Effect of a Polyhexanide-Based Antiseptic Composition on Dentin Microhardness and Mechanical Properties: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- Stage 1 (Backside leveling): P800 abrasive paper, counter-rotational mode, 6 min, disk speed 200 rpm, head speed 50 rpm, individual load 15 N, water cooling.
- Stage 2 (Investigated side grinding): P1200 abrasive paper, 2 min, counter-rotational mode, same parameters as Stage 1.
- Stage 3 (Polishing): Silk cloth with 1 µm monocrystalline diamond suspension, 6 min, unidirectional rotation, disk speed 200 rpm, head speed 50 rpm, individual load 10 N, dry polishing.
- Stage 4 (Final cleaning): Immersion of samples in chemically pure isopropyl alcohol (Propan-2-ol).
2.2. Microhardness Measurement and Chemical Treatment of Samples
- Group 1: 0.9% Sodium chloride solution for 1 h.
- Group 2: 3% Sodium hypochlorite (Belodez, VladMiVa, Belgorod, Russia) for 1 h.
- Group 3: 2% Chlorhexidine digluconate solution (Omega-Dent, Moscow, Russia) for 1 h.
- Group 4: 17% EDTA solution (MD Cleanser, META, Cheongju, Republic of Korea) for 1 h.
- Group 5: 0.1% Lavasept (polyhexanide-based solution) for 1 h.
- Group 6: 0.2% Lavasept (polyhexanide-based solution) for 1 h.
2.3. Statistical Analysis
3. Results
- Group 1: 0.9% sodium chloride,
- Group 2: 3% sodium hypochlorite (NaOCl),
- Group 3: 2% chlorhexidine digluconate,
- Group 4: 17% EDTA,
- Group 5: 0.1% Lavasept (polyhexanide-based solution),
- Group 6: 0.2% Lavasept (polyhexanide-based solution).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Jin, C.; Zhao, S.; Xie, H. Effects of sodium hypochlorite and ethylenediaminetetraacetic acid on proliferation, osteogenic/odontogenic differentiation, and mechanosensitive gene expression of human dental pulp stem cells. Tissue Cell. 2022, 79, 101955. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, Z.; Zhang, A.; Lin, F.; Fu, J.; Fok, A. Effects of concentration of sodium hypochlorite as an endodontic irrigant on the mechanical and structural properties of root dentine: A laboratory study. Int. Endod. J. 2022, 55, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Pascon, F.M.; Kantovitz, K.R.; Sacramento, P.A.; Nobre-Dos-Santos, M.; Puppin-Rontani, R.M. Effect of sodium hypochlorite on dentine mechanical properties: A review. J. Dent. 2009, 37, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Pandia, E.L.F.B.S.; Tanjung, D.S.; Purba, M.R. The differences in the effects of propolis, sodium hypochlorite, and EDTA as an irrigant solutions on the microhardness of root canal dentin: In-vitro study. J. Health Dent. Sci. 2025, 4, 255–264. [Google Scholar] [CrossRef]
- Elika, V.; Kunam, D.; Anumula, L.; Chinni, S.; Govula, K. Comparative evaluation of Chloroquick with Triphala, sodium hypochlorite, and ethylenediaminetetraacetic acid on the microhardness of root canal dentin: An in vitro study. J. Clin. Transl. Res. 2021, 7, 72–76. [Google Scholar]
- Kinney, J.H.; Marshall, S.J.; Marshall, G.W. The mechanical properties of human dentin: A critical review and re-evaluation of the dental literature. Crit. Rev. Oral Biol. Med. 2003, 14, 13–29. [Google Scholar] [CrossRef]
- Kinney, J.H.; Nalla, R.K.; Pople, J.A.; Breunig, T.M.; Ritchie, R.O. Age-related transparent root dentin: Mineral concentration, crystallite size, and mechanical properties. Biomaterials 2005, 26, 3363–3376. [Google Scholar] [CrossRef]
- Agarwal, S.; Mishra, L.; Singh, N.; Behera, R.; Kumar, M.; Nagaraja, R.; Sokolowski, K.; Łapińska, B. Effect of Different Irrigating Solutions on Root Canal Dentin Microhardness: A Systematic Review with Meta-Analysis. J. Funct. Biomater. 2024, 15, 132. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Perdigão, J.; Lopes, M.; Geraldeli, S.; Lopes, G.C.; García-Godoy, F. Effect of a sodium hypochlorite gel on dentin bonding. Dent. Mater. 2000, 16, 311–323. [Google Scholar] [CrossRef]
- Marending, M.; Luder, H.U.; Brunner, T.J.; Knecht, S.; Stark, W.J.; Zehnder, M. Effect of sodium hypochlorite on human root dentine: Mechanical, chemical and structural evaluation. Int. Endod. J. 2007, 40, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Balubaid, W.; Algarni, Y.; Barashid, A. Effect of BioPure MTAD, sodium hypochlorite and EDTA on dentin microhardness: In vitro study. Egypt. Dent. J. 2017, 63, 769–774. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.; Gao, Y.; Wang, M.; Wang, Y.; Sa, Y.; Jiang, T. Effects of different concentrations and exposure time of sodium hypochlorite on the structural, compositional and mechanical properties of human dentin. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Bakr, D.; Saleem, S.S.; Amin, B.K. Effect of sodium hypochlorite, chlorhexidine and EDTA on dentin microhardness. Zanco J. Med. Sci. 2016, 20, 1175–1179. [Google Scholar] [CrossRef]
- Sim, T.P.; Knowles, J.C.; Ng, Y.L.; Shelton, J.; Gulabivala, K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int. Endod. J. 2001, 34, 120–132. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kawashima, N.; Ichinose, S.; Nara, K.; Noda, S.; Okiji, T. EDTA treatment for sodium hypochlorite–treated dentin recovers disturbed attachment and induces differentiation of mouse dental papilla cells. J. Endod. 2017, 44, 256–262. [Google Scholar] [CrossRef]
- Ghisi, A.C.; Kopper, P.M.P.; Baldasso, F.E.R.; Stürmer, C.P.; Rossi-Fedele, G.; Steier, L.; de Figueiredo, J.A.P.; Morgental, R.D.; Vier-Pelisser, F.V. Effect of superoxidized water and sodium hypochlorite, associated or not with EDTA, on organic and inorganic components of bovine root dentin. J. Endod. 2015, 41, 925–930. [Google Scholar] [CrossRef]
- Khabadze, Z.; Generalova, Y.A.; Kulikova, A. The investigation of endodontic irrigants and polyhexanide-based solution action on smear layer. Endod. Today 2024, 22, 199–205. [Google Scholar] [CrossRef]
- Zurab, Z.S.; Generalova, Y.A.; Kulikova, A.; Umarov, A.; Badalov, F.V.; Wehbe, A.; Kakabadze, E.M. Analysis of the chemical interaction of polyhexanide with endodontic irrigants. Endod. Today 2024, 22, 319–334. [Google Scholar] [CrossRef]
- Kolosowski, K.P.; Sodhi, R.; Kishen, A.; Basrani, B. Qualitative time-of-flight secondary ion mass spectrometry analysis of root dentin irrigated with sodium hypochlorite, EDTA, or chlorhexidine. J. Endod. 2015, 41, 1672–1677. [Google Scholar] [CrossRef]
- Ramirez-Bommer, C.; Gulabivala, K.; Figueiredo, J.A.; Young, A. The influence of sodium hypochlorite and EDTA on the chemical composition of dentine. Int. Endod. J. 2007, 40, 404. [Google Scholar] [CrossRef]
- Campos, D.P.; Bragança, G.F.; de Oliveira, C.R.; Duarte, M.A.H.; Ordinola-Zapata, R. Effect of root canal irrigants on the microhardness and roughness of root dentin: A systematic review. Restor. Dent. Endod. 2020, 45. [Google Scholar]
- Rodrigues, C.H.; Ferraz, C.C.R.; Marciano, M.A.; Guimarães, L.F. Evaluation of root dentin hardness after instrumentation using different nickel-titanium systems and irrigating solutions. J. Endod. 2009, 35, 1004–1007. [Google Scholar]
- Barabashko, M.; Ponomarev, A.; Rezvanova, A.; Kuznetsov, V.; Moseenkov, S. Young’s Modulus and Vickers Hardness of the Hydroxyapatite Bioceramics with a Small Amount of the Multi-Walled Carbon Nanotubes. Materials 2022, 15, 5304. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N. Spatial Distribution of the Micro-Mechanical Properties in High-Translucent CAD/CAM Resin-Composite Blocks. Materials 2020, 13, 3352. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tanaka, M.; Furukimi, O. Hardness–Deformation Energy Relationship in Metals and Alloys. Materials 2021, 14, 7217. [Google Scholar] [CrossRef]
- Zaparolli, D.; Saquy, P.C.; Cruz-Filho, A.M. Effect of sodium hypochlorite and EDTA irrigation, individually and in alternation, on dentin microhardness at the furcation area. Braz. Dent. J. 2012, 23, 654–658. [Google Scholar] [CrossRef]
- Hegde, M.N.; Malhotra, S.; Dhingra, A. Influence of sodium hypochlorite and EDTA on fracture resistance of endodontically treated roots: An in vitro study. J. Conserv. Dent. 2017, 20, 237–240. [Google Scholar]
- Khabadze, Z.; Generalova, Y.A.; Taptun, Y.; Kozhevnikova, L.; Gadzhiev, F.; Dashtieva, M. The effect of the irrigation solutions on dentin organic components: Pilot study. Endod. Today 2024, 22, 19–24. [Google Scholar] [CrossRef]

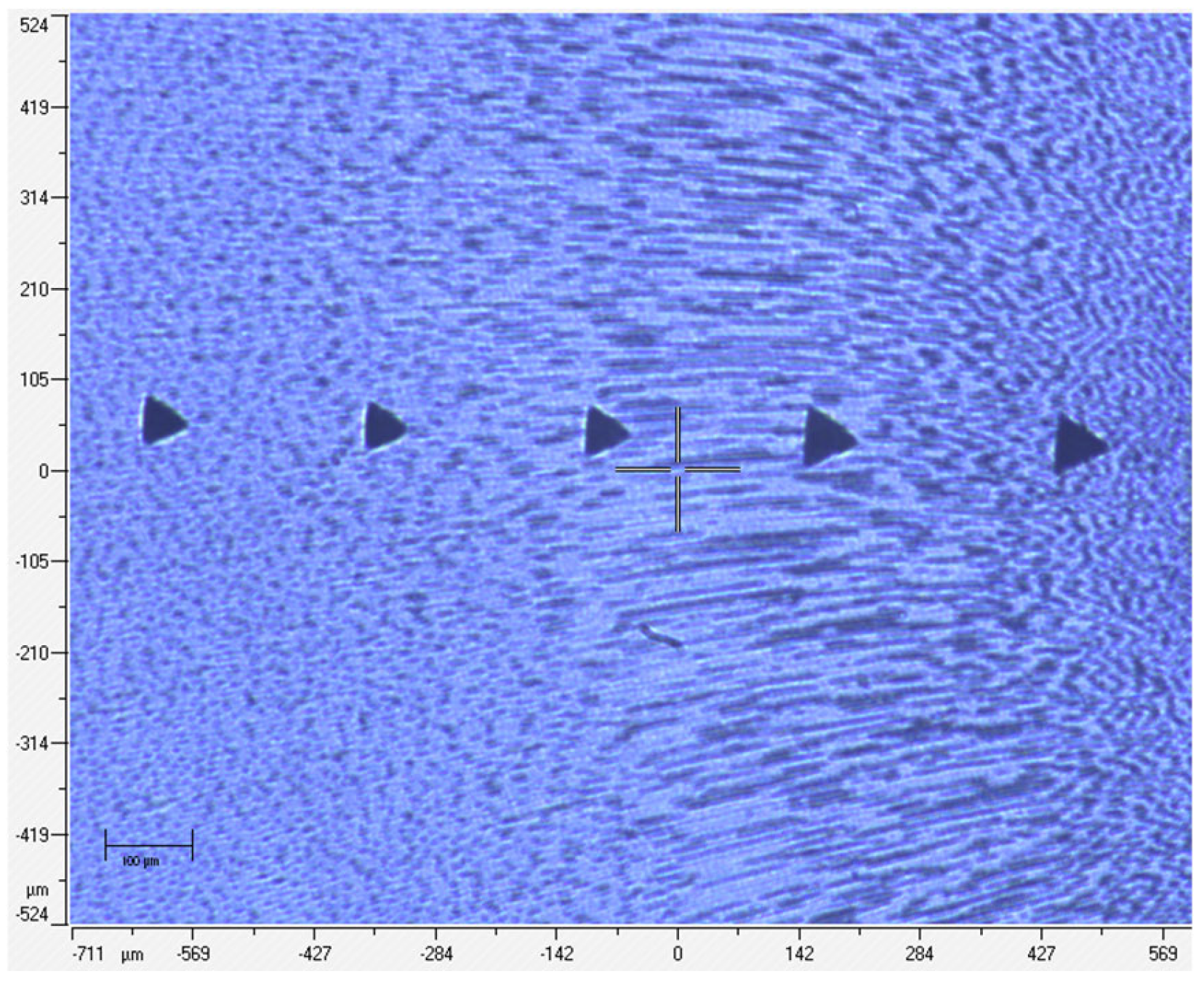
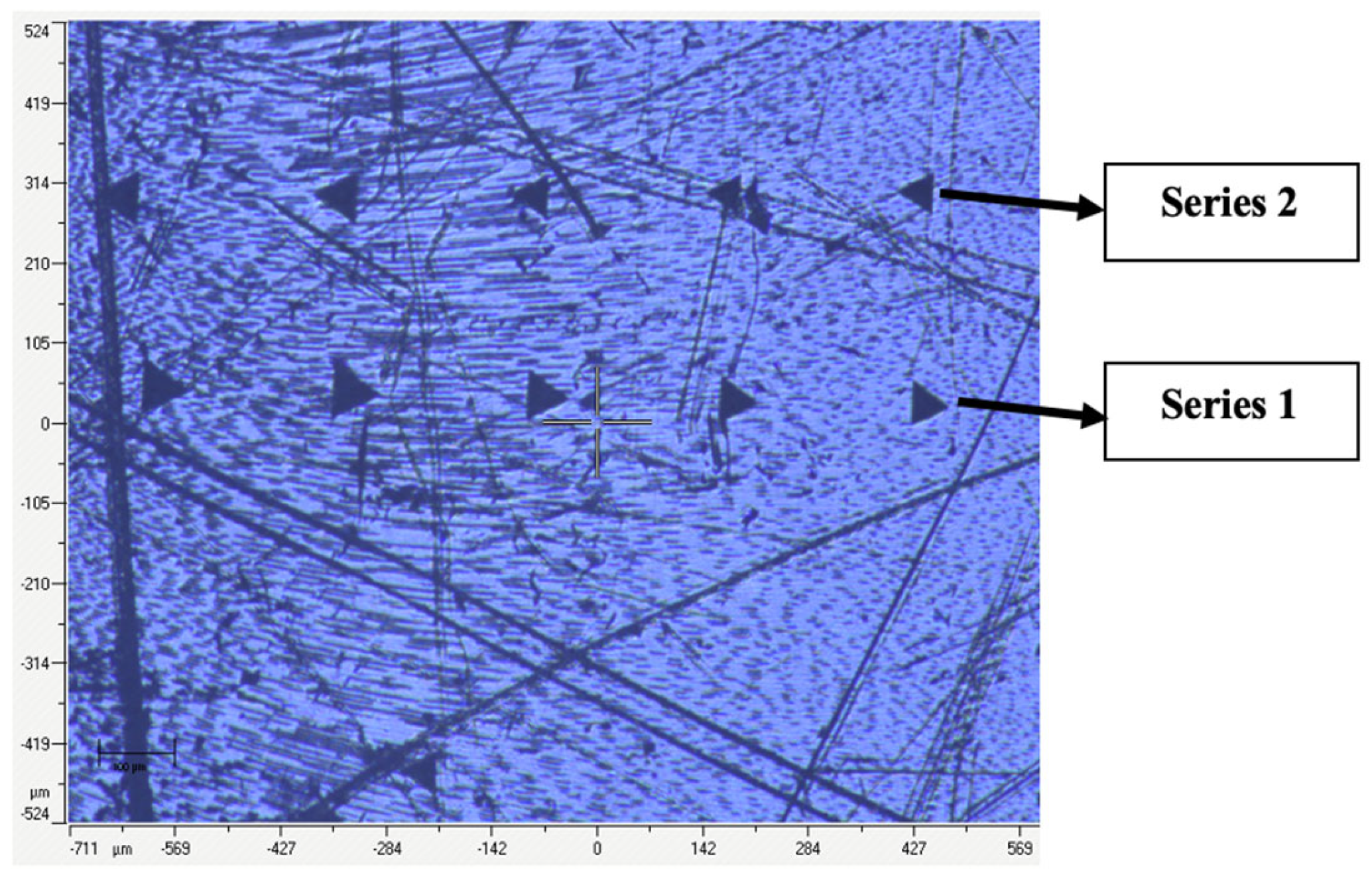
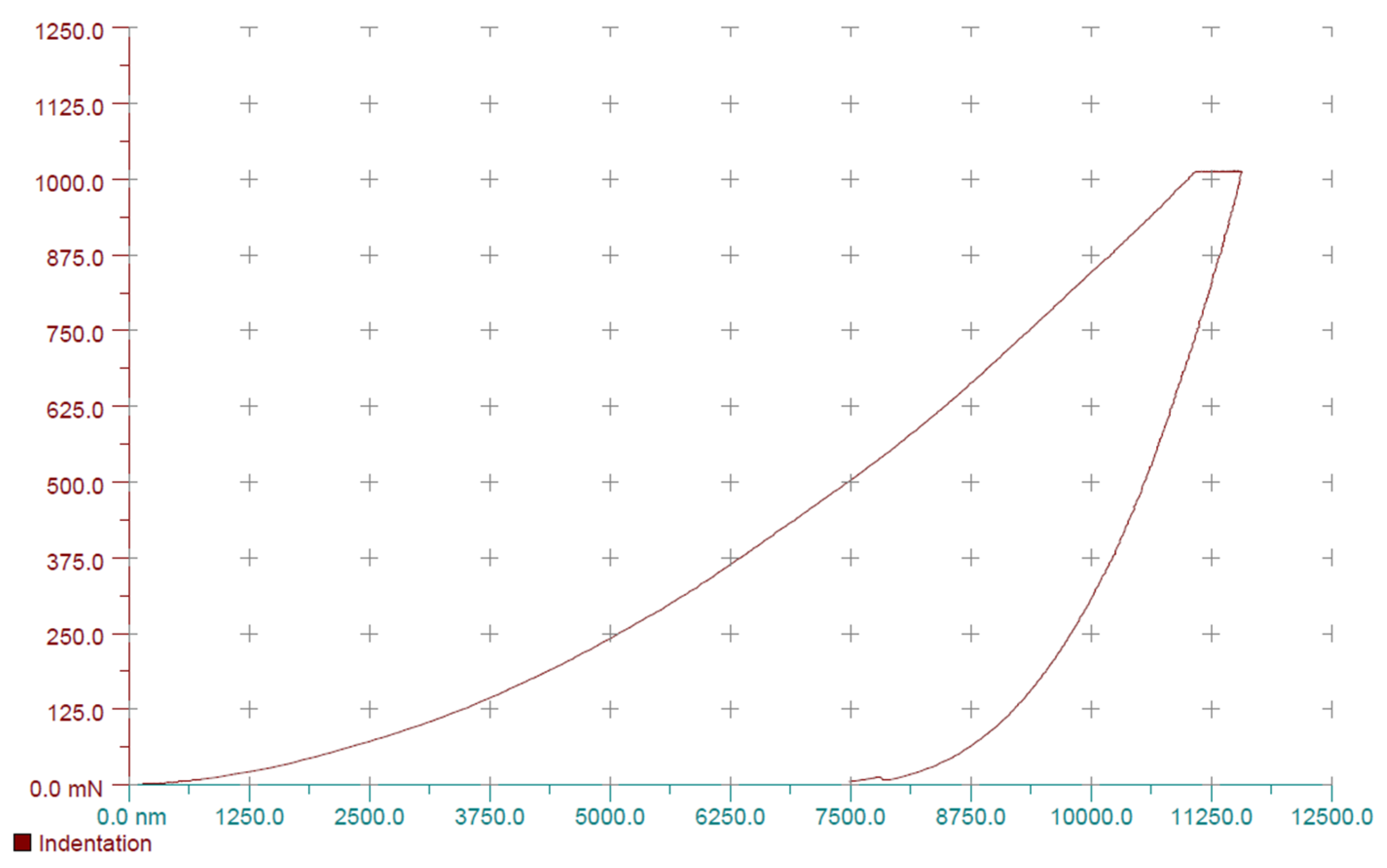
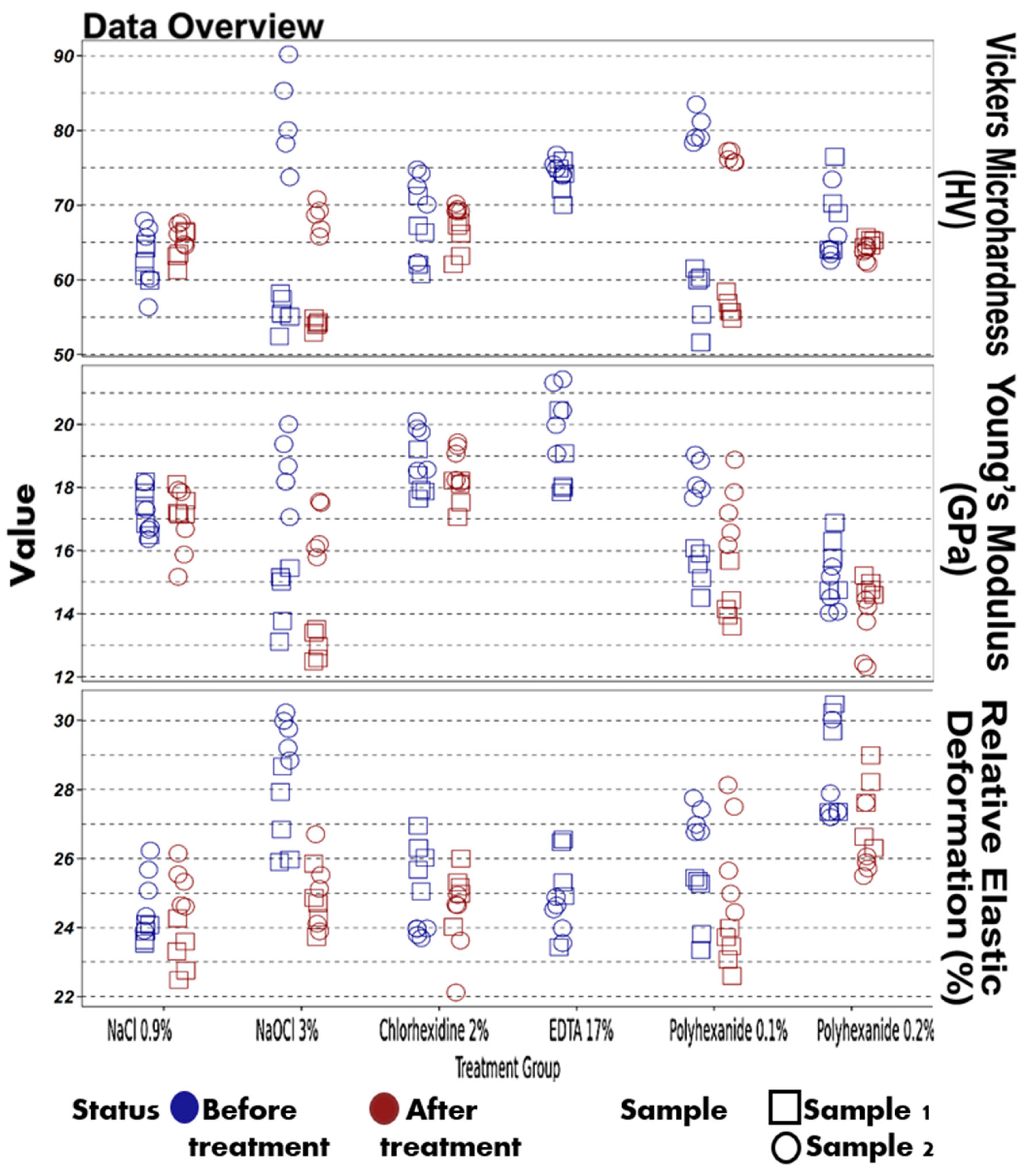
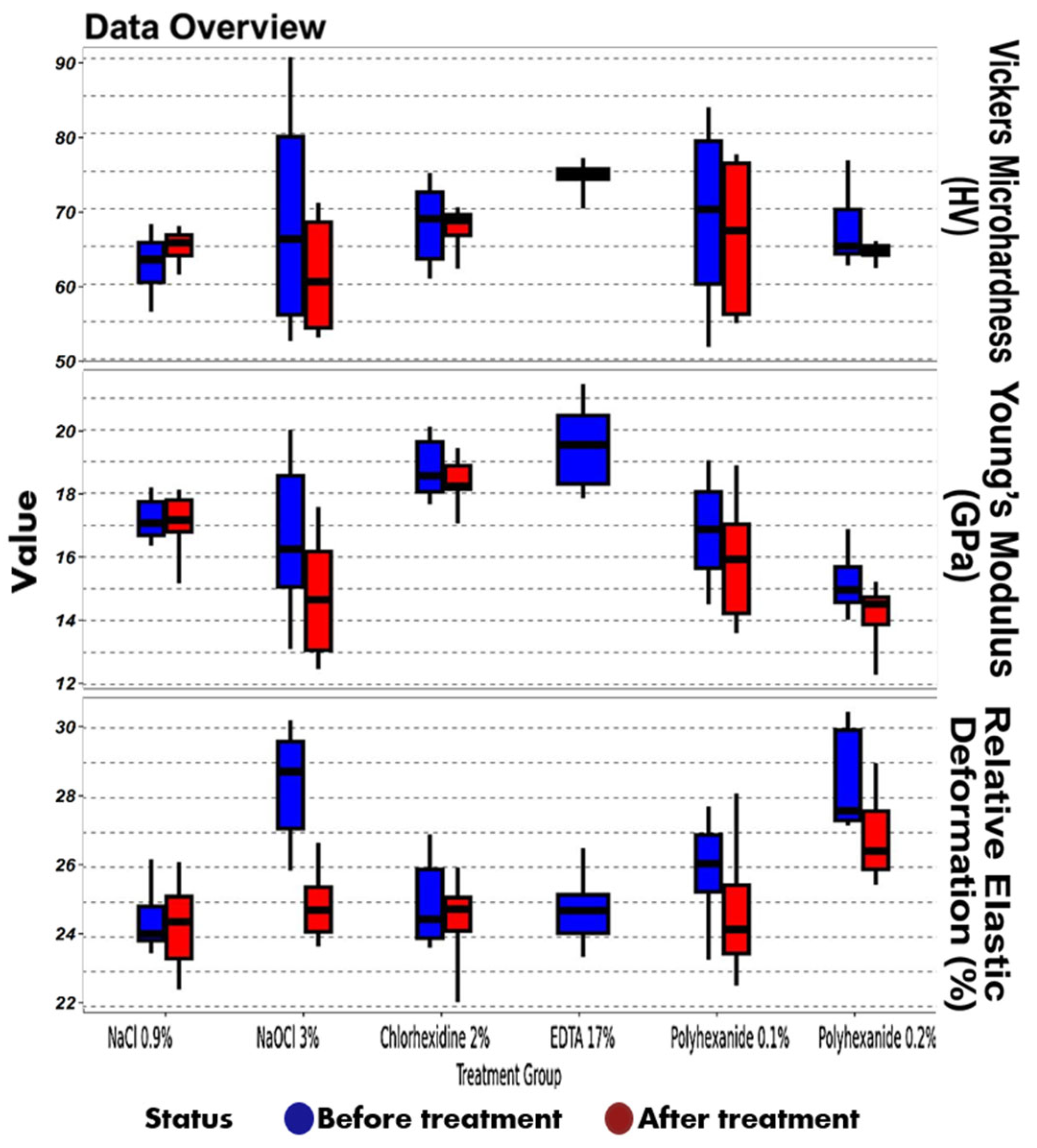
| Parameter | Treatment | Status | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Vickers microhardness (HV) | NaCl 0.9% | Before treatment | 56.34 | 60.26 | 63.28 | 65.5 | 67.96 |
| Vickers microhardness (HV) | NaCl 0.9% | After treatment | 61.29 | 63.77 | 65.47 | 66.5 | 67.7 |
| Vickers microhardness (HV) | NaOCl 3% | Before treatment | 52.44 | 55.94 | 65.98 | 79.6 | 90.19 |
| Vickers microhardness (HV) | NaOCl 3% | After treatment | 52.91 | 54.19 | 60.34 | 68.22 | 70.81 |
| Vickers microhardness (HV) | Chlorhexidine 2% | Before treatment | 60.77 | 63.33 | 68.67 | 72.23 | 74.79 |
| Vickers microhardness (HV) | Chlorhexidine 2% | After treatment | 62.09 | 66.46 | 68.43 | 69.21 | 70.21 |
| Vickers microhardness (HV) | EDTA 17% | Before treatment | 70.04 | 73.97 | 74.55 | 75.33 | 76.78 |
| Vickers microhardness (HV) | Lavasept 0.1% | After treatment | 51.62 | 60.04 | 69.93 | 79.03 | 83.49 |
| Vickers microhardness (HV) | Lavasept 0.1% | Before treatment | 54.8 | 56.03 | 67.09 | 76.06 | 77.28 |
| Vickers microhardness (HV) | Lavasept 0.2% | After treatment | 62.54 | 64.0 | 65.02 | 69.9 | 76.47 |
| Vickers microhardness (HV) | Lavasept 0.2% | Before treatment | 62.18 | 63.83 | 64.41 | 65.07 | 65.72 |
| Young’s modulus (GPa) | NaCl 0.9% | Before treatment | 16.36 | 16.69 | 17.07 | 17.73 | 18.18 |
| Young’s modulus (GPa) | NaCl 0.9% | After treatment | 15.17 | 16.8 | 17.17 | 17.79 | 18.11 |
| Young’s modulus (GPa) | NaOCl 3% | Before treatment | 13.12 | 15.06 | 16.25 | 18.55 | 20.0 |
| Young’s modulus (GPa) | NaOCl 3% | After treatment | 12.49 | 13.08 | 14.65 | 16.18 | 17.56 |
| Young’s modulus (GPa) | Chlorhexidine 2% | Before treatment | 17.65 | 18.04 | 18.55 | 19.62 | 20.11 |
| Young’s modulus (GPa) | Chlorhexidine 2% | After treatment | 17.07 | 18.13 | 18.22 | 18.86 | 19.43 |
| Young’s modulus (GPa) | EDTA 17% | Before treatment | 17.85 | 18.3 | 19.53 | 20.45 | 21.43 |
| Young’s modulus (GPa) | Lavasept 0.1% | After treatment | 14.5 | 15.65 | 16.87 | 18.04 | 19.04 |
| Young’s modulus (GPa) | Lavasept 0.1% | Before treatment | 13.6 | 14.22 | 15.93 | 17.04 | 18.88 |
| Young’s modulus (GPa) | Lavasept 0.2% | After treatment | 14.03 | 14.57 | 14.96 | 15.69 | 16.88 |
| Young’s modulus (GPa) | Lavasept 0.2% | Before treatment | 12.3 | 13.87 | 14.52 | 14.74 | 15.22 |
| Relative elastic deformation work (%) | NaCl 0.9% | Before treatment | 23.53 | 23.9 | 24.09 | 24.88 | 26.23 |
| Relative elastic deformation work (%) | NaCl 0.9% | After treatment | 22.48 | 23.38 | 24.44 | 25.16 | 26.15 |
| Relative elastic deformation work (%) | NaOCl 3% | Before treatment | 25.9 | 27.12 | 28.75 | 29.61 | 30.23 |
| Relative elastic deformation work (%) | NaOCl 3% | After treatment | 23.73 | 24.15 | 24.78 | 25.43 | 26.71 |
| Relative elastic deformation work (%) | Chlorhexidine 2% | Before treatment | 23.69 | 23.97 | 24.51 | 25.94 | 26.95 |
| Relative elastic deformation work (%) | Chlorhexidine 2% | After treatment | 22.12 | 24.18 | 24.81 | 25.13 | 26.0 |
| Relative elastic deformation work (%) | EDTA 17% | Before treatment | 23.43 | 24.12 | 24.76 | 25.21 | 26.55 |
| Relative elastic deformation work (%) | Lavasept 0.1% | After treatment | 23.35 | 25.29 | 26.11 | 26.93 | 27.76 |
| Relative elastic deformation work (%) | Lavasept 0.1% | Before treatment | 22.6 | 23.52 | 24.22 | 25.48 | 28.13 |
| Relative elastic deformation work (%) | Lavasept 0.2% | After treatment | 27.2 | 27.35 | 27.63 | 29.94 | 30.47 |
| Relative elastic deformation work (%) | Lavasept 0.2% | Before treatment | 25.5 | 25.94 | 26.47 | 27.62 | 28.99 |
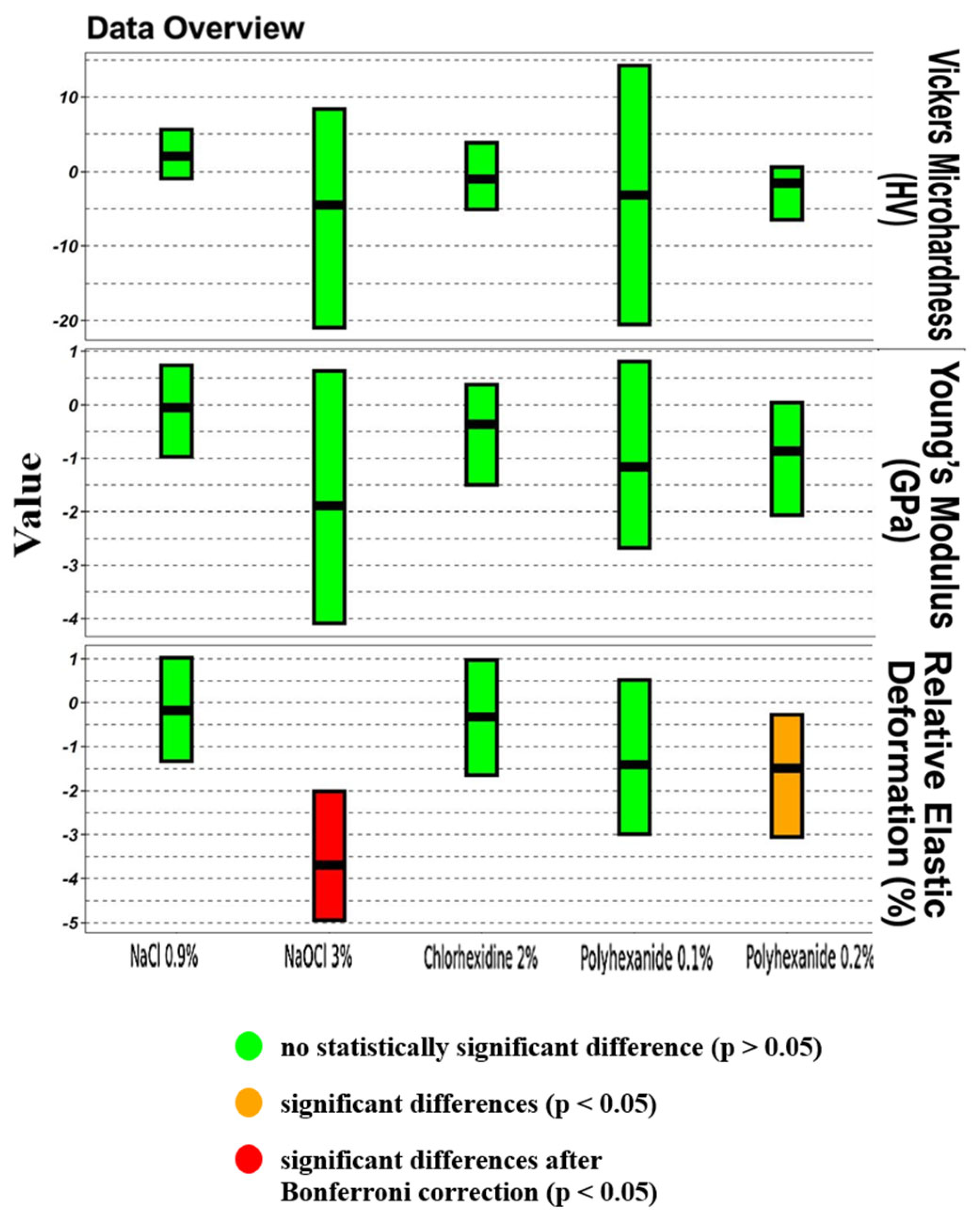
| Parameter | Treatment | p-Value | p-Value (Bonferroni Corrected) | Pseudomedian Difference | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Vickers microhardness (HV) | NaCl 0.9% | 0.1903 | 0.3816 | +2.07 | −0.94 | +5.61 |
| Vickers microhardness (HV) | NaOCl 3% | 0.1431 | 0.3816 | −4.49 | −20.94 | +8.39 |
| Vickers microhardness (HV) | Chlorhexidine 2% | 0.6842 | 0.7895 | −0.98 | −5.09 | +3.88 |
| Vickers microhardness (HV) | EDTA 17% | No data | No data | No data | No data | No data |
| Vickers microhardness (HV) | Lavasept 0.1% | 0.2475 | 0.3816 | −3.15 | −20.54 | +14.24 |
| Vickers microhardness (HV) | Lavasept 0.2% | 0.2799 | 0.3816 | −1.54 | −6.47 | +0.60 |
| Young’s modulus (GPa) | NaCl 0.9% | 0.9698 | 0.9698 | −0.05 | −0.97 | +0.73 |
| Young’s modulus (GPa) | NaOCl 3% | 0.1230 | 0.3816 | −1.89 | −4.09 | +0.63 |
| Young’s modulus (GPa) | Chlorhexidine 2% | 0.2799 | 0.3816 | −0.36 | −1.50 | +0.37 |
| Young’s modulus (GPa) | EDTA 17% | No data | No data | No data | No data | No data |
| Young’s modulus (GPa) | Lavasept 0.1% | 0.2176 | 0.3816 | −1.16 | −2.67 | +0.81 |
| Young’s modulus (GPa) | Lavasept 0.2% | 0.0753 | 0.3763 | −0.87 | −2.06 | +0.04 |
| Relative elastic deformation work (%) | NaCl 0.9% | 0.7959 | 0.8528 | −0.18 | −1.32 | +1.02 |
| Relative elastic deformation work (%) | NaOCl 3% | <0.0001 | 0.0006 | −3.70 | −4.94 | −2.01 |
| Relative elastic deformation work (%) | Chlorhexidine 2% | 0.6842 | 0.7895 | −0.32 | −1.64 | +0.97 |
| Relative elastic deformation work (%) | EDTA 17% | No data | No data | No data | No data | No data |
| Relative elastic deformation work (%) | Lavasept 0.1% | 0.1903 | 0.3816 | −1.40 | −2.99 | +0.52 |
| Relative elastic deformation work (%) | Lavasept 0.2% | 0.0355 | 0.2660 | −1.48 | −3.05 | −0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabadze, Z.; Generalova, Y.; Mordanov, O. Effect of a Polyhexanide-Based Antiseptic Composition on Dentin Microhardness and Mechanical Properties: An In Vitro Study. Materials 2025, 18, 2900. https://doi.org/10.3390/ma18122900
Khabadze Z, Generalova Y, Mordanov O. Effect of a Polyhexanide-Based Antiseptic Composition on Dentin Microhardness and Mechanical Properties: An In Vitro Study. Materials. 2025; 18(12):2900. https://doi.org/10.3390/ma18122900
Chicago/Turabian StyleKhabadze, Zurab, Yulia Generalova, and Oleg Mordanov. 2025. "Effect of a Polyhexanide-Based Antiseptic Composition on Dentin Microhardness and Mechanical Properties: An In Vitro Study" Materials 18, no. 12: 2900. https://doi.org/10.3390/ma18122900
APA StyleKhabadze, Z., Generalova, Y., & Mordanov, O. (2025). Effect of a Polyhexanide-Based Antiseptic Composition on Dentin Microhardness and Mechanical Properties: An In Vitro Study. Materials, 18(12), 2900. https://doi.org/10.3390/ma18122900






