Peripheral Cycloalkyl Functionalized Tetradentate Platinum(II) Phosphorescent Complex: Synthesis, Optical Tuning, and OLED Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
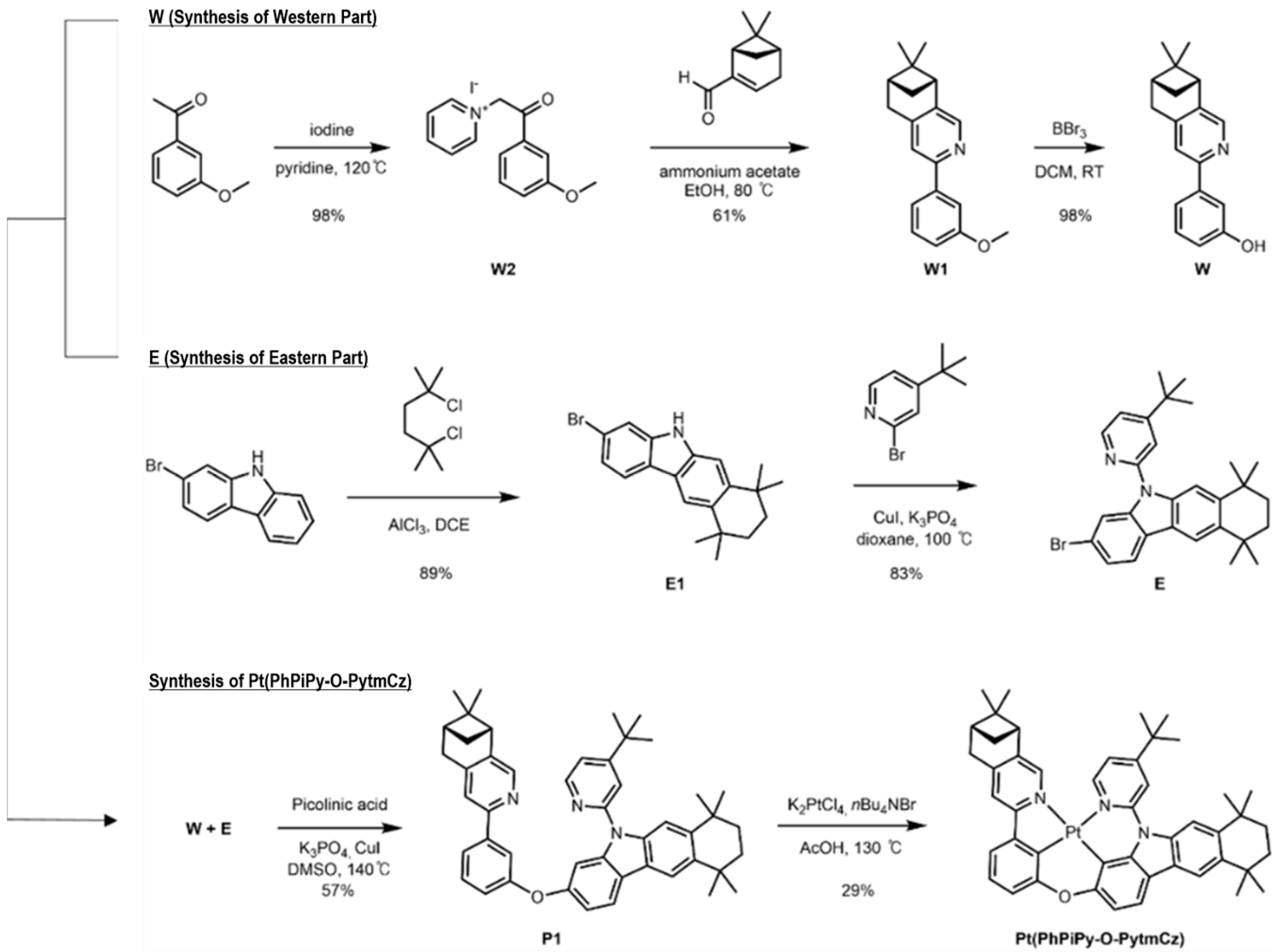
2.2. Synthesis
2.2.1. Synthesis of (6R,8R)-3-(3-Methoxyphenyl)-7,7-dimethyl-5,6,7,8-tetrahydro-6,8-methanoisoquinoline, (W1)
2.2.2. 3-((6R,8R)-7,7-Dimethyl-5,6,7,8-tetrahydro-6,8-methanoisoquinolin-3-yl)phenol, (W)
2.2.3. 3-Bromo-7,7,10,10-tetramethyl-7,8,9,10-tetrahydro-5H-benzo[b]carbazole, (E1)
2.2.4. 3-Bromo-5-(4-(tert-butyl)pyridin-2-yl)-7,7,10,10-tetramethyl-7,8,9,10-tetrahydro-5H-benzo[b]carbazole, (E)
2.2.5. 5-(4-(tert-Butyl)pyridin-2-yl)-3-(3-((6R,8R)-7,7-dimethyl-5,6,7,8-tetrahydro-6,8-methanoisoquinolin-3-yl)phenoxy)-7,7,10,10-tetramethyl-7,8,9,10-tetrahydro-5H-benzo[b]carbazole, (P1)
2.2.6. PhPiPy-O-PytmCz
3. Results and Discussion
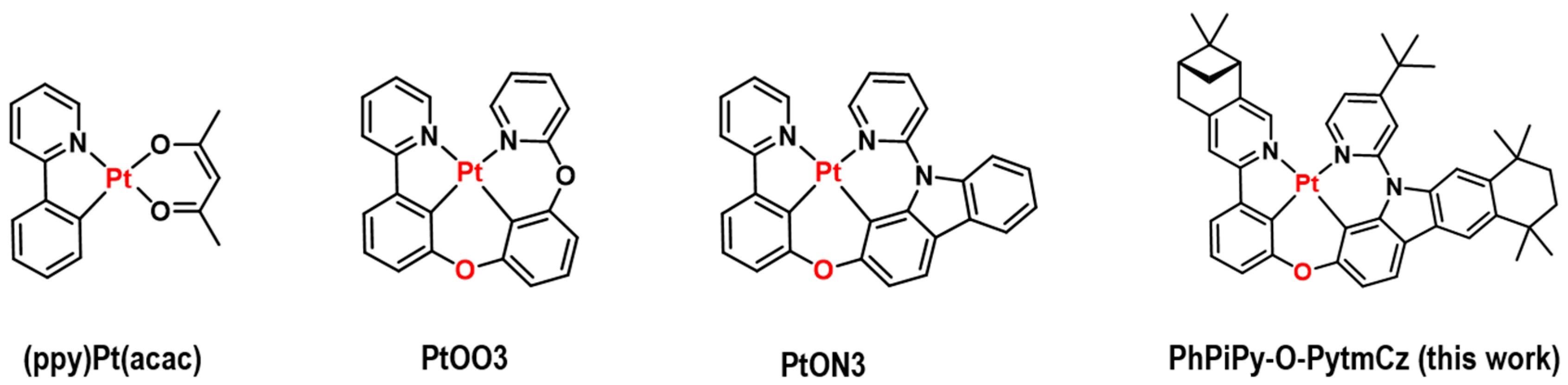
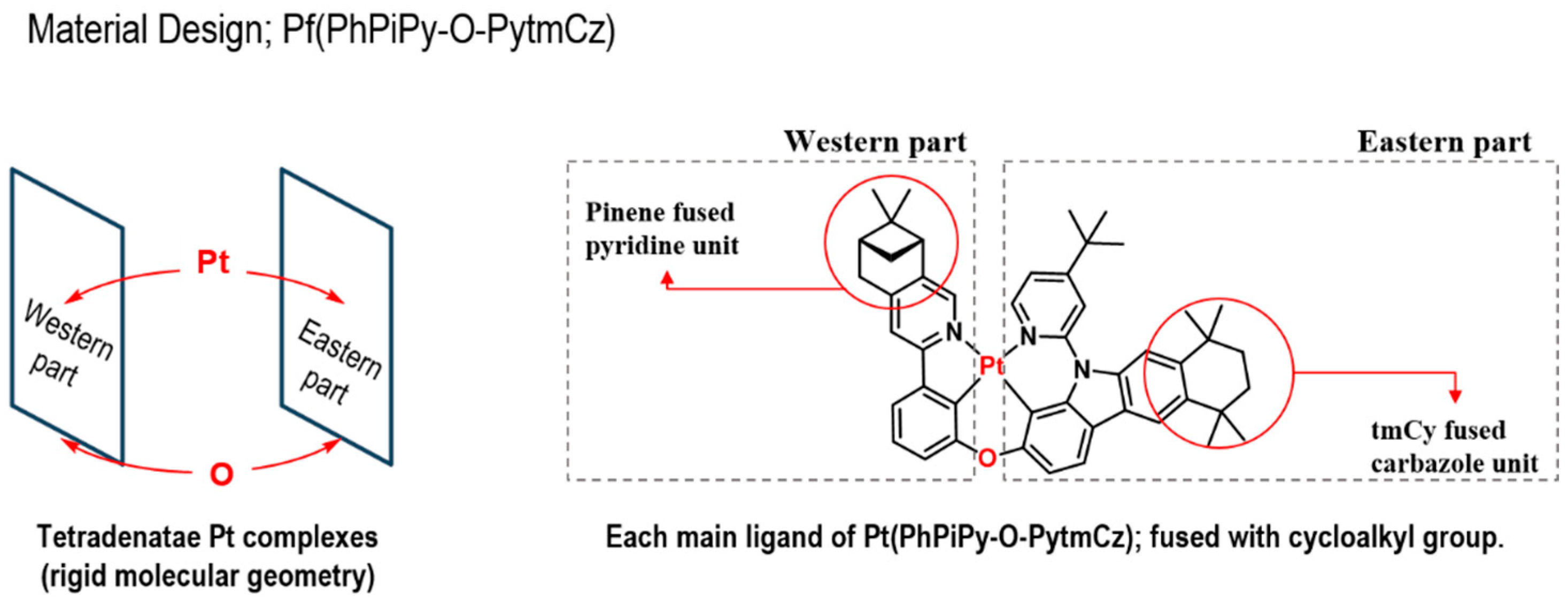
3.1. Molecular Design, Synthesis, and Characterization
3.2. Photophysical Properties
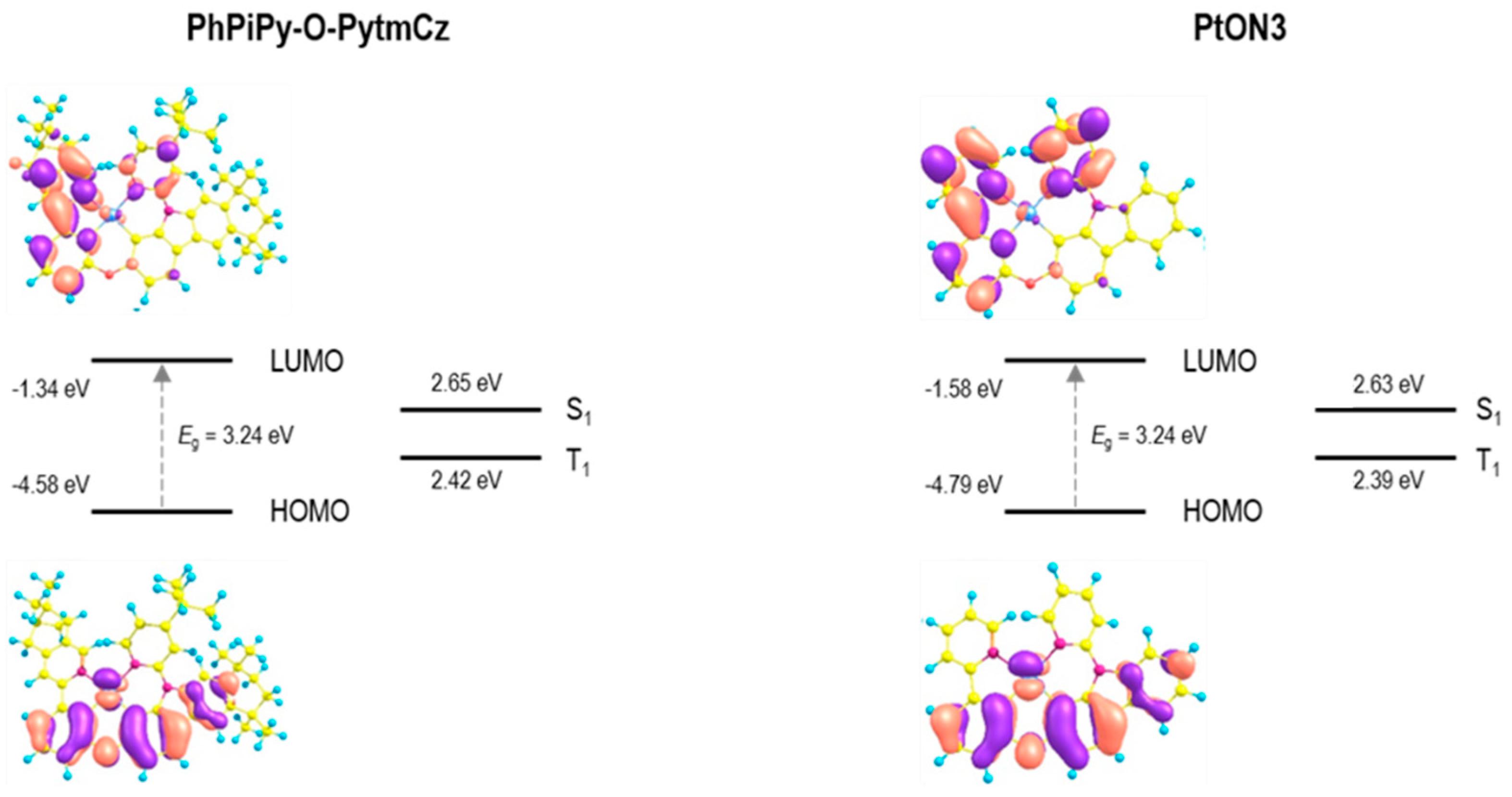
3.3. Theoretical Calculations
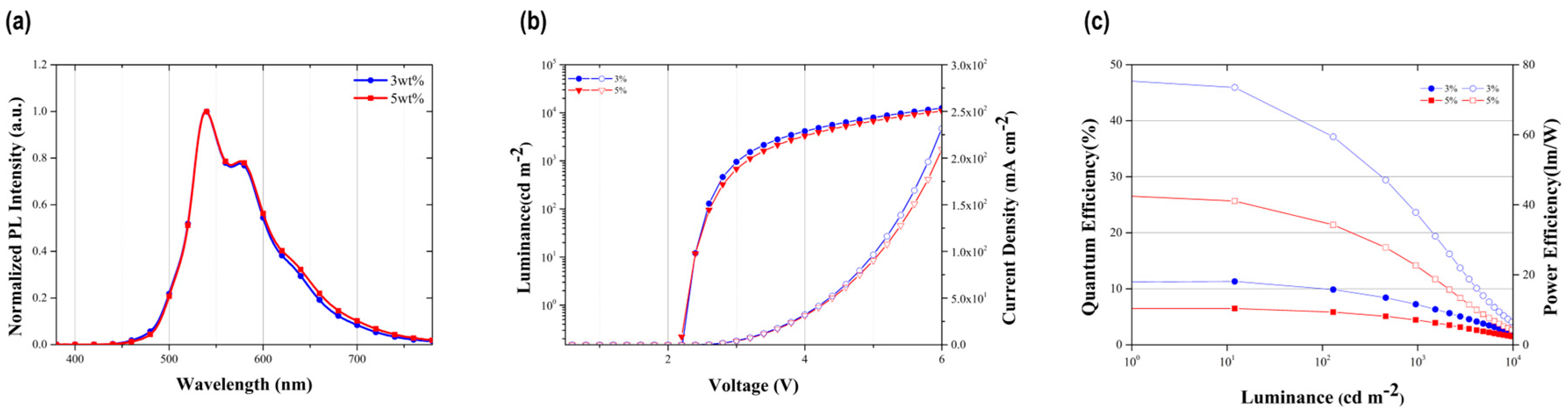
3.4. Electroluminescence (EL) Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudheendran Swayamprabha, S.; Dubey, D.K.; Shahnawaz, Y.; Yadav, R.A.K.; Nagar, M.R.; Sharma, A.; Tung, F.-C.; Jou, J.-H. Approaches for long lifetime organic light emitting diodes. Adv. Sci. 2020, 8, 2002254. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A brief history of OLEDs—Emitter development and industry milestones. Adv. Mater. 2021, 33, e2005630. [Google Scholar] [CrossRef]
- Tasaki, S.; Nishimura, K.; Toyoshima, H.; Masuda, T.; Nakamura, M.; Nakano, Y.; Itoi, H.; Kambe, E.; Kawamura, Y.; Kuma, H. Realization of ultra-high-efficient fluorescent blue OLED. J. Soc. Inf. Disp. 2022, 30, 441–451. [Google Scholar] [CrossRef]
- Kuang, C.; Li, S.; Murtaza, I.; Meng, Z.; Li, H.; Zhang, X.; Wu, C.; Tong, K.-N.; Shang, Y.; He, Y.; et al. Enhanced horizontal dipole orientation by novel penta-helicene anthracene-based host for efficient blue fluorescent OLEDs. Small 2024, 20, e2311114. [Google Scholar] [CrossRef]
- Lim, H.; Woo, S.-J.; Ha, Y.H.; Kim, Y.-H.; Kim, J.-J. Breaking the efficiency limit of deep-blue fluorescent OLEDs based on anthracene derivatives. Adv. Mater. 2022, 34, e2100161. [Google Scholar] [CrossRef] [PubMed]
- Tankelevičiūtė, E.; Samuel, I.D.W.; Zysman-Colman, E. The blue problem: OLED stability and degradation mechanisms. J. Phys. Chem. Lett. 2024, 15, 1034–1047. [Google Scholar] [CrossRef]
- Sree, V.G.; Maheshwaran, A.; Kim, H.; Park, H.-Y.; Kim, Y.; Lee, J.C.; Song, M.; Jin, S.-H. Synthesis and characterization of highly efficient solution-processable green Ir(III) complexes with high current efficiency and very low efficiency roll-off. Adv. Funct. Mater. 2018, 28, 1804714. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Feng, Z.; Liu, B.; Zhong, D.; Zhang, J.; Zhou, G.; Wu, Z. Highly efficient deep-red organic light-emitting devices based on asymmetric iridium(III) complexes with the thianthrene 5,5,10,10-tetraoxide moiety. ACS Appl. Mater. Interfaces 2019, 11, 26152–26164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Tang, R.; Xu, S.; Lam, T.-L.; Cheng, G.; Du, L.; Wan, Q.; Yang, J.; Hung, F.-F.; Low, K.-H.; Phillips, D.L.; et al. Highly robust CuI -TADF emitters for vacuum-deposited OLEDs with luminance up to 222 200 cd m−2 and device lifetimes (LT90) up to 1300 hours at an initial luminance of 1000 cd m−2. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203982. [Google Scholar] [CrossRef]
- Tang, R.; Xu, S.; Du, L.; Hung, F.-F.; Lam, T.-L.; Cheng, G.; Low, K.-H.; Wan, Q.; Wu, S.; Chen, Y.; et al. Au(I)-TADF emitters for high efficiency full-color vacuum-deposited OLEDs and TADF-sensitized fluorescent OLEDs with ultrahigh brightness and prolonged operational lifetime. Adv. Opt. Mater. 2023, 11, 2300950. [Google Scholar] [CrossRef]
- Chow, P.-K.; Cheng, G.; Tong, G.S.M.; Ma, C.; Kwok, W.-M.; Ang, W.-H.; Chung, C.Y.-S.; Yang, C.; Wang, F.; Che, C.-M. Highly luminescent palladium(II) complexes with sub-millisecond blue to green phosphorescent excited states. Photocatalysis and highly efficient PSF-OLEDs. Chem. Sci. 2016, 7, 6083–6098. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Hung, W.-Y.; Tso, J.-Y.; Ko, C.-L.; Wang, T.-H.; Chen, P.-T.; Hsu, H.-F.; Liu, S.-H.; Lee, G.-H.; Chou, P.-T.; et al. Functional pyrimidinyl pyrazolate Pt(II) Complexes: Role of Nitrogen Atom in Tuning the Solid-State Stacking and Photophysics. Adv. Funct. Mater. 2019, 29, 1900923. [Google Scholar] [CrossRef]
- Tuong Ly, K.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2017, 11, 63–68. [Google Scholar] [CrossRef]
- Kim, J.; Batagoda, T.; Lee, J.; Sylvinson, D.; Ding, K.; Saris, P.J.G.; Kaipa, U.; Oswald, I.W.H.; Omary, M.A.; Thompson, M.E.; et al. Systematic control of the orientation of organic phosphorescent Pt complexes in thin films for increased optical outcoupling. Adv. Mater. 2019, 31, e1900921. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Cheong, K.; Jiang, J.; Jeon, S.O.; Hong, W.P.; Lee, J.Y. Tetradentate Pt complexes for organic light-emitting diodes. Trends Chem. 2023, 5, 267–278. [Google Scholar] [CrossRef]
- Brooks, J.; Babayan, Y.; Lamansky, S.; Djurovich, P.I.; Tsyba, I.; Bau, R.; Thompson, M.E. Synthesis and characterization of phosphorescent cyclometalated platinum complexes. Inorg. Chem. 2002, 41, 3055–3066. [Google Scholar] [CrossRef]
- Li, G.; Fleetham, T.; Turner, E.; Hang, X.-C.; Li, J. Highly efficient and stable narrow-band phosphorescent emitters for OLED applications. Adv. Opt. Mater. 2015, 3, 390–397. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.; Han, G.R.; Mun, M.S.; Kang, S.; Hong, W.P.; Oh, H.Y.; Kim, T. Superbly efficient and stable ultrapure blue phosphorescent organic light-emitting diodes with tetradentate Pt(II) complex with vibration suppression effect. Adv. Mater. 2024, 36, e2409394. [Google Scholar] [CrossRef]
- Fleetham, T.; Li, G.; Li, J. Phosphorescent Pt(II) and Pd(II) Complexes for efficient, high-color-quality, and stable OLEDs. Adv. Mater. 2017, 29, 1601861. [Google Scholar] [CrossRef]
- Turner, E.; Bakken, N.; Li, J. Cyclometalated platinum complexes with luminescent quantum yields approaching 100%. Inorg. Chem. 2013, 52, 7344–7351. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, X.; Fleetham, T.; Chen, Q.; Zhan, F.; Zheng, J.; Yang, Y.-F.; Lou, W.; Yang, Y.; Fang, K.; et al. Tetradentate platinum(II) complexes for highly efficient phosphorescent emitters and sky blue OLEDs. Chem. Mater. 2020, 32, 537–548. [Google Scholar] [CrossRef]
- Li, G.; She, Y.; Cyclometalated, T. Tetradentate Cyclometalated Platinum(II) complexes for efficient and stable organic light-emitting diodes. In Light-Emitting Diode—An Outlook on the Empirical Features and Its Recent Technological Advancements; Thirumalai, J., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- You, Y. Pt(II) complexes with tetradentate ligands: Toward commercially applicable blue organic electroluminescence devices. Coord. Chem. Rev. 2025, 526, 216374. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, C.; Tsuboi, T.; Zhang, Q. Degradation mechanisms in blue organic light-emitting diodes. CCS Chem. 2020, 2, 1278–1296. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, G.S.; Muruganantham, S.; Kim, H.R.; Oh, J.H.; Ham, J.H.; Yadav, S.B.; Lee, J.H.; Chae, M.Y.; Kim, Y.-H.; et al. Modified t-butyl in tetradentate platinum (II) complexes enables exceptional lifetime for blue-phosphorescent organic light-emitting diodes. Nat. Commun. 2024, 15, 2977. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, C.; Batagoda, T.; Coburn, C.; Thompson, M.E.; Forrest, S.R. Hot excited state management for long-lived blue phosphorescent organic light-emitting diodes. Nat. Commun. 2017, 8, 15566. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Baek, G.W.; Lee, J.Y.; Kwak, J.; Park, J.-H. Advances in display technology: Augmented reality, virtual reality, quantum dot-based light-emitting diodes, and organic light-emitting diodes. J. Inf. Disp. 2024, 25, 219–234. [Google Scholar] [CrossRef]
- Sun, J.; Ahn, H.; Kang, S.; Ko, S.-B.; Song, D.; Um, H.A.; Kim, S.; Lee, Y.; Jeon, P.; Hwang, S.-H.; et al. Exceptionally stable blue phosphorescent organic light-emitting diodes. Nat. Photonics 2022, 16, 212–218. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Sun, Y.; Lou, W.; Yang, Y.-F.; She, Y. N-Heterocyclic carbene-based tetradentate platinum(II) complexes for phosphorescent OLEDs with high brightness. J. Mater. Chem. C 2021, 10, 210–218. [Google Scholar] [CrossRef]
- Cheong, K.; Jo, U.; Hong, W.P.; Lee, J.Y. Fused cycloalkyl unit-functionalized tetradentate Pt(II) Complexes for Efficient and Narrow-Emitting Deep Blue Organic Light-Emitting Diodes. Small Methods 2024, 8, e2300862. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Xu, Q.-L.; Han, H.-B.; Zhao, Y.; Zheng, Y.-X.; Zhou, L.; Zuo, J.-L.; Zhang, H. Highly efficient organic light-emitting diodes with low efficiency roll-off based on iridium complexes containing pinene sterically hindered spacer. Adv. Opt. Mater. 2016, 4, 1726–1731. [Google Scholar] [CrossRef]
- Tanaka, S.; Sato, K.; Ichida, K.; Abe, T.; Tsubomura, T.; Suzuki, T.; Shinozaki, K. Circularly polarized luminescence of chiral Pt(pppb)Cl (pppbH=1-pyridyl-3-(4,5-pinenopyridyl)benzene) aggregate in the excited state. Chem. Asian J. 2016, 11, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, Y.; Xu, K.; Zhang, C.; Chen, J.; Chu, Q.; Yang, Y.-F.; She, Y.; Tetradentate, P.B. Perimidocarbene-based tetradentate platinum(II) complexes with an unexpectedly negligible 3MLCT character. Inorg. Chem. 2024, 63, 6435–6444. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wolfe, A.; Brooks, J.; Zhu, Z.Q.; Li, J. Modifying emission spectral bandwidth of phosphorescent platinum(II) complexes through synthetic control. Inorg. Chem. 2017, 56, 8244–8256. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, D.; Li, J.; Wan, H.; Mei, Y. 2-phenylpyridine-based phosphorescent Ir(III) complexes for highly efficient greenish-blue organic light-emitting diodes with EQEs up to 33.5%. Dyes Pigment 2023, 210, 111032. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, Y.; Yang, X.; Sun, Y.; Zhong, D.; Deng, X.; Zhou, G.; Wu, Z. Manipulating MLCT transition character with ppy-type four-coordinate organoboron skeleton for highly efficient long-wavelength Ir-based phosphors in organic light-emitting diodes. J. Mater. Chem. C 2021, 9, 12650–12660. [Google Scholar] [CrossRef]
- Kim, S.C.; Hong, W.P.; Lee, J.Y. Cycloalkyl fused dibenzofuran derived green Ir(III) complexes possessing high horizontal emitting dipole orientation ratios and color stability at high doping concentrations. Adv. Opt. Mater. 2022, 10, 2201511. [Google Scholar] [CrossRef]
- Kim, E.; Park, J.; Jun, M.; Shin, H.; Baek, J.; Kim, T.; Kim, S.; Lee, J.; Ahn, H.; Sun, J.; et al. Highly efficient and stable deep-blue organic light-emitting diode using phosphor-sensitized thermally activated delayed fluorescence. Sci. Adv. 2022, 8, eabq1641. [Google Scholar] [CrossRef]
- Shin, S.K.; Han, S.H.; Lee, J.Y. High triplet energy exciplex host derived from a CN modified carbazole based n-type host for improved efficiency and lifetime in blue phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 10308–10314. [Google Scholar] [CrossRef]
- Iwasaki, H.; Majima, Y.; Izawa, S. Low-voltage turn-on in blue organic light-emitting diodes. Synth. Met. 2024, 309, 117772. [Google Scholar] [CrossRef]
- Bi, Y.; Wei, J.; Chen, S.; Zhao, H.; Zhang, X. Triphenylsilyl-promoted iridium complex for high-performance green-yellow phosphorescent organic light-emitting diodes. J. Phys. Chem. C 2021, 125, 24671–24684. [Google Scholar] [CrossRef]


| UV-vis [a] (nm) | λmax [a] (nm) | FWHM [a] (nm) | Φ [b] (%) | τ [a] (μs) | kr [c] (×105 s−1) | knr [d] (×104 s−1) | HOMO [e] (eV) | LUMO [e] (eV) | |
|---|---|---|---|---|---|---|---|---|---|
| Pt(PhPiPy-O-PytmCz) | 317, >420 | 516 (544) | 88 | 83.5 | 2.14 | 3.9 | 7.9 | −4.91 | −1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.; Lee, S.-j.; Kang, M.; Hong, W.P. Peripheral Cycloalkyl Functionalized Tetradentate Platinum(II) Phosphorescent Complex: Synthesis, Optical Tuning, and OLED Applications. Materials 2025, 18, 2942. https://doi.org/10.3390/ma18132942
Park G, Lee S-j, Kang M, Hong WP. Peripheral Cycloalkyl Functionalized Tetradentate Platinum(II) Phosphorescent Complex: Synthesis, Optical Tuning, and OLED Applications. Materials. 2025; 18(13):2942. https://doi.org/10.3390/ma18132942
Chicago/Turabian StylePark, Giheon, Seon-jin Lee, Minsoo Kang, and Wan Pyo Hong. 2025. "Peripheral Cycloalkyl Functionalized Tetradentate Platinum(II) Phosphorescent Complex: Synthesis, Optical Tuning, and OLED Applications" Materials 18, no. 13: 2942. https://doi.org/10.3390/ma18132942
APA StylePark, G., Lee, S.-j., Kang, M., & Hong, W. P. (2025). Peripheral Cycloalkyl Functionalized Tetradentate Platinum(II) Phosphorescent Complex: Synthesis, Optical Tuning, and OLED Applications. Materials, 18(13), 2942. https://doi.org/10.3390/ma18132942








