1. Introduction
Concrete structures in service in marine environments are subjected to salt erosion and pressure, making the problem of substantial durability failure more prominent. Under the combined effects of water pressure and load, cracks are more likely to occur inside the concrete structure, thus aggravating the water seepage problem. Groundwater in coastal areas has high chloride salt concentrations. Chloride ions enter the concrete through concrete cracks. The hydration products of the cement chemical reaction generate calcium trichloroaluminate and other expansive substances, causing the concrete to lose and destroy the passivation film on the surface of the steel reinforcement, leading to rapid corrosion of the steel reinforcement and resulting in the rapid deterioration of the concrete structure.
In recent years, scholars have conducted numerous theoretical and engineering studies on chloride transport in concrete structures in marine environments. Liu established a simplified formula for the quantitative index of the chloride ion permeability of concrete based on the cumulative chloride ion content, considering the temperature and dry–wet cycles, two typical marine environmental factors [
1]. Xiu et al. summarized the experimental methods for exploring chloride ion erosion in concrete in a marine salt spray environment, discussed the factors affecting chloride ion transport, and generalized a chloride ion transport model [
2]. Zhao and Gai summarized the chloride ion erosion process of static and repeated loads, proposed a chloride ion transport mechanism in concrete under load, and illustrated the practical value of the erosion model for the structural durability of concrete bridges subjected to chloride salt erosion [
3]. Ma et al. studied the transport characteristics of chloride ions at different hydrostatic pressures, mixing ratios, and pressure-action times, and found that under hydrostatic pressure, the chloride penetration depth and content increased with increasing hydrostatic pressure and action time [
4]. Liu et al. deduced a chloride diffusion erosion model of concrete considering linearly distributed stresses and proved that the established chloride erosion model could reflect the effect of linearly distributed stresses on chloride ion diffusion in the structural cross section [
5]. Jin and Wang investigated the diffusion of chloride ions in the protective layer of concrete and found that, at the same depth of the protective layer, as the load increases, the chloride ion content increases, and the corrosion rate of the reinforcement inside the concrete also increases [
6]. Ribeiro et al. conducted chloride erosion tests by varying the concrete water–cement ratio, cement type, mortar content, and the amount of mineral additives. The chloride diffusion coefficients were corrected based on test data to improve the accuracy of the existing model [
7].
Yu and Lin established a mesoscale finite element model for chloride ion transport within recycled concrete and analyzed the effects of factors such as the shape, location, substitution rate of recycled aggregate, and adhesion rate of old mortar on the transport of chloride ions within recycled concrete [
8]. Zhu et al. investigated the diffusion of chloride ions in concrete structures under cyclic impact loading using a series of tests and concluded that cyclic impact load has a pronounced accelerating effect on chloride diffusion [
9]. Pathan et al. presented a theoretical framework for modeling the service life of reinforced concrete structures in chloride environments using load factors and showed that the nature of the load (compression or tension) can lead to different diffusion results [
10]. Kim et al. conducted field tests on in-service offshore structures and concluded that the dry/wet ratio significantly affects chloride transport in concrete, suggesting that, owing to the complexity of the marine environment, the results of exposure tests may be affected by various factors and thus exhibit randomness [
11]. Wang et al. combined the Boltzmann–Matano method with measured chloride ion concentration data to establish a predictive model for the chloride ion diffusion coefficient in concrete, which can quantitatively analyze the variation rule of the chloride ion diffusion coefficient in concrete with exposure time and diffusion depth [
12]. Leung and Hou assumed that the diffusion coefficients of chloride ions in cracks and crack widths are independent of each other and further proposed an empirical formula for the macroscopic equivalent diffusion coefficients of chloride ions in cracked concrete by simulating the diffusion process of chloride ions in concrete containing a single crack [
13].
Bentz et al. investigated the effect of transverse cracking on the diffusion of chloride ions in concrete, adapted a predictive model using a graphical approach, and combined it with experimental data on saturated concrete to determine whether cement paste mixed with chloride ions plays a vital role in slowing the entry of chloride ions into the concrete [
14]. Studies show that even small cracks (0.1–0.3 mm) can drastically increase diffusion coefficients, leading to premature corrosion initiation [
15,
16,
17]. Homan et al. investigated the effect of moisture transport on chloride penetration in partially saturated concrete and modified the governing equation that describes the chloride transport in saturated concrete to account for the chloride movement induced by the moisture transport [
18]. Alsheet et al. proposed a chloride binding model consisting of physical adsorption and chemical ion exchange and determined that chloride transport by diffusion was limited to the region near the exposed surface, while in the concrete bulk it was dominated by electromigration [
19]. From a large amount of experimental data on the diffusion of chloride ions in concrete, Mangat and Molloy confirmed that the magnitude of the chloride diffusion coefficient is highly dependent on the exposure time of the concrete [
20]. Tegguer et al. prepared microcracks by axial precompression of standard and high-performance concrete, detected the degree of concrete damage using ultrasonic pulse velocity, and detected the change in the chloride diffusion coefficient of standard and high-performance concrete after chloride erosion [
21]. Rahman et al. investigated the effects of compressive stress-induced damage on chloride ion transport in concrete. This study demonstrated that the damage associated with ballast-induced stress significantly increased the diffusion of chloride ions in concrete [
22]. Al-Kutti et al. proposed a multiphysics field formulation to increase and quantify the chloride diffusivity of concrete owing to damage, expressing the quantitative damage index in terms of the degradation of the modulus of elasticity of concrete [
23].
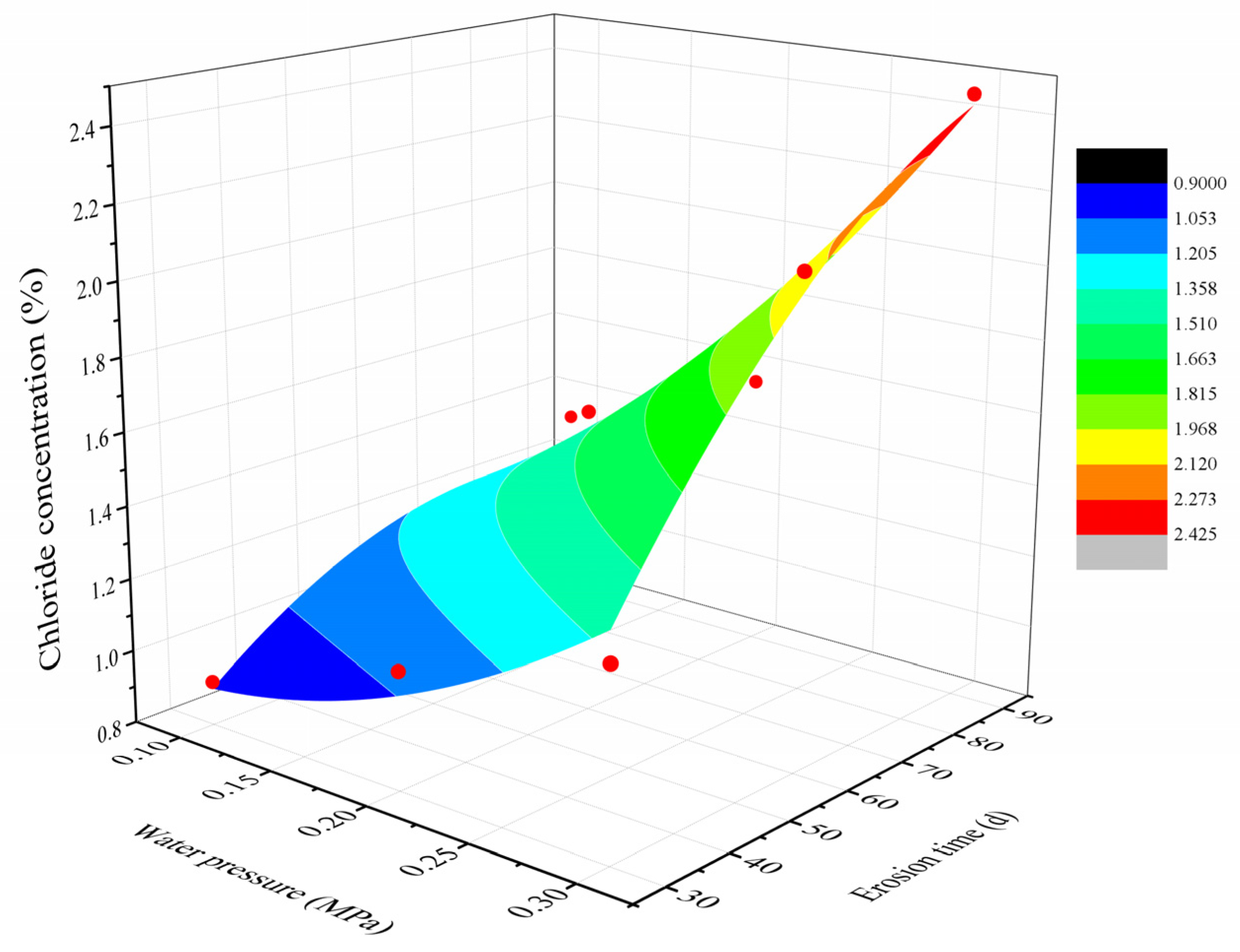
The above studies analyzed a single external factor rather than performing a comprehensive analysis of multiple factors, such as the erosion time, crack width, water pressure, and vertical load. The lack of a comparative analysis of the different influencing factors has led to certain limitations in the study of chloride erosion. Therefore, this study independently developed a set of test device systems according to the actual service state of an underwater concrete structure that were composed of a load loading device, dynamic water pressure device, and salt solution collection device. The systems could simultaneously consider the coupling effect of three factors: water pressure, vertical load, and erosion time. The aim of this study is to investigate the water pressure, vertical load, and erosion time on the chloride corrosion of concrete and establish the relationship between the chloride concentration and these factors.