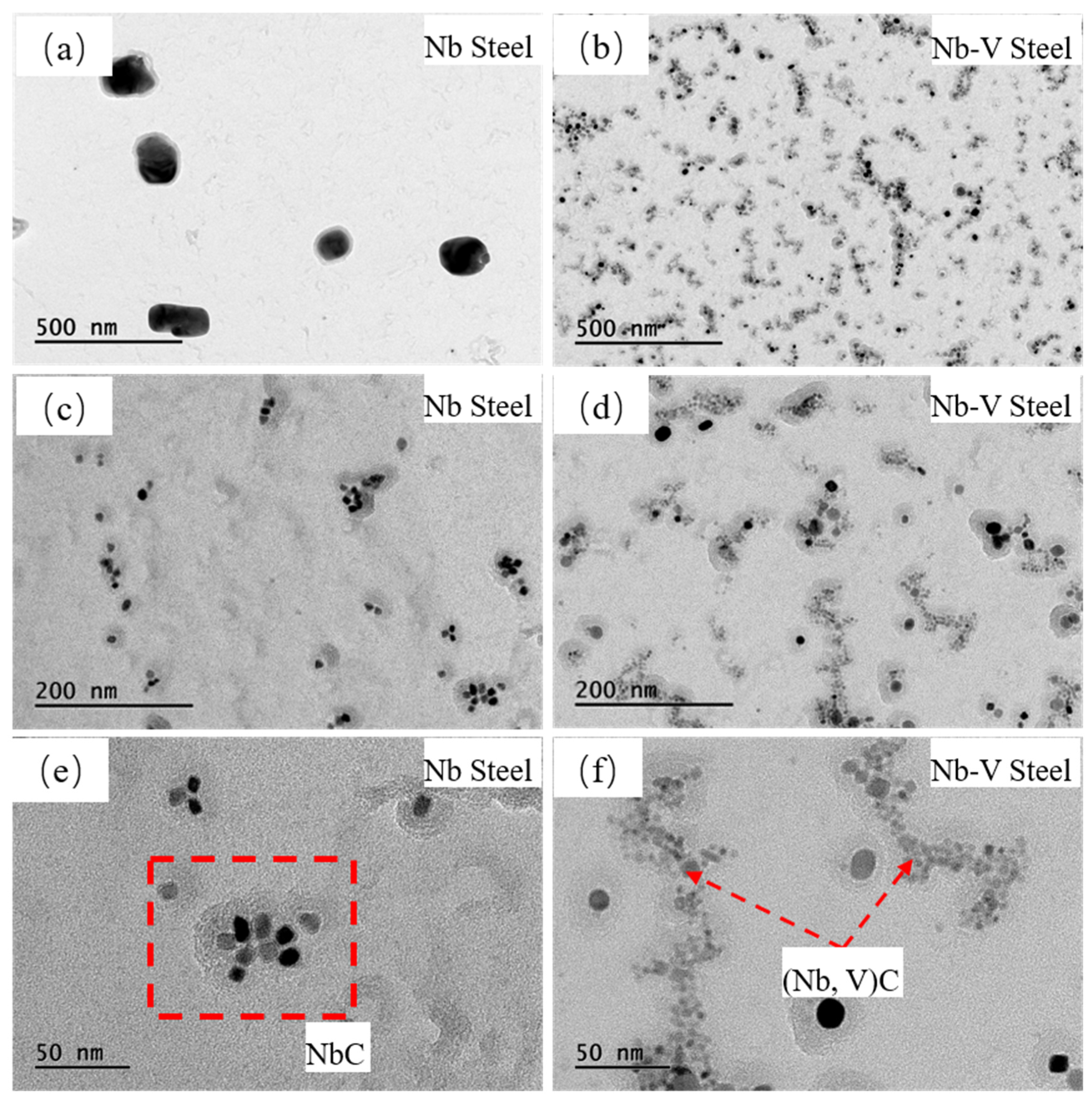
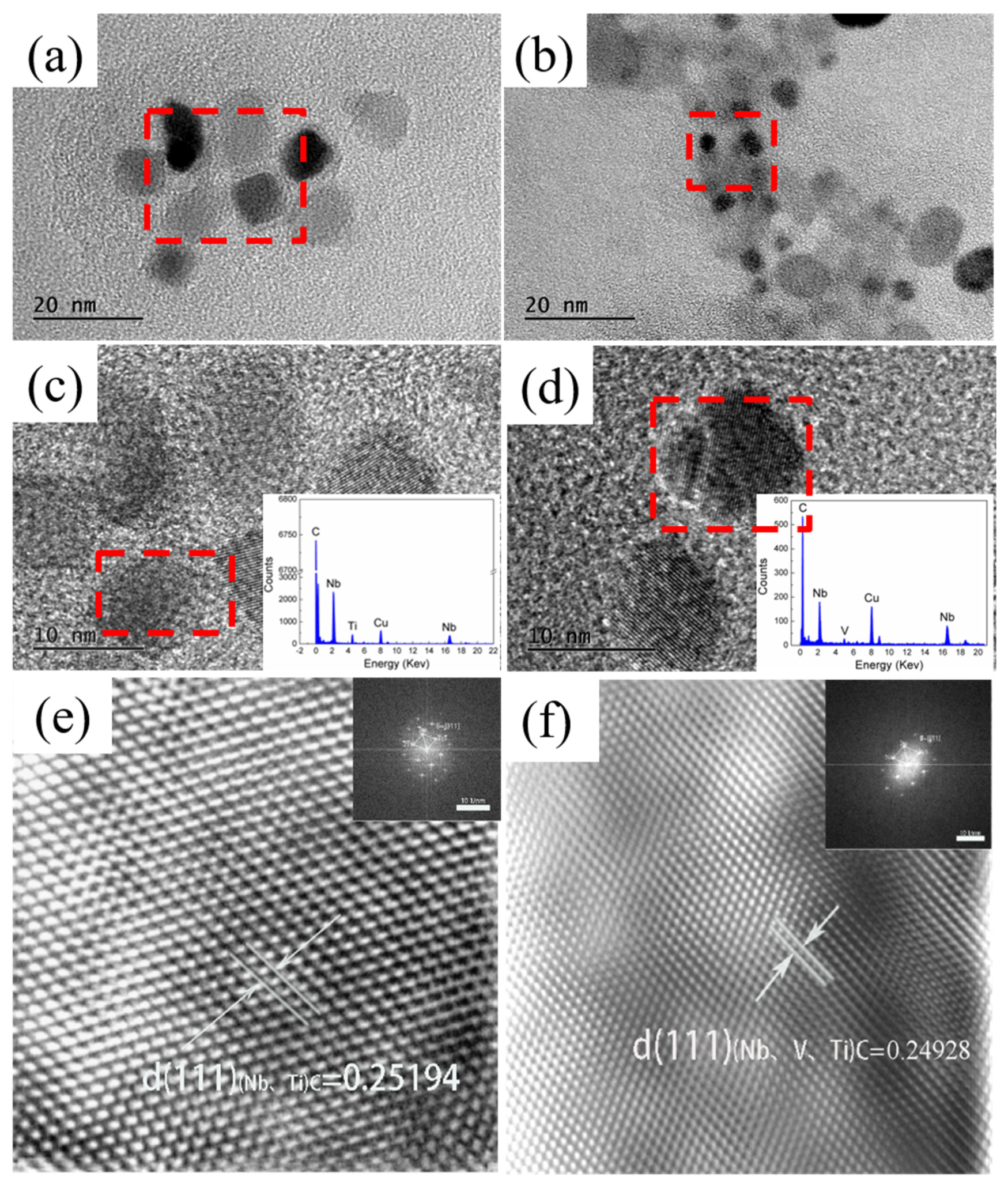
3.2. Effect of Nb-V Composite on the Precipitation of the Second Phase
4. Discussion
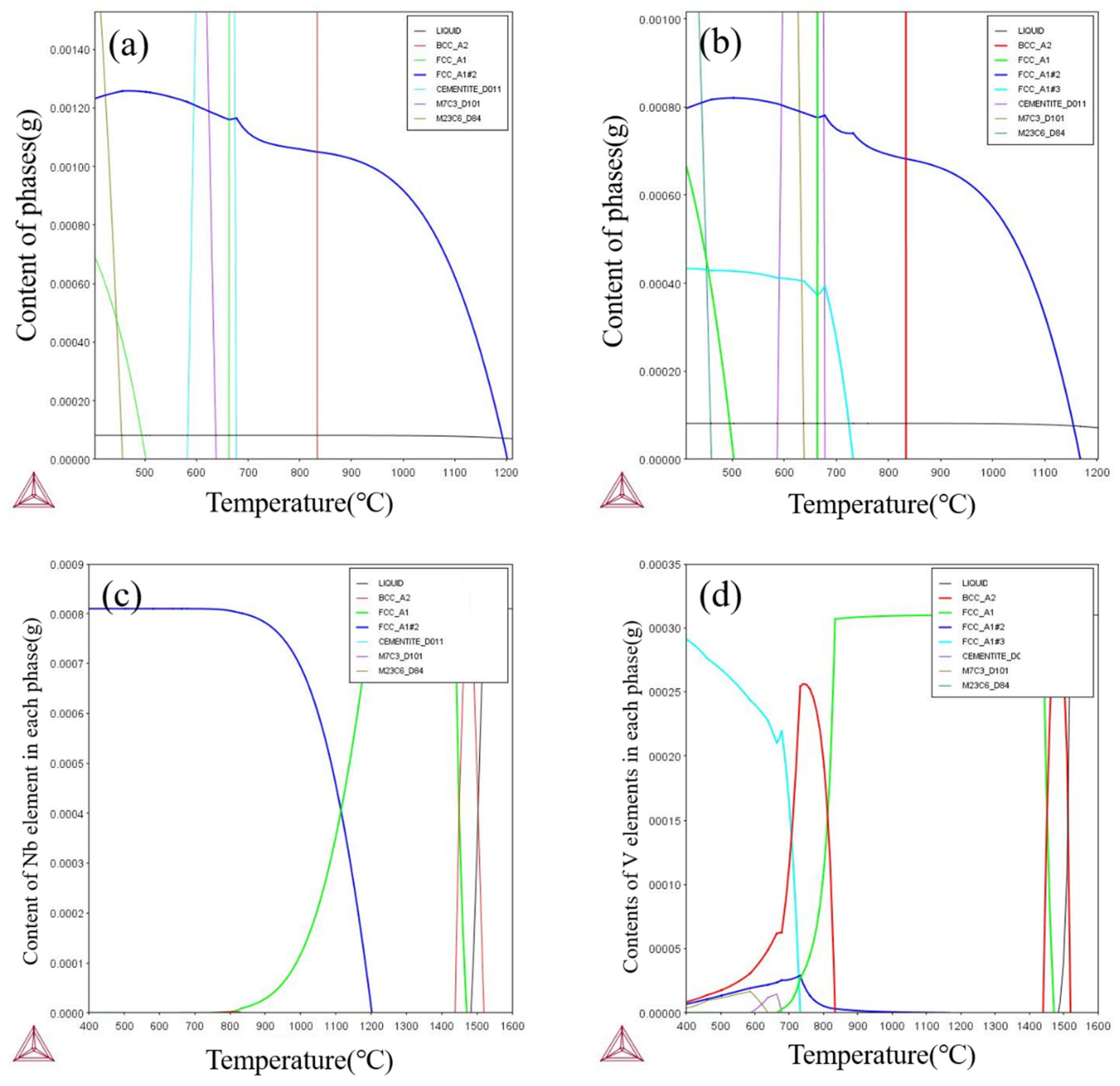
4.1. Thermodynamic Calculations for Experimental Steels
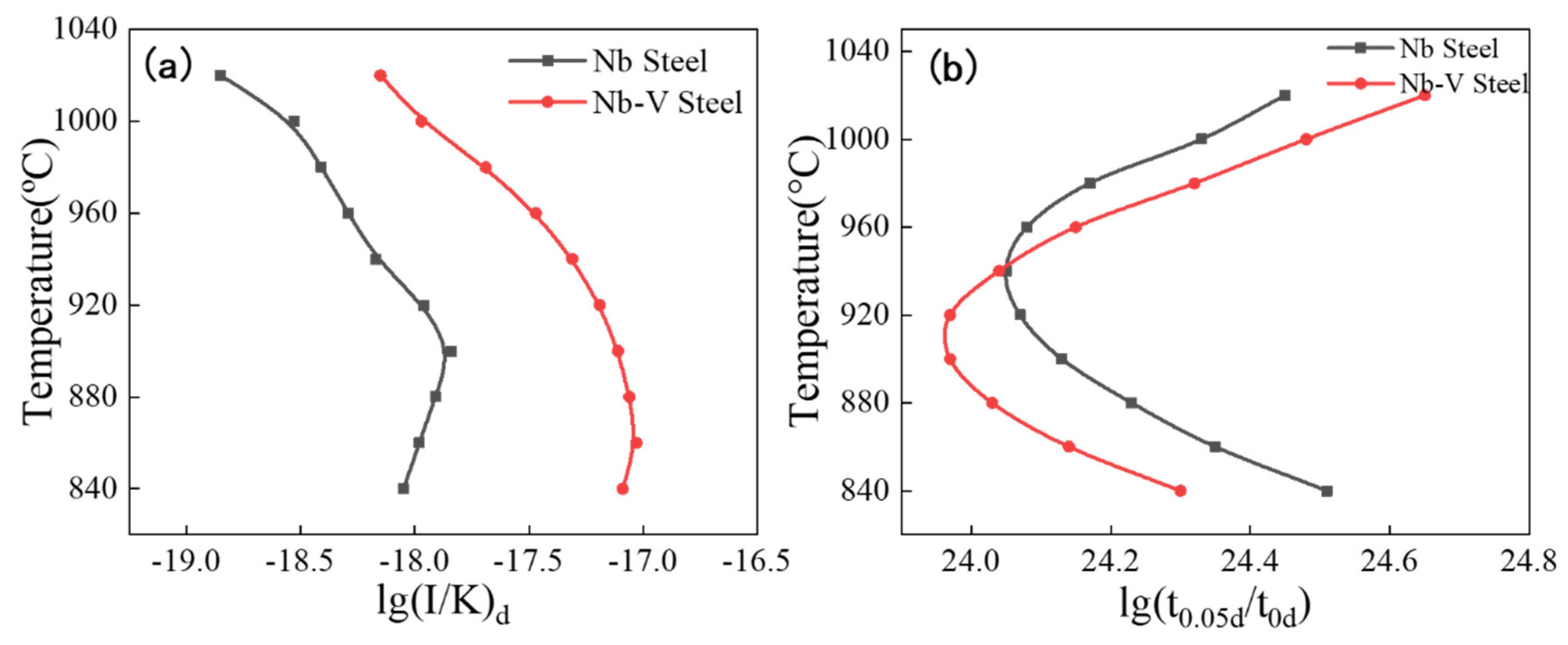
4.2. Dynamics Calculations for Experimental Steels
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Wang, Q.; Shi, G.; Yang, X.; Qiao, M.; Wu, J.; Zhang, F. In-depth understanding of the relationship between dislocation substructure and tensile properties in a low-carbon microalloyed steel. Mater. Sci. Eng. A 2022, 854, 143681. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Wang, S.; Gao, J.; Cao, R.; Feng, Q.; Li, H.; Mao, X. Research status and prospect of direct strip casting manufactured low-carbon microalloyed steel. J. Iron Steel Res. Int. 2022, 29, 17–33. [Google Scholar] [CrossRef]
- Chakraborty, A.; Webster, R.F.; Primig, S. Lath martensite substructure evolution in low-carbon microalloyed steels. J. Mater. Sci. 2022, 57, 10359–10378. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, G.; Dai, B.; Zhao, Y.; Wang, Z.; Jiang, B.; Liu, Y.; Wu, C. Improving strength-toughness of low carbon bainitic microalloyed steel via tailoring isothermal quenching process and niobium microalloying. J. Mater. Sci. Eng. A. 2024, 901, 146515. [Google Scholar] [CrossRef]
- Fu, H.; Chen, P.; Huang, X.; Zhang, W.; Wang, R.; Huang, Q.; Shan, Q. Effect of N and aging treatment on precipitation behavior, mechanical properties and wear resistance of Ti–V–Nb alloyed high manganese steel. J. Mater. Res. Technol. 2024, 29, 1949–1961. [Google Scholar] [CrossRef]
- Varanasi, R.S.; Gault, B.; Ponge, D. Effect of Nb micro-alloying on austenite nucleation and growth in a medium manganese steel during intercritical annealing. Acta Mater. 2022, 229, 117786. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Ma, Y.; Wang, P.; Zhu, F.; Yang, H.; Wang, C.; Wang, S. Effect of Nb on microstructure and mechanical properties between base metal and high heat input coarse-grain HAZ in a Ti-deoxidized low carbon high strength steel. J. Mater. Res. Technol. 2022, 18, 2399–2412. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Xiao, S.; Siyasiya, C.W.; Wei, T. Effects of final rolling temperature and coiling temperature on precipitates and microstructure of high-strength low-alloy pipeline steel. J. Iron Steel Res. Int. 2022, 29, 1236–1244. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Khan, M.; Khan, S.A.; Afzal, A.; Subbiah, R.; Ahmad, S.N.; Husain, M.; Butt, M.M.; Othman, A.R.; Bakar, E.A. Determination of Non-Recrystallization Temperature for Niobium Microalloyed Steel. Materials 2021, 14, 2639. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, Z.; Zhang, Q.; Xu, G. Towards excellent strength-ductility synergy via high temperature short time annealing in low-carbon ultrafine grain steel. Mater. Sci. Eng A. 2023, 886, 145674. [Google Scholar] [CrossRef]
- Singh, M.P.; Shukla, D.K.; Kumar, R.; Arora, K.S. The structural integrity of high-strength welded pipeline steels: A review. Int. J. Struct. Integr. 2021, 12, 470–496. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Chen, W.; Cao, J.; Lu, C.; Luo, H.; Zhang, W.; Guo, A. Effect of solution and precipitation of Nb on microstructure and strengthening mechanism of high-strength anti-seismic rebar. J. Mater. Eng. Perform. 2024, 34, 7773–7785. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.; Xu, X.; Lei, S.; Li, H.; Zhai, Q. Review on niobium application in microalloyed steel. J. Iron steel Res. Int. 2022, 29, 1513–1525. [Google Scholar] [CrossRef]
- Rakshe, B.; Patel, J.; Palmiere, E.J. Effect of niobium supersaturation in austenite on the static recrystallization behavior of carbon structural steels. Metall. Mater. Trans. A 2022, 53, 3143–3157. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, Y.; Zhang, L.J.; Ning, J.; Sun, Y.J.; Na, S.-J. Effect of niobium on the mechanical strength of the laser beam welding joints of molybdenum. Int. J. Refract. Met. Hard Mater. 2023, 113, 106207. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, G.; Hou, Y.; Lin, J.; Li, B.; Jia, S.; Liu, Q.; Liu, G.; Yang, P. Effect of Nb Addition and Heat Input on Heat-Affected Zone Softening in High-Strength Low-Alloy Steel. Materials 2022, 15, 4503. [Google Scholar] [CrossRef]
- Garcia, M.P.; Gervasy, A.; Lu, C.; Barbaro, F. Chemical composition and weld cooling time effects on heat-affected zone hardness of line pipe steels. Int. J. Press. Vessel. Pip. 2022, 200, 104837. [Google Scholar] [CrossRef]
- Zhao, T.; Rong, S.; Hao, X.; Wang, Y.; Chen, C.; Wang, T. Effect of Nb-V microalloying on hot deformation characteristics and microstructures of Fe-Mn-Al-C austenitic steel. Mater. Charact. 2022, 183, 111595. [Google Scholar] [CrossRef]
- Dai, Q.L.; Li, K.; Meng, K.R.; Fang, Z.; Chen, W.; Yang, T.B.; Feng, C.; Wu, J.M.; Misra, R.D.K. Effect of vanadium on the microstructure and mechanical properties of 2100 MPa ultra-high strength high plasticity spring steel processed by a novel online rapid-induction heat treatment. Met. Mater. Int. 2023, 29, 922–933. [Google Scholar] [CrossRef]
- Wu, Z.; Ushioda, K.; Fujii, H. Mechanism of suppressing HAZ softening in friction stir welded martensitic steel through hardening phenomenon initiated by adding vanadium. J. Mater. Res. Technol. 2023, 26, 1151–1167. [Google Scholar] [CrossRef]
- Li, B.; Liu, Q.; Jia, S.; Ren, Y.; Yang, P. Effect of V Content and Heat Input on HAZ Softening of Deep-Sea Pipeline Steel. Materials 2022, 15, 794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Lian, Y.; Ma, M.; Zhao, C.; Ye, H.; Li, G.; Zhang, C.; Huang, J. Effects of vanadium content on the carbides transformation and strengthening mechanism of MPS700V hot-work die steel at room and elevated temperatures. Mater. Sci. Eng. A 2021, 813, 141091. [Google Scholar] [CrossRef]
- Olasolo, M.; Uranga, P.; Rodriguez-Ibabe, J.M.; Lopez, B. Effect of austenite microstructure and cooling rate on transformation characteristics in a low carbon Nb–V microalloyed steel. Mater. Sci. Eng. A 2011, 528, 2559–2569. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Shang, Z.; Zhu, Y.; Li, G. The precipitation behavior of second phase in high titanium microalloyed steels and its effect on microstructure and properties of steel. High Temp. Mater. Process. 2022, 41, 111–122. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Z.; Wei, Z.; Wu, D.; Li, J.; Shao, Z. Microstructural features and precipitation behavior of Ti, Nb and V microalloyed steel during isothermal processing. Iron Steel Res. Int. 2019, 26, 102–111. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, X.; Li, Z.; Xu, K.; Jia, T.; Zhu, Z.H.; Ye, X.Y.; Kang, J.Y.; Yong, Q. Effect of Ti/V ratio on thermodynamics and kinetics of MC in γ/α matrices of Ti–V microalloyed steels. Iron Steel Res. Int. 2021, 28, 1019–1029. [Google Scholar] [CrossRef]
- Li, Z.; Xue, W.; Chen, Y.; Yu, W.; Xiao, K. Microstructure and grain boundary corrosion mechanism of pearlitic material. Mater. Eng. Perform. 2022, 31, 1–12. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Wang, W.Y.; He, Y.; Wang, J.; Han, M.; Wang, J.; Zhang, L.; Zhao, R.; Kou, H.; et al. Coupling effects of high magnetic field and annealing on the microstructure evolution and mechanical properties of additive manufactured Ti–6Al–4V. Mater. Sci. Eng. A 2021, 824, 141815. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, J.; Luo, X.; Yang, G.; Yin, H. Study on Mechanical Properties and Deformation Mechanism of TWIP Stainless Steel. Metals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Zhu, M.; Tian, J.; Su, X.; Zhang, Q.; Guo, A.; Xu, G. New insights to the metallurgical mechanism of niobium in high-carbon pearlitic steels. J. Mater. Res. Technol. 2023, 26, 1609–1623. [Google Scholar] [CrossRef]
- Najafkani, F.; Kheiri, S.; Pourbahari, B.; Mirzadeh, H. Recent advances in the kinetics of normal/abnormal grain growth: A review. Arch. Civ. Mech. Eng. 2021, 21, 29. [Google Scholar] [CrossRef]
- Xu, C.; Dai, W.J.; Chen, Y.; Qi, Z.X.; Zheng, G.; Cao, Y.D.; Zhang, J.P.; Bu, C.C.; Chen, G. Control of dislocation density maximizing precipitation strengthening effect. J. Mater. Sci. Technol. 2022, 127, 133–143. [Google Scholar] [CrossRef]
- Pan, X.; Yang, J.; Zhang, Y. Microstructure evolution in heat-affected zone of shipbuilding steel plates with Mg deoxidation containing different Nb contents. Metall. Res. Technol. 2021, 118, 409. [Google Scholar] [CrossRef]
- Mishra, B.; Singh, V.; Sarkar, R.; Mukhopadhyay, A.; Gopinath, K.; Madhu, V.; Mjnv, P. Dynamic recovery and recrystallization mechanisms in secondary B2 phase and austenite matrix during hot deformation of Fe–Mn–Al–C-(Ni) based austenitic low-density steels. Mater. Sci. Eng. A 2022, 842, 143095. [Google Scholar] [CrossRef]
- Kawahara, Y.; Tokuhisa, A.; Maeda, T.; Shirahata, H.; Uemori, R.; Kaneko, K. Formation of core-shell structured carbides via interphase precipitations in V-Nb microalloyed steels. Scr. Mater. 2024, 249, 116169. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Z.; Liang, R.; Song, Z.; Hou, H.; Zho, Y. Toughening mechanism and delayed fracture analysis of 4145H steel under intercritical quenching heat treatment process. J. Mater. Res. Technol. 2023, 27, 6863–6879. [Google Scholar] [CrossRef]
- Xiong, Z.; Timokhina, I.; Pereloma, E. Clustering, nano-scale precipitation and strengthening of steels. Prog. Mater. Sci. 2021, 118, 100764. [Google Scholar] [CrossRef]


| Steels | C | Mn | Si | Ni | Mo | Cr | Cu | Nb | V | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| Nb Steel | 0.054 | 1.73 | 0.214 | 0.103 | 0.155 | 0.262 | 0.095 | 0.080 | / | 0.012 |
| Nb-V Steel | 0.055 | 1.73 | 0.221 | 0.104 | 0.156 | 0.262 | 0.096 | 0.050 | 0.031 | 0.010 |
| Temperature (°C) | 940 | 960 | 980 | 1000 | 1020 | 1040 | 1060 | 1080 | 1100 |
|---|---|---|---|---|---|---|---|---|---|
| X | 0.886 | 0.891 | 0.895 | 0.900 | 0.903 | 0.909 | 0.916 | 0.927 | 0.933 |
| Carbide | Lattice Constant/nm | Linear Expansion Coefficient/k−1 |
|---|---|---|
| NbC | 0.4469 | 7.02 × 10−6 |
| VC | 0.4182 | 8.29 × 10−6 |
| Element | Qg/kJ | Qa/kJ |
|---|---|---|
| Nb | 267 | 252 |
| V | 264 | 241 |
| Temperature/°C | Nb Steel | Nb-V Steel | ||||
|---|---|---|---|---|---|---|
| d*d/nm | lg(I/K)d | lg(t0.05/t0) | d*d/nm | lg(I/K)d | lg(t0.05/t0) | |
| 940 | 0.59 | −18.22 | 24.51 | 0.58 | −17.09 | 24.3 |
| 960 | 0.60 | −18.13 | 24.35 | 0.59 | −17.03 | 24.14 |
| 980 | 0.62 | −17.95 | 24.23 | 0.60 | −17.06 | 24.03 |
| 1000 | 0.65 | −17.84 | 24.13 | 0.61 | −17.11 | 23.97 |
| 1020 | 0.67 | −17.96 | 24.07 | 0.63 | −17.19 | 23.97 |
| 1040 | 0.70 | −18.17 | 24.05 | 0.64 | −17.31 | 24.04 |
| 1060 | 0.71 | −18.29 | 24.08 | 0.66 | −17.47 | 24.25 |
| 1080 | 0.73 | −18.41 | 24.17 | 0.68 | −17.69 | 24.42 |
| 1100 | 0.75 | −18.53 | 24.33 | 0.69 | −17.97 | 24.68 |
| 1120 | 0.78 | −18.85 | 24.45 | 0.71 | −18.15 | 25.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Yin, S.; Shang, C.; Liu, Q.; Li, B.; Jia, S. Recrystallization and Second-Phase Precipitation in Nb-V Microalloyed Steels: A Thermal Simulation Study. Materials 2025, 18, 3069. https://doi.org/10.3390/ma18133069
Ma Q, Yin S, Shang C, Liu Q, Li B, Jia S. Recrystallization and Second-Phase Precipitation in Nb-V Microalloyed Steels: A Thermal Simulation Study. Materials. 2025; 18(13):3069. https://doi.org/10.3390/ma18133069
Chicago/Turabian StyleMa, Qilin, Shubiao Yin, Chengjia Shang, Qingyou Liu, Ba Li, and Shujun Jia. 2025. "Recrystallization and Second-Phase Precipitation in Nb-V Microalloyed Steels: A Thermal Simulation Study" Materials 18, no. 13: 3069. https://doi.org/10.3390/ma18133069
APA StyleMa, Q., Yin, S., Shang, C., Liu, Q., Li, B., & Jia, S. (2025). Recrystallization and Second-Phase Precipitation in Nb-V Microalloyed Steels: A Thermal Simulation Study. Materials, 18(13), 3069. https://doi.org/10.3390/ma18133069






