Developing NiAl-Strengthened ULCB Steels by Controlling Nanoscale Precipitation and Reversed Austenite
Abstract
1. Introduction
2. Methods
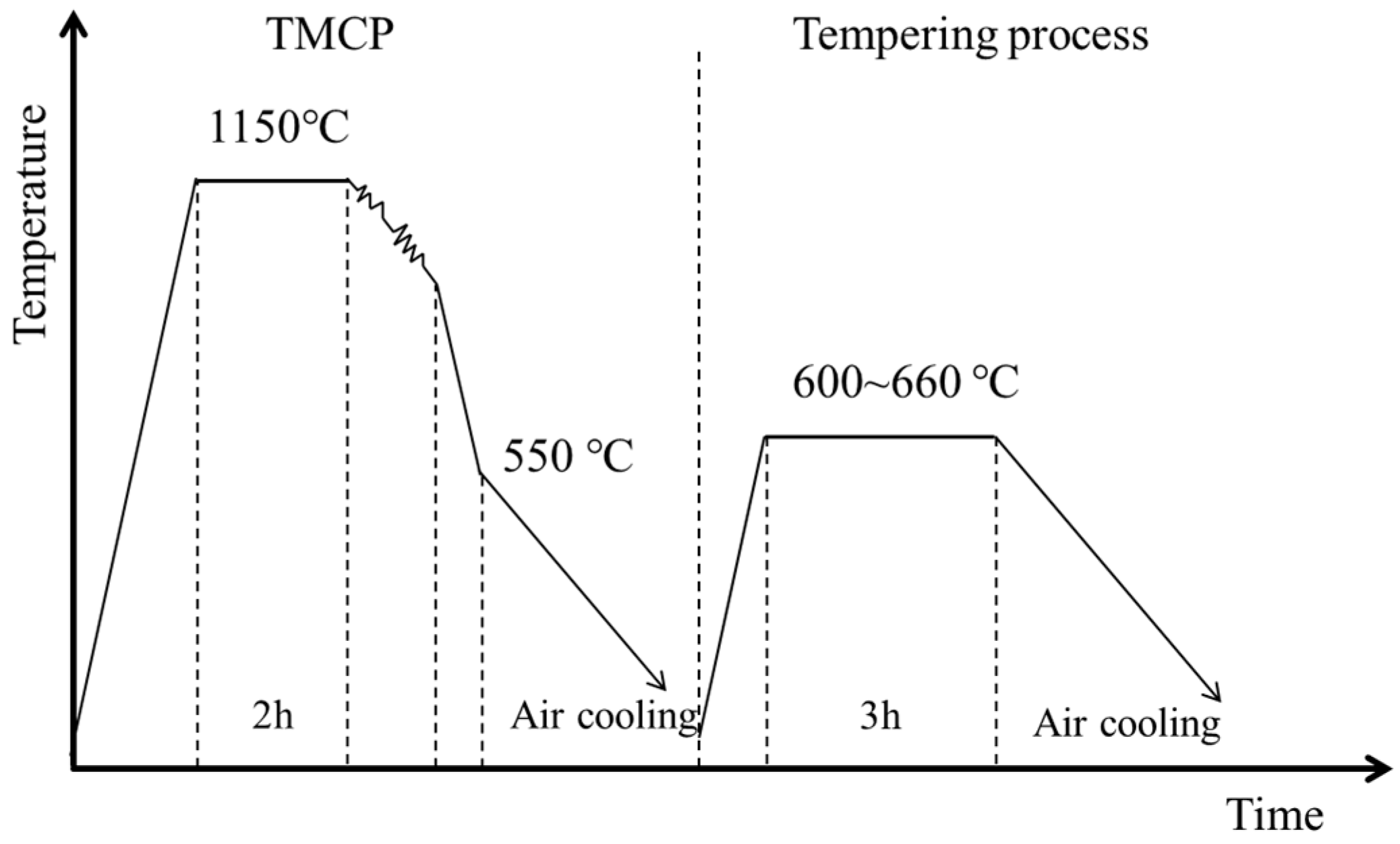
2.1. Production of the Experimental Alloy
2.2. Mechanical Characterization of Steel
2.3. Characterization of the Experimental Alloy
3. Results
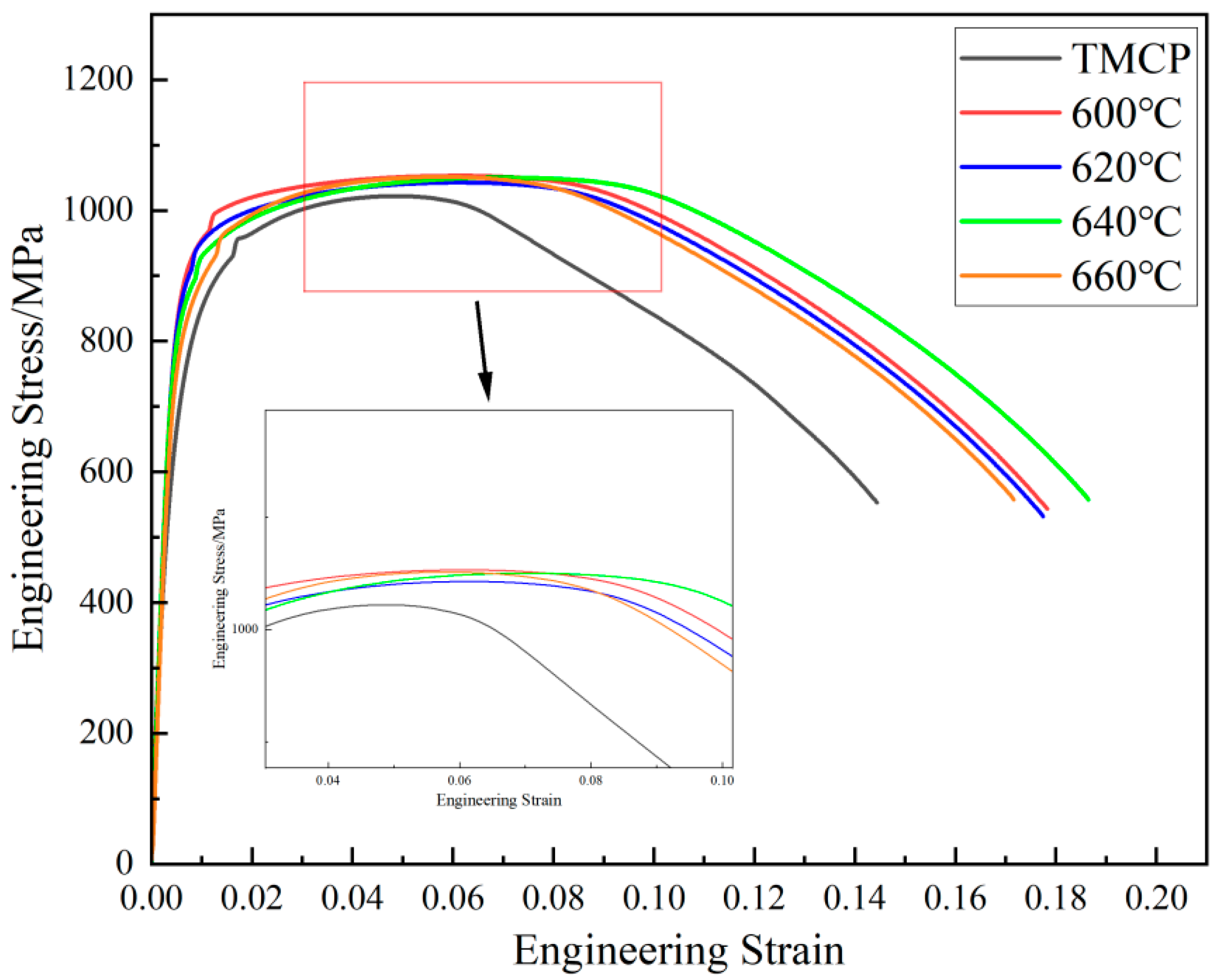
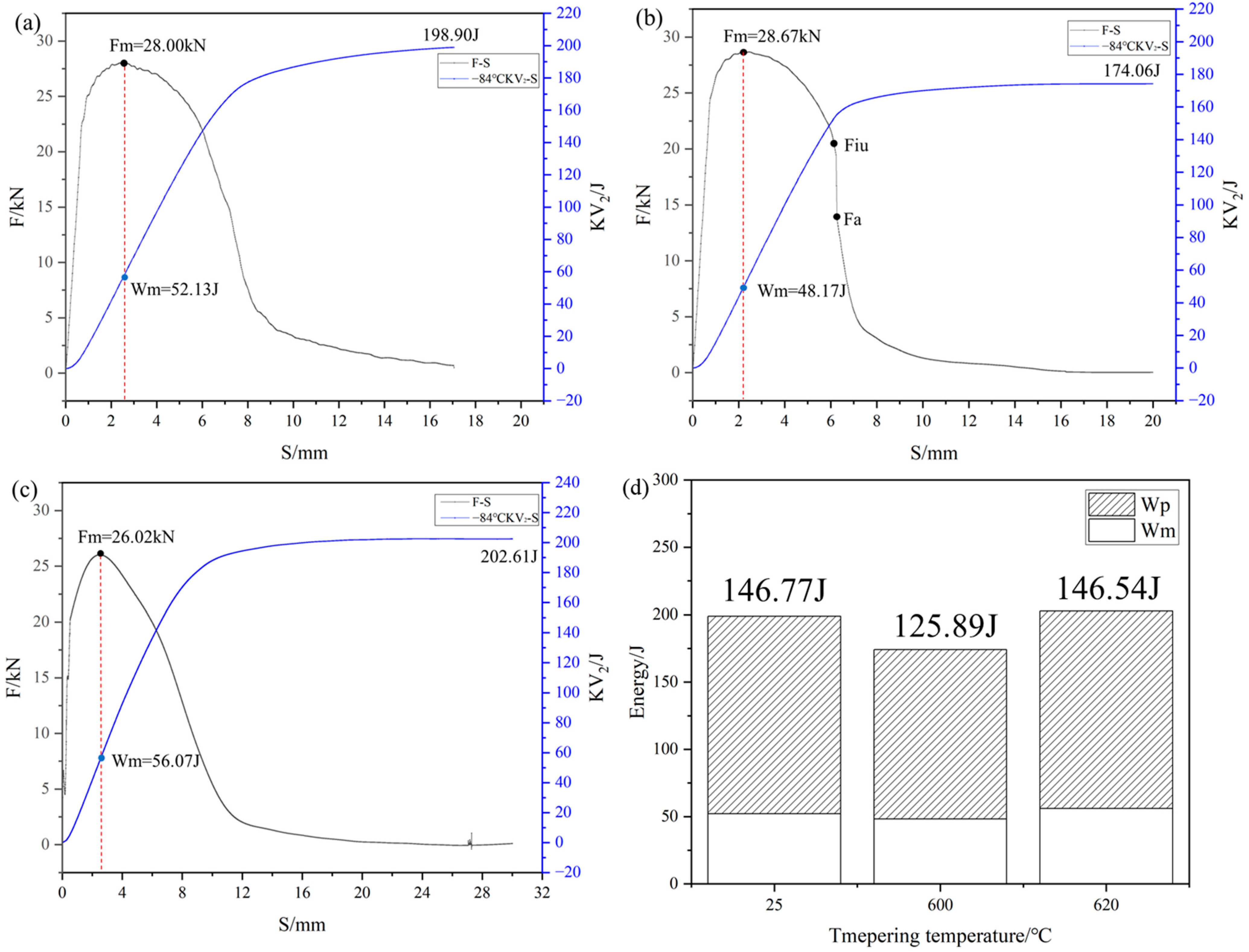
3.1. Mechanical Properties
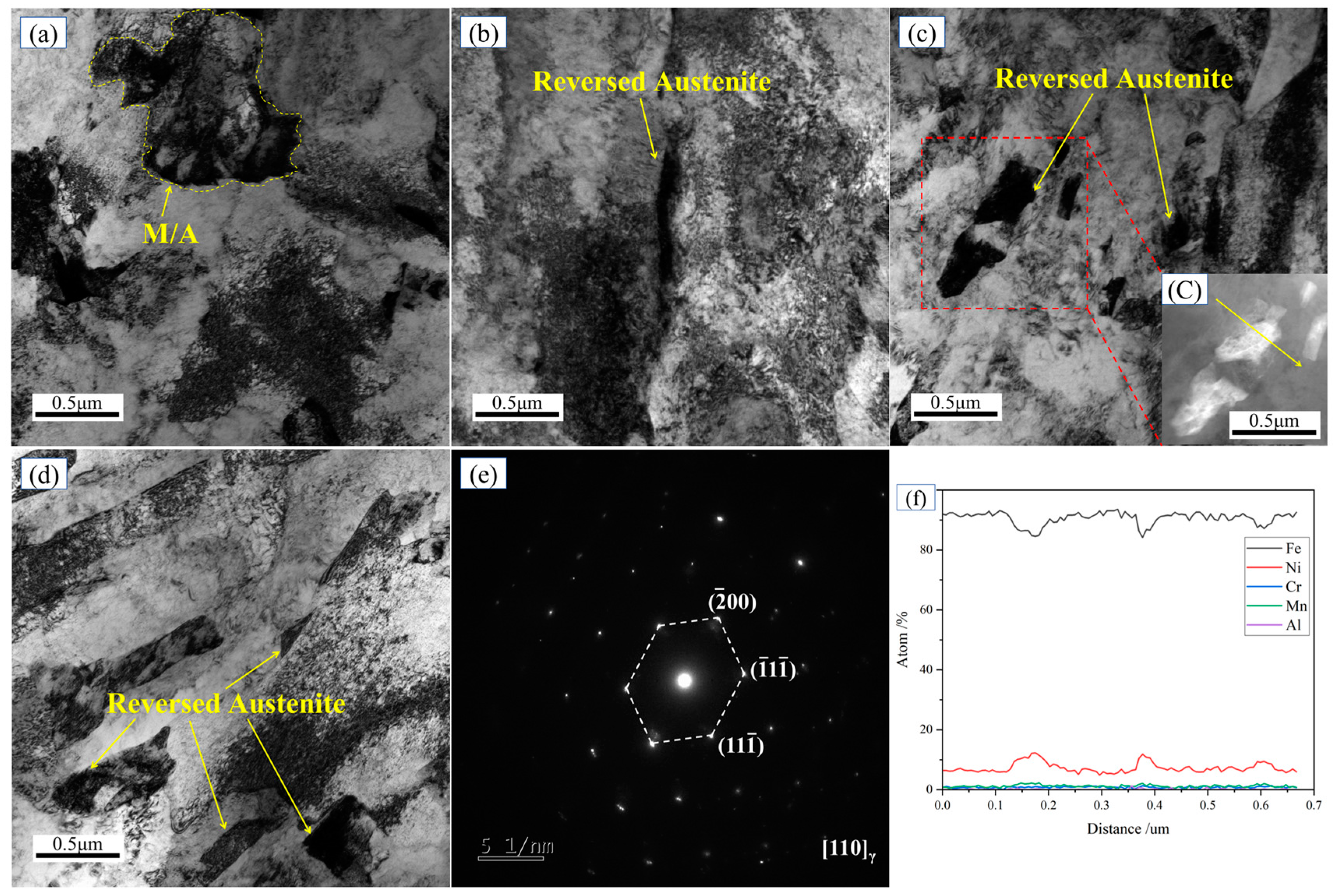
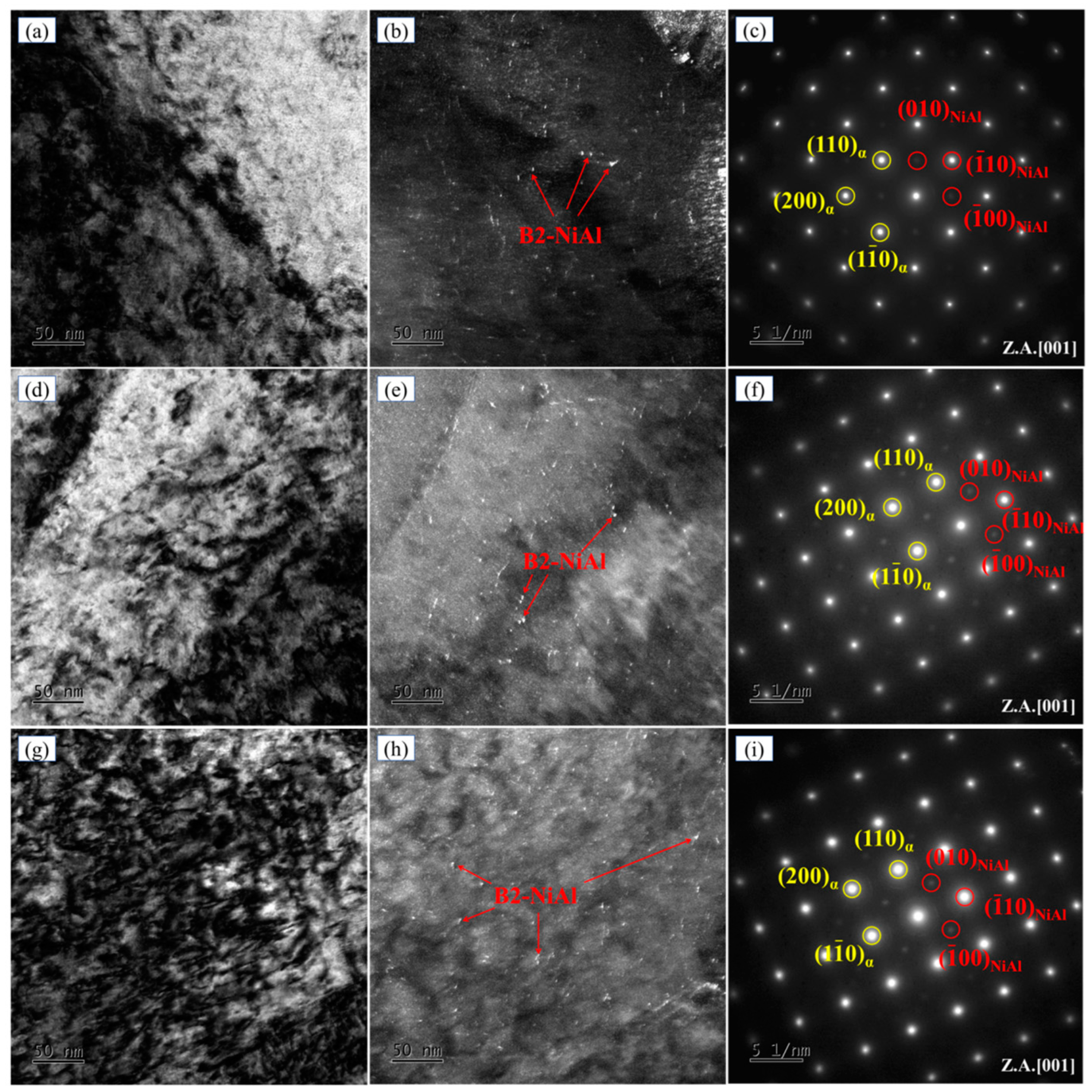
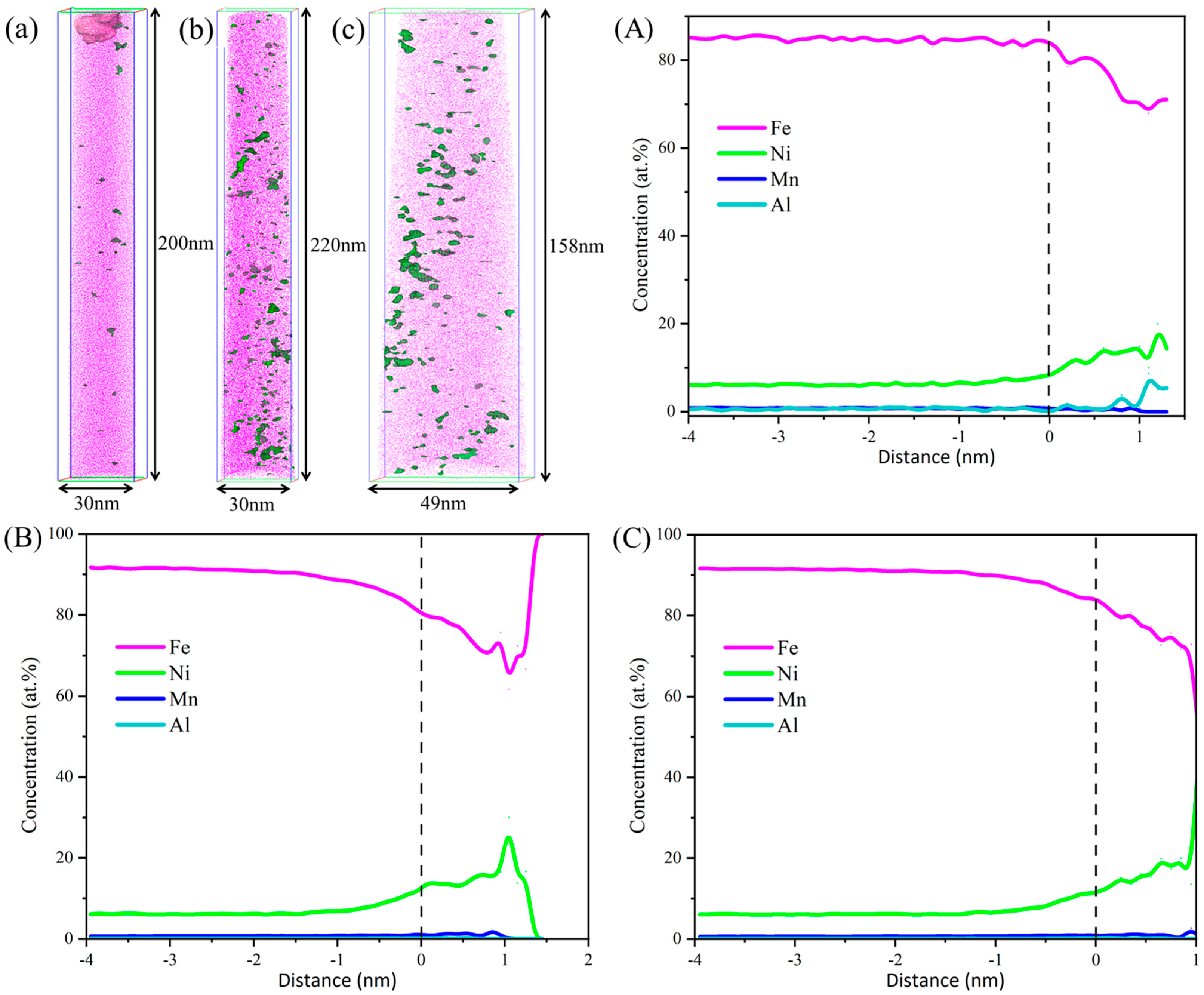
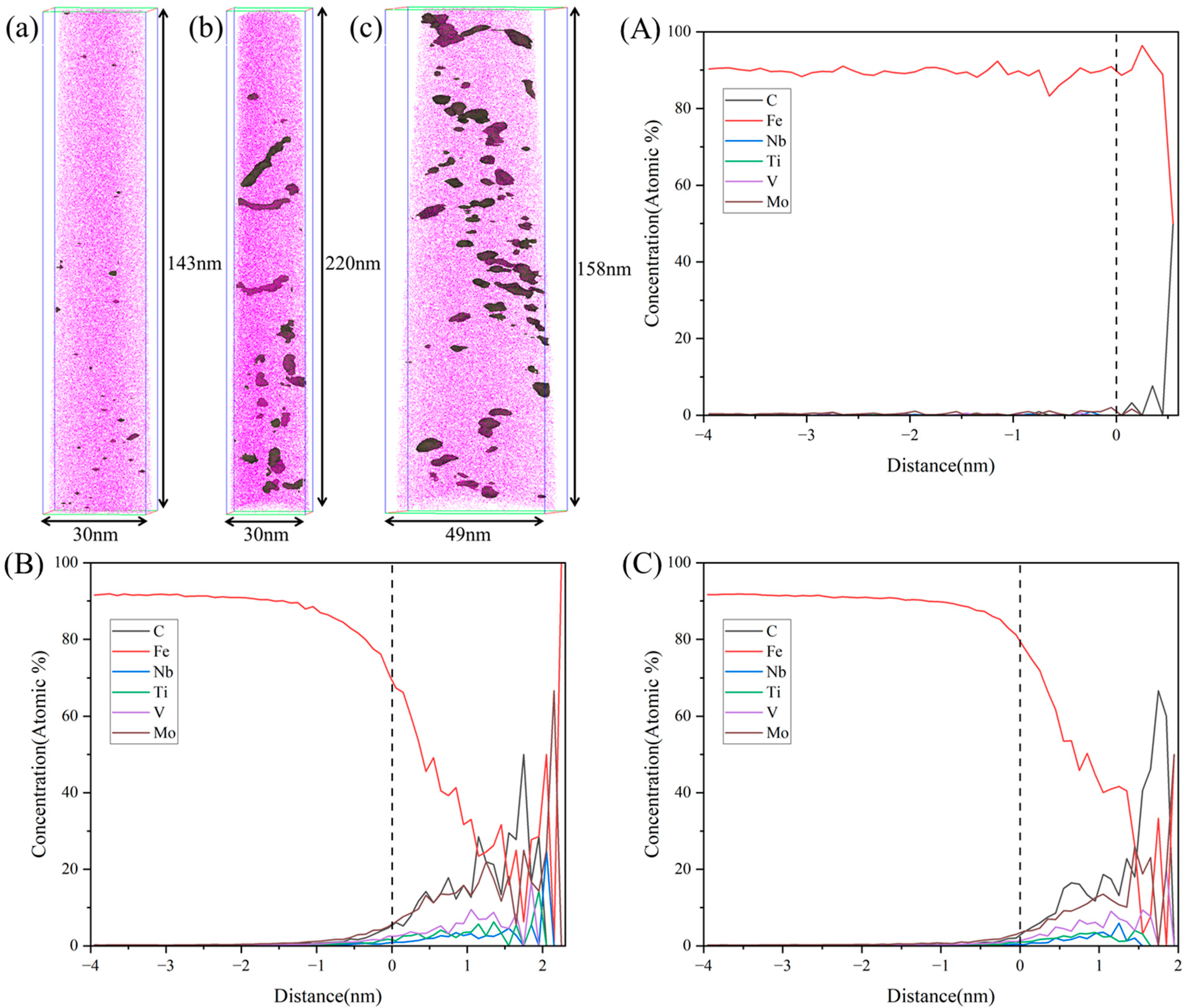
3.2. Microstructure
4. Discussion
4.1. Strengthening Model of ULCB Steel
4.2. Toughening Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zha, X.; Xiong, Y.; Zhou, T.; Ren, Y.; Hei, P.; Zhai, Z.; Kömi, J.; Huttula, M.; Cao, W. Impacts of Stress Relief Treatments on Microstructure, Mechanical and Corrosion Properties of Metal Active-Gas Welding Joint of 2205 Duplex Stainless Steel. Materials 2020, 13, 4272. [Google Scholar] [CrossRef] [PubMed]
- Imanian Ghazanlou, S.; Mobasher Amini, A.; Carrier, F.-A.; Sarkar, D.K.; Rehman, K.; Javidani, M. Study of the Microstructure and Mechanical Properties of Steel Grades for Ship Hull Construction. Materials 2024, 17, 5687. [Google Scholar] [CrossRef]
- Yu, L.; Guo, W.; Cao, C.; Li, M.; Wu, Z.; Wang, T.; Chen, H.; Pan, X. Experimental Study on the Fatigue Crack Propagation Rate of 925A Steel for a Ship Rudder System. Materials 2024, 17, 1808. [Google Scholar] [CrossRef]
- Hao, X. Effect of Isothermal Quenching near Ms Point on Microstructure and Properties of Medium-Low Carbon Steel. Master’s Thesis, Hebei University of Engineering, Handan, China, 2022. [Google Scholar]
- Su, C. Study on Microstructure and Plasticity-Toughness of 1000 MPa Grade Hot-Rolled Ultra-High Strength Steel. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2022. [Google Scholar]
- Han, C.; Liu, Q.; Cai, Z.; Sun, Q.; Huo, X.; Fan, M.; He, Y.; Li, K.; Pan, J. Effect of tempering heat treatment on the microstructure and impact toughness of a Ni-Cr-Mo-V steel weld metal. Mater. Sci. Eng. A Struct. Mater. Prop. Misrostructure Process. 2022, 850, 143521. [Google Scholar] [CrossRef]
- Li, Z.; Chai, F.; Luo, X.; Zhang, Z.; Yang, C.; Su, H. Effect of aging temperature on mechanical properties of Cu precipitation strengthened ultra-high strength marine steel. Mater. Rep. 2020, 34, 6132–6137. [Google Scholar]
- Li, Z.; Chai, F.; Yang, C.; Luo, X.; Yang, L.; Su, H. Effect of quenching process on mechanical properties of UHS marine steel. Chin. J. Mater. Res. 2018, 32, 889–897. [Google Scholar]
- Xiao, Y.; Peng, B.; Fan, A.; Jiang, S.; Wang, X. Complex precipitation behavior and strengthening mechanisms of Fe-Ni-Al ultra-high strength dual-phase steel. J. Iron Steel Res. 2023, 35, 873–880. [Google Scholar] [CrossRef]
- Jiao, Z.; Liu, J. Research and development of novel nano-strengthened ultra-high strength steels. Mater. China 2011, 30, 6–11+33. [Google Scholar]
- Gao, J.; Jiang, S.; Zhang, H.; Huang, Y.; Guan, D.; Xu, Y.; Guan, S.; Bendersky, L.A.; Davydov, A.V.; Wu, Y. Facile route to bulk ultrafine-grain steelsfor high strength and ductility. Nature 2021, 590, 262–267. [Google Scholar] [CrossRef]
- Zhang, X. Characterization of NiAl Nanoprecipitates and Strengthening-Toughening Mechanisms in Ultra-High Strength Hull Steel. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2024. [Google Scholar]
- Li, Z.; Chai, F.; Yang, L.; Luo, X.; Yang, C. Mechanical properties and nanoparticles precipitation behavior of multi-component ultra high strength steel. Mater. Des. 2020, 191, 108637. [Google Scholar] [CrossRef]
- Shikanai, N.; Kagawa, H.; Kurihara, M. Influence of Microstructure on Yielding Behavior of Heavy Gauge High Strength Steel Plates. ISIJ Int. 1992, 32, 335–342. [Google Scholar] [CrossRef][Green Version]
- Tong, M.W.; Venkatsurya, P.K.C.; Zhou, W.H.; Misra, R.D.K.; Guo, B.; Zhang, K.G.; Fan, W. Structure-mechanical property relationship in a high strength microalloyed steel with low yield ratio: The effect of tempering temperature. Mater. Sci. Eng. A 2014, 609, 209–216. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. State Administration for Market Regulation: Beijing, China, 2021.
- GB/T 229-2020; Metallic Materials—Charpy Pendulum Impact Test Method. State Administration for Market Regulation: Beijing, China, 2020.
- Miller, M.K. Atom Probe Tomography: Analysis at the Atomic Level; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Chen, J.; Lv, M.-Y.; Tang, S.; Liu, Z.-Y.; Wang, G.-D. Influence of cooling paths on microstructural characteristics and precipitation behaviors in a low carbon V-Ti microalloyed steel. Mater. Sci. Eng. A 2018, 731, 173–183. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steels; Metallurgy Industry Press: Beijing, China, 2006. [Google Scholar]
- Galindo-Nava, E.I.; Rainforth, W.M.; Rivera-Diaz-Del-Castillo, P.E.J. Predicting microstructure and strength of maraging steels:Elemental optimisation. Acta Mater. 2016, 117, 270–285. [Google Scholar] [CrossRef]
- Shibata, A.; Nagoshi, T.; Sone, M.; Morito, S.; Higo, Y. Evaluation of the block boundary and sub-block boundary strengths of ferrous lath martensite using a microbending test. Mater. Sci. Eng. A 2010, 527, 7538–7544. [Google Scholar] [CrossRef]
- Huang, M.; Rivera-Díaz-del-Castillo, P.E.J.; Bouaziz, O.; van der Zwaag, S. Modelling strength and ductility of ultrafine grained BCC and FCC alloys using irreversible thermodynamics. Mater. Sci. Technol. 2009, 25, 833–839. [Google Scholar] [CrossRef]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzaki, K.; Furuhara, T. Stress-strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta Mater. 2015, 83, 383–396. [Google Scholar] [CrossRef]
- Keh, A.S. Work hardening and deformation sub-structure in iron single crystals deformed in tension at 298k. Philos. Mag. 1965, 12, 9–30. [Google Scholar] [CrossRef]
- Zhou, T.; Faleskog, J.; Babu, R.P.; Odqvist, J.; Yu, H.; Hedström, P. Exploring the relationship between the microstructure and strength of fresh and tempered martensite in a maraging stainless steel Fe-15Cr-5Ni. Mater. Sci. Eng. A 2019, 745, 420–428. [Google Scholar] [CrossRef]
- Xu, S.S.; Zhao, Y.; Chen, D.; Sun, L.W.; Chen, L.; Tong, X.; Liu, C.T.; Zhang, Z.W. Nanoscale precipitation and its influence on strengthening mechanisms in an ultra-high strength low-carbon steel. Int. J. Plast. 2019, 113, 99–110. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Yu, C.Y.; Liu, C.T. Effects of Mn partitioning on nanoscale precipitation and mechanical properties of ferritic steels strengthened by NiAl nanoparticles. Acta Mater. 2015, 84, 283–291. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Zhang, Z.W.; Miller, M.K.; Liu, C.T. High-strength steels hardened mainly by nanoscale NiAl precipitates. Scr. Mater. 2014, 87, 45–48. [Google Scholar] [CrossRef]



| Element | C | Al | Si | Mn | Ni | Cr | Mo | V | Nb | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.044 | 0.24 | 0.21 | 0.68 | 7.10 | 0.51 | 0.61 | 0.061 | 0.043 | 0.020 | Bal. |
| at.% | 0.204 | 0.496 | 0.417 | 0.691 | 6.751 | 0.547 | 0.355 | 0.067 | 0.026 | 0.023 | Bal |
| States | Rp/nm | Nv/m−3 |
|---|---|---|
| TMCP | 0.67 ± 0.05 | 1.82 × 1023 |
| 600 °C 3 h | 0.78 ± 0.20 | 5.28 × 1024 |
| 620 °C 3 h | 0.76 ± 0.18 | 5.67 × 1023 |
| Strength (MPa) | σexperiment | σmodel | σ0 | σS | σHP | σd | σMC | σorder | σmodulus | σcoherency | σchemical |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TMCP | 697.0 | 701.8 | 87 | 161 | 132 | 318 | 0 | 32 | 43 | 1 | 5 |
| 600 °C | 879.5 | 856 | 87 | 161 | 124 | 230 | 252 | 237 | 248 | 8 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Chai, X.; Gong, S.; Wang, Z.; Pan, T. Developing NiAl-Strengthened ULCB Steels by Controlling Nanoscale Precipitation and Reversed Austenite. Materials 2025, 18, 2822. https://doi.org/10.3390/ma18122822
Guo J, Chai X, Gong S, Wang Z, Pan T. Developing NiAl-Strengthened ULCB Steels by Controlling Nanoscale Precipitation and Reversed Austenite. Materials. 2025; 18(12):2822. https://doi.org/10.3390/ma18122822
Chicago/Turabian StyleGuo, Jize, Xiyang Chai, Shuo Gong, Zemin Wang, and Tao Pan. 2025. "Developing NiAl-Strengthened ULCB Steels by Controlling Nanoscale Precipitation and Reversed Austenite" Materials 18, no. 12: 2822. https://doi.org/10.3390/ma18122822
APA StyleGuo, J., Chai, X., Gong, S., Wang, Z., & Pan, T. (2025). Developing NiAl-Strengthened ULCB Steels by Controlling Nanoscale Precipitation and Reversed Austenite. Materials, 18(12), 2822. https://doi.org/10.3390/ma18122822






