Understanding the Synthesis of Turbostratic/Flash Graphene via Joule Heating
Abstract
1. A Brief History of Graphene and Its Structure
2. Joule Effect and Heating: History and Applicability
3. How Does It Work and How It Is Applied
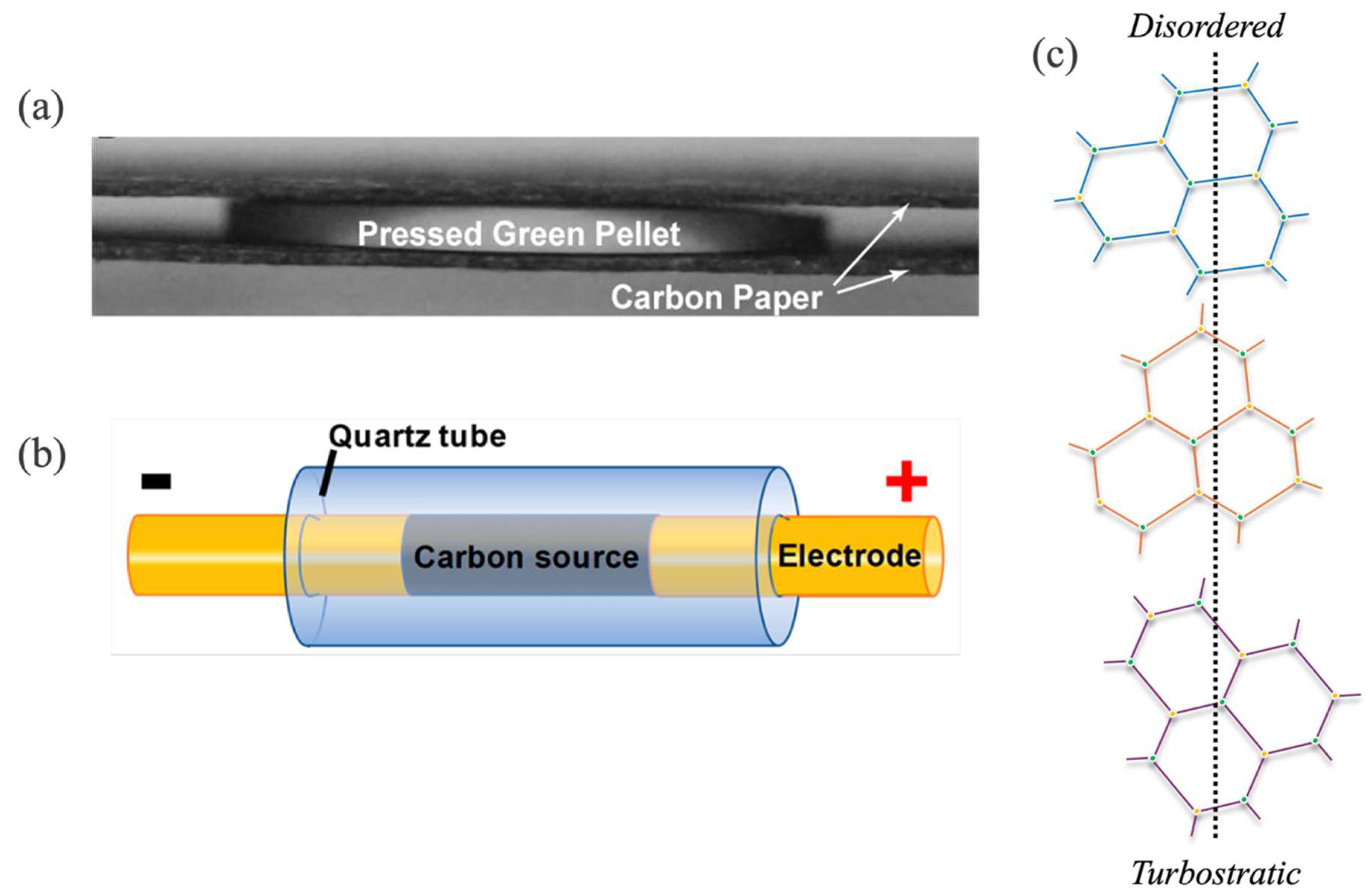
3.1. Plate-like Electrode Configuration
3.2. Rod-like Electrode Configuration
4. Characterizing Turbostratic/Flash Graphene
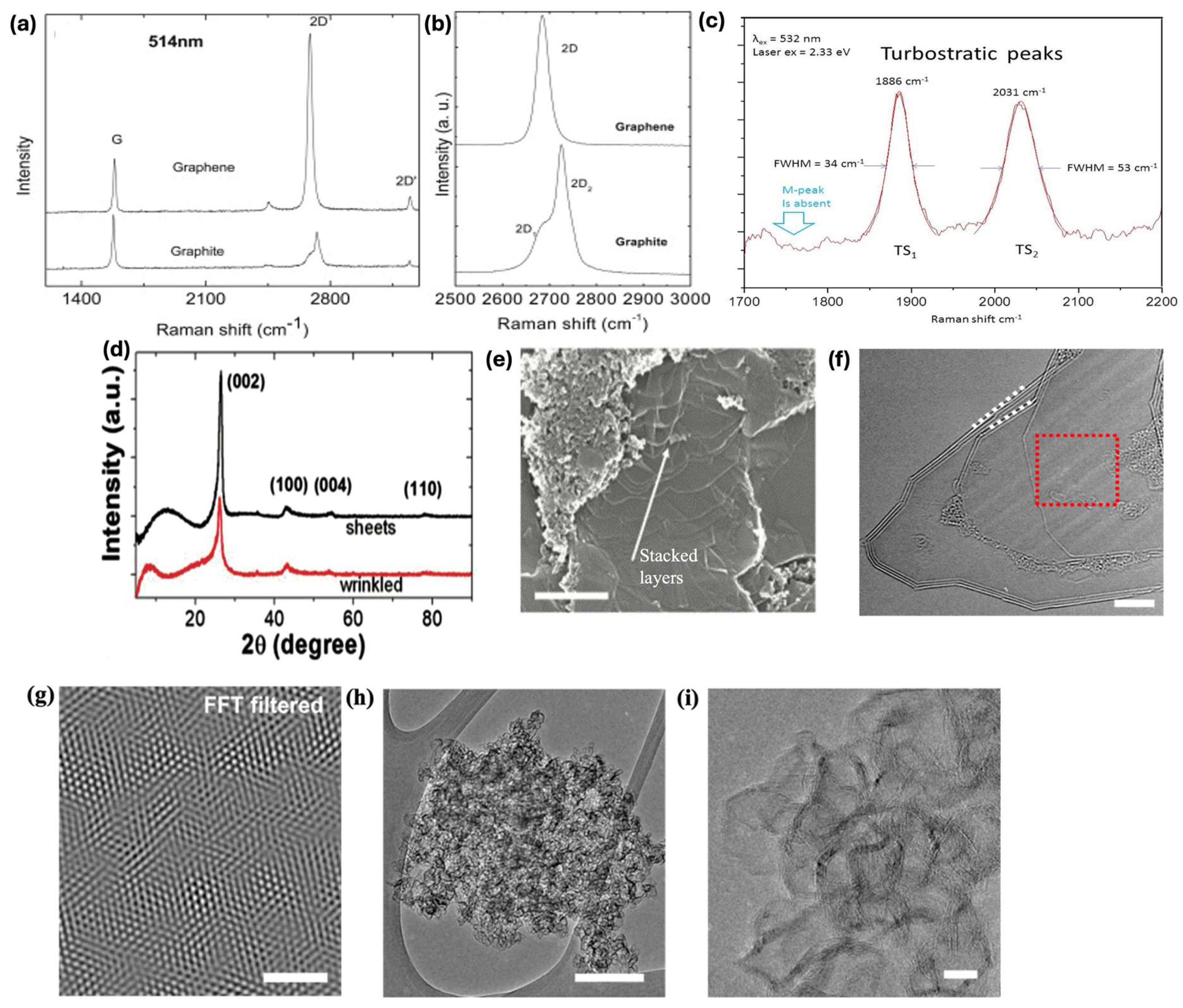
4.1. Raman Spectroscopy
4.2. X-Ray Diffraction (XRD)
4.3. Transmission Emission Microscopy (TEM)
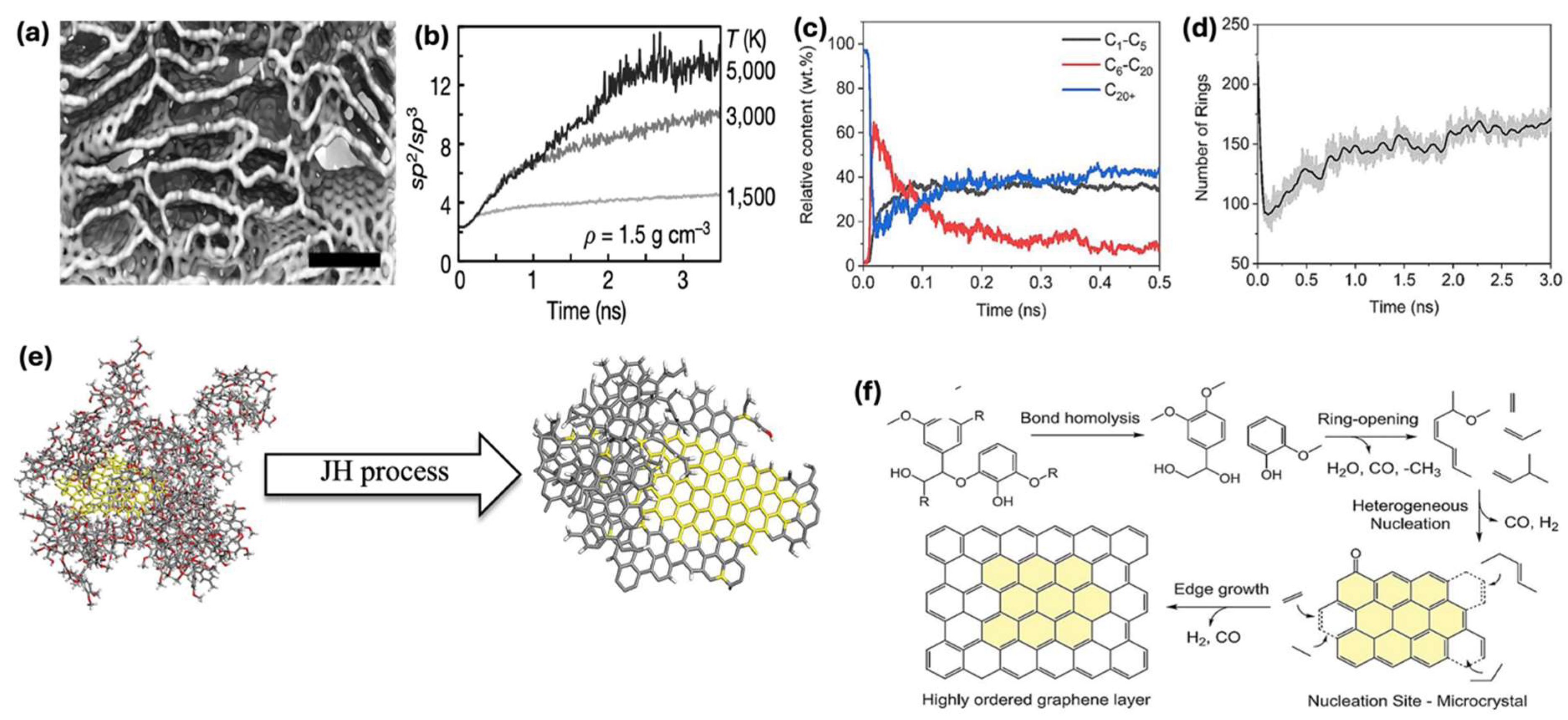
5. How Turbostratic Graphene Is Synthesized (Mechanism)
5.1. Simulation and Experimental Insights
5.2. Structural Transformation of Amorphous Carbon to Turbostratic Graphene
6. Perspective and Future Recommendations
Funding
Data Availability Statement
Conflicts of Interest
References
- Hass, J.; De Heer, W.A.; Conrad, E.H. The Growth and Morphology of Epitaxial Multilayer Graphene. J. Phys. Condens. Matter 2008, 20, 323202. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Cui, H.; Zhou, Z. Bi-Layer Graphene: Structure, Properties, Preparation and Prospects. Curr. Graphene Sci. 2018, 2, 97–105. [Google Scholar] [CrossRef]
- Shan, Y.; Li, Y.; Huang, D.; Tong, Q.; Yao, W.; Liu, W.T.; Wu, S. Stacking Symmetry Governed Second Harmonic Generation in Graphene Trilayers. Sci. Adv. 2018, 4, eaat0074. [Google Scholar] [CrossRef] [PubMed]
- Wyss, K.M.; Luong, D.X.; Tour, J.M. Large-Scale Syntheses of 2D Materials: Flash Joule Heating and Other Methods. Adv. Mater. 2022, 34, 2106970. [Google Scholar] [CrossRef]
- Mahmood, F.; Ashraf, S.; Shahzad, M.; Li, B.; Asghar, F.; Amjad, W.; Omar, M.M. Graphene Synthesis from Organic Substrates: A Review. Ind. Eng. Chem. Res. 2023, 62, 17314–17327. [Google Scholar] [CrossRef]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2022, 75, 614–630. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Chen, F.; Gu, Z. An Overview of Joule Heating in Energy Storage Materials and Applications. J. Mater. Chem. C Mater. 2024, 12, 14729–14753. [Google Scholar] [CrossRef]
- Wu, D.N.; Sheng, J.; Lu, H.G.; Li, S.D.; Li, Y. Mass Production of Graphene Using High-Power Rapid Joule Heating Method. Chem. Eng. J. 2025, 505, 159725. [Google Scholar] [CrossRef]
- Zafar, M.A.; Liu, Y.; Hernandez, F.C.R.; Varghese, O.K.; Jacob, M.V. Plasma-Based Synthesis of Freestanding Graphene from a Natural Resource for Sensing Application. Adv. Mater. Interfaces 2023, 10, 2202399. [Google Scholar] [CrossRef]
- Zafar, M.A.; Jacob, M.V. Instant Upcycling of Microplastics into Graphene and Its Environmental Application. Small Sci. 2024, 4, 2400176. [Google Scholar] [CrossRef]
- Zafar, M.A.; Jacob, M.V. Plasma-Based Synthesis of Graphene and Applications: A Focused Review. Rev. Mod. Plasma Phys. 2022, 6, 37. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards Wafer-Size Graphene Layers by Atmospheric Pressure Graphitization of Silicon Carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Dato, A.; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-Free Gas-Phase Synthesis of Graphene Sheets. Nano Lett. 2008, 8, 2012–2016. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-Scale Bottom-up Flash Graphene Synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Joule, J.P. On the Mechanical Equivalent of Heat. Abstr. Pap. Commun. R. Soc. Lond. 1851, 5, 839. [Google Scholar] [CrossRef]
- Guralnik, B.; Hansen, O.; Henrichsen, H.H.; Beltrán-Pitarch, B.; Østerberg, F.W.; Shiv, L.; Marangoni, T.A.; Stilling-Andersen, A.R.; Cagliani, A.; Hansen, M.F.; et al. 3ω Correction Method for Eliminating Resistance Measurement Error Due to Joule Heating. Rev. Sci. Instrum. 2021, 92, 94711. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X. Joule Heating in Electrokinetic Flow. Electrophoresis 2008, 29, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.K.; Villas-Boas, V. Nanocrystalline Magnetic Materials Obtained by Flash Annealing. Mater. Res. 1999, 2, 67–73. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A General Method to Synthesize and Sinter Bulk Ceramics in Seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef]
- Yang, S.; Yang, S.; Pang, R.; Zhao, X.; Fan, L.; Zhang, M.; An, L. Densification Behaviors of Al2O3 Ceramics during Flash Sintering. Int. J. Appl. Ceram. Technol. 2023, 20, 306–312. [Google Scholar] [CrossRef]
- Deng, B.; Luong, D.X.; Wang, Z.; Kittrell, C.; McHugh, E.A.; Tour, J.M. Urban Mining by Flash Joule Heating. Nat. Commun. 2021, 12, 5794. [Google Scholar] [CrossRef]
- Cologna, M.; Rashkova, B.; Raj, R. Flash Sintering of Nanograin Zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, C.; Dou, S.; Yuan, Q.; Xu, J.; Liu, W.-D.; Chen, Y.; Liu, Z.; Duan, C.; Dou, S.; et al. Ultrafast Porous Carbon Activation Promises High-Energy Density Supercapacitors. Small 2022, 18, 2200954. [Google Scholar] [CrossRef]
- Yu, J.; Ding, H.; Chen, B.; Sun, X.; Zhang, Y.; Zhou, Z. Changes in Current Transport and Regulation of the Microstructure of Graphene/Polyimide Films under Joule Heating Treatment. Materials 2024, 17, 2540. [Google Scholar] [CrossRef]
- Evseev, Z.I.; Prokopiev, A.R.; Dmitriev, P.S.; Loskin, N.N.; Popov, D.N. Fast Joule Heating for the Scalable and Green Production of Graphene with a High Surface Area. Materials 2024, 17, 576. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Mannering, J.; Huang, P.; Xu, Y.; Li, Q.; Li, H.; Qin, Y.; Kulak, A.N.; Menzel, R. Electrothermal Transformations within Graphene-Based Aerogels through High-Temperature Flash Joule Heating. J. Am. Chem. Soc. 2024, 146, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Upama, S.; Mikhalchan, A.; Arévalo, L.; Rana, M.; Pendashteh, A.; Green, M.J.; Vilatela, J.J. Processing of Composite Electrodes of Carbon Nanotube Fabrics and Inorganic Matrices via Rapid Joule Heating. ACS Appl. Mater. Interfaces 2023, 15, 5590–5599. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.G.; Bets, K.V.; Luong, D.X.; Advincula, P.A.; Chen, W.; Li, J.T.; Wang, Z.; McHugh, E.A.; Algozeeb, W.A.; Yakobson, B.I.; et al. Flash Graphene Morphologies. ACS Nano 2020, 14, 13691–13699. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, J.; Guo, Z.; Liu, Z.; Duan, C.; Dou, S.; Yuan, Q.; Liu, P.; Ji, K.; Zeng, C.; et al. Ultrafast Manufacturing of Ultrafine Structure to Achieve An Energy Density of Over 120 Wh Kg−1 in Supercapacitors. Adv. Energy Mater. 2023, 13, 2203061. [Google Scholar] [CrossRef]
- Eddy, L.; Xu, S.; Liu, C.; Scotland, P.; Chen, W.; Beckham, J.L.; Damasceno, B.; Choi, C.H.; Silva, K.; Lathem, A.; et al. Electric Field Effects in Flash Joule Heating Synthesis. J. Am. Chem. Soc. 2024, 146, 16010–16019. [Google Scholar] [CrossRef]
- Mahmood, F.; Mahmood, F.; Zhang, H.; Lin, J.; Wan, C. Laser-Induced Graphene Derived from Kraft Lignin for Flexible Supercapacitors. ACS Omega 2020, 5, 14611–14618. [Google Scholar] [CrossRef]
- Mahmood, F.; Zhang, C.; Xie, Y.; Stalla, D.; Lin, J.; Wan, C. Transforming Lignin into Porous Graphene via Direct Laser Writing for Solid-State Supercapacitors. RSC Adv. 2019, 9, 22713–22720. [Google Scholar] [CrossRef]
- Mahmood, F.; Sun, Y.; Wan, C. Biomass-Derived Porous Graphene for Electrochemical Sensing of Dopamine. RSC Adv. 2021, 11, 15410–15415. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T.L. Probing Layer Number and Stacking Order of Few-Layer Graphene by Raman Spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron–Phonon Coupling, Doping and Nonadiabatic Effects. Solid. State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Jorio, A.; Cançado, L.G. Raman Spectroscopy of Twisted Bilayer Graphene. Solid. State Commun. 2013, 175–176, 3–12. [Google Scholar] [CrossRef]
- Garlow, J.A.; Barrett, L.K.; Wu, L.; Kisslinger, K.; Zhu, Y.; Pulecio, J.F. Large-Area Growth of Turbostratic Graphene on Ni(111) via Physical Vapor Deposition. Sci. Rep. 2016, 6, 19804. [Google Scholar] [CrossRef]
- Lenski, D.R.; Fuhrer, M.S. Raman and Optical Characterization of Multilayer Turbostratic Graphene Grown via Chemical Vapor Deposition. J. Appl. Phys. 2011, 110, 013720. [Google Scholar] [CrossRef]
- Rehman, S.K.U.; Kumarova, S.; Memon, S.A.; Javed, M.F.; Jameel, M. A Review of Microscale, Rheological, Mechanical, Thermoelectrical and Piezoresistive Properties of Graphene Based Cement Composite. Nanomaterials 2020, 10, 2076. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-Ray Diffraction Patterns of Graphite and Turbostratic Carbon. Carbon. N. Y. 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Fujimoto, H. Theoretical X-Ray Scattering Intensity of Carbons with Turbostratic Stacking and AB Stacking Structures. Carbon. N. Y. 2003, 41, 1585–1592. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulova, V.; Dresselhaus, M.S.; Jing, K. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Dong, Z.; Jiang, H.; Chen, L.; Yang, H.; Li, T.; Yang, S.; Hua, D.; Shao, J.; Yu, J. Flash Joule Heating-Driven Lignin Conversion: Pyrolysis Mechanisms and Applications of Graphitic Carbon. Chem. Eng. J. 2025, 504, 158813. [Google Scholar] [CrossRef]
- Harris, P.J.F.; Tsang, S.C. High-Resolution Electron Microscopy Studies of Non-Graphitizing Carbons. Philos. Mag. A 1997, 76, 667–677. [Google Scholar] [CrossRef]
- Harris, P.J.F. New Perspectives on the Structure of Graphitic Carbons. Crit. Rev. Solid State Mater. Sci. 2005, 30, 235–253. [Google Scholar] [CrossRef]

| Method | Product | Layer Control | Scalability | Cost | Quality | Ref. |
|---|---|---|---|---|---|---|
| Chemical Vapor Deposition (CVD) | Thin films | Excellent (mono–few-layer) | Moderate | High | High (electronic grade) | [12] |
| Epitaxial Growth on SiC | Thin films | Good (few-layer) | Low | Very high | High | [13] |
| Laser-Induced Graphene (LIG) | Thin films/patterns | Poor–Moderate (multi-layer) | Moderate–High | Low–Moderate | Moderate–High | [14] |
| Spray/Spin Coating (from Graphene oxide (GO)/reduced GO (rGO) | Thin films | Poor (multi-layer) | High | Low | Low–Moderate | [15] |
| Liquid Phase Exfoliation (LPE) | Bulk | Poor (multi-layer) | High | Low | Moderate | [16] |
| Electrochemical Exfoliation | Bulk | Poor (few–multi-layer) | High | Low | Moderate | [17] |
| Chemical Reduction of GO | Bulk | Poor (multi-layer) | High | Low | Low–Moderate | [18] |
| Atmospheric Pressure Plasma (APP) | Thin films/bulk | Good (few–multi-layer) | High | Moderate | Moderate–High | [19] |
| Joule Heating (Flash Graphene) | Bulk | Poor (multi-layer) | Moderate | Low–Moderate | Moderate–High | [20] |
| Property | Flash/ Turbostratic Graphene [20] | Chemically Derived Graphene [18] | Laser-Induced Graphene (LIG) [14] | CVD Graphene [51] | Mechanically Exfoliated Graphene [52] |
|---|---|---|---|---|---|
| Production Method | Flash Joule heating; rapid pyrolysis of carbon feedstocks | Chemical reduction of graphene oxide (GO/rGO) | Laser writing of carbon precursors | Thermal decomposition of hydrocarbons on metal substrates | Mechanical peeling of graphite |
| Layer Stacking | Disordered; rotationally misaligned (turbostratic) | Typically, multi-layer with some disorder | Multi-layer; often porous and disordered | AB-stacked (Bernal) | Single to few-layer, typically high-order |
| Interlayer Coupling | Weak (due to misalignment) | Moderate to weak, varies with reduction quality | Weak–moderate due to porosity | Strong π–π interactions | Strong |
| Electrical Conductivity | High in-plane due to decoupling | Moderate (~102–103 S cm−1) | Moderate (~102–103 S cm−1) | Very high (~104 S cm−1) | Very high |
| Thermal Conductivity | High (variable with defects; up to ~2000 W m−1·K−1) | Lower (~100–500 W m−1·K−1) | Moderate–high (material-dependent) | Very high (~5000 W m−1·K−1 for monolayer) | Very high |
| Ease of Exfoliation | Easy due to weak interlayer bonding | Not applicable (not exfoliated) | Not applicable (direct-write process) | Hard (monolayer already formed on substrate) | Moderate (labor-intensive) |
| Crystallinity | Moderate; more defects than pristine graphene | Moderate; defect sites remain after reduction | Moderate; influenced by laser parameters | High; nearly defect-free in optimized systems | High |
| Cost and Scalability | Low cost; highly scalable from waste/biomass | Low cost; highly scalable | Low–moderate cost; highly scalable | High cost; moderate scalability due to CVD equipment | High cost; limited scalability |
| Applications | Batteries, supercapacitors, EMI shielding, composites | Flexible electronics, inks, coatings | Sensors, energy storage, antimicrobial coatings | High-performance electronics, sensors, flexible displays | Quantum devices, metrology standards |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, F.; Mbeugang, C.F.M.; Asghar, F.; Xie, X.; Lin, D.; Liu, D.; Li, B. Understanding the Synthesis of Turbostratic/Flash Graphene via Joule Heating. Materials 2025, 18, 2892. https://doi.org/10.3390/ma18122892
Mahmood F, Mbeugang CFM, Asghar F, Xie X, Lin D, Liu D, Li B. Understanding the Synthesis of Turbostratic/Flash Graphene via Joule Heating. Materials. 2025; 18(12):2892. https://doi.org/10.3390/ma18122892
Chicago/Turabian StyleMahmood, Faisal, Christian Fabrice Magoua Mbeugang, Furqan Asghar, Xing Xie, Dan Lin, Dongjing Liu, and Bin Li. 2025. "Understanding the Synthesis of Turbostratic/Flash Graphene via Joule Heating" Materials 18, no. 12: 2892. https://doi.org/10.3390/ma18122892
APA StyleMahmood, F., Mbeugang, C. F. M., Asghar, F., Xie, X., Lin, D., Liu, D., & Li, B. (2025). Understanding the Synthesis of Turbostratic/Flash Graphene via Joule Heating. Materials, 18(12), 2892. https://doi.org/10.3390/ma18122892








