Binding Properties of Methyltrimethoxysilane-Modified Silica Sol Particle Surfaces and Their Molecular Dynamics Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. MTMS Modified Silica Sol
2.3. Characterizations
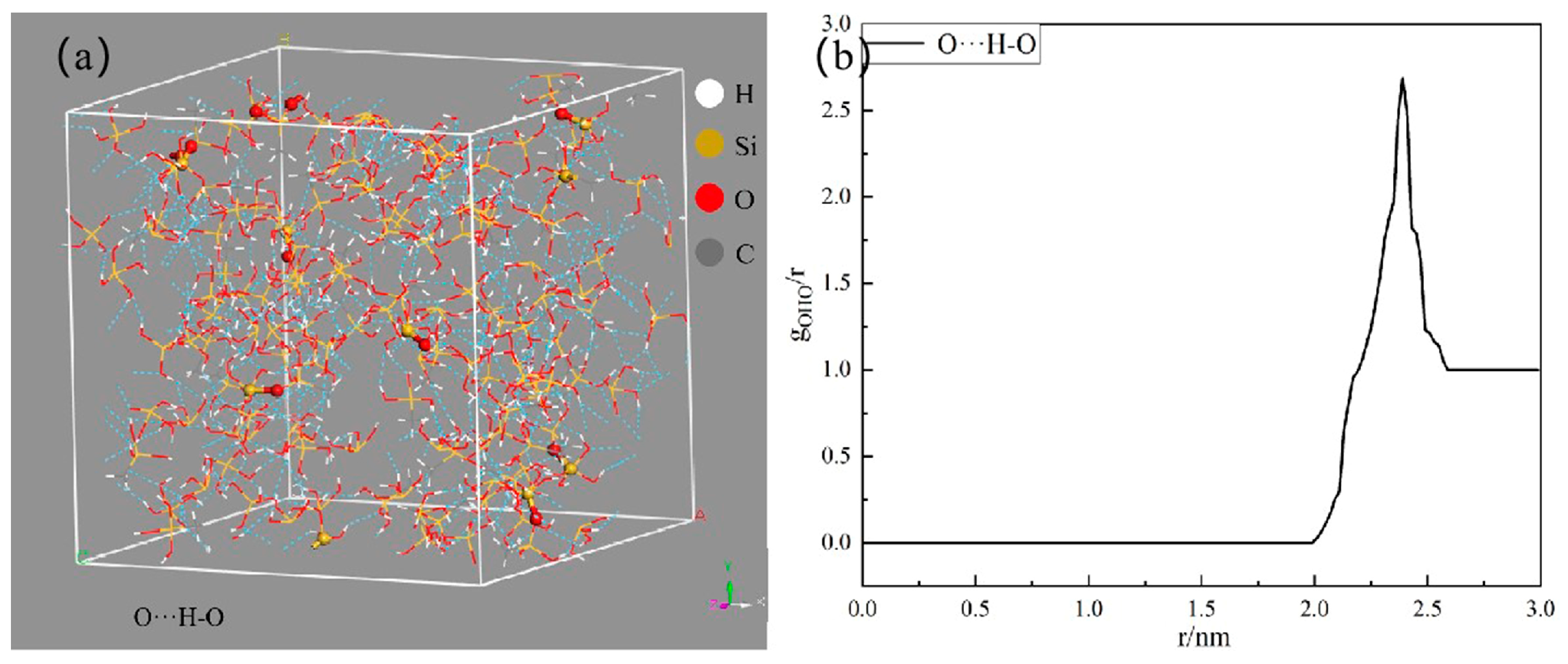
2.4. Model Building and Dynamics Simulation Details
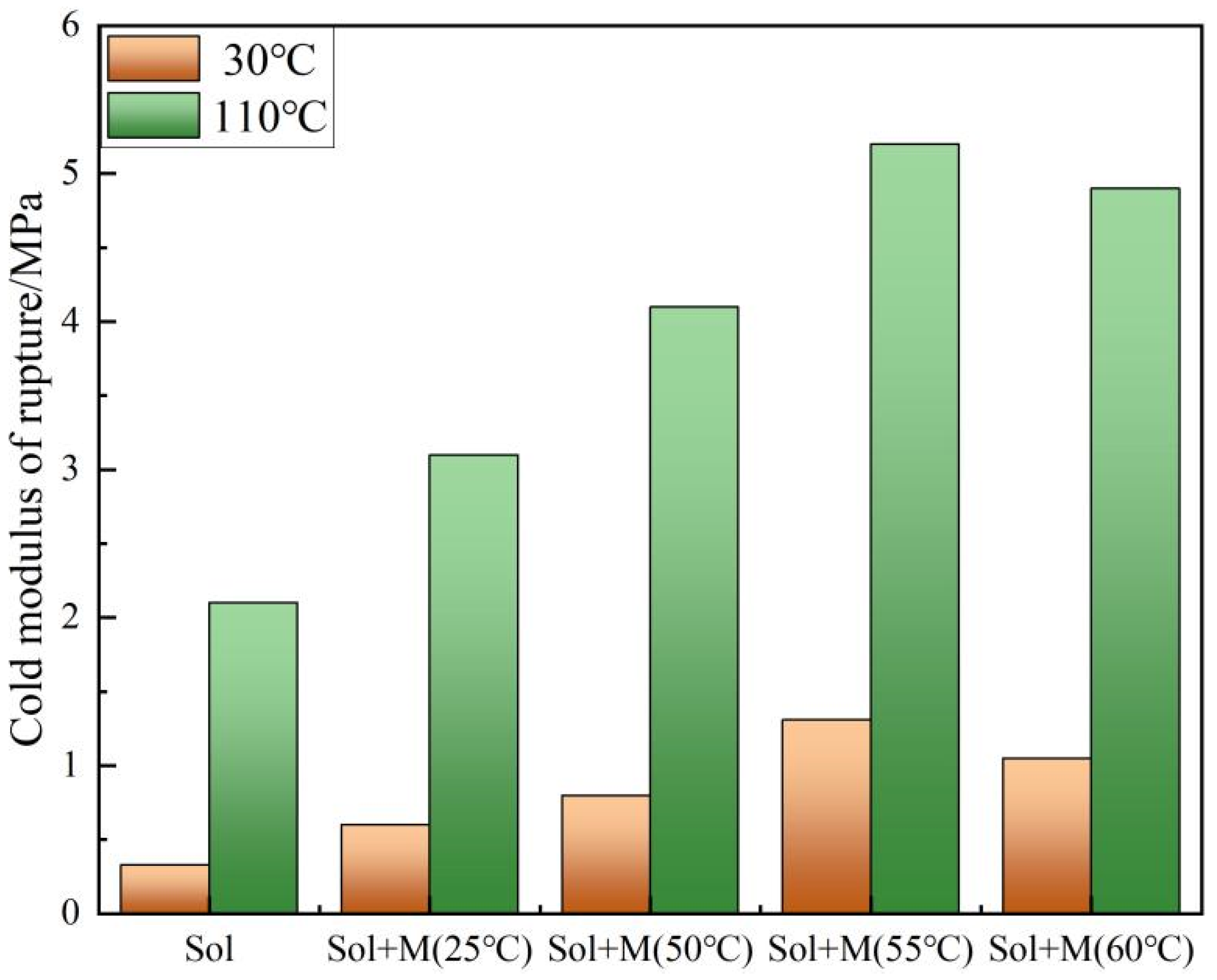
2.5. Verification Experiment
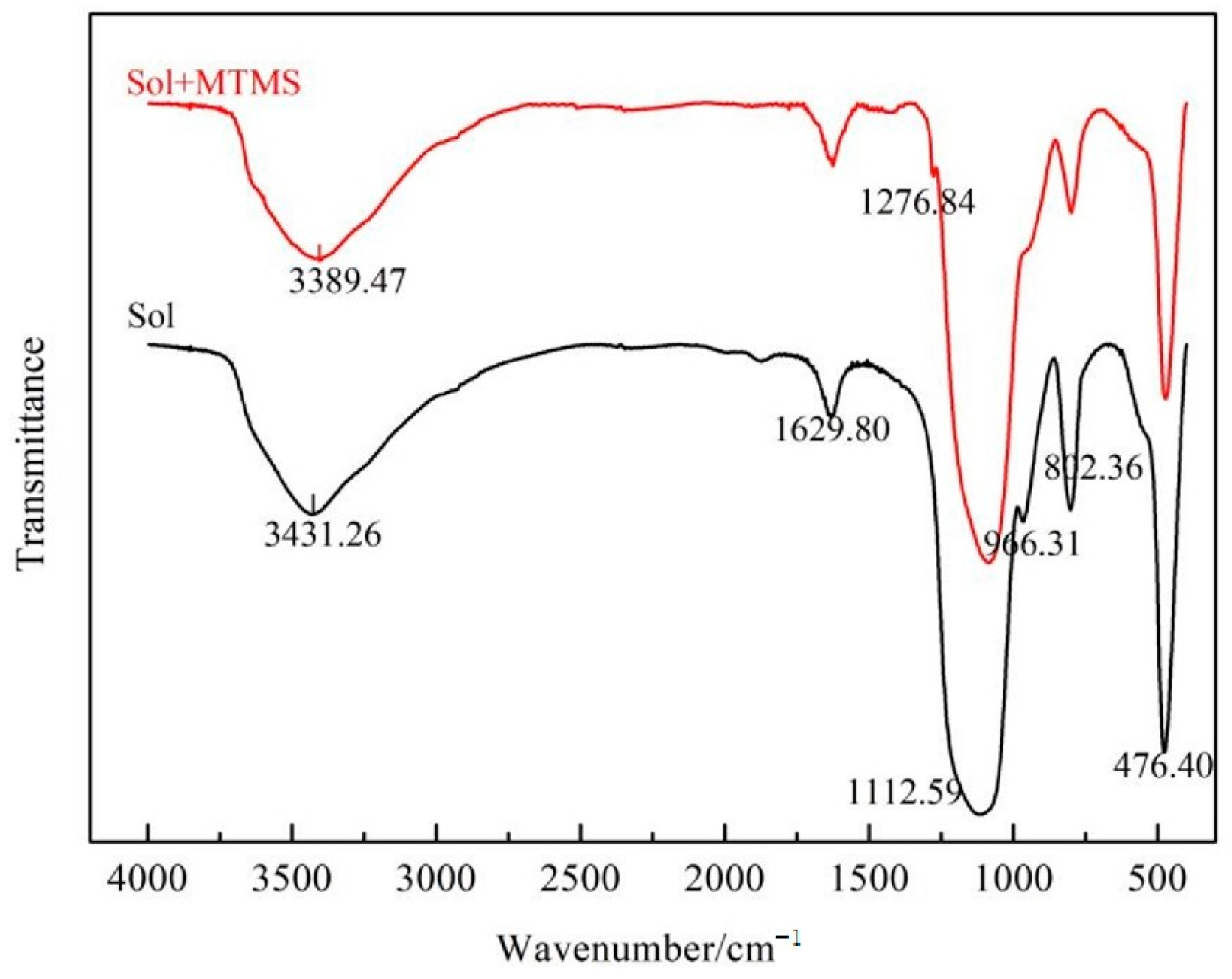
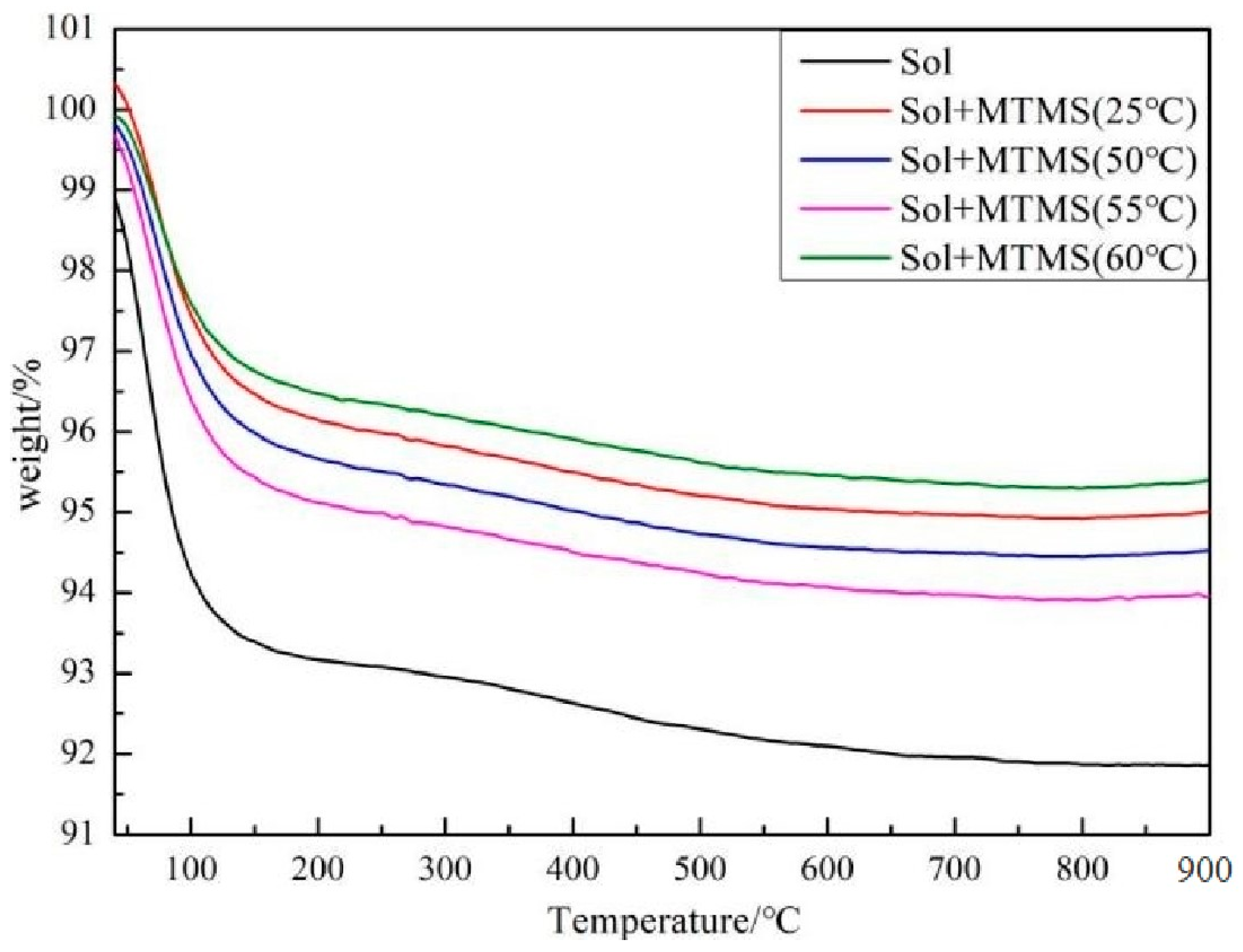
3. Results and Discussion



4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, G.; Ni, H.; Li, X.; Wang, Y.; Zhao, H. Eco-Friendly Fabrication of Highly Stable Silica Aerogel Microspheres with Core–Shell Structure. Polymers 2023, 15, 1882. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Zhu, M.; Yu, F.; Dai, B. Effect of Different Nano-Sized Silica Sols as Supports on the Structure and Properties of Cu/SiO2 for Hydrogenation of Dimethyl Oxalate. Catalysts 2017, 7, 75. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, L.; Yu, L.; Li, L.; Wu, Y.; Zheng, H. A study of high-strength and durable cotton fabric joining via laser-induced polymerisation of silica-sol photosensitive resin. J. Mater. Res. Technol. 2023, 27, 5507–5517. [Google Scholar] [CrossRef]
- Gąsiorek, J.; Gąsiorek, A.; Babiarczuk, B.; Jones, W.; Simka, W.; Detyna, J.; Kaleta, J.; Krzak, J. Anticorrosion properties of silica-based sol-gel coatings on steel—The influence of hydrolysis and condensation conditions. Ceram. Int. 2022, 48, 37150–37163. [Google Scholar] [CrossRef]
- Sögaard, C.; Funehag, J.; Abbas, Z. Silica sol as grouting material: A physio-chemical analysis. Nano Converg. 2018, 5, 6. [Google Scholar] [CrossRef]
- Li, J.; Lu, G. Reaction performance of partial oxidation of methane over Ni/SiO2 catalysts using monodisperse silica sol as supporting precursor. Appl. Catal. A Gen. 2004, 273, 163–170. [Google Scholar] [CrossRef]
- Nouri-Khezrabad, M.; Luz, A.; Salvini, V.; Golestani-Fard, F.; Rezaie, H.; Pandolfelli, V. Developing nano-bonded refractory castables with enhanced green mechanical properties. Ceram. Int. 2015, 41, 3051–3057. [Google Scholar] [CrossRef]
- Zhao, S.; Gan, Y.; Zhao, M.; Xie, J.; Yang, L.; Wang, T.-J. Dispersibility of γ-MPS-modified silica sol particles in an ethanol–water mixed solvent. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 134000. [Google Scholar] [CrossRef]
- Yang, D.; Yang, G.; Liang, G.; Guo, Q.; Li, Y.; Li, J. High-surface-area disperse silica nanoparticles prepared via sol-gel method using L-lysine catalyst and methanol/water co-solvent. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125700. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Kelkar, A.V.; Corti, D.S.; Franses, E.I. Effect of Interparticle Interactions on Agglomeration and Sedimentation Rates of Colloidal Silica Microspheres. Langmuir 2016, 32, 5111–5123. [Google Scholar] [CrossRef]
- Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Aggregation kinetics and colloidal stability of functionalized nanoparticles. Adv. Colloid Interface Sci. 2015, 222, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Rosace, G.; Colleoni, C.; Guido, E.; Malucelli, G. Phosphorus-Silica Sol-Gel Hybrid Coatings for Flame Retardant Cotton Fabrics. Tekstilec 2017, 60, 29–35. [Google Scholar] [CrossRef]
- Iler, R.K. Coagulation of colloidal silica by calcium ions, mechanism, and effect of particle size. J. Colloid Interface Sci. 1975, 53, 476–488. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Y.; Yin, Y.; Zhao, L.; Cai, M.; Nie, J. Enhancing the green mechanical strength of colloidal silica-bonded alumina castables using a silane coupling agent. Ceram. Int. 2016, 42, 11496–11499. [Google Scholar] [CrossRef]
- Das, S.; Jain, T.K.; Maitra, A. Inorganic–Organic Hybrid Nanoparticles from n-Octyl Triethoxy Silane. J. Colloid Interface Sci. 2002, 252, 82–88. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Jaroniec, M. An improved methodology for adsorption characterization of unmodified and modified silica gels. J. Colloid Interface Sci. 2003, 266, 168–174. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 23. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, X.; Ma, J.; Song, L.; Yang, Y.; Yang, Q. Interface of polyimide–silica grafted with different silane coupling agents: Molecular dynamic simulation. J. Appl. Polym. Sci. 2017, 135, 45725. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C.; Wang, X.; Zheng, W. Molecular dynamics simulation on the thermodynamic properties of insulating paper cellulose modified by silane coupling agent grafted nano-SiO2. AIP Adv. 2019, 9, 125134. [Google Scholar] [CrossRef]
- Du, T.; Li, H.; Sant, G.; Bauchy, M. New insights into the sol–gel condensation of silica by reactive molecular dynamics simulations. J. Chem. Phys. 2018, 148, 234504. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shi, Q.; Sha, H.; Liu, L.; Pan, J. Preparation of hydrophobic coatings with methyltrimethoxysilane modified Si-sol. CIESC J. 2009, 60, 2398. [Google Scholar] [CrossRef]
- Jia, G.Y.; Deng, Y.X. Investigation on the gelation process of silica sol. Bull. Chin. Ceram. Soc. 2004, 6, 91–93. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.H.; Chung, W.; Kim, J.Y.; Kim, S.H. Preparation and characterization of surface modified silica nanoparticles with organo-silane compounds. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 318–322. [Google Scholar] [CrossRef]
- Hou, C.; Kou, Y.; Cao, C. Preparation of super-hydrophobic surface via fluorinated epoxy resin and vano-SiO2. In Advanced Graphic Communication, Printing and Packaging Technology; Lecture Notes in Electrical Engineering; Springer: Singapore; Volume 600. [CrossRef]
- GB/T 5072-2008; Refractories—Determination of Cold Compressive Strength. National Standard of the People’s Republic of China: Beijing, China, 2008.
- Zhang, Y.; Liu, Y.; Liu, M.; Li, C. Preparation and performance study of modified silica sol/phenolic resin. BioResources 2021, 16, 6669–6683. [Google Scholar] [CrossRef]
- He, X.; Zou, G.; Xu, Y.; Zhu, H.; Jiang, H.; Jiang, X.; Xia, W. Nano-mechanical and tribological properties of copper matrix composites reinforced by graphene nanosheets. Prog. Nat. Sci. Mater. Int. 2018, 4, 416–421. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Kim, S.J. High-energy aqueous Li-ion hybrid capacitor based on metal-organic-framework-mimicking insertion-type copper hexacyanoferrate and capacitive-type graphitic carbon electrodes. J. Alloys Compd. 2018, 765, 1041–1048. [Google Scholar] [CrossRef]
- Sun, H.; He, X.; Tang, Q.; Li, X. Recyclable polyether–polyquaternium grafted SiO2 microsphere for efficient treatment of ASP flooding-produced water: Oil adsorption characteristics and mechanism. RSC Adv. 2020, 26, 15124–15131. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Shan, G.; Weng, Z. Curing polycondensation kinetics of multi-silanol. J. Chem. Ind. Eng. China 2007, 58, 908. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Venkateswara Rao, A. Effect of post-treatment (gel aging) on the properties of methyltrimethoxysilane based silica aerogels prepared by two-step sol–gel process. J. Sol-Gel Sci. Technol. 2009, 49, 53–59. [Google Scholar] [CrossRef]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Crystalline Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Kim, N.; Nguyen-Phan, T.-D.; Yoo, I.-K.; Shin, E.W. Adsorptive interaction of bisphenol A with mesoporous titanosilicate/reduced graphene oxide nanocomposite materials: FT-IR and Raman analyses. Nanoscale Res. Lett. 2014, 9, 462. [Google Scholar] [CrossRef]
- Shang, X.; Zhu, Y.; Li, Z. Surface modification of silicon carbide with silane coupling agent and hexadecyl iodiele. Appl. Surf. Sci. 2017, 394, 169–177. [Google Scholar] [CrossRef]
- Vansant, E.; Voort, P.; Vrancken, K. Chapter 1 Silica: Preparation and properties. Stud. Surf. Sci. Catal. 1995, 93, 3–30. [Google Scholar] [CrossRef]
- Salon, M.-C.B.; Bayle, P.-A.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N. Kinetics of hydrolysis and self condensation reactions of silanes by NMR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 83–91. [Google Scholar] [CrossRef]
- Du, D.; Tang, Y.; Yang, L.; Tang, C. Effects of Different Grafting Density of Amino Silane Coupling Agents on Thermomechanical Properties of Cross-Linked Epoxy Resin. Polymers 2020, 12, 1662. [Google Scholar] [CrossRef]
- Bernardi, R.; Gomes, D.; Gobato, R.; Taft, C.; Ota, A.; Pascutti, P. Molecular dynamics study of biomembrane/local anesthetics interactions. Mol. Phys. 2009, 107, 1437–1443. [Google Scholar] [CrossRef]
- Bernardi, R.C.; Gomes, D.E.B.; Ito, A.S.; Ota, A.T.; Pascutti, P.G.; Taft, C. Density functional and molecular dynamics simulations of local anesthetics in 0.9% NaCl solution. Mol. Simul. 2007, 33, 1135–1141. [Google Scholar] [CrossRef]
- Bernardi, R.C.; Gomes, D.E.B.; Pascutti, P.G.; Ito, A.S.; Taft, C.A.; Ota, A.T. Water solvent and local anesthetics: A computational study. Int. J. Quantum Chem. 2007, 107, 1642–1649. [Google Scholar] [CrossRef]






| Silicon Carbide | Silicon Powder | Silica Fume | Silica Sol (20 wt%) (Apposition) | |||
|---|---|---|---|---|---|---|
| 3–5 mm | 1–3 mm | ≤1 mm | 0.074 mm | |||
| 21 | 31 | 20 | 13 | 9 | 6 | 9 |
| Temperature | ∆m/% | Grafting Rate/% | ||
|---|---|---|---|---|
| <200 °C | 200–900 °C | |||
| Sol | - | 6.82 | 1.31 | - |
| Sol + MTMS | 25 | 3.86 | 1.13 | 8.38 |
| 50 | 4.34 | 1.14 | 9.43 | |
| 55 | 4.89 | 1.16 | 10.6 | |
| 60 | 3.61 | 1.11 | 7.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, H.; Wang, Z.; Liu, H.; Ma, Y.; Wang, X.; Jiang, P. Binding Properties of Methyltrimethoxysilane-Modified Silica Sol Particle Surfaces and Their Molecular Dynamics Simulations. Materials 2025, 18, 2974. https://doi.org/10.3390/ma18132974
Pang H, Wang Z, Liu H, Ma Y, Wang X, Jiang P. Binding Properties of Methyltrimethoxysilane-Modified Silica Sol Particle Surfaces and Their Molecular Dynamics Simulations. Materials. 2025; 18(13):2974. https://doi.org/10.3390/ma18132974
Chicago/Turabian StylePang, Hongxing, Zhoufu Wang, Hao Liu, Yan Ma, Xitang Wang, and Pengcheng Jiang. 2025. "Binding Properties of Methyltrimethoxysilane-Modified Silica Sol Particle Surfaces and Their Molecular Dynamics Simulations" Materials 18, no. 13: 2974. https://doi.org/10.3390/ma18132974
APA StylePang, H., Wang, Z., Liu, H., Ma, Y., Wang, X., & Jiang, P. (2025). Binding Properties of Methyltrimethoxysilane-Modified Silica Sol Particle Surfaces and Their Molecular Dynamics Simulations. Materials, 18(13), 2974. https://doi.org/10.3390/ma18132974






