Abstract
This study investigates the long-term durability and crystallization-induced degradation mechanisms of alkali-activated slag (AAS) mortars with varying water-to-binder ratios (w/b, 0.4, 0.45, 0.5) under semi-immersion in 5 wt.% sodium sulfate solution. Through 360 d of exposure, the evolution of physical–mechanical properties (mass change, open porosity, compressive/flexural strength) and ion migration patterns (SO42−, Na+, Ca2+) were analyzed to unravel the interplay between pore structure, ion transport, and crystallization-induced deterioration. Results demonstrated that higher w/b ratios exacerbated surface crystallization and spalling due to accelerated ion transport and pore coarsening. Early-stage strength gains (up to 25.15% at 120–180 d) stemmed from pore refinement via sulfate deposition and continued slag hydration. However, prolonged exposure triggered microstructural degradation, with open porosity increasing by 58.9% and strength declining by 30.6% at 360 d for a w/b of 0.5 compared to a w/b of 0.4. This was driven by crystallization pressure and the decalcification of hydration products. Ion migration analysis revealed SO42− enrichment in evaporation area and outward Na+ diffusion, establishing supersaturation gradients that aligned with crystallization damage progression. These findings provide critical insights for optimizing AAS mortar formulations to mitigate sulfate crystallization risks in semi-immersed environments.
1. Introduction
Concrete infrastructures in sulfate-rich environments, such as coastal zones and saline soils, are prone to severe durability degradation due to sulfate attack [1,2,3,4]. The Ordinary Portland cement-based materials often suffer from chemical sulfate corrosion, leading to expansion and cracking via the formation of ettringite and gypsum [5,6,7], and also its own alkali–silica reaction will cause it to swell and crack [8]. In contrast, Alkali-Activated Slag Cement (AASC), a sustainable alternative synthesized from granulated blast furnace slag (GBFS) and alkali-activators, exhibits excellent resistance to chemical sulphate attack by avoiding such expansive products [9,10]. However, recent studies highlight a critical vulnerability of AASC under cyclic wet-dry conditions, where sodium sulfate crystallization induces physical damage through pore-wall pressure, even without chemical corrosion products [11]. This paradoxical combination of excellent chemical resistance and a high risk of crystalline damage poses significant challenges for AASC applications in semi-immersed environments like tidal zones.
Under semi-immersion, capillary transport and evaporation synergistically drive sulfate ion enrichment, accelerating supersaturation and surface crystallization [12]. The AASC inherent characteristics exacerbate this process: its unsaturated water absorption rate is 2–3 times higher than Ordinary Portland cement concrete (OPC), and its pore structure dominated by sub-20 nm capillaries, amplifies crystallization pressure due to the inverse relationship between pore size and crystallization stress [13,14]. Furthermore, the high alkali ion concentration (e.g., Na+) in AASC pore solutions promotes sodium sulfate supersaturation, increasing crystallization potential [15]. Under cyclic wet-dry conditions, capillary transport and evaporation synergistically concentrate dissolved ions at exposed surfaces, leading to supersaturation and crystallization of salts like Na2SO4. This process exerts physical stress on pore walls, causing spalling and strength loss [16,17]. Unlike chemical sulfate attack, efflorescence in AAMs is primarily a physical degradation mechanism, exacerbated by their fine capillary pores (<20 nm) and high alkali content. Despite these risks, systematic investigations into the interplay between w/b ratios, ion migration, and microstructural evolution under semi-immersion remain scarce. Existing studies primarily focus on full-immersion scenarios or dry-wet cycles, overlooking the unique ion transport dynamics in partially saturated systems.
Recent advances highlight material-specific strategies: calcined clay-geopolymer hybrids reduce Na+ mobility through ion-exchange capacity [18], while hybrid alkali-activated cements (HAC) blend slag with OPC to enhance crystallization resistance via pore refinement [19]. However, neither approach resolves the intrinsic trade-off between workability (governed by w/b) and crystallization durability in pure AAS systems—the core focus of this study.
Previous work by our group revealed severe surface spalling (15% mass loss) in AAS mortars after 450 d of semi-immersion in 5 wt.% Na2SO4 solution, far exceeding damage under full immersion [11,13]. This underscores the critical role of evaporation-driven ion enrichment in crystallization damage. However, the mechanism linking the w/b ratio, a key parameter controlling the pore structure and transport properties, to the kinetics of sulphate crystallization and mechanical degradation has yet to be deciphered. Higher w/b ratios typically increase porosity and ion mobility [20,21], potentially accelerating sulfate accumulation and damage progression. Conversely, optimized ratios might mitigate these effects by refining pore networks. Resolving this trade-off is essential for designing durable AASC structures in sulfate-exposed environments.
This study addresses these gaps by evaluating AAS mortars with w/b ratios of 0.4, 0.45, and 0.5 during 360 d semi-immersion in sodium sulfate. Mechanical properties (compressive/flexural strength), pore structure evolution (open porosity, water absorption), and ion distribution (SO42−, Na+, Ca2+) were analyzed to unravel crystallization damage mechanisms. Key objectives include: (1) quantifying the impact of w/b ratios on surface crystallization and spalling; (2) correlating pore structure changes with strength degradation; and (3) mapping ion migration patterns to establish supersaturation gradients driving crystallization. The findings aim to provide actionable insights for optimizing AASC formulations, balancing workability and durability in semi-immersed applications.
2. Experimental
2.1. Materials
The granulated blast furnace slag (GBFS) was produced from Chongqing Iron and Steel Group (Chongqing, China), with a specific surface area of 398.7 m2/kg by Blain method, and the main compositions measured by X-ray Fluorescence Spectrometer (XRF) are shown in Table 1. The fine aggregate was Yueyang river sand with a fineness modulus of 2.6, apparent density of 2.53 g/cm3, and bulk density of 1.79 g/cm3, according to the Chinese standard GB/T-14684-2022 [22]. Sodium sulphate was anhydrous sodium sulphate produced by Chengdu Kelong Chemicals Co. (Chengdu, China) with a content of sodium sulphate of more than 99%.

Table 1.
Compositions of GBFS (wt%).
2.2. Preparation of AAS Mortars
The AAS mortars with water-to-binder ratios (w/b) of 0.4, 0.45, and 0.5 were prepared using a constant alkali content of 4% Na2O and a binder-to-sand ratio of 1:3. The alkali activator solution (SiO2:Na2O molar ratio = 1.5) was prepared by dissolving sodium hydroxide pellets in deionized water, followed by mixing with sodium silicate solution.
The slag and sand were dry-mixed for 2 min in a planetary mixer. The alkali activator was added gradually, followed by 3 min of mixing. The mixtures were cast into 40 × 40 × 160 mm3 steel molds within 5 min of mixing completion, vibrated with cement mortar vibration compaction equipment to remove entrapped air, and covered with plastic film. Specimens were demolded after 24 h and cured at 20 ± 2 °C under >95% relative humidity (RH) for 28 d.
After curing, specimens were vertically semi-immersed to a depth of 4 cm in 5 wt.% Na2SO4 solution maintained at 20 ± 2 °C. The exposure test was conducted in a controlled environment (20 ± 2 °C, 60 ± 5% RH) to simulate tidal zone conditions. The solution was replenished every 60 d to maintain consistent ion concentration. The samples were labeled as AAS-0.4, AAS-0.45, and AAS-0.5 based on their w/b ratios. Detailed mix proportions are summarized in Table 2.

Table 2.
Summarizes mix proportions and curing parameters.
2.3. Experiment of the Semi-Immersion Form of AAS Sodium Sulphate Erosion
2.3.1. Surface Crystallization and Mass Change
During the semi-immersion in sodium sulphate, crystalline features on the mortar surface were documented via digital imaging every 60 d. The mass of the mortar was determined using an electronic balance (RC-FA-1262SEM, Shanghai Sunyu Hengping Scientific Instrument Co., Shanghai, China, ±0.1 mg) after removing the crystalline material from the surface of the specimen after the photographs were taken, and the rate of change in the mass of the mortar during the erosion process was calculated according to Equation (1).
where: Mt was the mass change ratio, mt referred to the mass of the AAS mortar after semi-immersion in a specific age, and m0 represented the initial mass of mortar before erosion.
2.3.2. Mechanical Property
The flexural and compressive tests were carried out using DYE-10A Flexural Testing Machine for Mortar and NYL-600 Pressure Testing Machine (Beijing North Jianyi Technology Co., Beijing, China). The loading rates of flexural strength and compressive strength were 50 N/s and 2.4 kN/s according to the Chinese standard GB/T17671-2021 [24], and the test results were the average values of three specimens and six specimens, respectively.
2.3.3. Pore Structure Analysis
The open porosity of the mortar was measured following the standard ASTM C642 [25,26]. Specimens were saturated in water for 72 h, surface-dried with a towel, and weighed in saturated surface-dry (ms) and submerged (mw) states. After oven-drying at 40 °C to constant weight, the dry mass (md) was recorded. Total open porosity (φt) was calculated as:
To determine the water absorption coefficient (S), s the specimens were dried to constant weight at 40 °C, sealed on four sides with aluminum foil, and partially immersed in water (3–5 mm depth). Mass gain was monitored every 15 min, and S was derived by fitting the data to:
where I was the cumulative water absorption per unit area, and t was the absorption time.
2.4. Erosion Resistance Mechanism of AAS
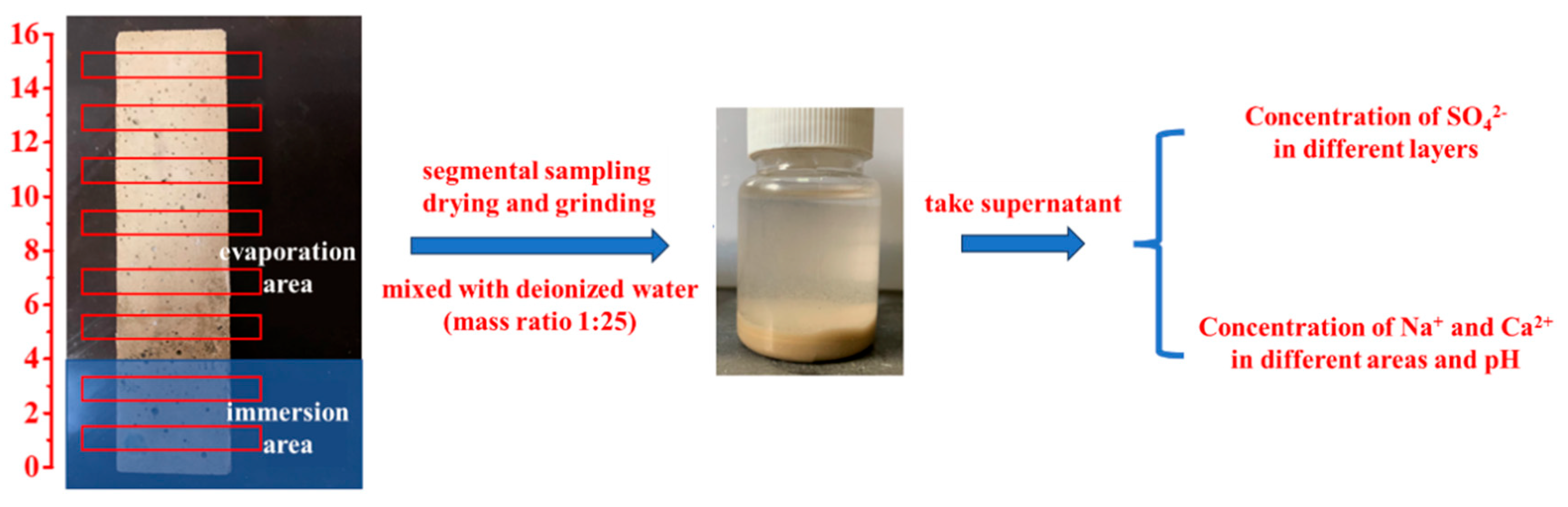
To analyze ion migration and pH evolution during erosion, SO42−, Na+, and Ca2+ concentrations in leachates were quantified using a UV spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan), ICP-OES (ICAP6300, Thermo Fisher Scientific, Shanghai, China), and a pH meter (TP310, Beijing Times New Dimension Measurement and Control Equipment Co., Beijing, China). Mortar specimens at designated ages (0–360 d) were retrieved, and surface crystallization products were mechanically removed. Before testing, seal all four sides of the mortar with aluminum foil tape and cover the top with a plastic sheet to prevent water evaporation. Then the specimens (40 × 40 × 160 mm3) were vertically semi-immersed to 4 cm depth in 5 wt.% Na2SO4 solution. As illustrated in Figure 1, the immersion and evaporation areas were separately sectioned using a concrete powder mill. Powder samples were collected at 1–15 cm intervals from the specimen base, sieved (160 mesh), and dried to constant weight. The sieved powder was mixed with deionized water (1:25 mass ratio), sealed for 24 h, and filtered to obtain leachates for testing.
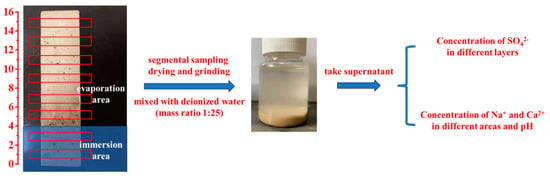
Figure 1.
SO42−, Na+, Ca2+ ion concentrations and pH measurement.
3. Results and Discussion
3.1. Macroscopic Performance Evolution Under Sulfate Attack
This section systematically evaluates the macroscopic degradation of AAS mortars under semi-immersion in sodium sulfate, focusing on surface deterioration, mass dynamics, mechanical degradation, and pore structure evolution.
3.1.1. Surface Crystallization and Morphological Deterioration
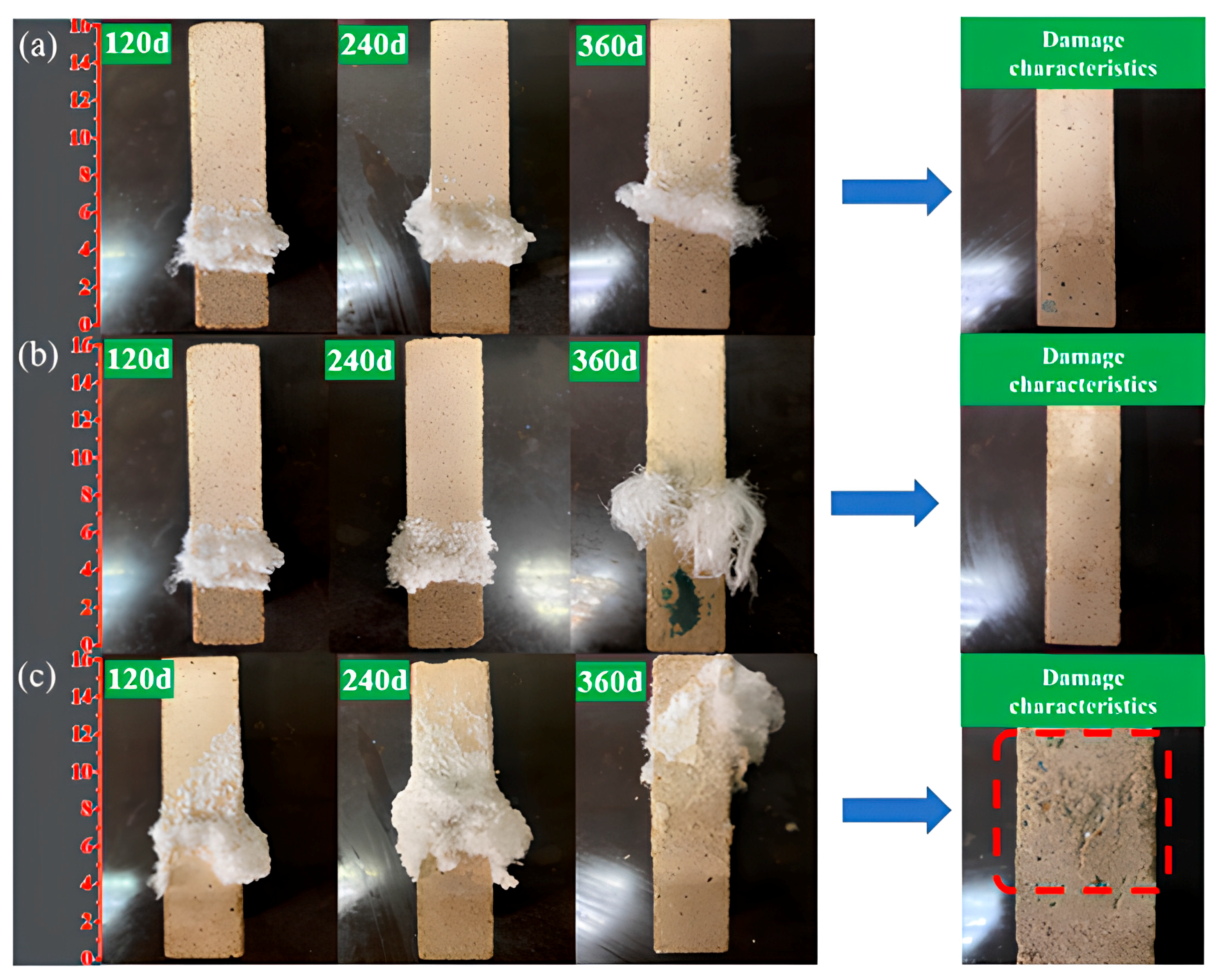
The surface crystallization behavior of AAS mortars under semi-immersion in sodium sulfate solution exhibited significant dependency on the water-to-binder (w/b) ratio. As illustrated in Figure 2, sodium sulfate crystals progressively accumulated on the evaporation area of the mortar surface, with crystallization intensity escalating as the w/b ratio increased. At 120 d of exposure, the AAS-0.5 specimen (w/b = 0.5) displayed extensive crystal coverage (about 35% of the evaporation area), whereas AAS-0.4 (w/b = 0.4) and AAS-0.45 (w/b = 0.45) exhibited smaller crystallization (about 20% coverage). The AAS-0.5 surface was almost entirely encrusted with crystalline deposits at 360 d (Figure 2c), accompanied by severe spalling in the evaporation area, while AAS-0.4 and AAS-0.45 retained structural integrity despite localized crystal formation.
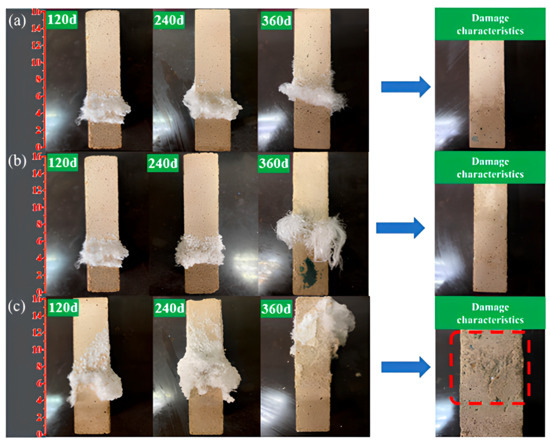
Figure 2.
Apparent crystalline behavior and damage characteristics of AAS mortars with different w/b ratios at immersion in 5 wt.% Na2SO4 solution ((a): AAS-0.4, (b): AAS-0.45, (c): AAS-0.5).
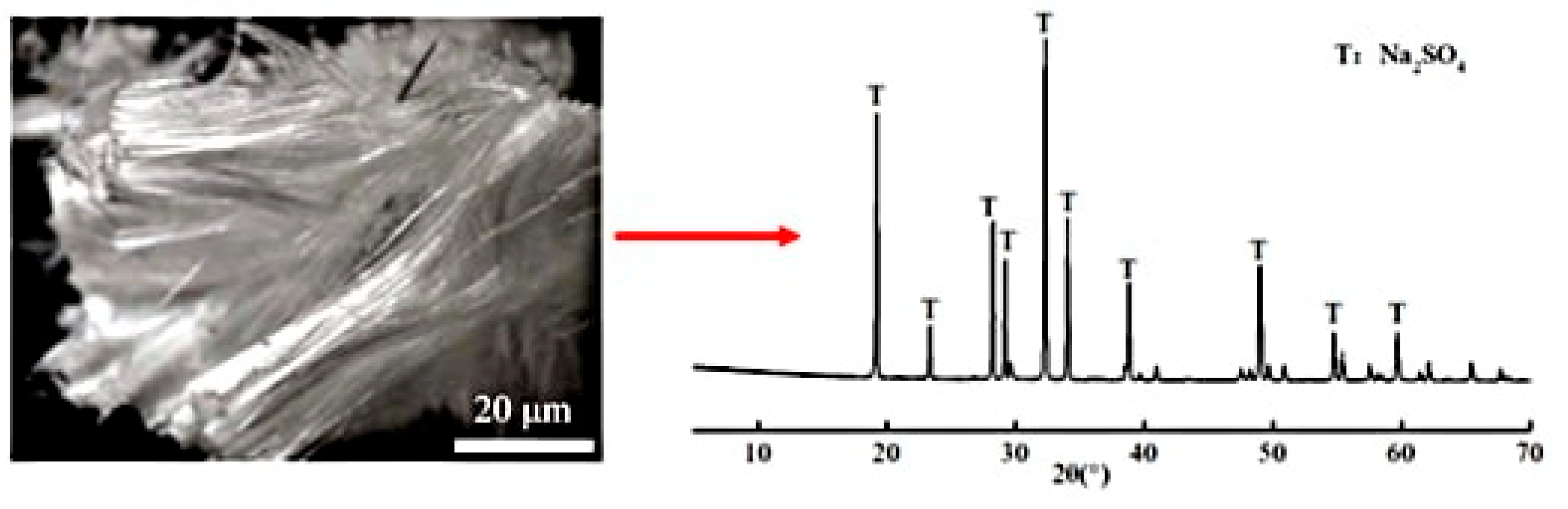
XRD analysis (Figure 3) confirmed the crystalline deposits as anhydrous sodium sulfate (Na2SO4), formed through supersaturation driven by capillary-evaporation synergy. The sodium sulfate solution infiltrated the mortar matrix via capillary action, with subsequent evaporation concentrating ions at the air-exposed surface [11,27]. Higher w/b ratios amplified this process due to accelerated ion mobility and coarsened pore structures, which facilitated rapid sulfate transport and prolonged supersaturation.
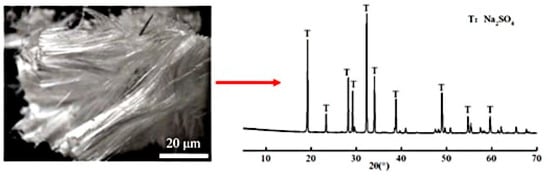
Figure 3.
Crystalline products on the mortar surface and the XRD patterns.
The spalling severity in high w/b specimens (e.g., AAS-0.5) arose from crystallization pressure exerted within pores. As Na2SO4 crystals nucleated and grew, the resultant stress exceeded the strength of the mortar matrix, particularly in the evaporation area where porosity and water absorption were elevated. Conversely, lower w/b ratios (0.4 and 0.45) mitigated damage through refined pore networks, which restricted ion ingress and distributed crystallization stresses more uniformly.
These observations are in agreement with prior studies [9,28], confirming that AAS mortars resist chemical sulfate attack (e.g., ettringite formation) but remain vulnerable to physical crystallization damage under semi-immersion. The interplay between w/b ratio, pore structure, and ion transport dynamics underscores the critical role of formulation optimization in balancing durability and crystallization resistance.
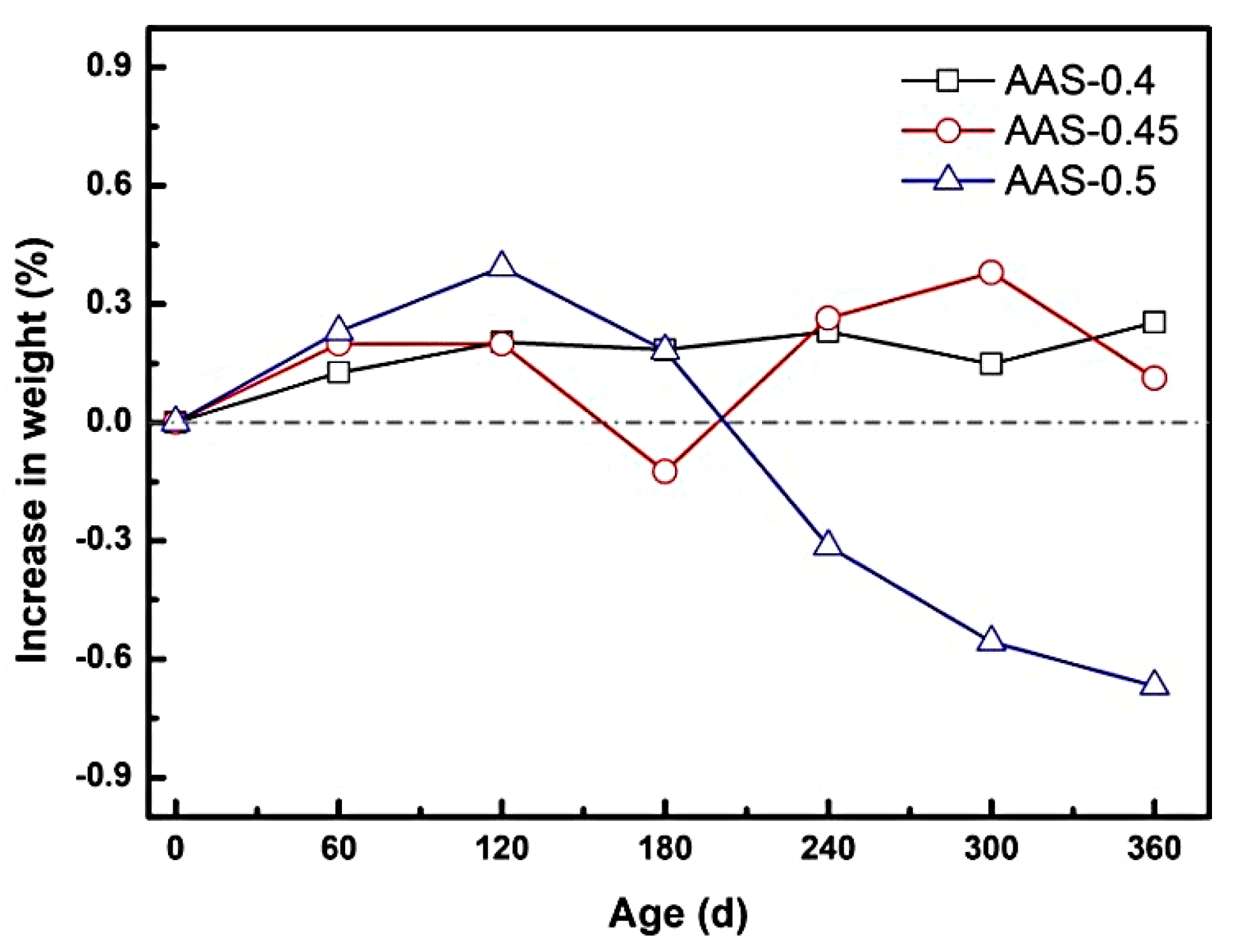
3.1.2. Mass Change and Spalling Behavior
The mass evolution of AAS mortars during semi-immersion in sodium sulfate solution revealed distinct phases of sulfate accumulation and crystallization-induced spalling, strongly influenced by the water-to-binder (w/b) ratio. As depicted in Figure 4, mortars with lower w/b ratios (0.4 and 0.45) exhibited a gradual mass gain during the erosion process, where the increases at 360 d were 0.25% and 0.13%, respectively. This trend reflects the progressive infiltration and deposition of sodium sulfate within capillary pores, enhanced by the continuous hydration of unreacted slag particles [29].
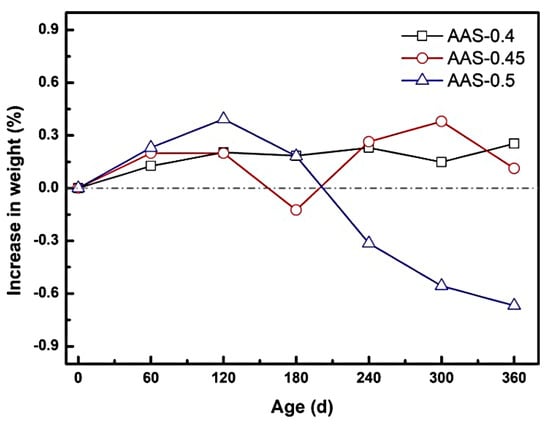
Figure 4.
Mass change of AAS mortar with different w/b ratios during the immersion process.
In contrast, the AAS-0.5 specimen (w/b = 0.5) displayed a biphasic mass response: an initial rapid gain of 0.39% at 120 d, followed by a sharp decline to a net loss of 0.67% by 360 d. The pore structure was decisive for this process, as evidenced by the water absorption coefficient of AAS-0.5 much higher than that of AAS-0.4 and AAS-0.45, indicating significantly enhanced pore connectivity. The early mass increase stemmed from accelerated sulfate ingress due to the coarser pore structure and elevated water absorption coefficient, which facilitated capillary-driven ion transport. This high connectivity promoted rapid SO42−/Na+ migration to the evaporation area, concentrating ions for crystallization. However, prolonged exposure led to supersaturation of Na2SO4 in the evaporation area, triggering extensive crystallization. The resultant crystallization pressure, amplified by the inverse relationship between pore size and stress intensity, exceeded the mortar strength, with the interconnected pore network further aggravating matrix exfoliation after crystal formation, causing surface spalling and subsequent mass loss [30,31].
The change in mass of AAS mortar reflects its spalling condition. The spalling severity correlated directly with w/b ratio. For w/b = 0.5, macroscopic flaking of the surface layer in the evaporation area (Figure 2c), while lower w/b ratios (0.4 and 0.45) showed no visible spalling. This divergence arises from the interplay between pore refinement and transport kinetics: lower w/b mortars restricted sulfate accumulation and distributed crystallization stresses more uniformly, delaying damage initiation.
3.1.3. Mechanical Degradation: Compressive and Flexural Strength
The mechanical performance of AAS mortars under semi-immersion in sodium sulfate solution exhibited dual-phase behavior: initial strength enhancement followed by progressive degradation, with the severity of deterioration governed by the water-to-binder (w/b) ratio.
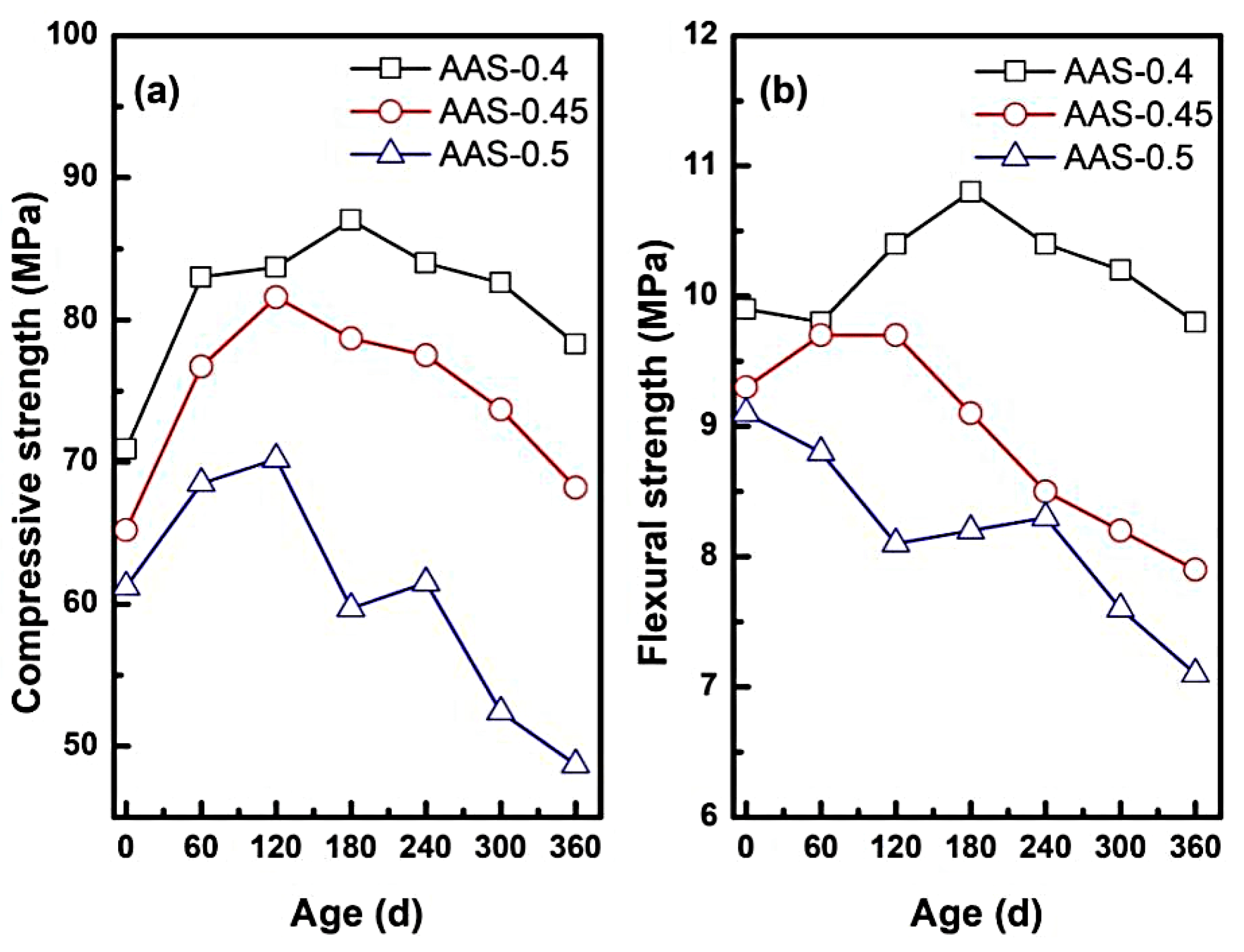
As shown in Figure 5a, compressive strength initially increased for all w/b ratios, peaking at 180 d for AAS-0.4 (+22.71%), 120 d for AAS-0.45 (+25.15%), and 120 d for AAS-0.5 (+14.71%). Early strength gains align with slag hydration kinetics [32] and sulphate-induced [9,33] pore refinement. In this process, residual slag reaction and sodium sulphate deposition work together to enhance matrix density. (1) Pore refinement: sulfate ions (SO42−) migrating into capillary pores precipitated as Na2SO4 crystals, reducing pore volume and enhancing matrix compactness. (2) Continued slag hydration [29,32]: residual unhydrated slag particles reacted with alkaline activators, forming additional C-(N)-A-S-H gel that strengthened the binder network. However, prolonged exposure triggered strength loss, with compressive strength declining by 10% (w/b = 0.4), 16.42% (w/b = 0.45), and 30.63% (w/b = 0.5) at 360 d (Figure 5a). This degradation might be correlated with two mechanisms: (1) crystallization pressure [34]: supersaturated Na2SO4 in coarser pores (w/b = 0.5) generated internal stresses exceeding the tensile strength of the matrix, inducing microcracks and porosity expansion. (2) Decalcification of hydration products [35]: leaching of Ca2+ from C-(N)-A-S-H gel weakened the gel structure, particularly in high w/b mortars, where transport properties accelerated ion exchange.
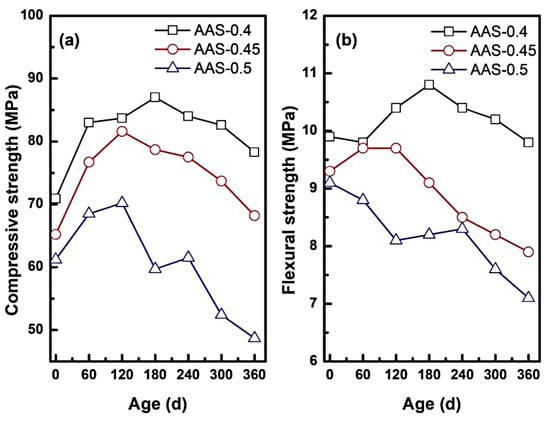
Figure 5.
Mechanical properties of AAS mortar with different w/b ratios during erosion ((a): compressive strength, (b): flexural strength).
Flexural strength followed similar biphasic trends for w/b = 0.4 and 0.45, peaking at 120 d and 180 d before declining (Figure 5b). In contrast, w/b = 0.5 exhibited monotonic flexural strength reduction (26.38% at 360 d). The reason for this discrepancy could be: (1) silica-rich gel bridging [11]: decalcification of C-(N)-A-S-H gel produced silica-rich phases that temporarily bridged microcracks, mitigating early flexural loss in lower w/b mortars. (2) pore coarsening: higher w/b ratios (0.5) exacerbated pore coarsening, diminishing the reinforcing effect of silica-rich gel and amplifying stress concentrations under bending loads [36].
These results demonstrated that the refined pore network with low w/b (0.4–0.45) slowed down the sulfate intrusion and crystalline stress distribution, and retarded the strength loss. However, the coarsened pores with high w/b (0.5) accelerated the ion migration, which exacerbated the supersaturation gradient and microstructural damage.
3.1.4. Pore Structure Evolution and Water Absorption Dynamics
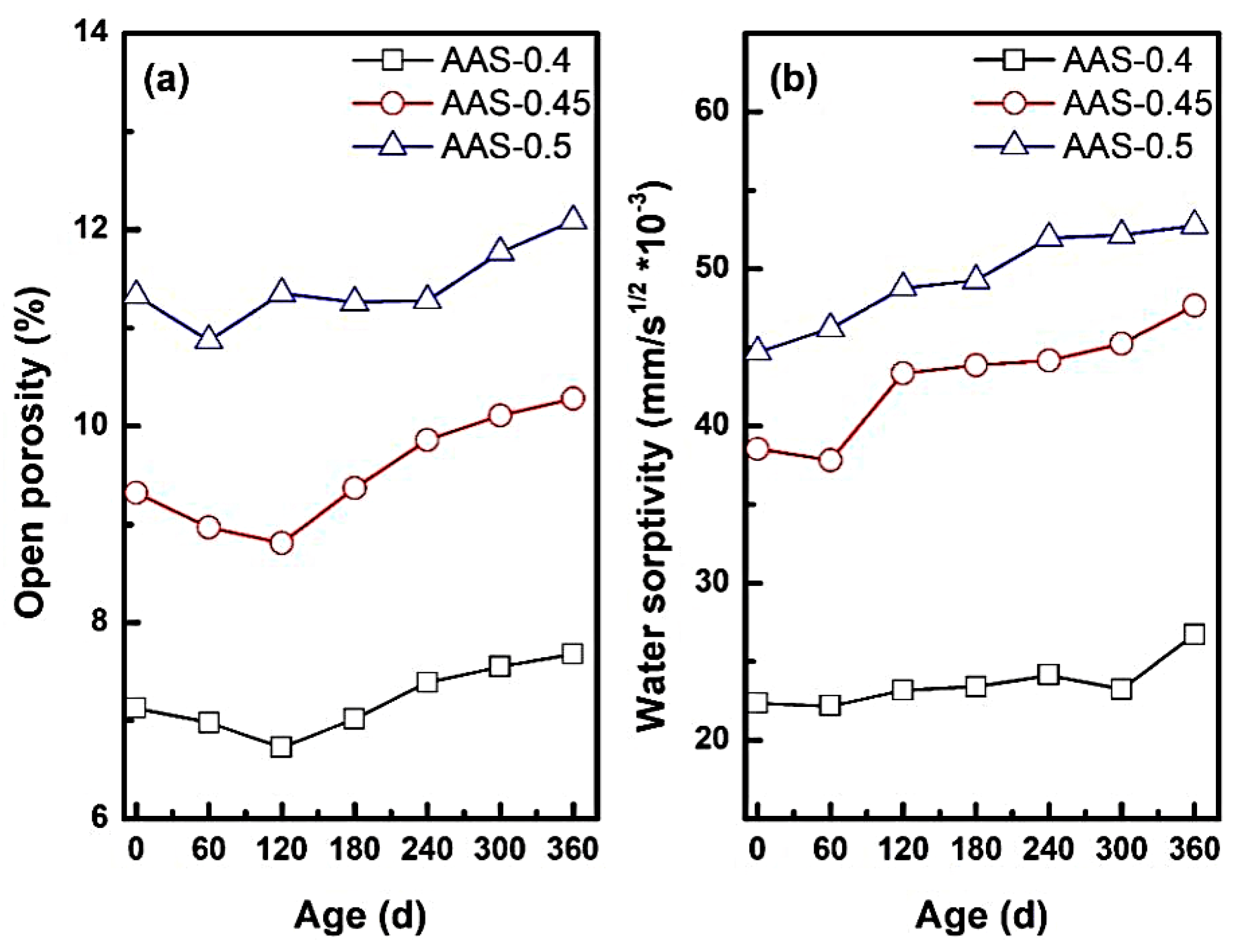
The open porosity and water absorption coefficient could reflect the change of internal pore structure of AAS mortar. It also determined the transport rate of sodium sulfate solution inside the AAS mortar, reflecting the internal microstructural changes of the mortar during the erosion process. Figure 6 presents the variation of open porosity and water absorption coefficient of AAS mortar with different w/b ratios during the erosion process.
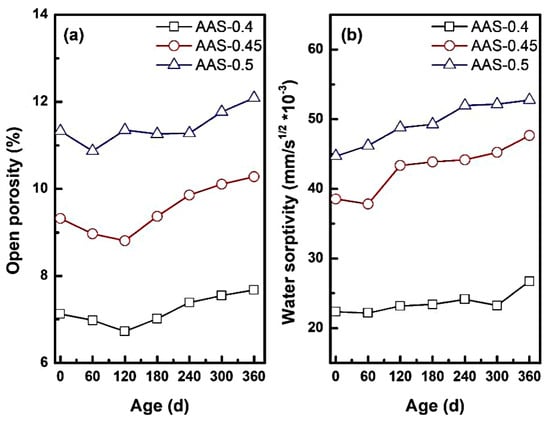
Figure 6.
Open porosity and water absorption rate coefficient of AAS mortar with different w/b ratio ((a): open porosity, (b): water absorption coefficient).
As shown in Figure 6a, open porosity exhibited a biphasic trend during erosion: initial reduction followed by significant expansion. For example, AAS-0.4 and AAS-0.45 reached a porosity minimum at 180 d, attributed to pore refinement via sulfate deposition and continued slag hydration [37,38]. Sodium sulfate crystals filled capillary pores, reducing connectivity and enhancing matrix compactness. However, prolonged exposure reversed this trend, with open porosity increasing by 6.6% for w/b = 0.5 compared to its initial state. This degradation stemmed from crystallization pressure within coarser pores (dominant in high w/b mortars), which generated microcracks and expanded pore networks.
The w/b ratio critically modulated porosity dynamics. Mortars with w/b = 0.5 exhibited the highest open porosity, nearly 1.6 times that of w/b = 0.4. This disparity arose from the coarser pore structure in high w/b mortars, which accelerated sulfate ingress and localized stress concentrations during crystallization.
Water absorption coefficients (Figure 6b) increased progressively with erosion time, reflecting pore structure degradation. For w/b = 0.5, the absorption coefficient surged by 23.52% (from 44.72 to 52.77 g/m2·s−1) at 360 d. This might be attributed to: (1) pore connectivity: higher w/b ratios amplified pore interconnectivity, creating preferential pathways for water and ion transport. (2) Hydration of anhydrous Na2SO4: during drying cycles, anhydrous Na2SO4 in pores absorbed moisture to form hydrated phases (e.g., Na2SO4·10H2O), further increasing water uptake.
These findings underscore the critical role of w/b ratio in balancing pore structure refinement against crystallization risks, providing actionable guidelines for optimizing AAS formulations in sulfate-rich environments.
3.2. Mechanistic Insights into Sulfate-Induced Degradation
3.2.1. Analysis of SO42− Ion Migration Behavior
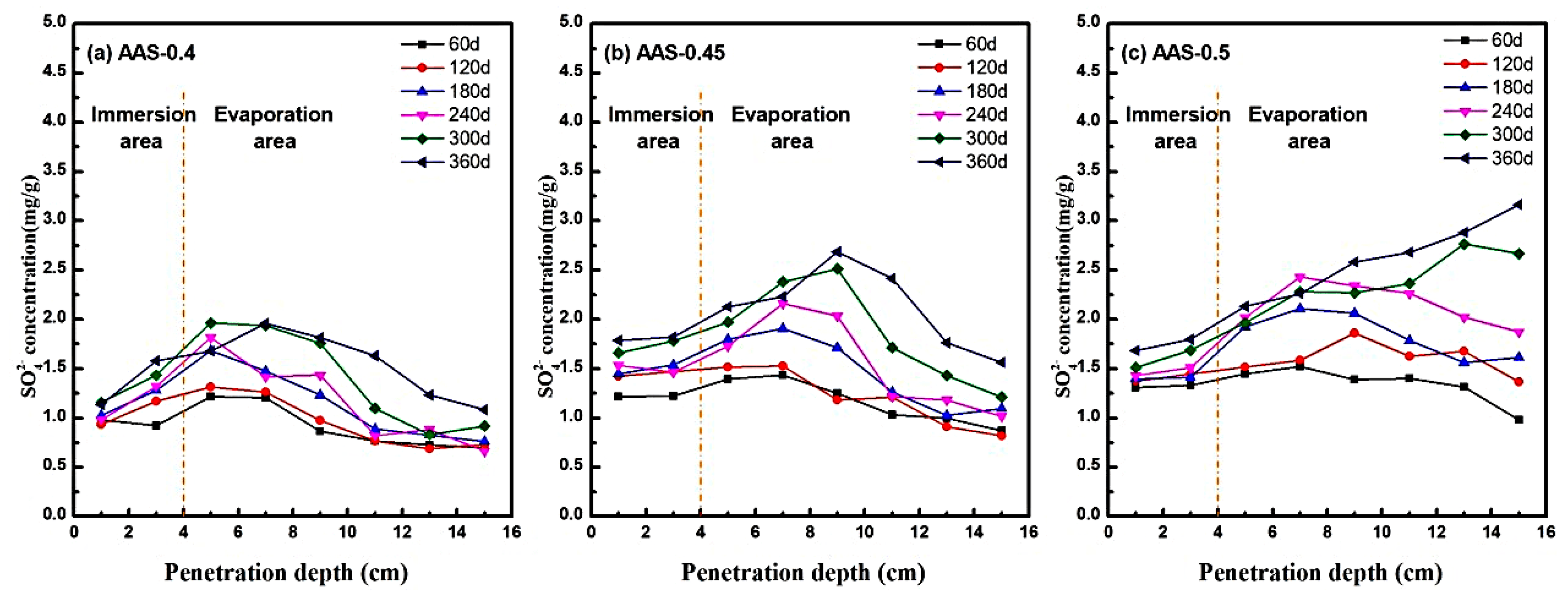
The migration and enrichment of SO42− ions within AAS mortars under semi-immersion were systematically analyzed by measuring spatial ion concentrations at incremental depths (1–15 cm from the base) using UV spectrophotometry. Figure 7 illustrates the SO42− distribution profiles across immersion and evaporation areas for mortars with varying w/b ratios (0.4, 0.45, 0.5) at 360 d of exposure.
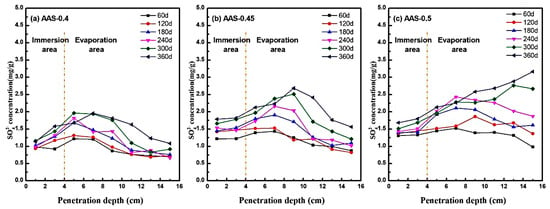
Figure 7.
SO42− distribution characteristics of AAS mortars with different w/b ratios at different erosion ages ((a): AAS-0.4, (b): AAS-0.45, (c): AAS-0.5).
In low w/b mortars (e.g., AAS-0.4), SO42− exhibited limited transport capacity, peaking at 2.0 mg/g at 7 cm from the base (evaporation area) before declining sharply to 1.0–1.25 mg/g at greater distances (Figure 7a). This localized enrichment aligns with capillary-evaporation dynamics: finer pore networks restricted ion mobility, causing sulfate accumulation in the mid-evaporation region. Conversely, higher w/b ratios amplified transport kinetics. For AAS-0.5 (Figure 7c), SO42− concentrations increased monotonically with distance, reaching 3.2 mg/g at the top of the evaporation area. The coarser pore structure facilitated rapid capillary rise and prolonged ion retention, enabling sulfate to permeate the entire evaporation area.
The biphasic concentration trends, which rise in the immersion area and peak in the mid-evaporation regions, reflect the balance between the ingress driven by capillaries and the supersaturation driven by evaporation [39,40]. This mechanism is consistent with Fickian diffusion models [41], where ion flux (J) is proportional to the concentration gradient (∇C) and diffusivity (D): . Higher w/b ratios elevated D due to increased pore connectivity, accelerating sulfate ingress. Concurrently, evaporation at the surface established a supersaturation gradient, promoting Na2SO4 crystallization in regions with sufficient ion accumulation.
Notably, the spatial mismatch between SO42− enrichment and crystallization damage highlight the role of pore size distribution. In AAS-0.4, sulfate is concentrated in sub-20 nm pores, where crystallization stress inversely scales with pore radius. Despite lower total sulfate content, localized stress exceeded matrix strength in coarser pores, initiating microcracks. In contrast, AAS-0.5 broader pore distribution dispersed stress but allowed deeper sulfate penetration, exacerbating surface spalling.
These findings underscore the critical trade-off between transport kinetics and crystallization resistance. Lower w/b ratios mitigate damage by refining pore networks, while higher ratios amplify sulfate ingress and supersaturation risks.
3.2.2. Na+ Ion Redistribution and Crystallization Influence
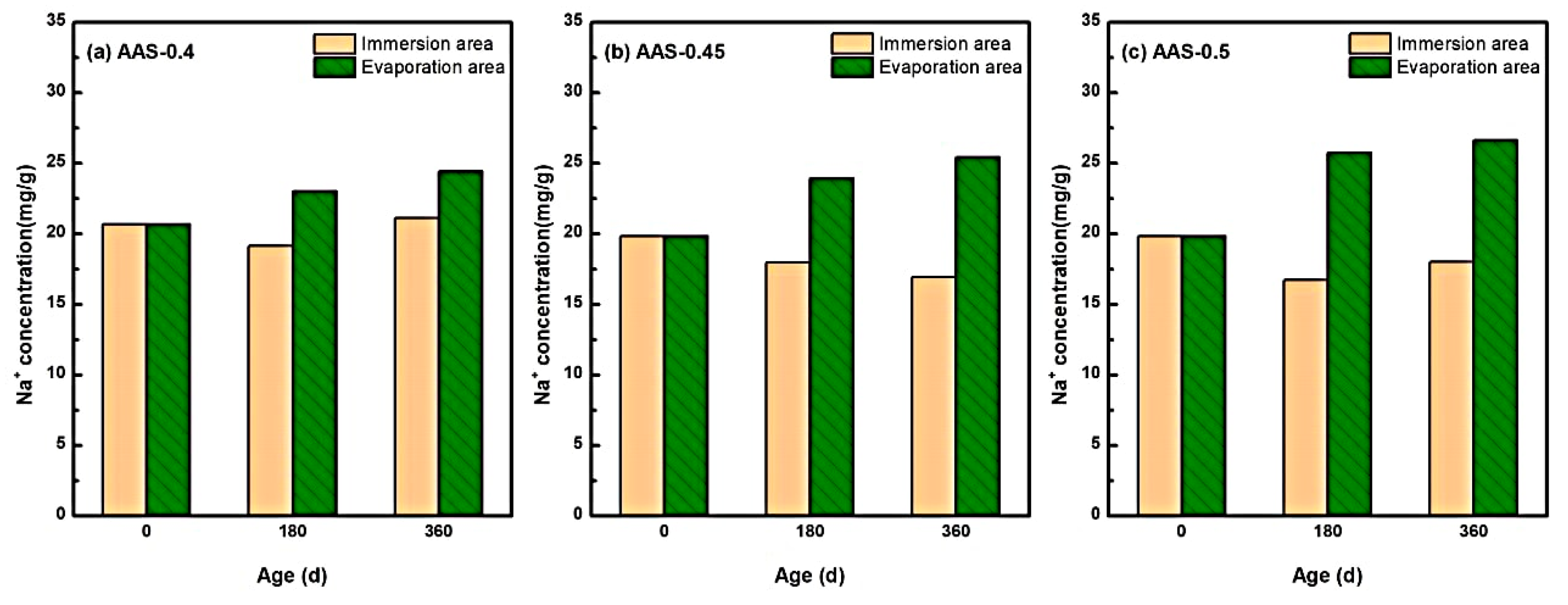
The redistribution of Na+ ions within AAS mortars under semi-immersion played a pivotal role in governing sodium sulfate crystallization dynamics, particularly due to the inherently high Na+ content in alkali-activated systems.
Figure 8 delineates the spatial-temporal evolution of Na+ concentrations across immersion and evaporation areas for mortars with varying w/b ratios (0.4, 0.45, 0.5). Notably, Na+ exhibited a bidirectional migration pattern: outward diffusion from the immersion area (saturated region) and upward capillary-driven transport toward the evaporation area (unsaturated region). For instance, in AAS-0.5 (w/b = 0.5), Na+ concentration in the immersion area decreased by 9.10% over 360 d, while the evaporation area exhibited a 34.26% increase. In contrast, lower w/b ratios (0.4 and 0.45) displayed attenuated Na+ migration, with evaporation area concentrations rising by only 19.03% and 28.16%, respectively.
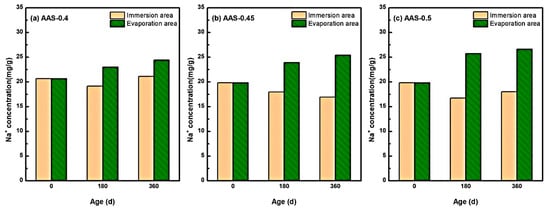
Figure 8.
Characteristics of Na+ distribution of AAS mortars with different w/b ratios at different erosion ages ((a): AAS-0.4, (b): AAS-0.45, (c): AAS-0.5).
This behavior stems from the interplay of three mechanisms: (1) Concentration-driven diffusion [42]: the initial Na+ concentration in AAS pore solutions exceeded that of the external sodium sulfate solution (5 wt.%), establishing a concentration gradient that drove outward Na+ diffusion from the immersion area. (2) Capillary-evaporation synergy: in the evaporation area, continuous water loss elevated ionic concentrations, creating localized supersaturation [43]. Concurrently, capillary rise transported Na+ and SO42− from the immersion area, further amplifying ion accumulation. (3) Pore structure modulation: Higher w/b ratios (e.g., 0.5) exacerbated pore coarsening, accelerating ion mobility and prolonging Na+ retention in the evaporation area.
These findings align with studies by, which emphasize that Na+ migration governs crystallization kinetics in alkali-activated systems. However, this work uniquely quantifies the role of w/b ratios in modulating Na+ transport pathways and crystallization regimes, providing a mechanistic basis for optimizing pore structures to mitigate damage.
3.2.3. pH Evolution and Alkalinity Loss
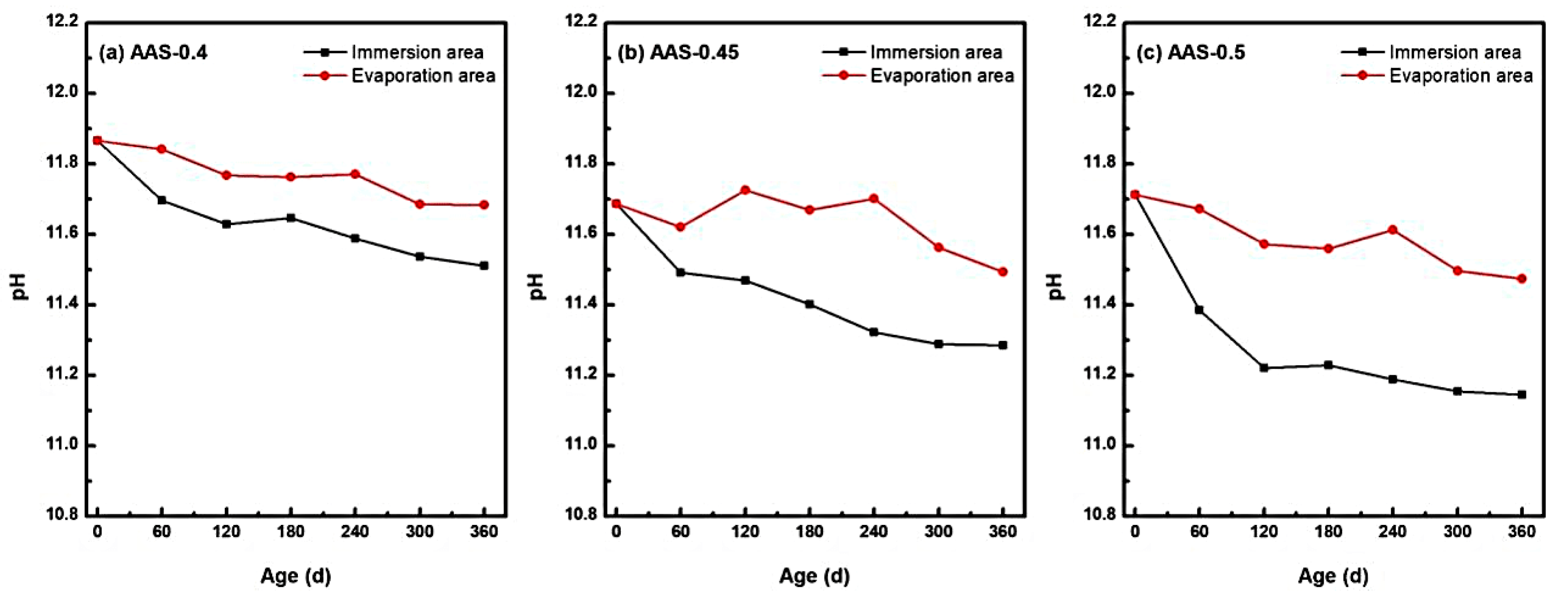
The long-term stability of hydration products in alkali-activated slag (AAS) mortars is intrinsically linked to their high-alkalinity environment, which was critically influenced by semi-immersion in sodium sulfate solution [44]. As shown in Figure 9, pH values in both immersion and evaporation areas exhibited a progressive decline over 360 d of exposure, with distinct spatial and compositional dependencies tied to w/b ratios.
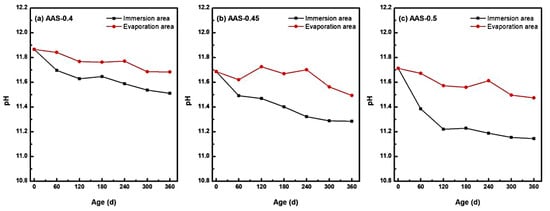
Figure 9.
pH changes of AAS mortars with different w/b ratios at different erosion ages ((a): AAS-0.4, (b): AAS-0.45, (c): AAS-0.5).
The immersion zone, characterized by continuous water saturation, experienced the most pronounced alkalinity loss. For AAS-0.5 (w/b = 0.5), pH decreased by 4.85% (from 11.712 to 11.144), while AAS-0.4 (w/b = 0.4) and AAS-0.45 (w/b = 0.45) showed reductions of 3.06% and 3.47%, respectively. This accelerated pH declined in high w/b mortars arose from enhanced ion mobility through coarser pore networks, facilitating outward leaching of hydroxyl ions (OH−) and alkali ions (Na+) into the external solution. The sustained ionic exchange destabilized hydration products, particularly C-(N)-A-S-H gel, promoting decalcification reactions and free Ca2+ release [5].
In contrast, the evaporation area exhibited milder pH reductions (e.g., 2.04% for AAS-0.5), attributed to limited water availability and localized ion enrichment. Evaporation-driven concentration effects partially counteracted OH− leaching, yet residual moisture still enabled gradual alkalinity loss. Notably, the pH gradient between immersion and evaporation areas widened with higher w/b ratios, reflecting divergent transport kinetics.
The pronounced alkalinity reduction in immersion area underscores the vulnerability of AAS mortars to chemical destabilization under semi-immersion. Lower w/b ratios mitigated this degradation by refining pore structures, thereby preserving the high-pH environment essential for hydration product stability. These findings highlight the necessity of optimizing w/b ratios to balance ionic mobility and alkalinity retention in sulfate-rich environments.
3.2.4. Decalcification and Free Ca2+ Release
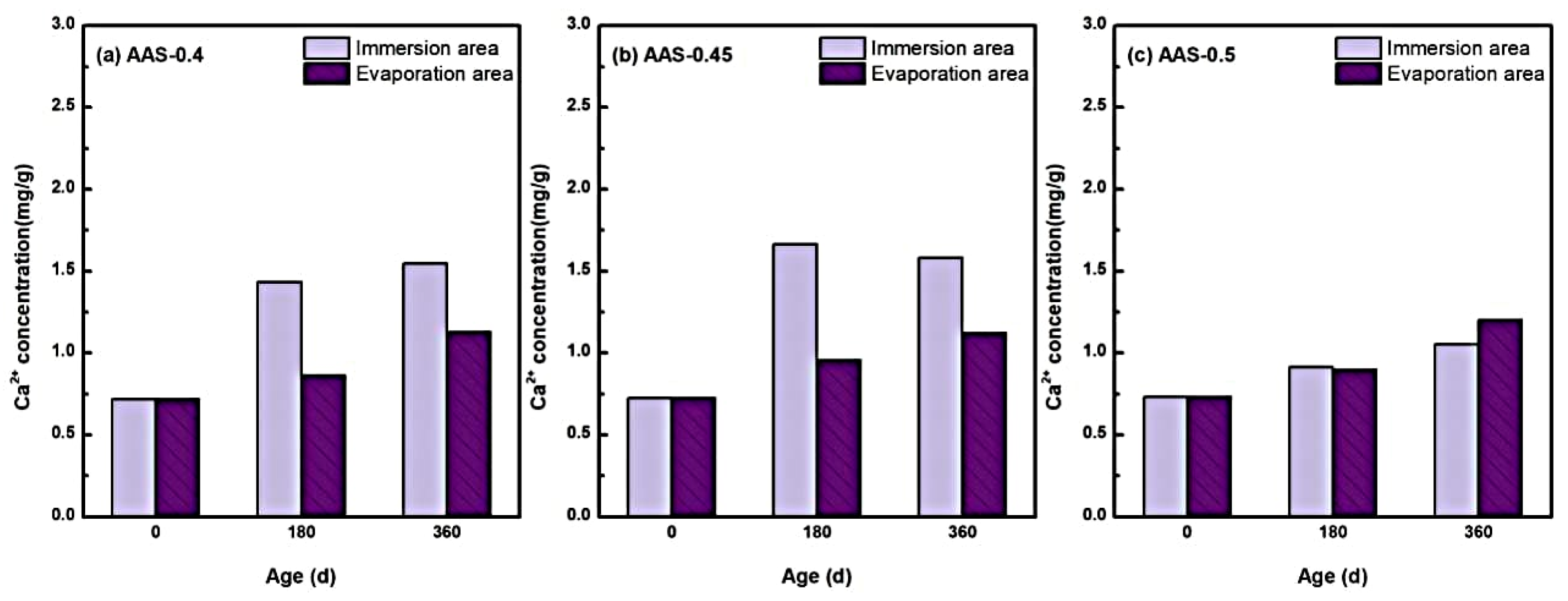
To further investigate the chemical stability of the hydration products during the erosion process, the changes in the internal free Ca2+ concentration of AAS mortars with different w/b ratios were analyzed during the immersion process, and the results are shown in Figure 10.
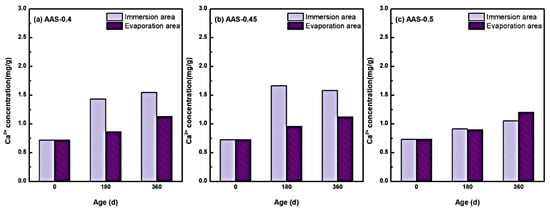
Figure 10.
Variation of free Ca2+ concentration in the interior of AAS mortar with different w/b ratio ((a): AAS-0.4, (b): AAS-0.45, (c): AAS-0.5).
Free Ca2+ concentrations exhibited a marked increase across all w/b ratios during the 360 d erosion period, with pronounced spatial heterogeneity between immersion and evaporation areas. For instance, in AAS-0.4 (w/b = 0.4), Ca2+ concentration in the immersion area surged by 115.30% (from 0.719 to 1.548 mg/g), compared to a milder 57.02% increase (from 0.719 to 1.129 mg/g) in the evaporation zone. This disparity underscores the dominant role of continuous water saturation in the immersion area, which facilitated ionic dissolution and decalcification of hydration products, particularly C-(N)-A-S-H gel [45].
The decalcification process intensified with higher w/b ratios but exhibited nonlinear behavior. AAS-0.45 (w/b = 0.45) showed the most significant Ca2+ accumulation in the immersion area, reflecting enhanced ion mobility through moderately coarsened pores. However, for AAS-0.5 (w/b = 0.5), despite severe pH reduction in the immersion area, Ca2+ concentration increased only to 1.112 mg/g, which lower than expected. This anomaly arose from the interplay between accelerated decalcification and outward Ca2+ migration [46]: coarser pore networks enabled rapid leaching of liberated Ca2+ ions into the external solution, reducing their retention within the mortar matrix.
In contrast to the immersion area, Ca2+ enrichment in the evaporation area remained limited (e.g., 1.129 mg/g for w/b = 0.4 at 360 d). Restricted water availability and localized sulfate crystallization suppressed sustained decalcification, while evaporation-driven ion concentration partially offset Ca2+ leaching. Notably, higher w/b ratios marginally amplified Ca2+ accumulation in the evaporation area (e.g., 1.233 mg/g for w/b = 0.5), consistent with broader ion transport pathways.
These findings reveal a dual role of w/b ratios in governing decalcification: (1) Lower ratios (0.4–0.45) restricted ion mobility, localizing Ca2+ release within the immersion area and exacerbating microstructural damage [47,48]. (2) Higher ratios (0.5) accelerated Ca2+ leaching, depleting internal concentrations despite intensified decalcification [49]. This trade-off underscores the importance of optimizing w/b ratios to balance pore structure refinement and ion retention, thereby mitigating long-term chemical degradation in sulfate-rich environments.
4. Discussion
The key degradation metrics across w/b ratios at 360 d are consolidated in Table 3 for direct comparison.

Table 3.
Consolidates key performance metrics at 360 d.
The degradation of AAS mortars in a semi-immersed state could be attributed to two synergistic mechanisms: physical crystallization pressure and chemical decalcification. Higher w/b ratios (0.5) accelerated both processes due to coarsened pores, increasing ion mobility by 58.03% and enabling deeper sulphate penetration. This is consistent with the findings of references [50,51], which noted that capillary-driven ion enrichment in coarser pores exacerbates efflorescence.
Notably, the biphasic strength evolution (an initial gain followed by a loss) contrasted with OPC systems, in which sulfate attack usually caused a steady deterioration via ettringite expansion [52,53,54]. In the AAS, the initial strength increase was due to pore-filling crystallization and an ongoing slag reaction, which was consistent with the findings of Rashad et al. [55]. However, prolonged exposure caused strength loss of over 30% in high w/b mortars, which was greater than the losses reported for hybrid alkali-activated cements [56] or fly ash-slag blends [57].
The alkali activation fundamentally reorganized the structure of slag by breaking Ca–O–Si bonds and releasing Ca2+ and silicate monomers, which then formed a C-(N)-A-S-H gel [58,59]. At an optimal dosage of 4% Na2O, this yielded a gel with a Ca/Si ratio of approximately 1.2 and an Al/Si ratio of approximately 0.3, achieving a balance of strength and durability. However, increasing the alkali concentration over-dissolved the slag glassy phase, resulting in increased free Na+ and pore coarsening [60], which exacerbated sulfate crystallization. In contrast, clay-based geopolymers fractured Al–O–Si bonds under alkali attack to form nanoporous N-A-S-H gels that immobilized Na+ via ion-exchange sites [61]. This structural distinction explained why AAS was more vulnerable to crystallization despite similar alkali dosages. The severity of efflorescence in AAS was three times higher than in clay-based geopolymers [61], which was attributable to the higher mobility of Na+ in AAS. Optimizing w/b to 0.4–0.45 reduced spalling by refining pores, a strategy similar to those proposed for mitigating efflorescence in natural pozzolan-based AAMs.
5. Conclusions
The following conclusions were drawn from the investigation of AAS mortars under semi-immersion in sodium sulfate solution:
The higher water-cement ratio (0.5) significantly exacerbated surface crystallization and spalling, with almost 100% Na2SO4 coverage in the evaporation area after 360 d. This damage originated from accelerated ion transport and pore coarsening, with local crystallization stresses exceeding the tensile strength of the mortar. Lower water-cement ratios (0.4–0.45) can maintain structural integrity by refining the pore network and delaying stress accumulation.
Early strength gains (up to 25.15%) were driven by pore refinement and slag hydration, while prolonged exposure caused strength losses (up to 30.63%) due to microcracking from crystallization pressure and decalcification of hydration products. Flexural strength trends further highlighted the role of silica-rich gel bridging in mitigating damage at lower w/b ratios.
The SO42− ion enrichment in evaporation area and outward Na+ diffusion established supersaturation gradients, aligning with crystallization progression. Higher w/b ratios amplified sulfate penetration depth (3.2 mg/g at 15 cm for w/b = 0.5), while lower ratios restricted transport (2.0 mg/g at 7 cm for w/b = 0.4).
The alkalinity loss (pH reduction up to 4.85%) and free Ca2+ release (115.3% increase in immersion area) underscored the vulnerability of hydration products to decalcification, exacerbated by coarser pore structures in high w/b mortars.
This study demonstrated that optimizing the w/b ratio (0.4–0.45) effectively balances pore refinement, ion retention, and stress distribution, providing a viable strategy for improving the durability of AAS mortars in sulphate-rich semi-immersed environments. Although a w/b ratio of 0.4–0.45 minimizes the risk of sulfate crystallization, real-world applications require control of alkali leaching (with an evaporation area of Na+ < 30% of the baseline) and monitoring of carbonation (with a pH level of >11.5). ASTM C642 pore data capture transport-relevant capillaries, but not gel-scale effects.
Author Contributions
Methodology, Y.Q. and J.Y.; Software, P.L.; Writing—original draft, Z.Z. and K.Y.; L.Y.: Conceptualization, Validation, Supervision, Writing—review & editing; C.Y.: Resources, Data curation, Project administration, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from National Natural Science Foundation of China (No. 52278242), and The Lets Innovation Foundation (KZJSX.JS20230262KZJSX.TO.JS20230008) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to containing proprietary formulations protected by industrial partners.
Conflicts of Interest
Author Zhenwei Zhou was employed by the company China Communications Construction Company Ltd.; Authors Yuetao Qiu and Peng Liu were employed by the company Chongqing Jianyan Kezhijie New Material Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Martins, M.C.; Langaro, E.A.; Macioski, G.; Medeiros, M.H.F. External ammonium sulfate attack in concrete: Analysis of the current methodology. Constr. Build. Mater. 2021, 277, 122252. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I. Numerical assessment of external sulfate attack in concrete structures. A review. Cem. Concr. Res. 2019, 121, 91–105. [Google Scholar] [CrossRef]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Shen, C.; Tang, D.; Lyu, Z.; Yang, C.; Yu, L. Distribution of coarse aggregate and steel fibers in ultra-high performance concrete containing coarse aggregate (UHPC-CA) and their effect on the flexural performance. Constr. Build. Mater. 2024, 421, 135666. [Google Scholar] [CrossRef]
- Yang, B.; Hu, X.; Li, A.; Zhao, T.; Sun, Y.; Fu, H. Degradation mechanism of cast-in-situ concrete under long-term sulfate saline soil attack. Constr. Build. Mater. 2025, 478, 141401. [Google Scholar] [CrossRef]
- Sun, L.; Lan, S.; Wang, C. Durability and microstructural evolution of seawater sea-sand coral concrete under different exposure environments of sulfate attack. Constr. Build. Mater. 2025, 478, 141399. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, M.; Zou, D.; Liu, T.; Li, Y. Mesoscale modeling of mechanical deterioration in sulfate-attacked concrete. Eng. Fract. Mech. 2025, 323, 04025154. [Google Scholar] [CrossRef]
- Banik, D.; He, R.; Lu, N.; Feng, Y. Mitigation mechanisms of alkali silica reaction through the incorporation of colloidal nanoSiO2 in accelerated mortar bar testing. Constr. Build. Mater. 2024, 422, 135834. [Google Scholar] [CrossRef]
- Gong, K.; White, C.E. Nanoscale Chemical Degradation Mechanisms of Sulfate Attack in Alkali-activated Slag. J. Phys. Chem. C 2018, 122, 5992–6004. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Yang, K.; Zhu, X.; Gevaudan, J.P.; Yang, C.; Basheer, M. The long-term failure mechanisms of alkali-activated slag mortar exposed to wet-dry cycles of sodium sulphate. Cem. Concr. Compos. 2021, 116, 103893. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Yang, C. An alternative admixture to reduce sorptivity of alkali-activated slag cement by optimising pore structure and introducing hydrophobic film. Cem. Concr. Compos. 2019, 95, 183–192. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Yang, K.; Mu, S.; Zhang, Z.; Magee, B.; Yang, C.; Basheer, M. Characterisation of temporal variations of alkali-activated slag cement property using microstructure features and electrical responses. Constr. Build. Mater. 2020, 261, 119884. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem. Concr. Res. 2024, 34, 139–148. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Walter, J. Autoclaving of alkali-activated materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012012. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Korniejenko, K.; Łach, M.; Mikuła, J.; Krzywda, J. Effect of Coffee Grounds Addition on Efflorescence in Fly Ash-based Geopolymer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012035. [Google Scholar] [CrossRef]
- Kuang, L.; Li, G.; Ma, W.; Liu, L.; Cui, X. Effect of metakaolin on the chloride diffusion properties of alkali-activated slag in an acidic environment. Appl. Clay Sci. 2024, 260, 107544. [Google Scholar] [CrossRef]
- Olatoyan, O.J.; Okeyinka, O.M.; Oluwafemi, B.; Oyewo, S.T.; Olayanju, O.K.; Kareem, M.A.; Adebanjo, A.U.; Salami, M.O. Investigation of tensile strength performance of green concrete incorporating steel slag. Hybrid Adv. 2024, 6, 100186. [Google Scholar] [CrossRef]
- Liu, J.; Yan, C.; Zhang, J.; Li, J.; Zhao, J.; Shen, X.; Cao, F.; Zhang, G.; Meng, X. Characteristics of AC impedance and pore structure in solid waste-based cementitious materials with different water-to-binder ratios. Constr. Build. Mater. 2025, 467, 140342. [Google Scholar] [CrossRef]
- Zheng, R.; Xia, J.; Liu, E.; Zhou, Q.; Wang, H. A high water-cement ratio aerated concrete using carbon dioxide as the foaming agent: Mix design, pore structure and hydration products. J. Build. Eng. 2025, 108, 112926. [Google Scholar] [CrossRef]
- GB/T 14684-2022; Sand for Construction. State Administration for Market Regulation: Beijing, China, 2022.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). State Administration for Market Regulation: Beijing, China, 2021.
- Wang, Y.; Jin, S.; Li, Y.; Zhao, B.; Chen, D.; Li, J.; Zhang, T.C. Polyvinyl alcohol fiber reinforced electrolytic manganese slag based geopolymer mortar: Mechanical properties and microstructure. Constr. Build. Mater. 2025, 477, 141390. [Google Scholar] [CrossRef]
- Wulandari, R.; Meysara; Emiliana; Sunarno; Heltina, D.; Khairat; Amri, A. Structural and physicomechanical properties of rice husk Ash-based geopolymer mortar with the addition of graphene nanosheets. Mater. Today Proc. 2023, 87, 70–78. [Google Scholar] [CrossRef]
- ASTM C642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Xue, L.; Ni, Z.; Zhou, Z.; Zhang, Z.; Xiong, H.; Wang, H.; Zhuge, X.; Liu, H. Effects of desulfurized gypsum on shrinkage behavior of alkali-activated slag (AAS) and hybrid alkali-activated cement (HAC). Case Stud. Constr. Mater. 2025, 22, e04320. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2012, 46, 361–373. [Google Scholar] [CrossRef]
- Yin, K.; Luo, Z.; Liu, X.; Pan, H.; Zhi, T.; Feng, H.; Song, Y.; Su, Y. Preparation and application of Na2SiO3@EC microcapsules for self-healing alkali-activated slag. Constr. Build. Mater. 2023, 400, 132651. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.X.; Khennane, A. Numerical modelling of degradation of cement-based materials under leaching and external sulfate attack. Comput. Struct. 2015, 158, 1–14. [Google Scholar] [CrossRef]
- Ran, B.; Li, K.; Fen-Chong, T.; Omikrine-Metalssi, O.; Dangla, P. Spalling rate of concretes subject to combined leaching and external sulfate attack. Cem. Concr. Res. 2022, 162, 106951. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef]
- Guan, X.; Hou, X.; Hao, X.; Liu, Y.; Li, F.; Hou, P. Dynamic energy performance and pore structure of CGSC under sulfate attack: An SHPB-based study. Constr. Build. Mater. 2024, 457, 139457. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Deng, D.; Xie, Y.; Long, G.; Tang, X. Physical sulfate attack on concrete lining–A field case analysis. Case Stud. Constr. Mater. 2017, 6, 206–212. [Google Scholar] [CrossRef]
- Jia, Z.; Cao, R.; Xiong, Y.; Zhang, S.; Jia, L.; Zhang, Y.; Gao, Y. Understanding the deterioration of hydration products in alkali-activated slag/Portland cement exposure to sulfate attack. J. Build. Eng. 2024, 95, 110292. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Phung, Q.T.; Jacques, D.; Neji, M.; Dauzeres, A.; Elsen, J.; Pontikes, Y. Microstructure, water permeability and micromechanical properties of alkali activated slag subjected to accelerated leaching. Mater. Des. 2024, 238, 112706. [Google Scholar] [CrossRef]
- Emminger, Y.H.; Ladner, L.; Ruiz-Agudo, C. Comparative study of the early stages of crystallization of calcium silicate hydrate (C-S-H) and calcium aluminate silicate hydrate (C-A-S-H). Cem. Concr. Res. 2025, 193, 107873. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Provis, J.L. Phase diagrams for alkali-activated slag binders. Cem. Concr. Res. 2017, 95, 30–38. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, D.; Schutter, G.D.; Yu, Z. Chemical sulfate attack performance of partially exposed cement and cement+fly ash paste. Constr. Build. Mater. 2012, 28, 230–237. [Google Scholar] [CrossRef]
- Gentilini, C.; Franzoni, E.; Bandini, S.; Nobile, L. Effect of salt crystallisation on the shear behaviour of masonry walls: An experimental study. Constr. Build. Mater. 2012, 37, 181–189. [Google Scholar] [CrossRef]
- Yan, S.; Liu, Y.; Liang, Y. A space fractal derivative crack model for characterizing chloride ions superdiffusion in concrete in the marine tidal zone. Constr. Build. Mater. 2024, 450, 138585. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Liu, W.; Fan, M.; Zhang, X.; Han, X.; Jin, G.; Xu, C. Continuous separation of alkali and salt through pressure-concentration double driven diffusion dialysis and its mathematic model. Sep. Purif. Technol. 2021, 272, 118934. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Z.; Wang, X.; Qi, H.; Shan, D.; Guo, B.; Xu, J. Anisotropic capillary-driven evaporation performance on laser textured prismatic micropillars. Int. J. Heat Mass Transf. 2025, 246, 126972. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Džunuzović, N.; Komljenović, M.; Nikolić, V.; Ivanović, T. External sulfate attack on alkali-activated fly ash-blast furnace slag composite. Constr. Build. Mater. 2017, 157, 737–747. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.; Zhang, D.; Zhang, J.; Jiao, Y.; Fang, C.; Tang, W.; Zhao, Z. Durability of geopolymer cementitious materials synergistically stimulated by Ca2+ and Na+. Case Stud. Constr. Mater. 2024, 21, e03514. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Fu, S.Y.; Wei, K.; Liu, X.; Chen, Y.K.; Wang, R.K.; Yu, R. Clarify the impact of chloride ion migration in different concentration fields on the hydration and microstructure characteristics of ultra-low water/binder ratio cement-based composites under submerged conditions. Cem. Concr. Compos. 2025, 160, 106014. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Z.; Dong, E.; Fan, D.; Yu, R. Impact of freezing conditions on the characteristics of ultra-low water binder ratio cementitious composites (ULCC): Towards to hydration mechanism and molecular migration model. Constr. Build. Mater. 2024, 435, 136878. [Google Scholar] [CrossRef]
- Ying, J.; Deng, C.; Qian, S.; Chen, B. Effect of water-to-binder ratio on chloride ion diffusion in concrete under continuous biaxial compression. Constr. Build. Mater. 2024, 416, 135308. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Liu, M.; Suo, S.; Wu, J.; Gan, Y.; Hanaor, D.A.; Chen, C.Q. Tailoring porous media for controllable capillary flow. J. Colloid Interface Sci. 2019, 539, 379–387. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, D.; Qin, S.; Zhang, X.; Liu, T. Decoupling chemical and physical sulfate attack on OPC and L.SAC concrete under wet-dry cycles. J. Build. Eng. 2025, 99, 111637. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Liao, H.; Zou, D.; Liu, T. Deterioration mechanism of OPC mortar under combined chemical and physical sulfate attack in partial immersion. Constr. Build. Mater. 2025, 475, 141187. [Google Scholar] [CrossRef]
- Ohno, M.; Maekawa, K. Fully coupled physicochemical-mechanical modeling of sulfate attack-induced expansion in cement-based materials. Cem. Concr. Compos. 2025, 161, 106076. [Google Scholar] [CrossRef]
- Rashad, A.M.; Mokhtar, M.M.; El-Nashar, M.H.; Mohamed, R.A.-E. Waste marble powder emerges as a promising material choice to enhance the properties of alkali-activated slag cement. Sustain. Chem. Pharm. 2025, 43, 101864. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Pang, K.; Neupane, A.; Xie, L.; Shen, D. Degradation of alkali-activated slag pastes exposed to high-concentration chloride, sulfate and magnesium multi-salt solutions. Constr. Build. Mater. 2025, 464, 140182. [Google Scholar] [CrossRef]
- Abdalqader, A.; Jin, F.; Al-Tabbaa, A. Performance of magnesia-modified sodium carbonate-activated slag/fly ash concrete. Cem. Concr. Compos. 2019, 103, 160–174. [Google Scholar] [CrossRef]
- Chen, P. Alkali-activated slag material. In High-Volume Mineral Admixtures in Cementitious Binders; Woodhead Publishing: Sawston, UK, 2025; pp. 167–194. [Google Scholar]
- Park, J.; Wang, L. The Structure and Properties of Silicate Slags. In Treatise Process Metall; Elsevier: Amsterdam, The Netherlands, 2024; pp. 113–200. [Google Scholar]
- Sun, B.; Ye, G.; de Schutter, G. A review: Reaction mechanism and strength of slag and fly ash-based alkali-activated materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- Ferrante, F.; Bertini, M.; Ferlito, C.; Lisuzzo, L.; Lazzara, G.; Duca, D. A computational and experimental investigation of halloysite silicic surface modifications after alkaline treatment. Appl. Clay Sci. 2023, 232, 106813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).