Study on the Wear Resistance of 6061 Aluminum Alloy Bipolar Plasma Electrolytic Oxidation Ceramic Coating by the Addition of K2ZrF6
Abstract
1. Introduction
2. Methods and Materials
2.1. Experiment Preparation
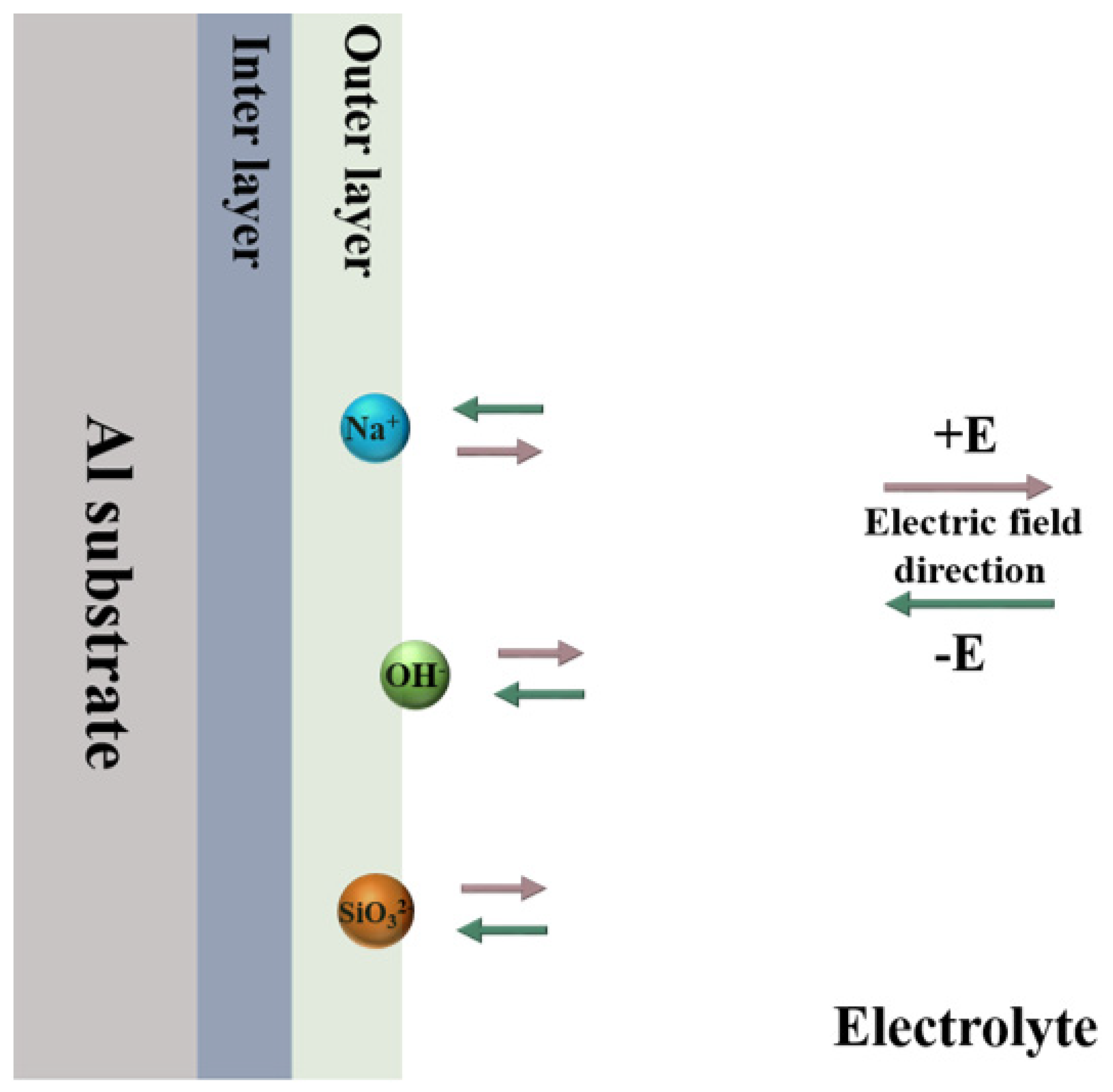
2.2. Plasma Electrolytic Oxidation
2.3. Coating Performance Test Methods
3. Results and Discussion
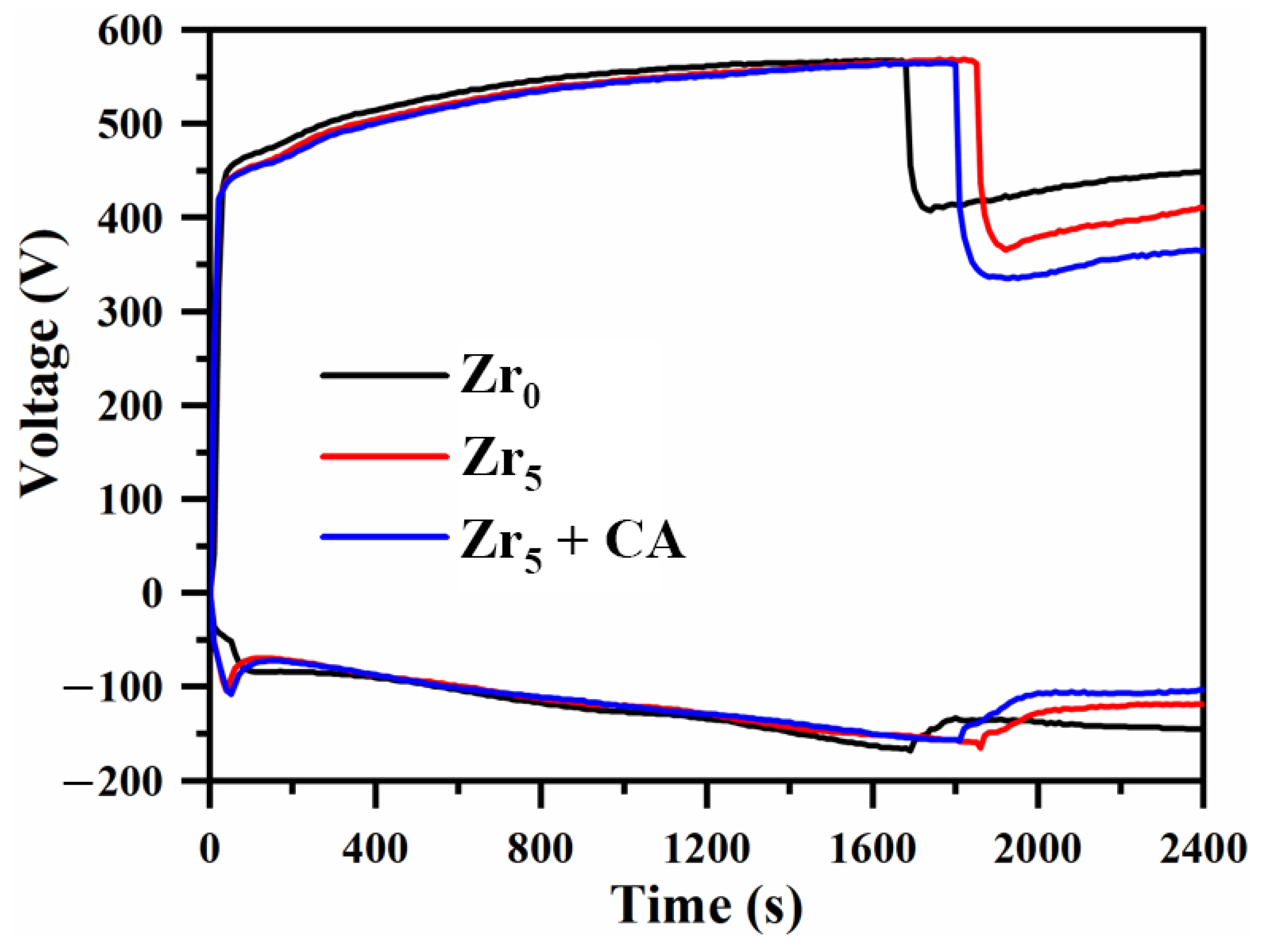
3.1. Effect of K2ZrF6 Addition on the Time-Dependent Voltage Profile
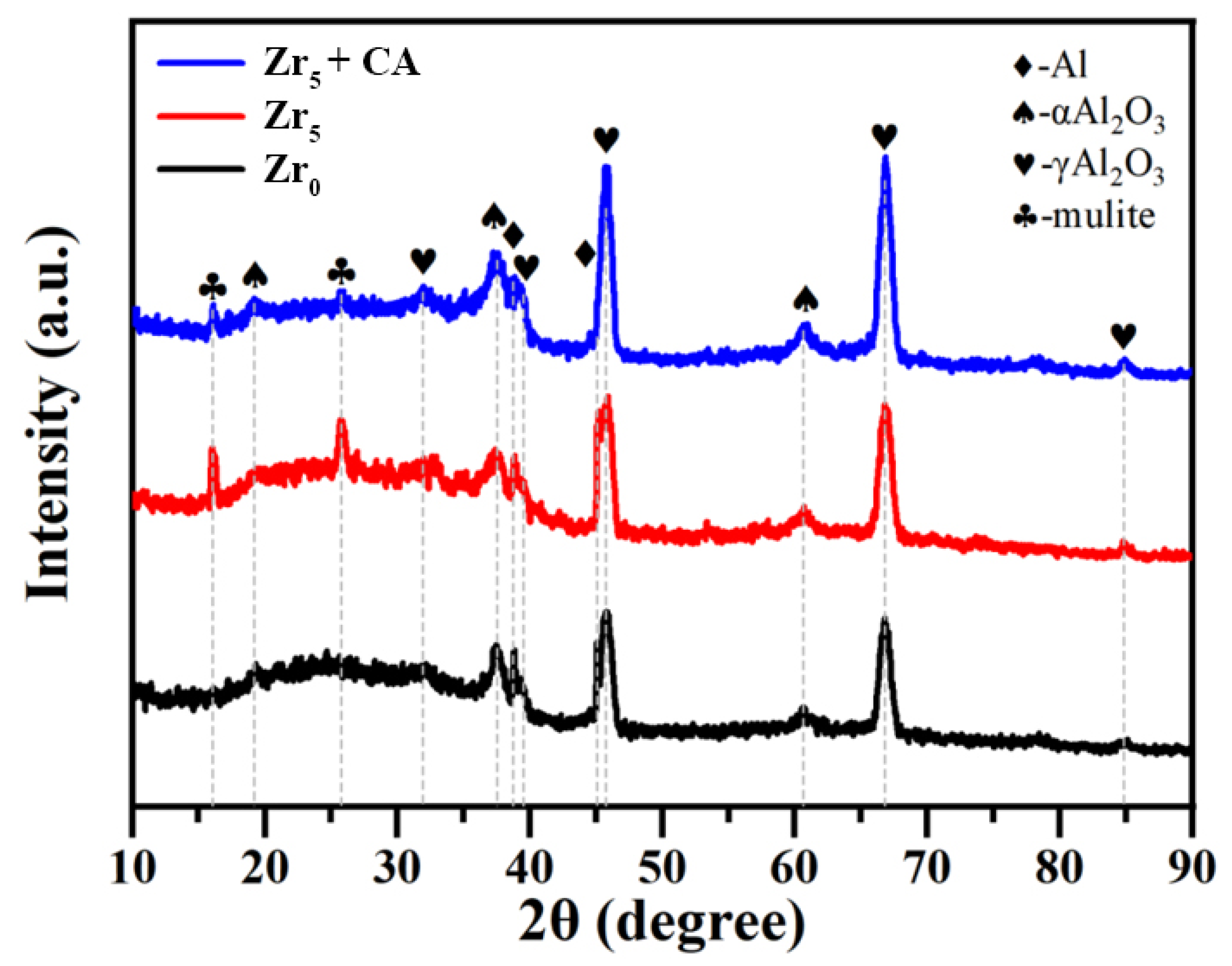
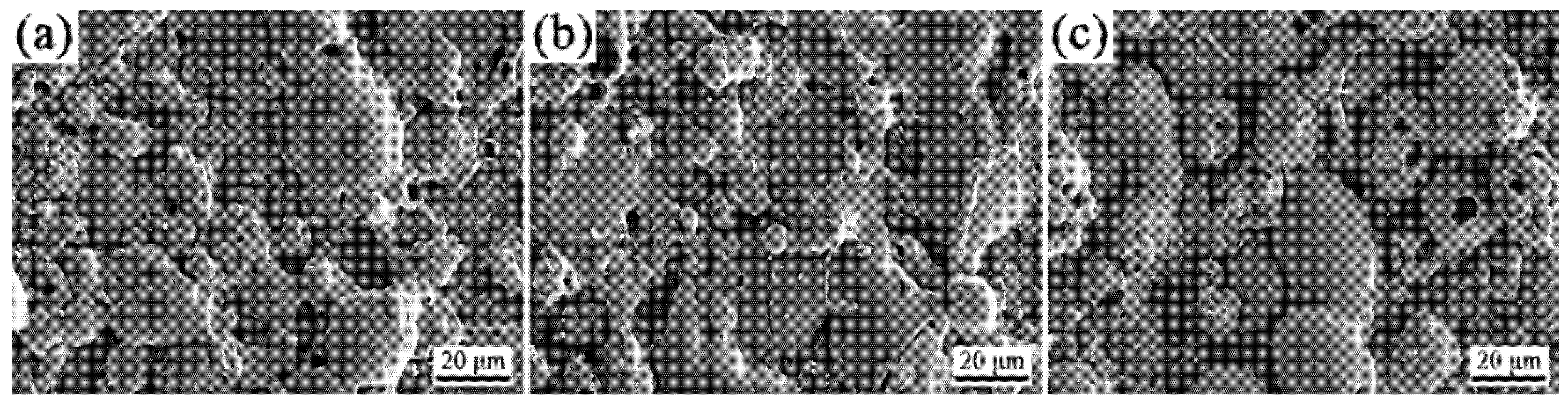
3.2. Phase Composition and Microscopic Morphology of Coatings
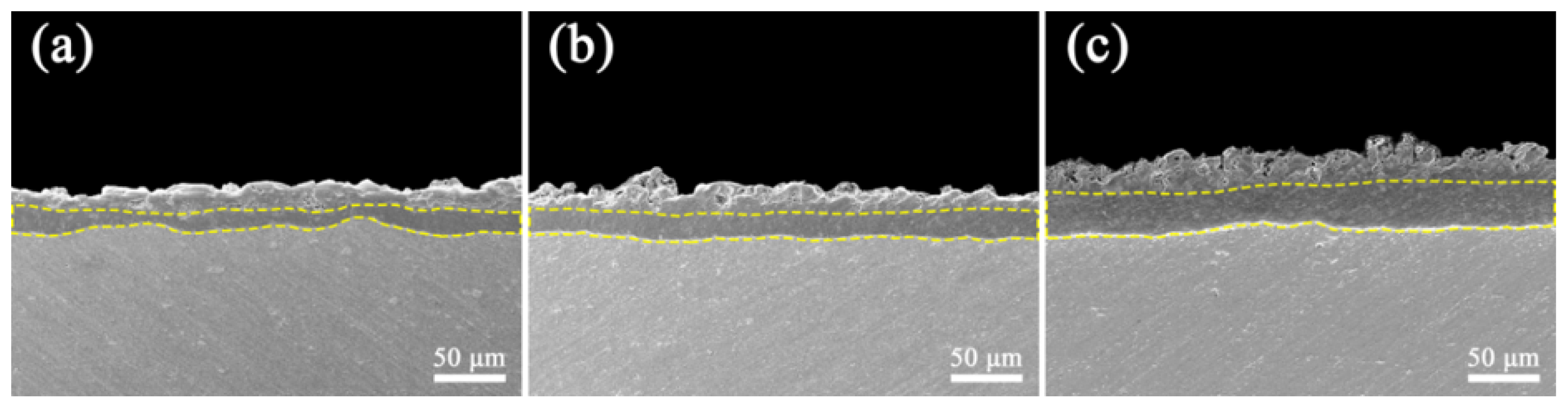
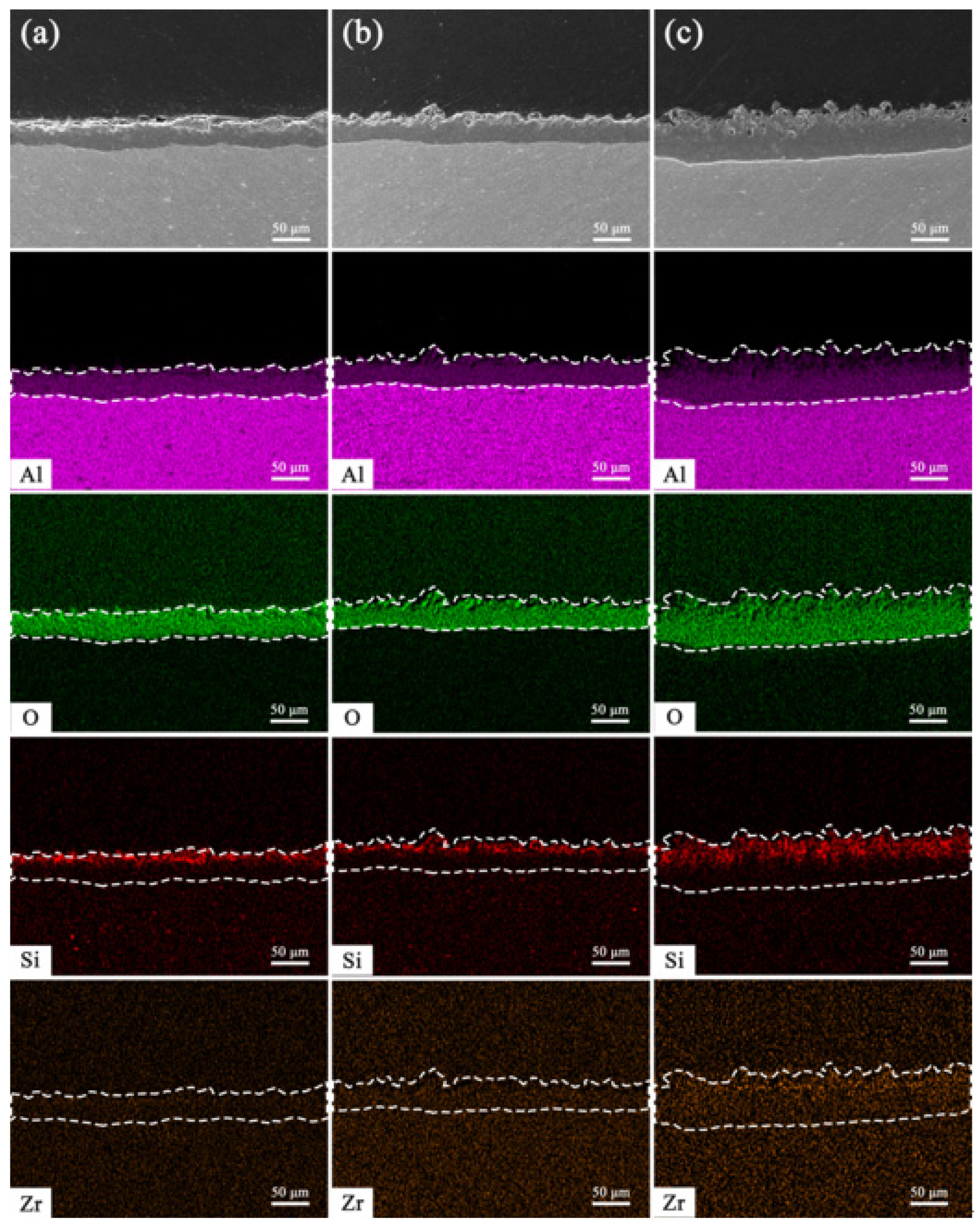
3.3. EDS Analysis and Elemental Maps of Coated Cross-Sections
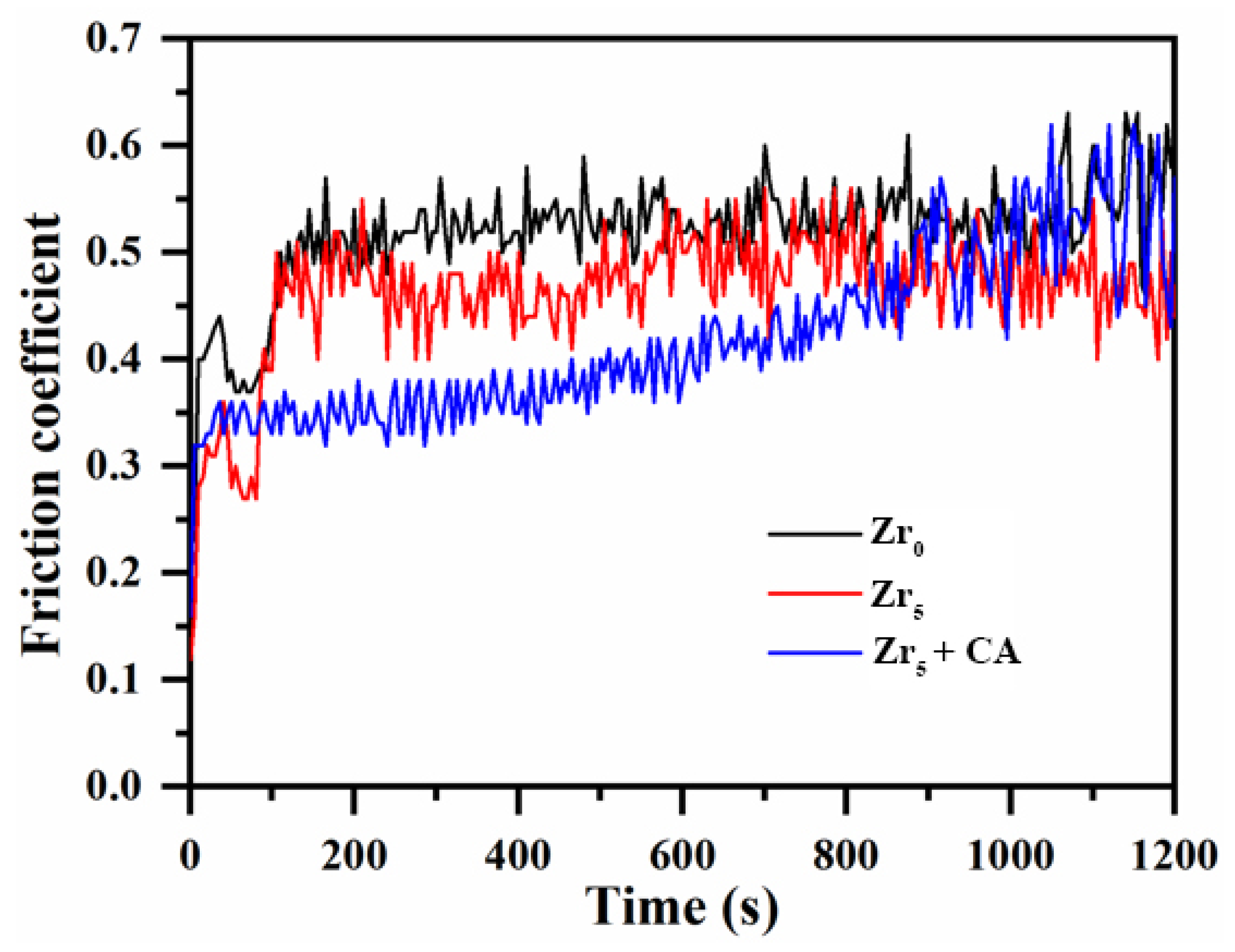
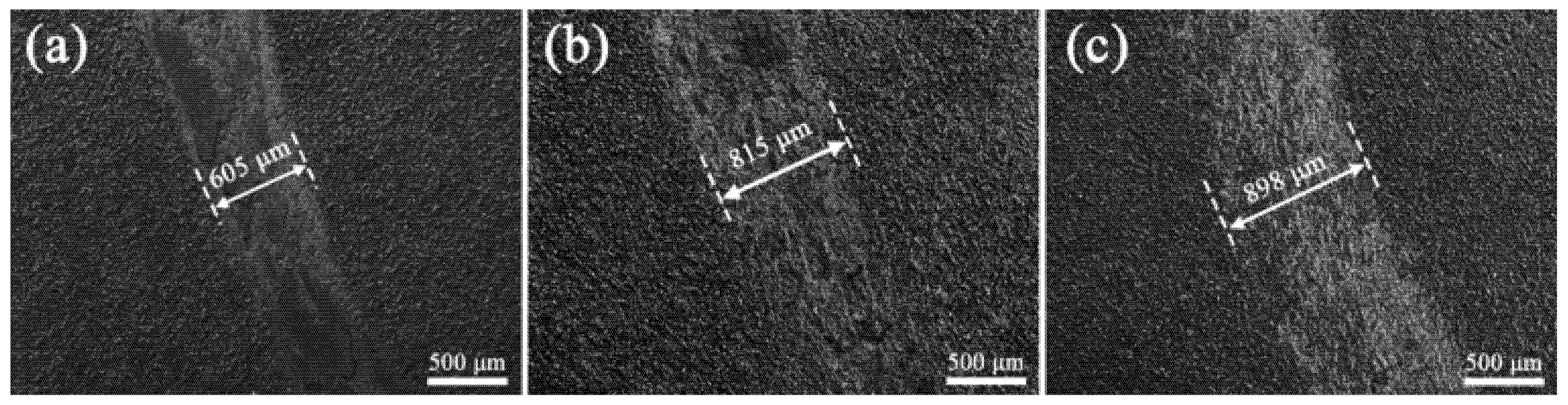
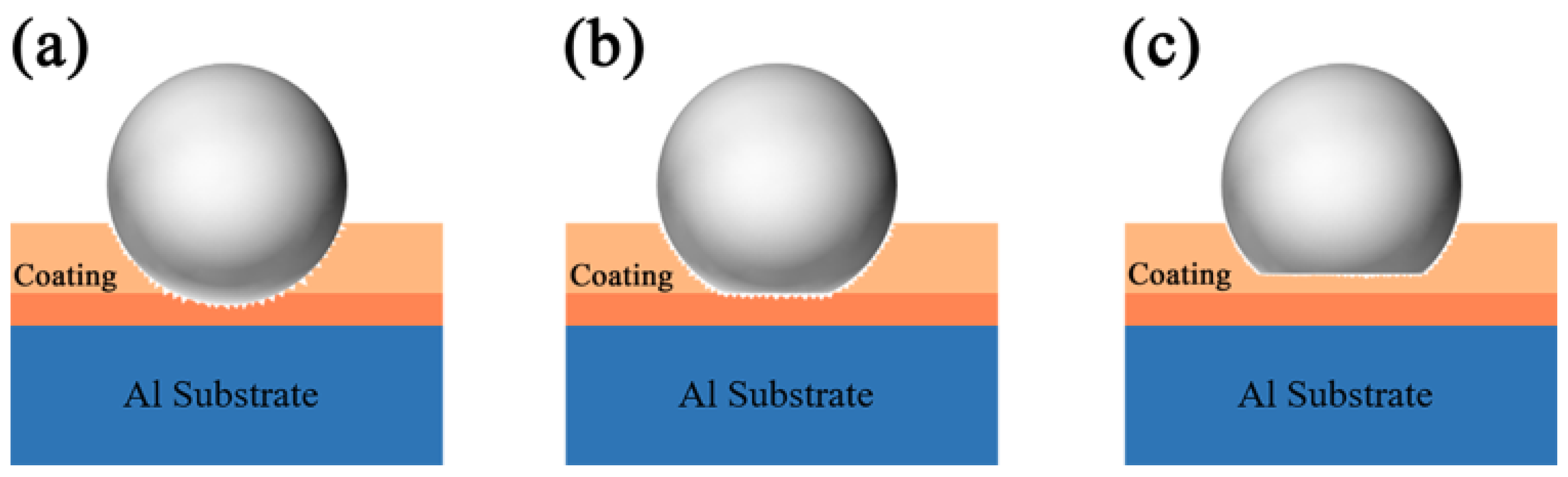
3.4. Tribological Behavior of the Coating
4. Conclusion
- (1)
- The addition of K2ZrF6 delays the appearance of soft plasma discharge and increases the thickness of the PEO coatings by 37.3% and the roughness by 27.6% at the S3 parameter compared to S1.
- (2)
- The addition of K2ZrF6 facilitates the formation of a mullite phase and improves the densification of the PEO coating.
- (3)
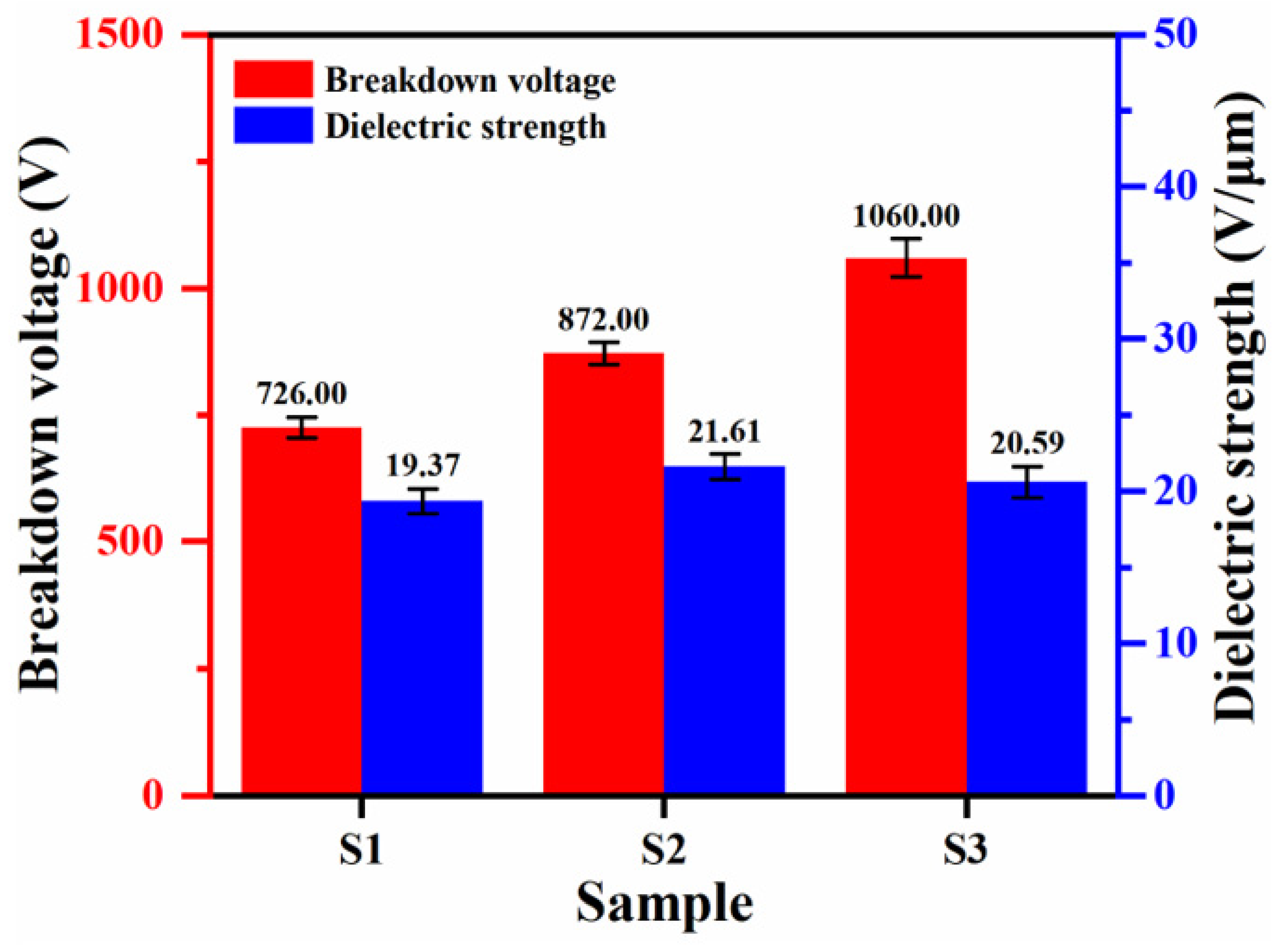
- The addition of K2ZrF6 facilitated the improvement of the breakdown voltage of the coating, which was increased by 46.0% at the S3 parameter compared with S1; it also exhibited excellent wear resistance, with a 41.8% reduction in mass wear rate compared with S1.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z.; Domblesky, J.P.; Han, J.; Li, Z.; Liu, X. Investigation of through thickness microstructure and mechanical properties in friction stir welded 7N01 aluminum alloy plate. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 760, 316–327. [Google Scholar] [CrossRef]
- Shen, D.; Li, G.; Guo, C.; Zou, J.; Cai, J.; He, D.; Ma, H.; Liu, F. Microstructure and corrosion behavior of micro-arc oxidation coating on 6061 aluminum alloy pre-treated by high-temperature oxidation. Appl. Surf. Sci. 2013, 287, 451–456. [Google Scholar] [CrossRef]
- Zhu, M.H.; Cai, Z.B.; Lin, X.Z.; Ren, P.D.; Tan, J.; Zhou, Z.R. Fretting wear behaviour of ceramic coating prepared by micro-arc oxidation on Al-Si alloy. Wear 2007, 263, 472–480. [Google Scholar] [CrossRef]
- Arslan, E.; Totik, Y.; Demirci, E.E.; Vangolu, Y.; Alsaran, A.; Efeoglu, I. High temperature wear behavior of aluminum oxide layers produced by ac micro arc oxidation. Surf. Coat. Technol. 2009, 204, 829–833. [Google Scholar] [CrossRef]
- Sundararajan, G.; Krishna, L.R. Mechanisms underlying the formation of thick alumina coatings through the MAO coating technology. Surf. Coat. Technol. 2003, 167, 269–277. [Google Scholar] [CrossRef]
- Krishna, L.R.; Somaraju, K.R.C.; Sundararajan, G. The tribological performance of ultra-hard ceramic composite coatings obtained through microarc oxidation. Surf. Coat. Technol. 2003, 163, 484–490. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Mao, M.; Xie, H.; Skeldon, P. Key factors determining the development of two morphologies of plasma electrolytic coatings on an Al-Cu-Li alloy in aluminate electrolytes. Surf. Coat. Technol. 2016, 291, 239–249. [Google Scholar] [CrossRef]
- Jaspard-Mecuson, F.; Czerwiec, T.; Henrion, G.; Belmonte, T.; Dujardin, L.; Viola, A.; Beauvir, J. Tailored aluminium oxide layers by bipolar current adjustment in the plasma electrolytic oxidation (PEO) process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, 44458. [Google Scholar] [CrossRef] [PubMed]
- Matykina, E.; Arrabal, R.; Scurr, D.J.; Baron, A.; Skeldon, P.; Thompson, G.E. Investigation of the mechanism of plasma electrolytic oxidation of aluminium using 18O tracer. Corros. Sci. 2010, 52, 1070–1076. [Google Scholar] [CrossRef]
- Rogov, A.B.; Matthews, A.; Yerokhin, A. Role of cathodic current in plasma electrolytic oxidation of Al: A quantitative approach to in-situ evaluation of cathodically induced effects. Electrochim. Acta 2019, 317, 221–231. [Google Scholar] [CrossRef]
- Yilmaz, M.S.; Sahin, O. Applying high voltage cathodic pulse with various pulse durations on aluminium via micro-arc oxidation (MAO). Surf. Coat. Technol. 2018, 347, 278–285. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Santamaria, M.; Asghari, M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 al in Na2WO4 containing solution: From unipolar to soft-sparking regime. Electrochim. Acta 2018, 284, 618–629. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Imshinetskiy, I.M.; Puz’, A.V. Plasma electrolytic oxidation of the magnesium alloy MA8 in electrolytes containing tin nanoparticles. J. Mater. Sci. Technol. 2017, 33, 461–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Miao, C.; Tu, X.; Yu, J.; Li, J. Effect of tungsten carbide particles on the characteristics of PEO coatings formed on AZ31B magnesium alloy in alkaline electrolyte. Int. J. Electrochem. Sci. 2018, 13, 7923–7929. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Investigation of the growth processes of coatings formed by ac plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Kocagoez, S.; Arnholt, C.; Huet, R.; Ueno, M.; Walter, W.L. Advances in zirconia toughened alumina biomaterials for total joint replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 107–116. [Google Scholar] [CrossRef]
- De Aza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef]
- Zadorozhnaya, O.Y.; Khabas, T.A.; Tiunova, O.V.; Malykhin, S.E. Effect of grain size and amount of zirconia on the physical and mechanical properties and the wear resistance of zirconia-toughened alumina. Ceram. Int. 2020, 46, 9263–9270. [Google Scholar] [CrossRef]
- Singh, A.K.; Drunka, R.; Smits, K.; Vanags, M.; Iesalnieks, M.; Joksa, A.A.; Blumbergs, I.; Steins, I. Nanomechanical and electrochemical corrosion testing of nanocomposite coating obtained on AZ31 via plasma electrolytic oxidation containing tin and sic nanoparticles. Crystals 2023, 13, 508. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Chen, D.-F.; Gong, C.-Z.; Yang, S.-Q.; Tian, X.-B. Modulation effects of K2ZrF6 additive on microstructure and heat resistance of micro-arc oxide coatings fabricated on LY12 aluminum alloy. J. Inorg. Mater. 2010, 25, 865–870. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.G.; Tian, J.; Liu, H.W.; Zhou, J.F.; Xu, T. Effect of potassium fluoride in electrolytic solution on the structure and properties of microarc oxidation coatings on magnesium alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Li, J.; Song, R.G.; Qi, X.; Wang, C.; Jiang, B. Effects of polyvinylidene fluoride sealing on micro-arc oxidation coating of 7075 aluminum alloy. Anti-Corros. Methods Mater. 2022, 69, 1–8. [Google Scholar] [CrossRef]
- Qian, C.; Chen, B.; Li, H.; Shi, R.; Yang, Z.; Zhang, N.; Song, S.; Liu, C.; Yang, B. Growth behavior and wear resistance of PEO coatings in bipolar mode. Trans. Indian Inst. Met. 2023, 76, 2263–2271. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chen, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Shen, D.; Yu, S.; Nash, P. Micro-structures and growth mechanisms of plasma electrolytic oxidation coatings on aluminium at different current densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- Wu, Y.-k.; Yang, Z.; Wang, R.-q.; Wu, G.-r.; Chen, D.; Wang, D.-d.; Liu, X.-t.; Li, D.-l.; Guo, C.-h.; Yu, S.-x.; et al. An investigation of microstructure evolution for plasma electrolytic oxidation (PEO) coated al in an alkaline silicate electrolyte. Surf. Coat. Technol. 2018, 351, 136–152. [Google Scholar] [CrossRef]
- Xu, J.L.; Xiao, Q.F.; Mei, D.D.; Zhong, Z.C.; Tong, Y.X.; Zheng, Y.F.; Li, L. Preparation and characterization of amorphous SiO2 coatings deposited by mirco-arc oxidation on sintered NdFeb permanent magnets. J. Magn. Magn. Mater. 2017, 426, 361–368. [Google Scholar] [CrossRef]


| Sample | K2ZrF6 (g·L−1) | CA (g·L−1) |
|---|---|---|
| S1 | 0 | 0 |
| S2 | 5 | 0 |
| S3 | 5 | 5 |
| Friction Test Parameters | |
|---|---|
| Load | 4 N |
| Liner speed | 0.42 m/s |
| Time | 20 min |
| Frictional pair, spherical | Φ 5 mm (GCr15) |
| Track diameter | 10 mm |
| Sample | Percentage of Element Content (at%) | |||
|---|---|---|---|---|
| Al | O | Si | Zr | |
| S1 | 28.82 | 58.53 | 12.65 | 0.00 |
| S2 | 22.63 | 59.46 | 17.84 | 0.08 |
| S3 | 22.27 | 59.43 | 17.05 | 1.26 |
| Sample | Thickness (μm) | Hardness (HV0.1) | Wear Rate (g) |
|---|---|---|---|
| S1 | 37.48 ± 1.7 | 965 ± 71.8 | 0.0091 |
| S2 | 40.34 ± 2.2 | 1105 ± 73.4 | 0.0074 |
| S3 | 51.47 ± 2.6 | 1195 ± 84.2 | 0.0053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, R.; Zhou, S.; Li, H.; Tao, X.; Chen, J. Study on the Wear Resistance of 6061 Aluminum Alloy Bipolar Plasma Electrolytic Oxidation Ceramic Coating by the Addition of K2ZrF6. Materials 2025, 18, 2962. https://doi.org/10.3390/ma18132962
Tong R, Zhou S, Li H, Tao X, Chen J. Study on the Wear Resistance of 6061 Aluminum Alloy Bipolar Plasma Electrolytic Oxidation Ceramic Coating by the Addition of K2ZrF6. Materials. 2025; 18(13):2962. https://doi.org/10.3390/ma18132962
Chicago/Turabian StyleTong, Rui, Shiquan Zhou, Hongtao Li, Xiang Tao, and Jian Chen. 2025. "Study on the Wear Resistance of 6061 Aluminum Alloy Bipolar Plasma Electrolytic Oxidation Ceramic Coating by the Addition of K2ZrF6" Materials 18, no. 13: 2962. https://doi.org/10.3390/ma18132962
APA StyleTong, R., Zhou, S., Li, H., Tao, X., & Chen, J. (2025). Study on the Wear Resistance of 6061 Aluminum Alloy Bipolar Plasma Electrolytic Oxidation Ceramic Coating by the Addition of K2ZrF6. Materials, 18(13), 2962. https://doi.org/10.3390/ma18132962






