1. Introduction
Energetic materials, which are distinguished by their extraordinarily high energy-release rates and the simultaneous generation of significant acoustic, optical, and thermal effects during energy discharge, have seen extensive adoption across civilian and military domains since their inception [
1,
2,
3,
4,
5].
In recent years, the advancement of target application fields and innovations in material synthesis methodologies have driven the compositional diversification of energetic materials, accompanied by continuous improvements in their explosive performance [
6,
7,
8,
9]. Undoubtedly, the development of high-energy materials with inherent safety remains a central focus in energetic materials research, irrespective of their application scenarios (e.g., primary explosives or high-energy explosives). Being widely acknowledged, ionic energetic compounds and co-crystalline materials have emerged as promising strategies for designing high-stability, high-energy compounds (
Figure 1) [
10,
11,
12,
13,
14,
15,
16,
17]. However, these approaches are challenged by fundamental limitations, such as density degradation and the scarcity of structurally robust co-crystals, which collectively hinder the rapid development of novel high-energy–high-stability energetic compounds. Against this scientific backdrop, a novel multicomponent architectural design—integrating the advantages of dense hydrogen-bonding networks in ionic compounds and the high-density features of MOF-type materials—has been explored for synthesizing high-stability–high-energy systems, specifically energetic coordination complexes [
18,
19,
20,
21,
22,
23]. These complexes exhibit remarkable diversity in physicochemical properties, which is attributed to the synergistic effects of abundant intracomplex hydrogen-bonding networks, coordination bonding, and the tunable nature of ligand/metal ion combinations. With the increasing prevalence of in-depth research into energetic complexes, their applications have expanded to primary explosives, ammonium perchlorate decomposition catalysts, and high-energy low-sensitivity explosives. Notably, previous studies predominantly employed oxygen-rich oxyacid anions or nitrogen-rich anions as primary ligands—a strategy that often compromises the oxygen balance of the complexes and consequently limits their maximum detonation performance.
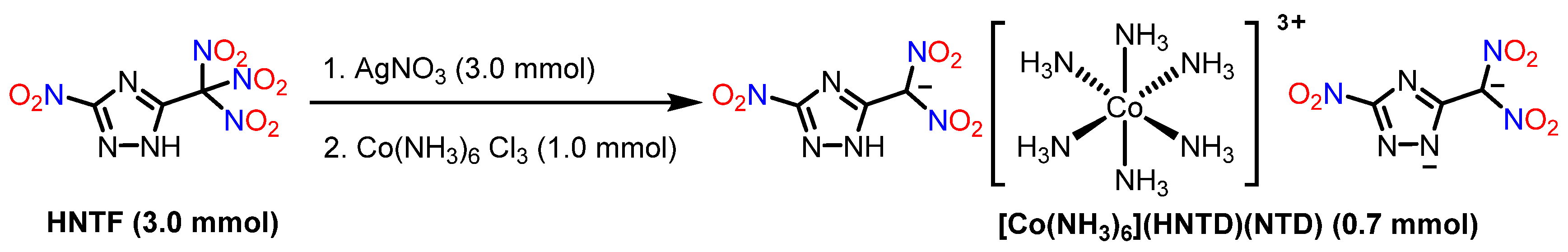
Building upon the aforementioned research landscape, we synthesized a novel high-energy complex, [Co(NH3)6](HNTD)(NTD)·H2O, utilizing 5-nitro-3-(trinitromethyl)-1,2,4-triazole (HNTF) as the starting material. The complex demonstrates a low mechanical sensitivity, which is attributed to the dense hydrogen-bonding network within its crystal lattice. Notably, although the structure of the complex contains a large number of ammonia molecules, the inherent high density of both 5-nitro-3-(dinitromethyl)-1,2,4-triazole (H2NTD) and cobalt ions collectively imparts the complex with a relatively high overall density. This study not only enriches the research scope of high-energy complexes but also provides a reference for the development of novel low-sensitivity energetic materials.
2. Results and Discussion
2.1. Synthesis
The synthetic pathway for complex [Co(NH
3)
6](HNTD)(NTD)·H
2O is outlined in
Scheme 1. Equimolar amounts of compound HNTF [
24] and silver nitrate were stirred in deionized water at room temperature for 12 h. The resulting precipitate was collected by filtration to afford the intermediate (NTF-Ag). This intermediate was then reacted with Co(NH
3)
6Cl
3 in deionized water under stirring for 8 h. The reaction mixture was filtered and the filtrate was evaporated to yield complex [Co(NH
3)
6](HNTD)(NTD)·H
2O.
2.2. Crystal Structures
Similarly to most energetic compounds bearing highly labile N-H protons, HNTF exhibits a pronounced hygroscopicity, rendering it difficult to isolate corresponding crystals via the evaporation of its alcoholic, ethyl acetate, or acetonitrile solutions. To obtain detailed crystallographic data for HNTF, we employed water-immiscible dichloromethane as the solvent and performed a slow evaporation of its dichloromethane solution within a desiccator, ultimately achieving the growth of suitable single crystals.
Although HNTF does not exhibit a planar geometry (
Figure 2a), its molecular packing forms highly ordered wave-like layered stacks (
Figure 2b,c). The nitro groups, which are positioned at both termini of the molecular framework, facilitate the close proximity of nitro moieties during stacking. Notably, the minimum distance between nitro oxygen atoms in interlayer molecules is 3.662 Å (
Figure 2c), whereas the intralayer distance is significantly shorter at 2.863 Å (
Figure 2d). Such a compact nitro group arrangement is typically correlated with an increased mechanical sensitivity in energetic materials. Additionally, due to the single hydrogen atom in the molecule, HNTF crystals contain a limited number of hydrogen bonds. However, it is noteworthy that the crystal density of HNTF at room temperature is 1.95 g/cm
3, which aligns well with the reported experimental value (1.94 g/cm
3 [
24]).
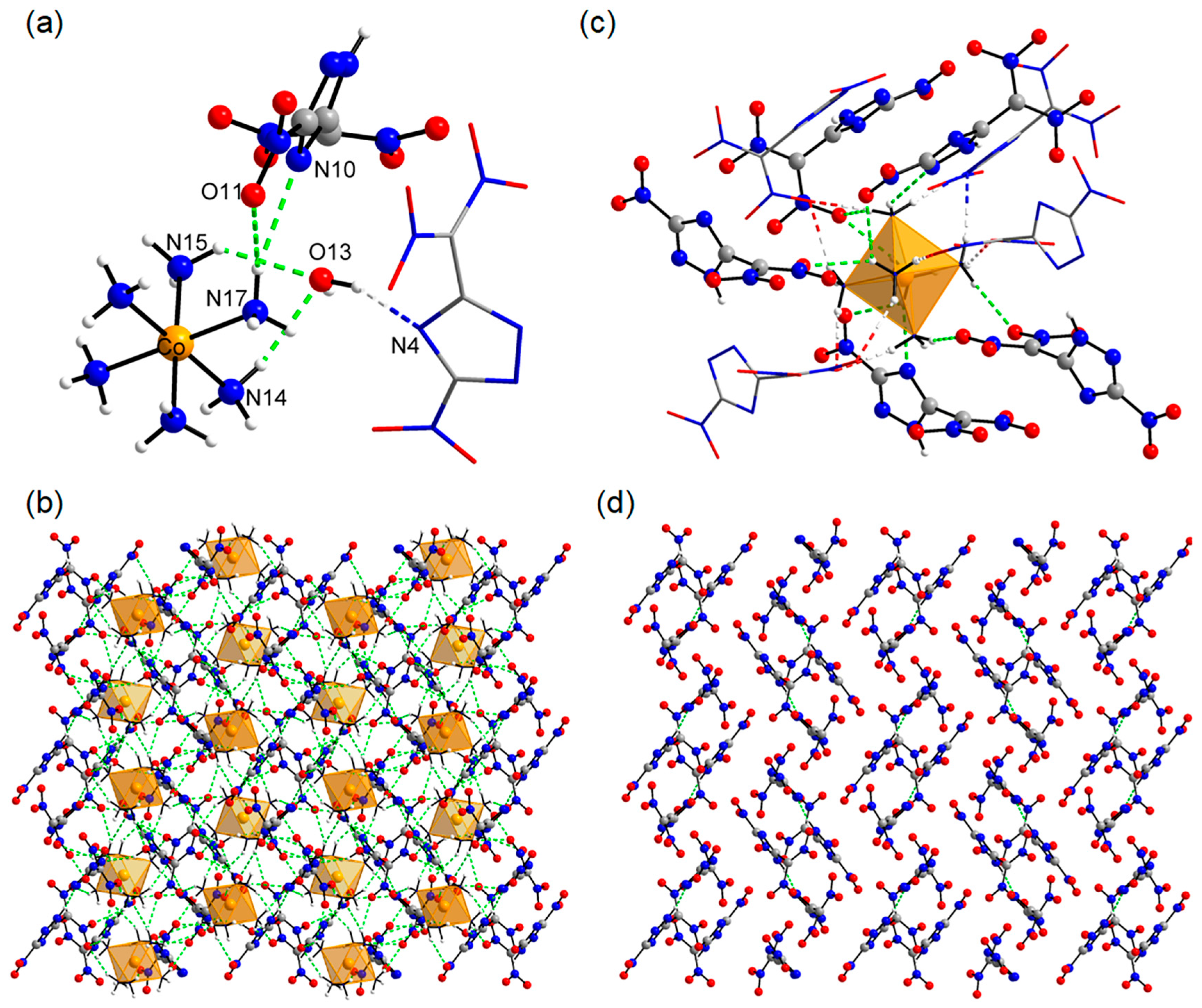
Crystallographic analysis reveals that in [Co(NH
3)
6](HNTD)(NTD)·H
2O, coordination bonds and hydrogen bonds alternately link cobalt ions, ammonia molecules, and energetic (HNTD
− and NTD
2−) moieties, giving rise to a distinctive “core–shell structure” within the unit cell (
Figure 3c). In this architecture, nine high-energy ions (among them, there are five HNTD
− and four NTD
2−) encapsulate a non-energetic Co(NH
3)
6 core. Notably, energetic moieties exist in two valence states within the structure—monovalent C
3N
6O
6H (HNTD
−) and divalent C
3N
6O
6 (NTD
2−) (
Figure 3a). This valence diversity facilitates the formation of a limited hydrogen-bonding network among HNTD
− and NTD
2− (
Figure 3d). Despite HNTD
− and NTD
2− possessing one fewer nitro group than HNTF, as well as the inclusion of one crystallization water molecule in the [Co(NH
3)
6](HNTD)(NTD)·H
2O crystal, its room-temperature density reaches 1.886 g/cm
3.
2.3. Thermal Stability and Mechanical Sensitivity
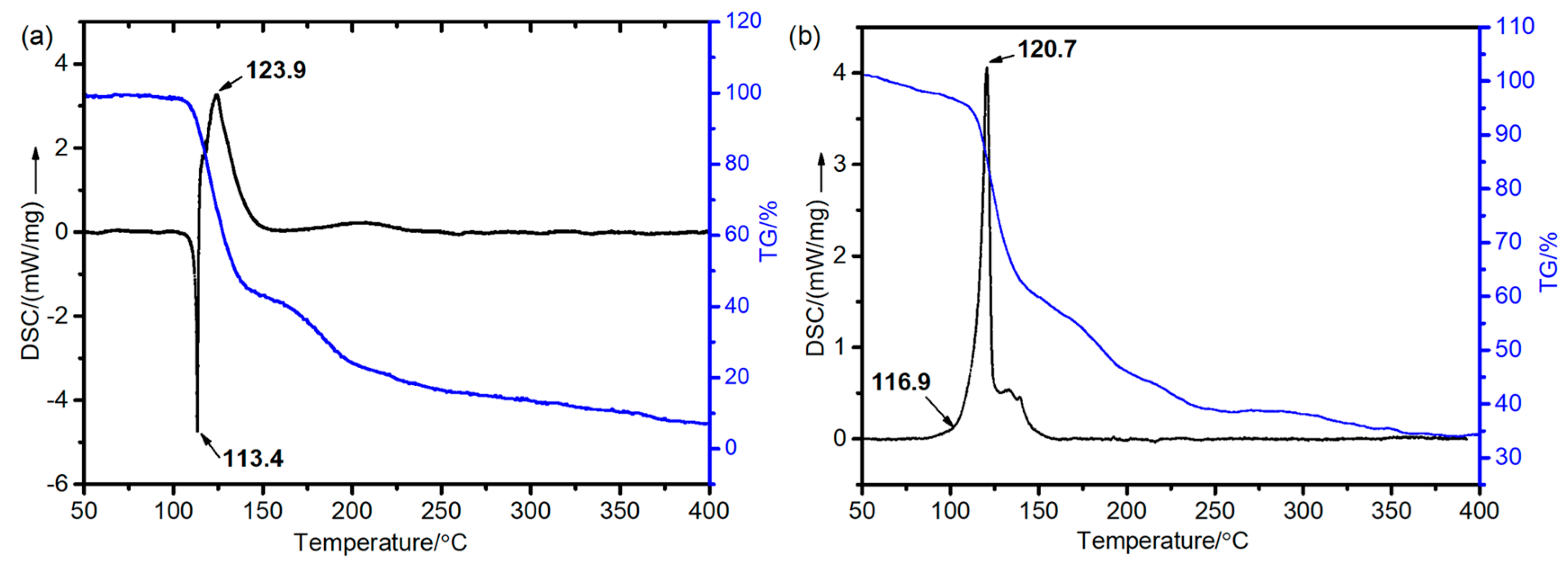
Thermal stability is a critical parameter for evaluating the stability of energetic compounds. In this study, the thermal decomposition behaviors of HNTF and [Co(NH
3)
6](HNTD)(NTD)·H
2O were characterized using TG-DSC under a nitrogen atmosphere at a heating rate of 5 °C min
−1 (
Figure 4). The DSC curve of HNTF reveals two distinct thermal stages—an endothermic phase followed by an exothermic phase. The corresponding TG analysis indicates that HNTF initiates decomposition at the endothermic peak, with an initial decomposition temperature of 113.4 °C. In contrast, [Co(NH
3)
6](HNTD)(NTD)·H
2O exhibits a single exothermic decomposition process, with an initial decomposition temperature of 116.9 °C, which is 3.5 °C higher than that of HNTF. Notably, the presence of the heavy metal Co in [Co(NH
3)
6](HNTD)(NTD)·H
2O results in a significantly higher solid residue (34.5%) after complete decomposition compared to HNTF.
Furthermore, non-isothermal kinetic studies were carried out at four different heating rates to obtain the sensitivity data, including the thermal explosion temperature (
Tpb), the self-accelerating decomposition temperature (
TSADT), and the activation energy (
E), of [Co(NH
3)
6](HNTD)(NTD)·H
2O to temperature changes (
Figure 5). The experimental results show that the activation energy (calculated using Kissinger’s method) of the compound [Co(NH
3)
6](HNTD)(NTD)·H
2O is
Ek = 114.7 kJ/mol (
SE = 0.16), and the
TSADT and
Tpb are 110.5 and 110.7 °C, respectively. These data indicate the adaptability of the compound [Co(NH
3)
6](HNTD)(NTD)·H
2O under the application environmental conditions of energetic compounds.
In terms of mechanical sensitivity, the impact sensitivity (IS) and friction sensitivity (FS) of HNTF measured using the standard BAM impact sensitivity tester and friction sensitivity tester are 4 J and 80 N, respectively. The impact sensitivity and friction sensitivity of [Co(NH3)6](HNTD)(NTD)·H2O are 10 J and 140 N, respectively, which shows that its mechanical sensitivity is significantly lower compared to HNTF.
2.4. Theoretical Analysis of Stability
To theoretically rationalize the substantial stability disparity between HNTF and [Co(NH
3)
6](HNTD)(NTD)·H
2O, Hirshfeld surface [
25] analysis coupled with fingerprint plots was employed to characterize atomic-level interactions. For HNTF, hydrogen-bonding interactions (H···O/N) constitute a minor fraction of the total intermolecular forces, whereas O···O interactions dominate at 51.9%—a direct consequence of densely packed nitro groups (
Figure 6a). This nitro–nitro interaction dominance mechanistically explains HNTF’s low stability, as close-proximity oxygen atoms in nitro groups enhance electrostatic repulsion and thermal sensitivity [
26,
27].
In contrast, [Co(NH
3)
6](HNTD)(NTD)·H
2O exhibits a 50% increase in H···O/N interactions, which is indicative of a more extensive hydrogen-bonding network. The [Co(NH
3)
6]
3+ core introduces steric hindrance, physically separating HNTD
− and NTD
2− anions and reducing O···O interactions to 13.6% (vs. 51.9% in HNTF) (
Figure 6b). This structural confinement diminishes nitro group clustering, thereby dampening mechanical and thermal sensitivities. The synergistic effects of enhanced hydrogen bonding and steric shielding from the cobalt complex collectively stabilize the crystal lattice, rationalizing the superior stability of [Co(NH
3)
6](HNTD)(NTD)·H
2O relative to HNTF.
2.5. Detonation Performance
For energetic materials, density, molecular composition, enthalpy of formation (Δ
fH), and oxygen balance (OB) are critical metrics for evaluating detonation performance. In this study, the Δ
fH values of HNTF and [Co(NH
3)
6](HNTD)(NTD)·H
2O were determined using the isodesmic equation and combustion calorimetry, respectively, (detailed experimental protocols are provided in the
Supporting Information). The detonation velocity and pressure (
Table 1) of the two compounds were calculated using EXPLO5 v6.05.04 software [
28] and the K-J equation, respectively, incorporating the experimentally measured molecular composition, density, and enthalpy of formation data.
The oxygen balance of [Co(NH3)6](HNTD)(NTD)·H2O is relatively low at −28.7%, primarily due to two key factors. Firstly, the energetic ion (HNTD− and NTD2−) in [Co(NH3)6](HNTD)(NTD)·H2O lacks one nitro group compared to HNTF; Secondly, the [Co(NH3)6]3+ moiety consumes a significant amount of oxygen atoms. Nevertheless, this value remains higher than that of most energetic complexes. Additionally, attributed to the inherently high energy performance of the HNTD− and NTD2− moieties and the high density of [Co(NH3)6](HNTD)(NTD)·H2O, the compound exhibits an excellent detonation performance. Its detonation velocity and detonation pressure reach 8030 m s−1 and 29.2 GPa, respectively, which are significantly higher than those of most energetic complexes.
3. Conclusions
In general, in this study, a novel polynitro energetic complex—[Co(NH3)6](HNTD)(NTD)·H2O—was prepared using the oxygen-rich and high-energy compound HNTF as the raw material. Its structure, stability, and energy performance were investigated. As a polynitro energetic complex, [Co(NH3)6](HNTD)(NTD)·H2O exhibits a good stability (IS = 10 J and FS = 140 N) and a higher energy performance (Vd = 8030 m s−1 and P = 29.2 GPa) compared with complexes that use non-energetic acid radicals as the oxygen-rich structure. Theoretical studies have shown that in [Co(NH3)6](HNTD)(NTD)·H2O, on the one hand, the [Co(NH3)6]3+ structure hinders the formation of intermolecular nitro interactions that are likely to lead to high mechanical sensitivity. On the other hand, with NH3 serving as the intermediate layer of the core–shell structure, the energetic ions HNTD− and NTD2− are connected together to form an energetic shell through a tight hydrogen-bonding network, thereby enabling [Co(NH3)6](HNTD)(NTD)·H2O to have a relatively low mechanical sensitivity while ensuring a high energy performance.
Author Contributions
F.Y.: investigation, data curation, validation, writing—original draft, and funding acquisition. Z.H.: visualization, data curation, and formal analysis. X.W.: investigation, data curation, and validation. M.L.: conceptualization and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (NSFC) (No. 22305120).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/
Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, J.; Staples, R.J.; Shreeve, J.M. Coordination-driven safer and sustainableenergetic materials. J. Mater. Chem. A 2025, 13, 11475–11485. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Y.; Yang, F.; Wang, P.; Lin, Q.; Huang, H.; Lu, M. A review of ultra-high temperature heat-resistant energetic materials. Def. Technol. 2024, 38, 33–57. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Pang, S.; Shreeve, J.M. Nitroimino as an energetic group in designing energetic materials for practical use, a tautomerism from nitroamino. J. Mater. Chem. A 2023, 11, 13876–13888. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Liu, Y. Data-Driven Combinatorial Design of Highly Energetic Materials. Acc. Mater. Res. 2024, 6, 64–76. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Fershtat, L.; Zhang, Q. Synthesis, design and development of energetic materials: Quo Vadis? Chem. Eng. J. 2024, 486, 150410. [Google Scholar] [CrossRef]
- Barton, L.M.; Edwards, J.T.; Johnson, E.C.; Bukowski, E.J.; Sausa, R.C.; Byrd, E.F.C.; Orlicki, J.A.; Sabatini, J.J.; Baran, P.S. Impact of Stereo- and Regiochemistry on Energetic Materials. J. Am. Chem. Soc. 2019, 141, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Staples, R.J.; Shreeve, J.M. A Dihydrazone as a Remarkably Nitrogen-Rich Thermostable and Insensitive Energetic Material. Org. Lett. 2023, 25, 6082–6086. [Google Scholar] [CrossRef]
- Yadav, A.K.; Ghule, V.D.; Dharavath, S. Promising Thermally Stable Energetic Materials with the Combination of Pyrazole–1,3,4-Oxadiazole and Pyrazole–1,2,4-Triazole Backbones: Facile Synthesis and Energetic Performance. ACS Appl. Mater. Interfaces 2022, 14, 49898–49908. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.C.; Staples, R.J.; Zhang, J.H.; Shreeve, J.M. Synthesis of insensitive, high-density energetic materials through molecular self-assembly. J. Mater. Chem. A 2025, 13, 5164–5168. [Google Scholar] [CrossRef]
- Bennion, J.C.; Matzger, A.J. Development and Evolution of Energetic Cocrystals. Acc. Chem. Res. 2021, 54, 1699–1710. [Google Scholar] [CrossRef]
- Gamekkanda, J.C.; Sinha, A.S.; Aakeröy, C.B. Cocrystals and Salts of Tetrazole-Based Energetic Materials. Cryst. Growth Des. 2020, 20, 2432–2439. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Wu, Y.; Jin, S.; Wang, X.; Wang, Y.; Shang, F.; Chen, K.; Du, J.; Shu, Q. A novel cocrystal composed of CL-20 and an energetic ionic salt. Chem. Commun. 2018, 54, 13268–13270. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Das, P.; Kumar, D. Engaging Two Anions with Single Cation in Energetic Salts: Approach for Optimization of Oxygen Balance in Energetic Materials. ACS Appl. Mater. Interfaces 2024, 16, 64846–64857. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xia, Y.; Zhang, H.; Su, D.; Lai, Q.; Yin, P.; Pang, S. Dinitramide salts based on nitropyrazole-diaminotriazole hybrid: Novel ionic energetic materials with high-energy and low-sensitivity. Phys. Chem. Chem. Phys. 2025, 27, 3463–3468. [Google Scholar] [CrossRef]
- Kent, R.V.; Wiscons, R.A.; Sharon, P.; Grinstein, D.; Frimer, A.A.; Matzger, A.J. Cocrystal Engineering of a High Nitrogen Energetic Material. Cryst. Growth Des. 2017, 18, 219–224. [Google Scholar] [CrossRef]
- Zhu, N.; Bian, C.; Yang, F.; Yang, X.; Ma, Z.; Li, X. Insensitive energetic compounds based on regioisomeric nitro-substituted fused triazole. J. Mol. Struct. 2024, 1298, 137029. [Google Scholar] [CrossRef]
- Meng, F.; Zhou, R.; Xu, Z.; Wang, P.; Xu, Y.; Lu, M. A Promising Strategy toward the Development of C–C- and C–N-Linked Tricyclic Tetrazole Energetic Materials with High Energy Density. J. Org. Chem. 2025, 90, 3964–3973. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Jiang, S.; Lin, Q.; Wang, P.; Xu, Y.; Lu, M. Lithium-Promoted Formation of M-2AZTO-Li (M = N2H5+ or NH3OH+ and AZTO = Anion of 1-Hydroxytetrazole-5-hydrazide)-Type “Quaternary” Complexes with Nitrogen-Rich Characteristics: Construction of Novel Insensitive Energetic Materials. ACS Appl. Mater. Interfaces 2022, 15, 1601–1609. [Google Scholar] [CrossRef]
- Dong, W.S.; Zhang, P.P.; Xu, M.Q.; Lu, Z.J.; Li, Z.M.; Wang, K.; Yu, Q.Y.; Zhang, J.G. One-Pot Synthesis of Innovative Multicomponent Complexes as Catalysts for Enhanced Decomposition of Ammonium Perchlorate. Small 2025, 21, 2411382. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Dong, K.; Su, H.; Zhang, S.; Li, S.; Pang, S. Taming Dinitramide Anions within an Energetic Metal–Organic Framework: A New Strategy for Synthesis and Tunable Properties of High Energy Materials. Chem. Mater. 2016, 28, 1472–1480. [Google Scholar] [CrossRef]
- Xu, J.G.; Sun, C.; Zhang, M.J.; Liu, B.W.; Li, X.Z.; Lu, J.; Wang, S.H.; Zheng, F.K.; Guo, G.C. Coordination Polymerization of Metal Azides and Powerful Nitrogen-Rich Ligand toward Primary Explosives with Excellent Energetic Performances. Chem. Mater. 2017, 29, 9725–9733. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Xu, M.; Kuang, B.; Xie, Z.; Lu, Z.; Yi, Z.; Li, Y.; Zhang, J. The combined effect of pyrazole and amino groups: Preparation of novel energetic coordination compounds with high ClO4− content. Inorg. Chem. Front. 2023, 10, 5311–5319. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Li, D.; Wang, P.; Lin, Q.; Lu, M. Hexaamminecobalt(III) Cation as Multiple Hydrogen Bond Donors: Synthesis, Characterization and Energetic Properties of cyclo-Pentazolate and Azide Based Complexes. Cryst. Growth Des. 2022, 23, 811–819. [Google Scholar] [CrossRef]
- Thottempudi, V.; Shreeve, J.n.M. Synthesis and Promising Properties of a New Family of High-Density Energetic Salts of 5-Nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-Bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole. J. Am. Chem. Soc. 2011, 133, 19982–19992. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Xie, W.; Jiang, X.; Sun, M.; Zhang, X.; Yin, P.; Lai, Q. Assembling of Nitropyrazoles into Tetranitroacetimidic Acid (TNAA): A Pathway to High-Performance Energetic Oxidizers through Dual C/N-Functionalization. Org. Lett. 2024, 26, 6591–6596. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Jiang, S.; Yang, F.; Li, D.; Wang, P.; Lin, Q.; Lu, M. 4,4′-Bis(trinitromethyl)-3,3′-azo/azoxy-furazan: High-energy dense oxidizers. Chem. Eng. J. 2023, 454, 140358. [Google Scholar] [CrossRef]
- Sućeska, M. EXPLO5, V6.05.04; Brodarski Institute: Zagreb, Croatia, 2020. [Google Scholar]
- Ma, X.; Cai, C.; Sun, W.; Song, W.; Ma, Y.; Liu, X.; Xie, G.; Chen, S.; Gao, S. Enhancing Energetic Performance of Multinuclear Ag(I)-Cluster MOF-Based High-Energy-Density Materials by Thermal Dehydration. ACS Appl. Mater. Interfaces 2019, 11, 9233–9238. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Qi, C.; Zhao, X.; Zhang, J.; Zhang, S.; Pang, S. 3D Energetic Metal–Organic Frameworks: Synthesis and Properties of High Energy Materials. Angew. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Deng, H.; Shang, L.; Chen, D.; Li, Y.; Zhu, S.; Li, H. Evolution of Oxidizing Inorganic Metal Salts: Ultrafast Laser Initiation Materials Based on Energetic Cationic Coordination Polymers. ACS Appl. Mater. Interfaces 2019, 11, 41523–41530. [Google Scholar] [CrossRef]
- Wurzenberger, M.H.H.; Szimhardt, N.; Stierstorfer, J. Copper(II) Chlorate Complexes: The Renaissance of a Forgotten and Misjudged Energetic Anion. J. Am. Chem. Soc. 2018, 140, 3206–3209. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 09, Revision a. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhang, J.H.D.; Wang, F. DFT Studies on a High Energy Density Cage Compound 4-Trinitroethyl-2,6,8,10,12-pentanitrohezaazaisowurtzitane. J. Phys. Chem. A 2011, 24, 6617–6621. [Google Scholar] [CrossRef]
- Atkins, P.W. Physical Chemistry; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Politzer, P.J.; Murray, S.; Brinck, T.; Lan, P. Immunoanalysis of Agrochemicals; ACS Sympsium Series 586; American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).