Mechanical Properties and Material Characteristics of 3D-Printed Titanium Capsules for Cancer Drug Delivery Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Results of Mechanical Testing
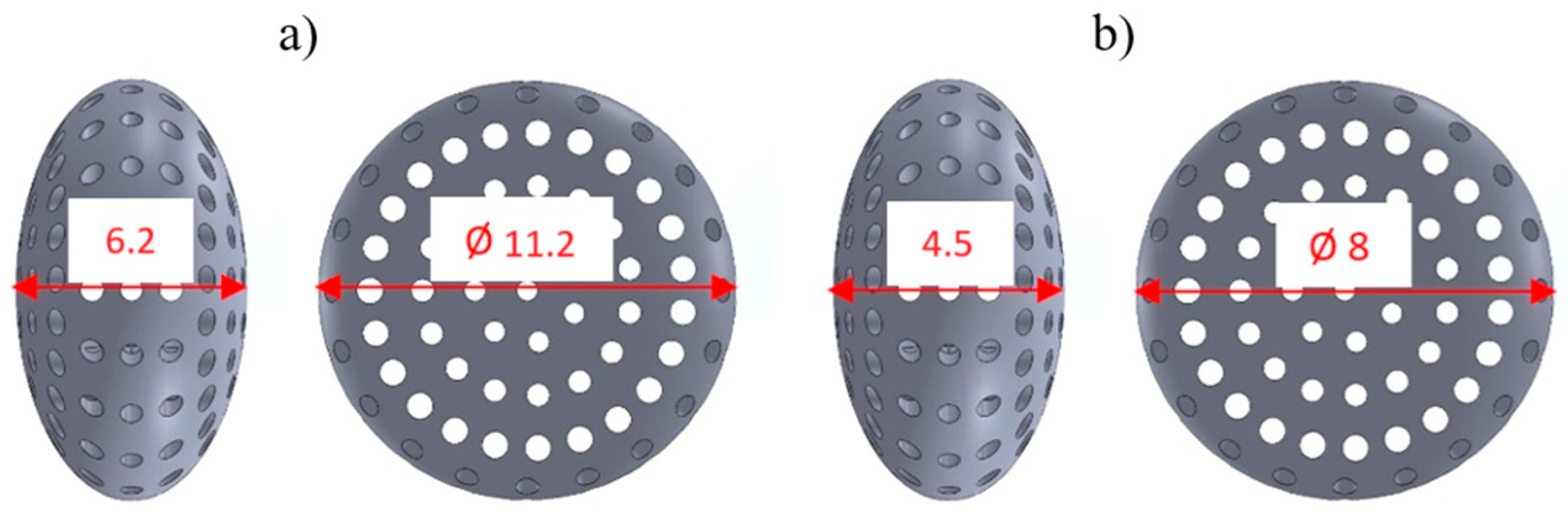
3.2. Characterization of Titanium Capsule
4. Summary and Conclusions
- It is a widely adopted method for producing components from titanium powder;
- The resulting surface (high surface roughness, Rz = 118.9 µm) significantly limits uncontrolled migration of the implanted capsules (as no experimental data on capsule migration are available, this remains a preliminary hypothesis pending future validation);
- It provides sufficient compressive force, ranging for KTD capsules from 5460.15 N (capsules printed at 0° and loaded along the longitudinal axis) to 8416.92 N (capsules printed at 45° and loaded along the short axis), and for KTM capsules from 2674.81 N (printed at 0° and loaded along the longitudinal axis) to 4047.99 N (printed at 45° and loaded along the short axis).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadaun, D.; Dhaker, S.; Ghadge, M.; Jain, V.; Sharma, R.; Dhaiya, R. A review on 3D printing technology in pharmaceuticals. Int. J. Eng. Appl. Sci. Technol. 2024, 9, 177–180. [Google Scholar] [CrossRef]
- Caballero-Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Three-dimensional printed drug delivery systems. In Engineering Drug Delivery Systems; Woodhead Publishing: Cambridge, UK, 2020; pp. 147–162. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, C.; Wang, H.; Wei, Q.; Cai, H.; Wei, F.; Bian, Z.; Liu, W.; Wang, X.; Liu, Z. Fabrication of a composite 3D-printed titanium alloy combined with controlled in situ drug release to prevent osteosarcoma recurrence. Mater. Today Bio 2023, 20, 100683. [Google Scholar] [CrossRef]
- Cui, X.; Chen, H.; Ye, Q.; Cui, X.; Cui, X.; Cui, H.; Shen, G.; Li, M.; Lin, J.; Sun, Y. Porous Titanium Dioxide Spheres for Drug Delivery and Sustained Release. Front. Mater. 2021, 8, 649237. [Google Scholar] [CrossRef]
- Zuo, F.; Zhu, Y.; Wu, T.; Li, C.; Liu, Y.; Wu, X.; Ma, J.; Zhang, K.; Ouyang, H.; Qiu, X.; et al. Titanium Dioxide Nanomaterials: Progress in Synthesis and Application in Drug Delivery. Pharmaceutics 2024, 16, 1214. [Google Scholar] [CrossRef]
- Turner, M. Design Validation Testing—Drug Delivery Devices. 2017. Available online: https://www.ondrugdelivery.com/design-validation-testing-drug-delivery-devices/ (accessed on 1 February 2025).
- Li, R.; Ting, Y.-H.; Youssef, S.H.; Song, Y.; Garg, S. Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies. Pharmaceuticals 2021, 14, 787. [Google Scholar] [CrossRef]
- Yang, J.; Qin, C.; Lu, J.; Shi, X.; Shi, K.; Cui, Y.; Xiong, X.; Wan, K.; Shen, M. Investigating mechanical properties of 3D printed porous titanium scaffolds for bone tissue engineering. Mater. Res. Express 2024, 11, 075404. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Li, D.; Zhang, Z.-Y.; Liu, G.; Liang, H.; Qin, Y.; Yu, J.; Li, Y. Fabrication of bioactive 3D printed porous titanium implants with Sr ions-incorporated zeolite coatings for bone ingrowth. J. Mater. Chem. B 2018, 6, 3254–3261. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Shi, X.; Shen, M.; Shi, K.; Shen, L.; Yang, C. Design and analysis of three-dimensional printing of a porous titanium scaffold. BMC Musculoskelet. Disord. 2021, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, Q.; Zhou, H.; Jing, Z. Three-dimensional-printed individualized porous implants: A new “implant-bone” interface fusion concept for large bone defect treatment. Bioact. Mater. 2021, 6, 3659–3670. [Google Scholar] [CrossRef]
- Jing, Z.; Yuan, W.; Wang, J.; Ni, R.; Qin, Y.; Mao, Z.; Wei, F.; Song, C.; Zheng, Y.; Cai, H.; et al. Simvastatin/hydrogel-loaded 3D-printed titanium alloy scaffolds suppress osteosarcoma via TF/NOX2-associated ferroptosis while repairing bone defects. Bioact. Mater. 2024, 34, 463–465. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, A.; Wang, Z.; Liu, H.; Wang, J.; Li, C. Advanced Surface Modification for 3D-Printed Titanium Alloy Implant Interface Functionalization. Front. Bioeng. Biotechnol. 2022, 10, 850110. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Ikeda, M.; Mori, S.; Doi, K.; Hisashi, K.; Terauchi, S. Mechanical Properties of Additively Manufactured Porous Titanium with Sub-Millimetre Structural Units. Mater. Trans. 2019, 60, 1792–1798. [Google Scholar] [CrossRef]
- He, S.; Zhu, J.; Jing, Y.; Long, S.; Tang, L.; Cheng, L.; Shi, Z. Effect of 3D-Printed Porous Titanium Alloy Pore Structure on Bone Regeneration: A Review. Coatings 2024, 14, 253. [Google Scholar] [CrossRef]
- Song, P.; Hu, C.; Pei, X.; Sun, J.; Sun, H.; Wu, L.; Jiang, Q.; Fan, H.; Yang, B.; Zhou, C.; et al. Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration. J. Mater. Chem. B 2019, 7, 2865–2877. [Google Scholar] [CrossRef]
- Fry, M.; Ren, W.; Bou-Ak, T.; Wu, B.; Pawlitz, P.; Markel, D.C. Influence of Porosities of 3D Printed Titanium Implants on the Tensile Properties in a Rat Tendon Repair Model. Spartan Med. Res. J. 2024, 9, 123410. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, T.; Wei, Q.; Cai, H.; Cheng, Y.; Tian, Y.; Leng, H.; Wang, C.; Feng, S.; Liu, Z. Surface treatment of 3D printed porous Ti6Al4V implants by ultraviolet photofunctionalization for improved osseointegration. Bioact. Mater. 2022, 7, 26–38. [Google Scholar] [CrossRef]
- Karolewska, K.; Szala, G.; Trepczyńska-Łent, M.; Ligaj, B. Improving the mechanical properties of structural elements made of titanium, aluminum alloys, and steel through the AM application. Adv. Sci. Technol. Res. J. 2025, 19, 48–71. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Junak, A.; Gołkowska, A.; Dyba, A.; Nartowski, K.P. The use of FDM 3D printing technology in obtaining pediatric drugs. Farm. Pol. 2021, 77, 251–261. (In Polish) [Google Scholar] [CrossRef]
- Eskandari, H.; Lashgari, H.R.; Zangeneh, S.; Kong, C.; Ye, L.; Eizadjou, M.; Wang, H. Microstructural characterization and mechanical properties of SLM-printed Ti–6Al–4V alloy: Effect of build orientation. J. Mater. Res. 2022, 37, 2645–2660. [Google Scholar] [CrossRef]
- Cojocaru, V.; Frunzaverde, D.; Nedelcu, D.; Miclosina, C.-O.; Marginean, G. Study Regarding the Influence of the Printing Orientation Angle on the Mechanical Behavior of Parts Manufactured by Material Jetting. Mater. Plast. 2021, 58, 198–209. [Google Scholar] [CrossRef]
- Dang, L.C.; Nguyen, C.V.; Le, A.H.; Bui, D.T. A study on the influence of printing orientation in metal printing using material extrusion technology on the mechanical properties of 17-4 stainless steel products. J. Mach. Eng. 2023, 23, 89–100. [Google Scholar] [CrossRef]
- Meier, B.; Godja, N.; Warchomicka, F.; Belei, C.; Schäfer, S.; Schindel, A.; Palcynski, G.; Kaindl, R.; Waldhauser, W.; Sommitsch, C. Influences of Surface, Heat Treatment, and Print Orientation on the Anisotropy of the Mechanical Properties and the Impact Strength of Ti 6Al 4V Processed by Laser Powder Bed Fusion. J. Manuf. Mater. Process. 2022, 6, 87. [Google Scholar] [CrossRef]
- Ren, S.; Chen, Y.; Liu, T.; Qu, X. Effect of Build Orientation on Mechanical Properties and Microstructure of Ti-6Al-4V Manufactured by Selective Laser Melting. Metall. Mater. Trans. A 2019, 50, 4388–4409. [Google Scholar] [CrossRef]
- Celles, C.A.S.; Teixeira, A.B.V.; da Costa Valente, M.L.; Sangali, M.; Rodrigues, J.F.Q.; Caram, R.; dos Reis, A.C. Effect of post-processing and variation of the building angle of Ti-6Al-4V disks obtained by selective laser melting: A comparison of physical, chemical and mechanical properties to machined disks. Mater. Today Commun. 2024, 39, 108700. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Casas, M.; Ferrero, C.; Linares, V.; Caraballo, I. 3D Printing Direct Powder Extrusion in the Production of Drug Delivery Systems: State of the Art and Future Perspectives. Pharmaceutics 2024, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Yu, Z.; Yu, H.; Meng, D.; Zhu, L.; Li, H. Direct 3D printing of triple-responsive nanocomposite hydrogel microneedles for controllable drug delivery. J. Colloid Interface Sci. 2024, 670, 1–11. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, N.; Misra, S.K. Suitably Incorporated Hydrophobic, Redox-Active Drug in Poly Lactic Acid-Graphene Nanoplatelet Composite Generates 3D-Printed Medicinal Patch for Electrostimulatory Therapeutics. Langmuir 2024, 40, 11858–11872. [Google Scholar] [CrossRef]
- ISO 25178-601:2025; Geometrical Product Specifications (GPS)—Surface Texture: Areal. ISO: Geneva, Switzerland, 2025.











 | Maximum Compressive Load (N) | Displacement at Maximum Compressive Load (mm) | ||
|---|---|---|---|---|
| 0° | 45° | 0° | 45° | |
| 1 | 5255.98 | 6571.38 | 1.22 | 0.84 |
| 2 | 4881.01 | 7134.22 | 0.88 | 0.85 |
| 3 | 6243.46 | 5596.12 | 1.08 | 0.83 |
| Mean | 5460.15 | 6433.91 | 1.06 | 0.84 |
| Standard Deviation | 703.798 | 778.211 | 0.171 | 0.010 |
| p value | 0.183 | 0.090 | ||
 | ||||
| 1 | 7044 | 9166.2 | 0.95 | 1.14 |
| 2 | 7123.44 | 7812.83 | 1 | 1.2 |
| 3 | 7141.58 | 8271.72 | 0.97 | 1.06 |
| Mean | 7103.01 | 8416.92 | 0.97 | 1.13 |
| Standard Deviation | 51.900 | 688.269 | 0.025 | 0.070 |
| p value | 0.030 | 0.021 | ||
 | Maximum Compressive Load (N) | Displacement at Maximum Compressive Load (mm) | ||
|---|---|---|---|---|
| 0° | 45° | 0° | 45° | |
| 1 | 2997.61 | 2974.29 | 0.92 | 0.71 |
| 2 | 2120.72 | 5219.36 | 0.53 | 0.94 |
| 3 | 2906.11 | 3708.63 | 0.66 | 0.74 |
| Mean | 2674.81 | 3967.43 | 0.70 | 0.80 |
| Standard Deviation | 482.035 | 1144.691 | 0.199 | 0.125 |
| p value | 0.146 | 0.529 | ||
 | ||||
| 1 | 4124.84 | 4819.17 | 0.75 | 0.89 |
| 2 | 3155.02 | 3581.42 | 0.66 | 0.69 |
| 3 | 3992.08 | 3743.38 | 0.76 | 0.72 |
| Mean | 3757.31 | 4047.99 | 0.72 | 0.77 |
| Standard Deviation | 525.808 | 672.753 | 0.055 | 0.108 |
| p value | 0.587 | 0.569 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierska-Drobny, K.; Szala, G.; Musiał, J.; Macko, M.; Karasiewicz, T.; Lewandowski, J. Mechanical Properties and Material Characteristics of 3D-Printed Titanium Capsules for Cancer Drug Delivery Applications. Materials 2025, 18, 2969. https://doi.org/10.3390/ma18132969
Kazimierska-Drobny K, Szala G, Musiał J, Macko M, Karasiewicz T, Lewandowski J. Mechanical Properties and Material Characteristics of 3D-Printed Titanium Capsules for Cancer Drug Delivery Applications. Materials. 2025; 18(13):2969. https://doi.org/10.3390/ma18132969
Chicago/Turabian StyleKazimierska-Drobny, Katarzyna, Grzegorz Szala, Janusz Musiał, Marek Macko, Tomasz Karasiewicz, and Jakub Lewandowski. 2025. "Mechanical Properties and Material Characteristics of 3D-Printed Titanium Capsules for Cancer Drug Delivery Applications" Materials 18, no. 13: 2969. https://doi.org/10.3390/ma18132969
APA StyleKazimierska-Drobny, K., Szala, G., Musiał, J., Macko, M., Karasiewicz, T., & Lewandowski, J. (2025). Mechanical Properties and Material Characteristics of 3D-Printed Titanium Capsules for Cancer Drug Delivery Applications. Materials, 18(13), 2969. https://doi.org/10.3390/ma18132969







