Phase Transformation Kinetics During Post-Weld Heat Treatment in Weldments of C-250 Maraging Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Base Material
2.2. Welding
2.3. Microstructural Characterization, Local Chemical Analysis and Microhardness Profiles
2.4. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
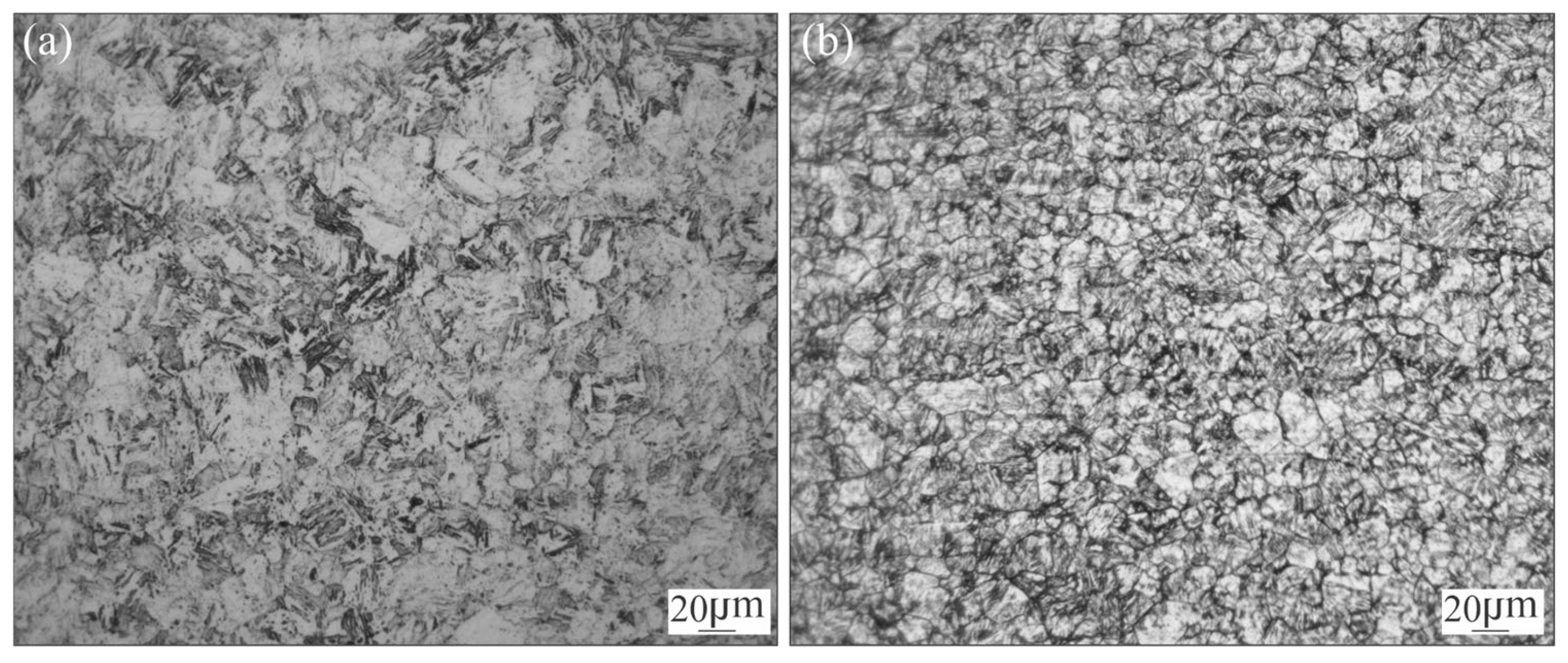
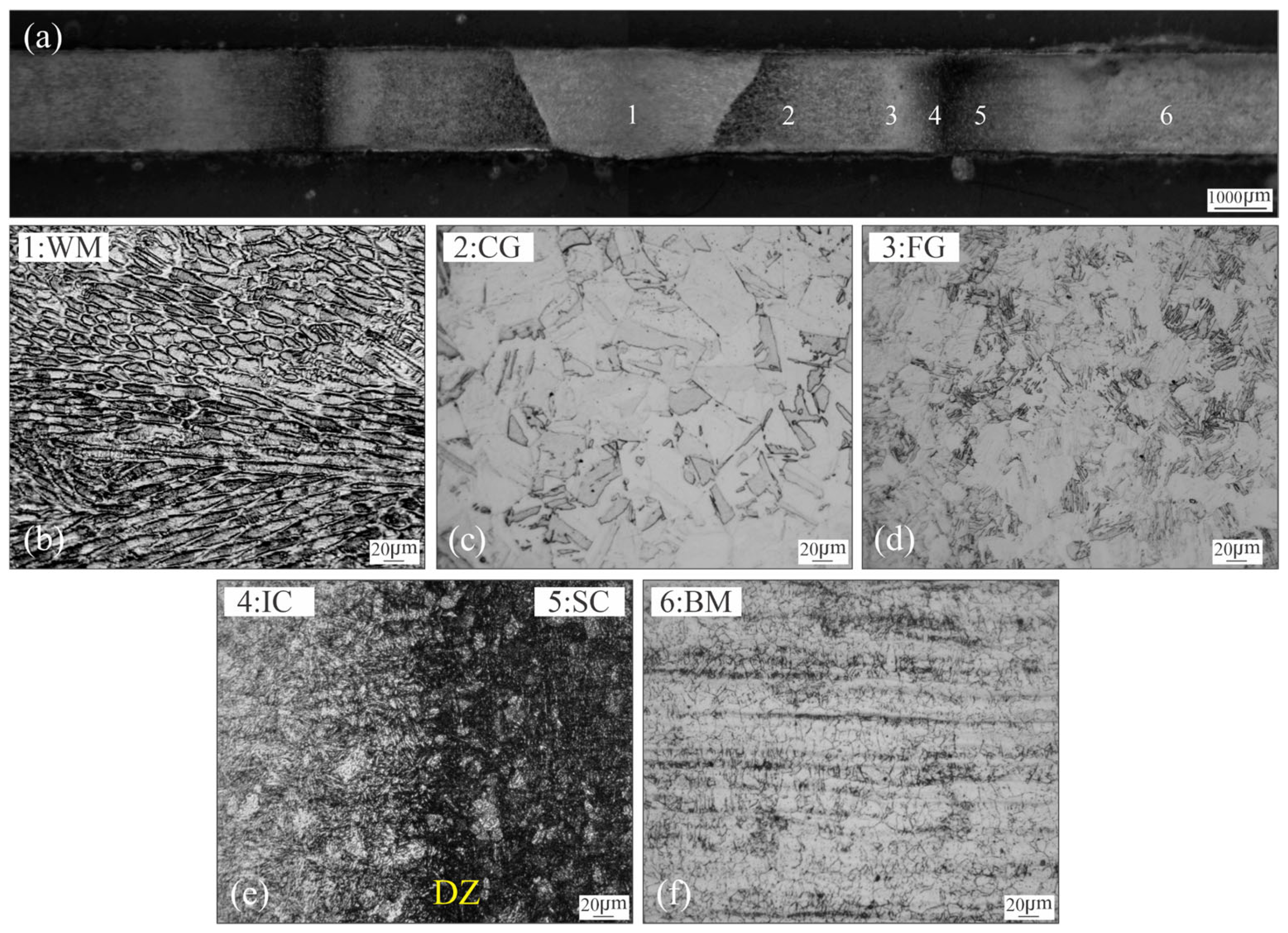
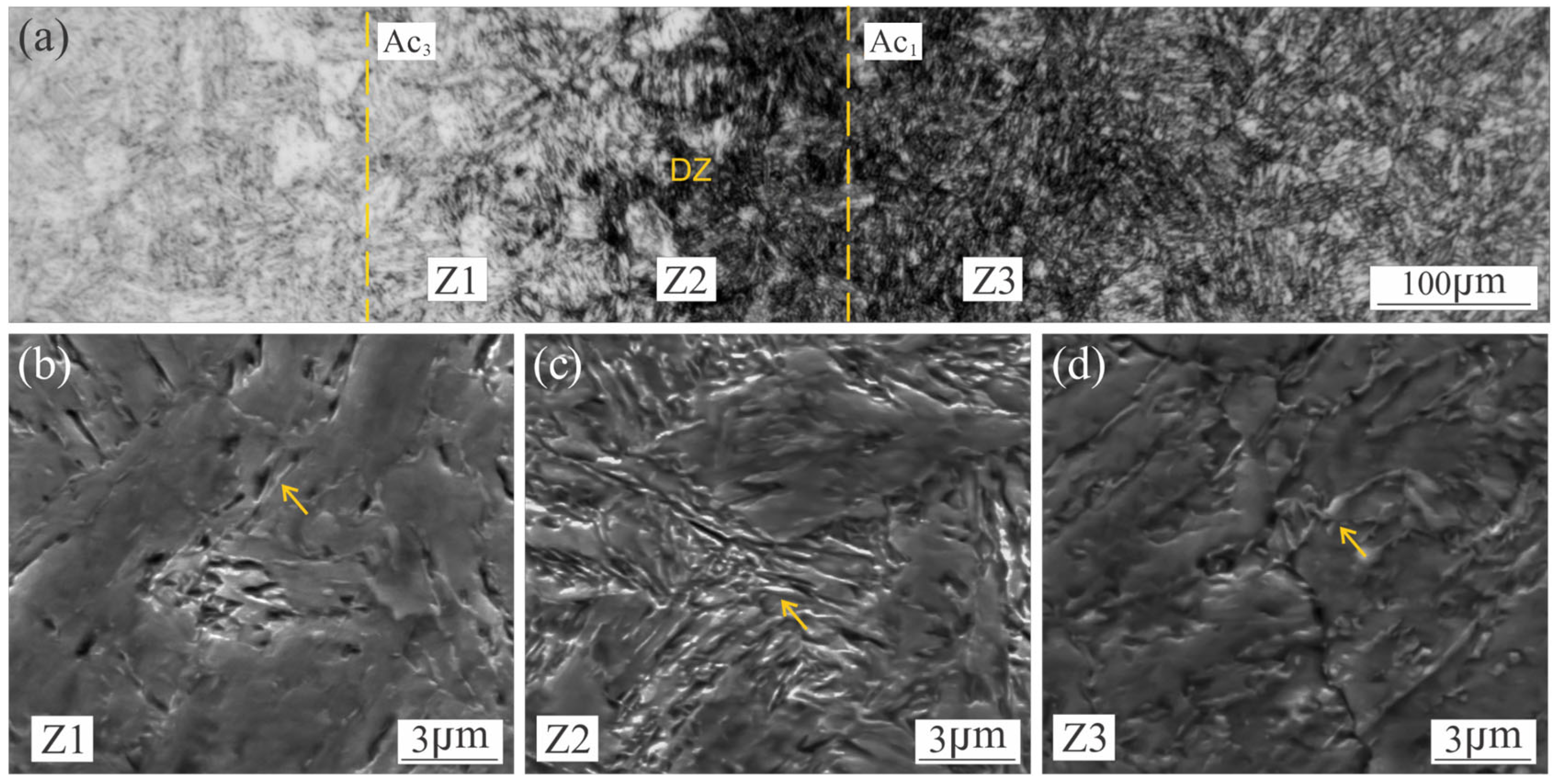
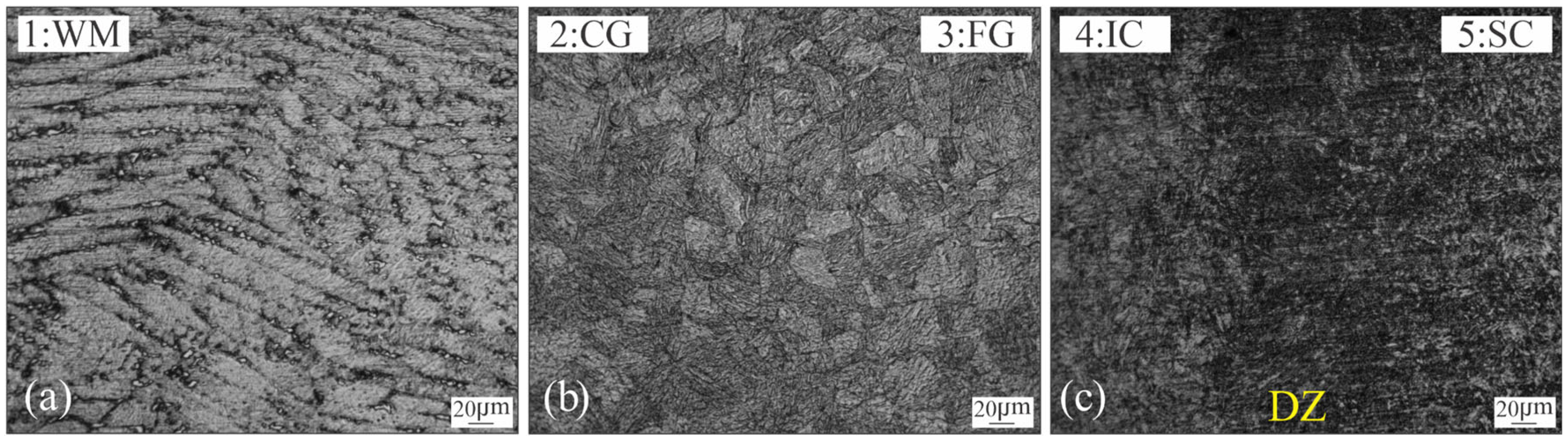
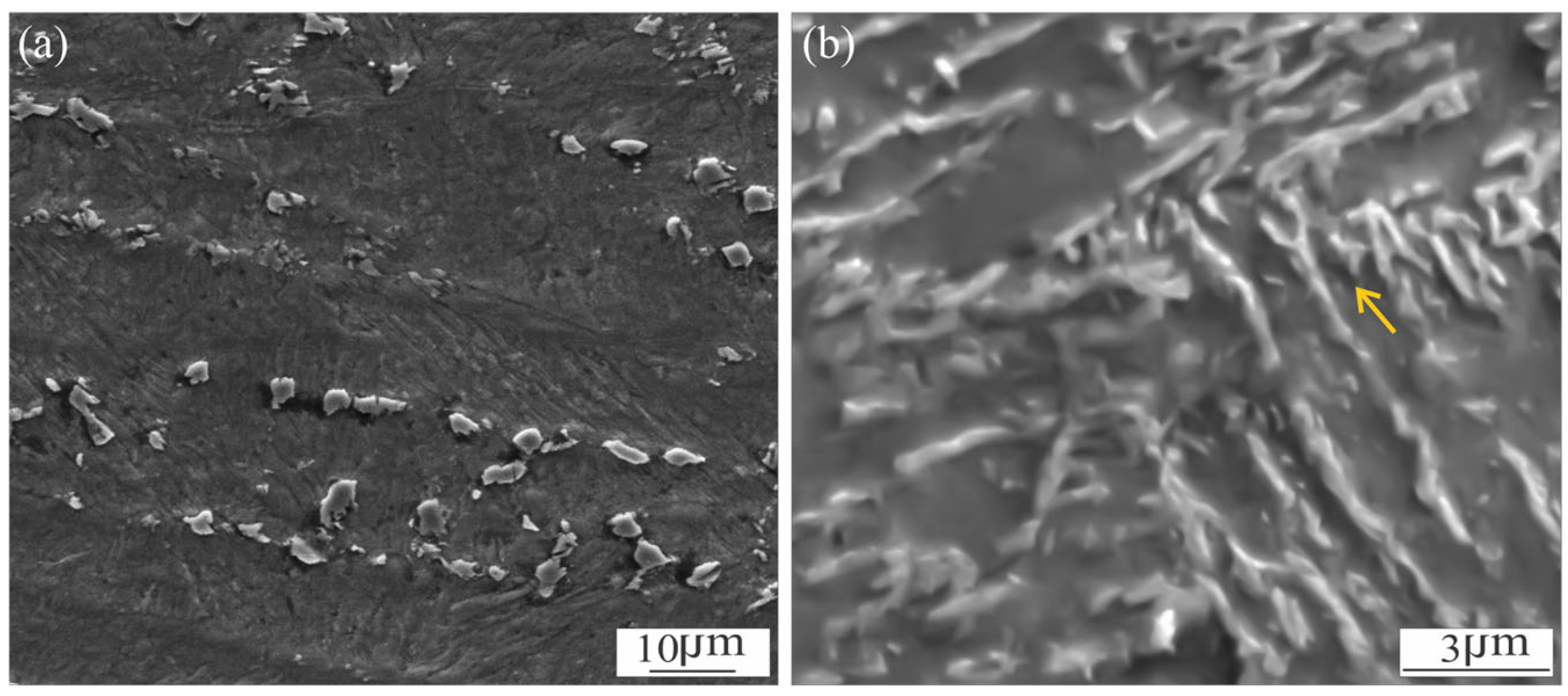
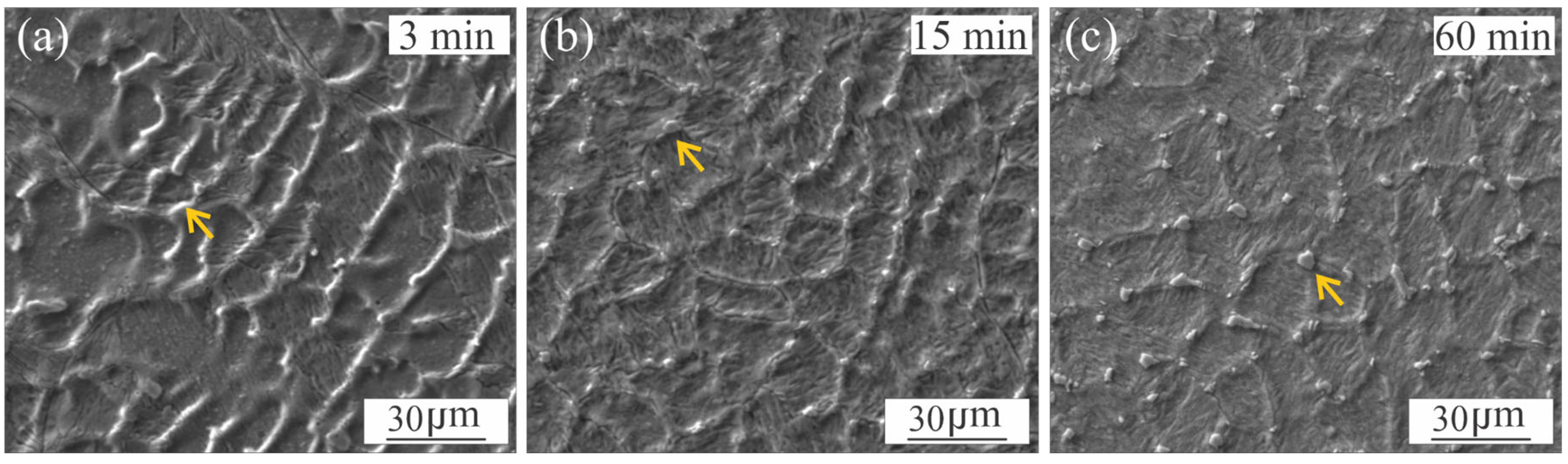
3.1. Microstructural Characterization of Welded Joint
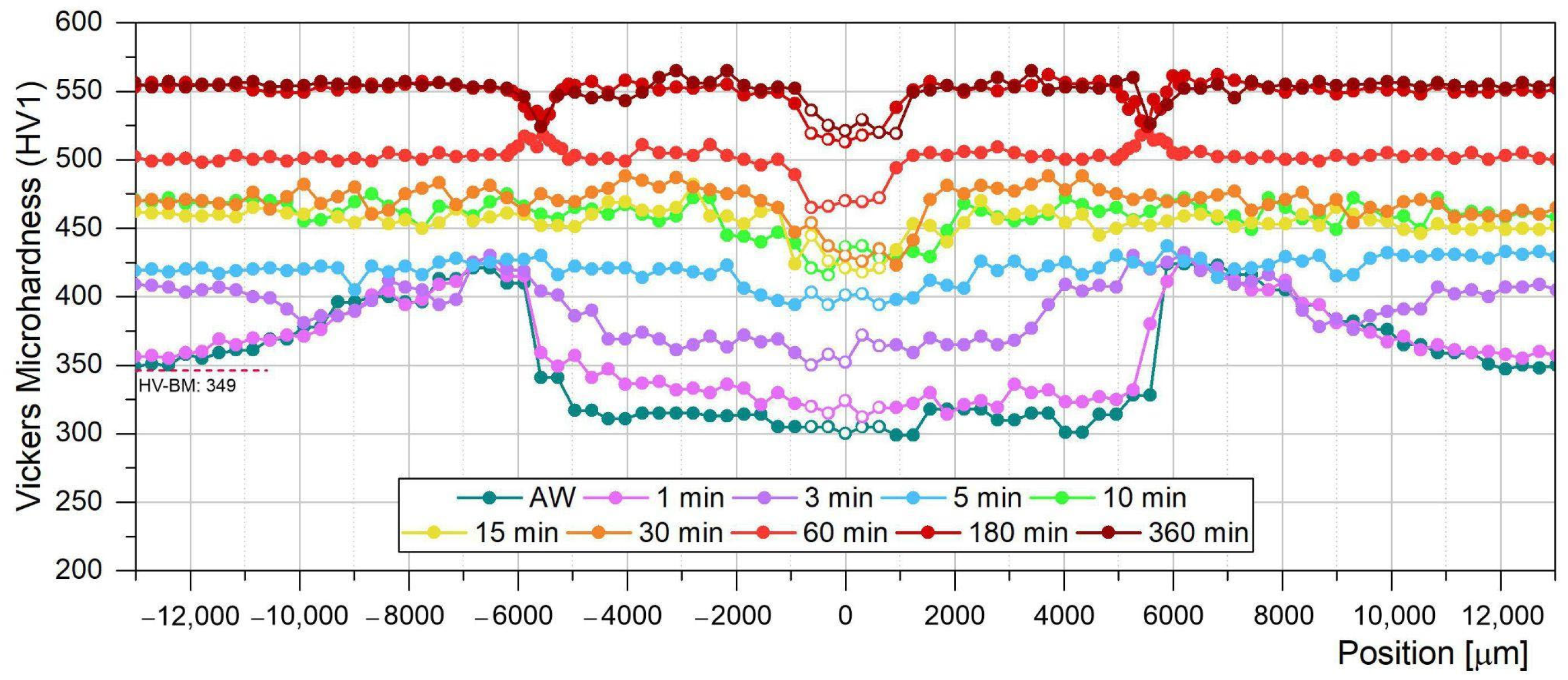
3.2. Microhardness Evolution of the Welded Joint During PWHT
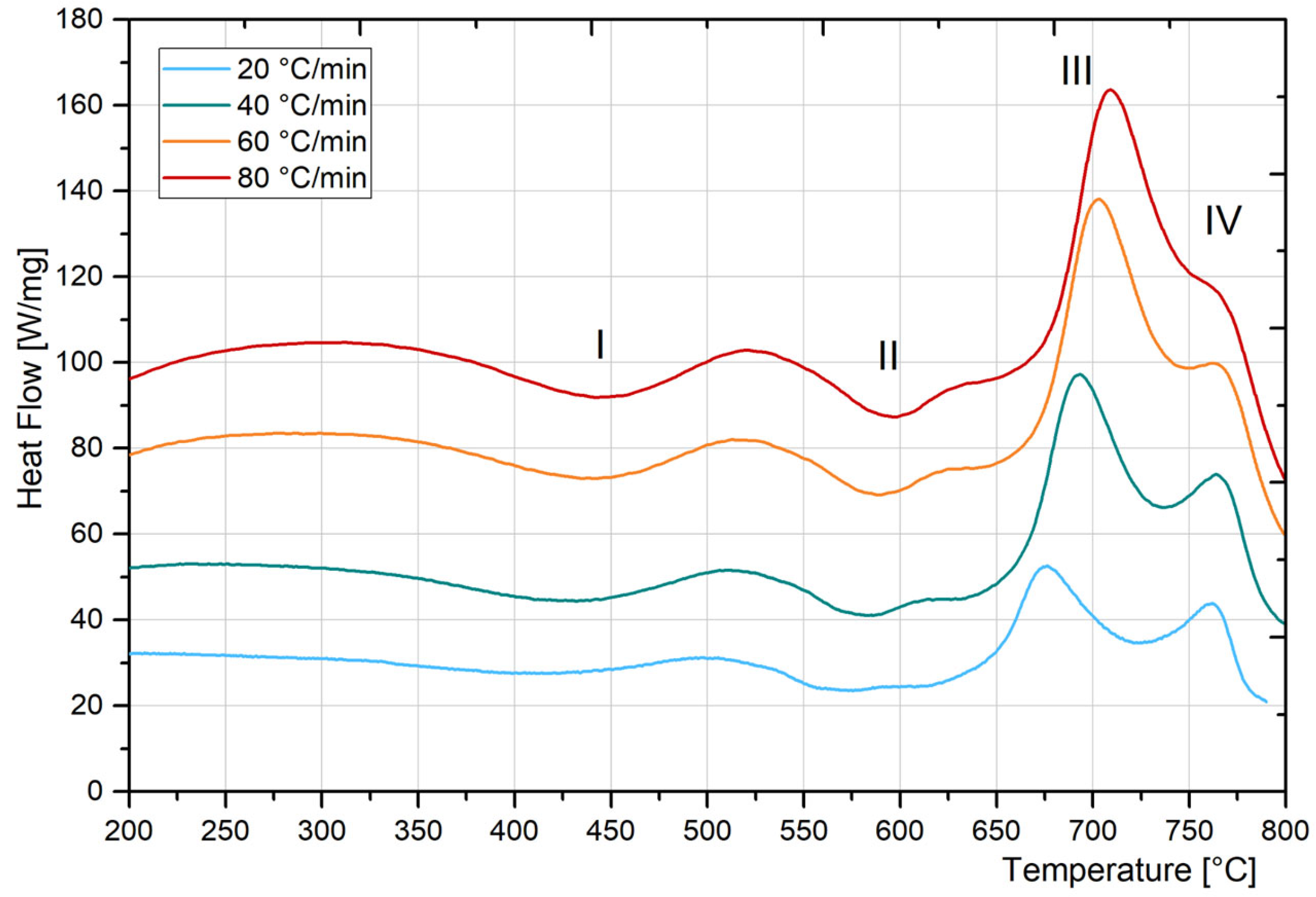
3.3. DSC Analysis
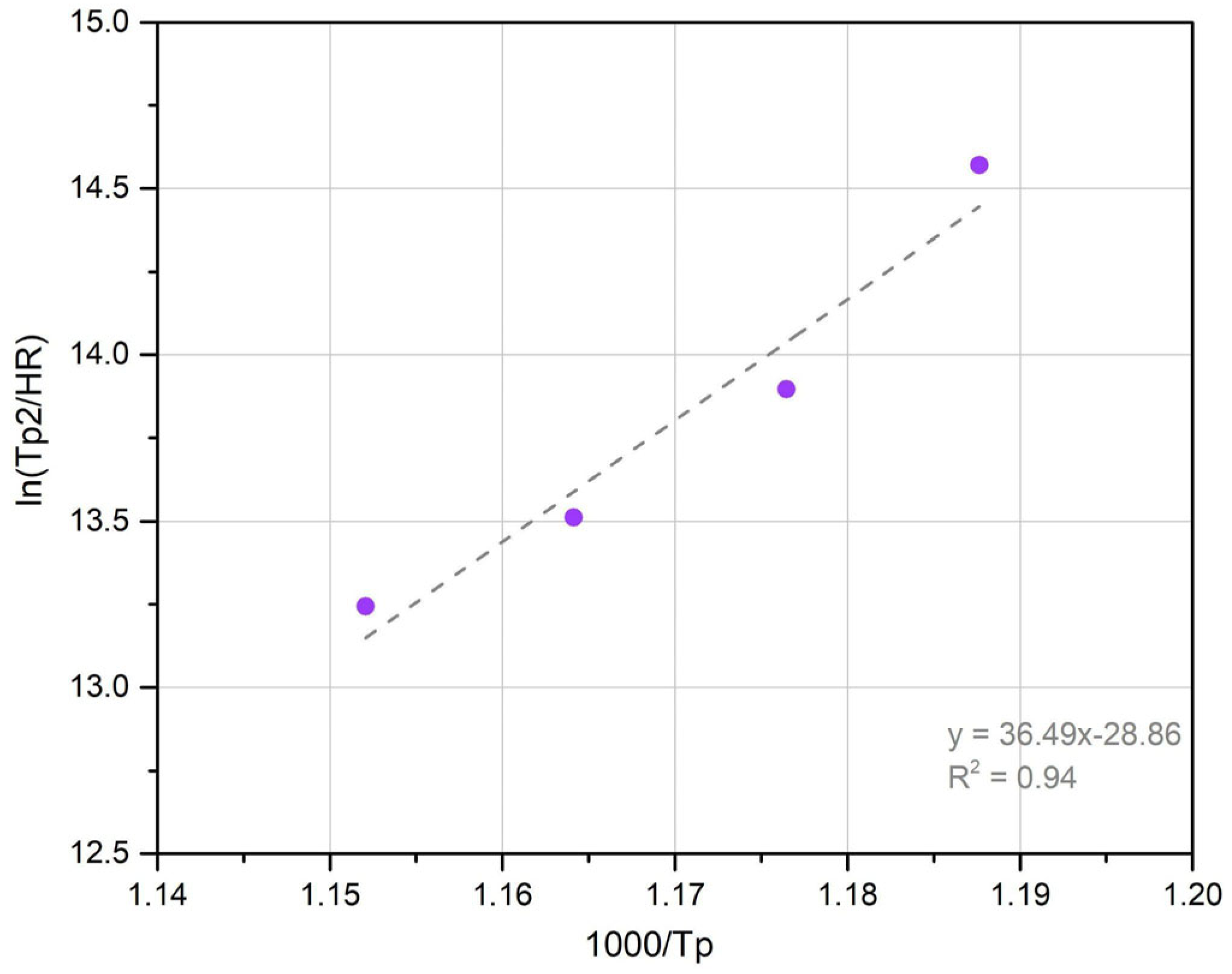
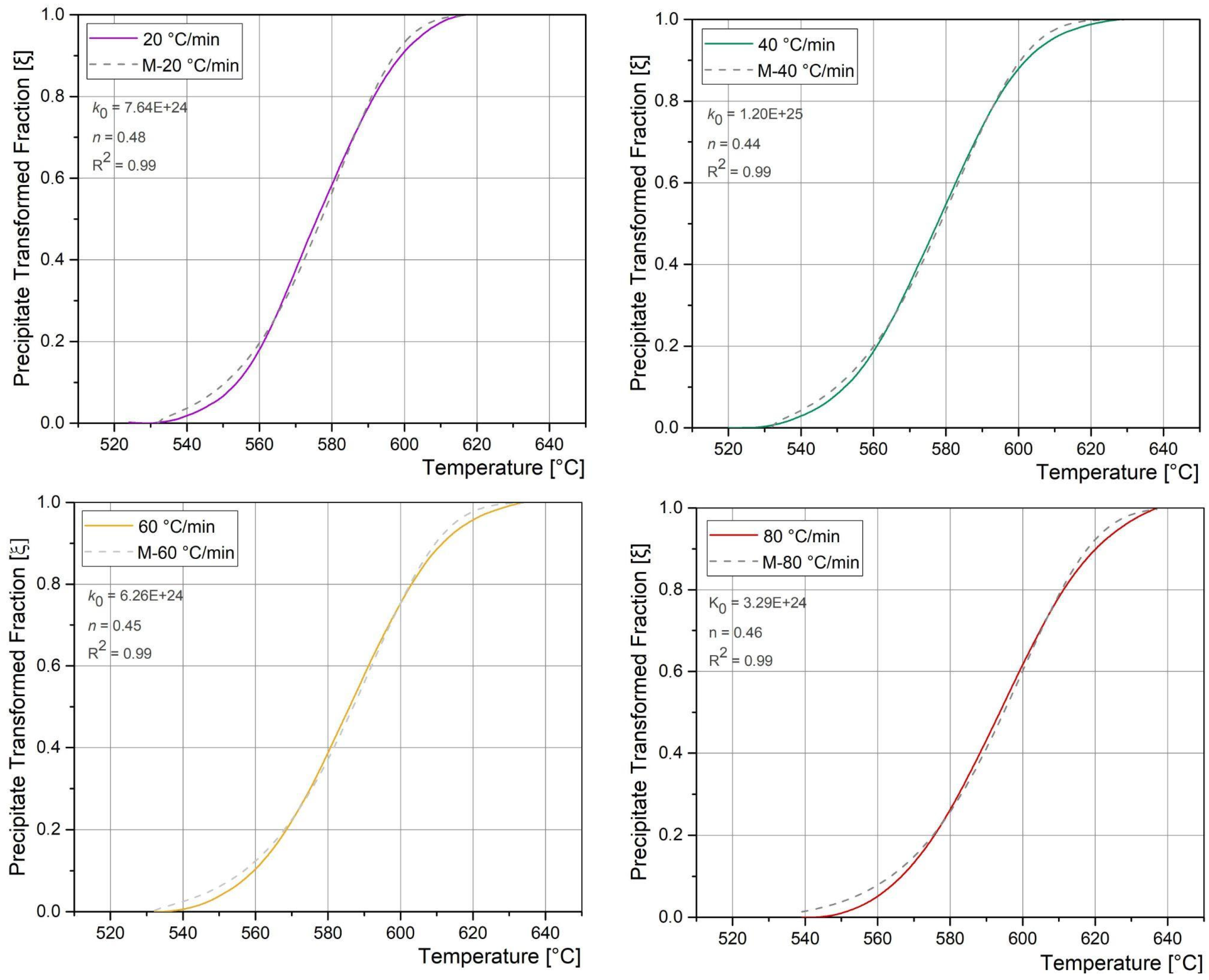
3.4. Precipitation Reaction Kinetics
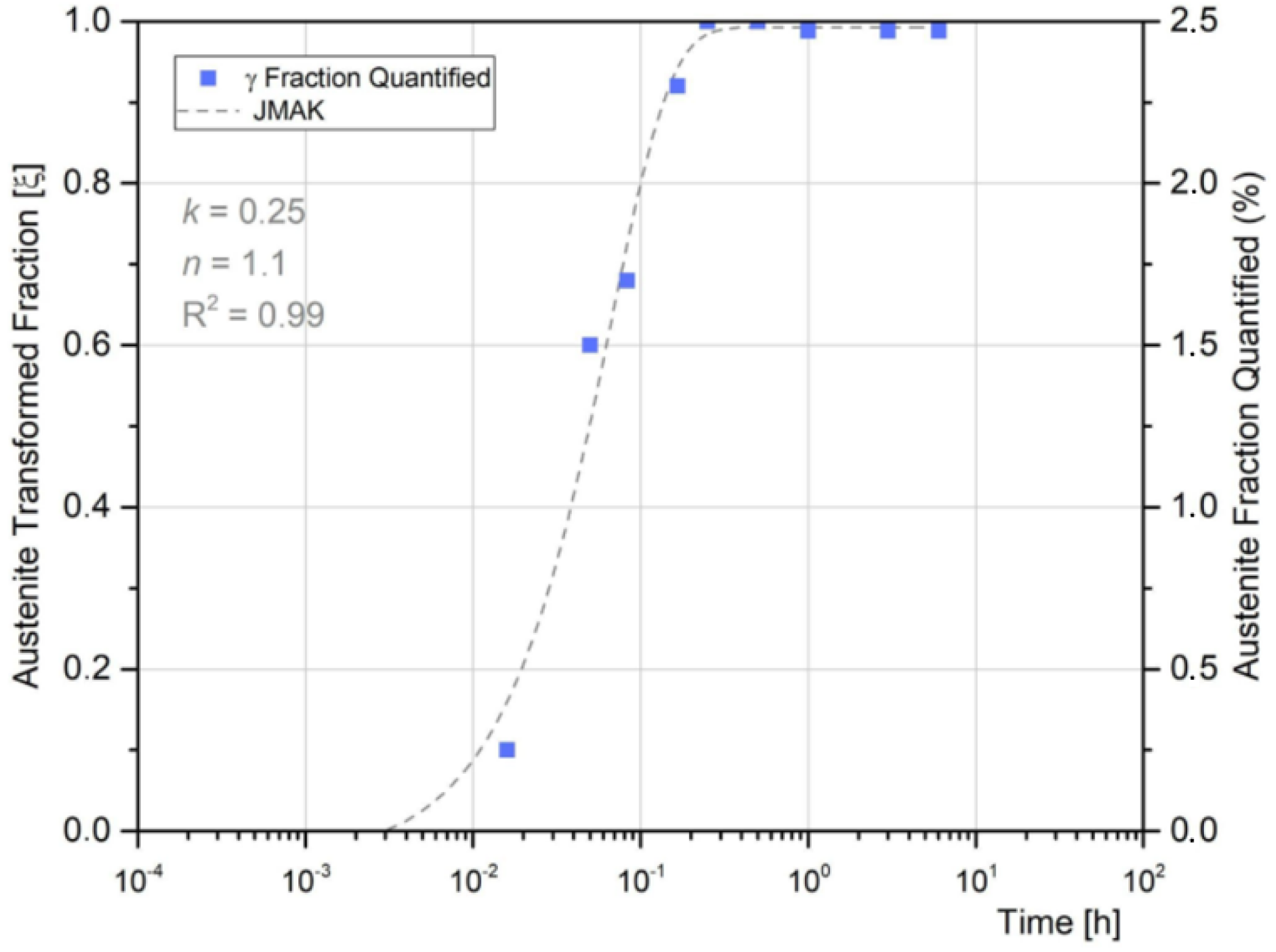
3.5. Austenite Reversion in the Weld Metal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GTAW-P | Gas Tungsten Arc Welding—Pulsed |
| AW | As Welded |
| WM | Weld Metal |
| R-HAZ | Recrystallized Heat Affected Zone |
| CG-HAZ | Coarse Grain Heat Affected Zone |
| FG-HAZ | Fine Grain Heat Affected Zone |
| IC-HAZ | Intercritical Heat Affected Zone |
| SC-HAZ | Subcritical Heat Affected Zone |
| DZ | Dark Zone |
| BM | Base Material |
| PWHT | Post Weld Heat Treatment |
| WCL | Weld Central Line |
| DSC | Differential Scanning Calorimetry |
| EDS | Energy Dispersive Spectroscopy |
| JMAK | Johnson-Mehl-Avrami-Kolmogorov |
| M-JMAK | Modified Johnson-Mehl-Avrami-Kolmogorov |
| γr | Reverted Austenite |
| MA | Maraging |
| SRM | Solid Rocket Motor |
| AR | As Received |
| HV | Hardness Vickers |
| AVH | Average Vickers Hardness |
| HI | Heat Input |
| HT | Heat Treatment |
| Ea | Activation Energy |
| Tp | Temperature of the precipitation peak |
| HR | Heating Rate |
| n | Avrami Exponent |
| k0 | Reaction Rate |
| k | Constant |
| T0 | Onset Temperature |
References
- Sha, W.; Malinov, S. Maraging Steels–Modelling of Microstructure, Properties and Applications; Sha, W., Guo, Z., Eds.; Woodhead Publishing: Cambridge, UK, 2009; p. 194. [Google Scholar]
- Sundaresan, S.; Manirajan, M.; Rao, B.N. On the Fracture Toughness Evolution in Weldments of Maraging Steel Rocket Motor Case. Mater. Des. 2010, 31, 4921–4926. [Google Scholar] [CrossRef]
- Freeman, R. New Welding Techniques for Aerospace Materials. In Welding and Joining for Aerospace Materials, 2nd ed.; Chaturvedi, M., Ed.; Woodhead Publishing: Cambridge, UK, 2020; p. 452. [Google Scholar]
- Kumar, B.D.; Nayana, B.S.; Shree, D.S. Design and Structural Analysis of Solid Rocket Motor Casing Hardware Used in Aerospace Applications. J. Aeronaut. Aerosp. Eng. 2016, 5, 2168–9792. [Google Scholar] [CrossRef]
- Moshka, O.; Pinkas, M.; Brosh, E.; Ezersky, V.; Meshi, L. Addressing the Issue of Precipitates in Maraging Steels—Unambiguous Answer. Mater. Sci. Eng. A 2015, 638, 232–239. [Google Scholar] [CrossRef]
- Floreen, S. The Physical Metallurgy of Maraging Steel. Metall. Rev. 1968, 13, 115–128. [Google Scholar] [CrossRef]
- Lang, F.H.; Kenyon, N. Welding of Maraging Steel; Lang, F.H., Kenyon, N., Eds.; Welding Research Council, Engineering Foundation: New York, NY, USA, 1971; p. 40. [Google Scholar]
- Shamantha, C.; Narayanan, R.; Iyer, K.; Radhakrishnan, V.; Seshadri, S.; Sundararajan, S.; Sundaresan, S. Microstructural Changes During Welding and Subsequent Heat Treatment of 18Ni (250-grade) Maraging Steel. Mater. Sci. Eng. A 2000, 287, 43–51. [Google Scholar] [CrossRef]
- Kapoor, R.; Kumar, L.; Batra, I. A Dilatometric Study of the Continuous Heating Transformations in 18 wt.% Ni Maraging Steel of Grade 350. Mater. Sci. Eng. A 2003, 352, 318–324. [Google Scholar] [CrossRef]
- Li, K.; Shan, J.; Wang, C.; Tian, Z. Influence of Aging Temperature on Strength and Toughness of Laser-Welded T-250 Maraging Steel Joint. Mater. Sci. Eng. A 2016, 669, 58–65. [Google Scholar] [CrossRef]
- Gupta, R.N.; Raja, V. Environmentally Assisted Cracking Susceptibility of Dark Etched HAZ/Parent Metal Interface Region of 18Ni 250 Maraging Steel Weldment. Mater. Sci. Eng. A 2020, 774, 138911. [Google Scholar] [CrossRef]
- Rao, V.V.; Reddy, G.M.; Raju, A.V.S. Influence of Post-weld Heat Treatments on Microstructure and Mechanical Properties of Gas Tungsten Arc Maraging Steel Weldments. Mater. Sci. Technol. 2010, 26, 1459–1468. [Google Scholar] [CrossRef]
- Svoboda, H.G.; Duran, M.; Belzunce, F.J.; Rodríguez, C. Estimation of Local Mechanical Properties by Small Punch Test in Welded Joints of Maraging C250 Steel. J. Mater. Eng. Perform. 2024, 33, 9405–1059. [Google Scholar] [CrossRef]
- Ahmed, B.; Tariq, F.; Naz, N.; Baloch, R.A. How Multiple Weld Repairs Impact Maraging Steel. Weld. J. 2012, 91, 380–47. Available online: https://www.researchgate.net/publication/259659159 (accessed on 29 April 2025).
- Shamantha, C.; Narayanan, R.; Iyer, K.; Radhakrishnan, V.; Seshadri, S.; Sundararajan, S.; Sundaresan, S. Tensile Properties and Fracture Toughness of 18Ni (250 grade) Maraging Steel Weldments. Sci. Technol. Weld. Join. 2000, 5, 329–337. [Google Scholar] [CrossRef]
- Jose, B.; Manoharan, M.; Natarajan, A.; Muktinutalapati, N.R.; Reddy, G.M. Development of a Low Heat-Input Welding Technique for Joining Thick Plates of 250 Grade Maraging Steel to Fabricate Rocket Motor Casing. Mater. Lett. 2022, 326, 132984. [Google Scholar] [CrossRef]
- Sakai, P.; Lima, M.; Fanton, L.; Gomes, C.; Lombardo, S.; Silva, D.; Abdalla, A. Comparison of Mechanical and Microstructural Characteristics in Maraging 300 Steel Welded by three different processes: LASER, PLASMA and TIG. Procedia Eng. 2016, 114, 291–297. [Google Scholar] [CrossRef]
- Tariq, F.; Baloch, R.A.; Ahmed, B.; Naz, N. Investigation into Microstructure of Maraging Steel 250 Weldments and Effect of Post-Weld Heat Treatments. J. Mater. Perform. 2009, 19, 264–273. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Li, D. Quantification of Phase Transformation Kinetics of 18 wt. % Ni C250 Maraging Steel. Mater. Sci. Eng. A 2004, 373, 10–20. [Google Scholar] [CrossRef]
- Feitosa, A.L.M.; Escobar, J.; Ribamar, G.G.; Avila, J.A.; Padilha, A.F. Direct Observation of Austenite Reversion During Aging of 18Ni (350 Grade) Maraging Steel Through In-Situ Synchrotron X-Ray Diffraction. Met. Mater Trans A 2022, 53, 420–431. [Google Scholar] [CrossRef]
- Conde, F.; Escobar, J.; Oliveira, J.; Jardini, A.; Filho, W.B.; Avila, J. Austenite Reversion Kinetics and Stability During Tempering of an Additively Manufactured Maraging 300 Steel. Addit. Manuf. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Gao, P.; Jing, G.; Lan, X.; Li, S.; Zhou, Y.; Wang, Y.; Yang, H.; Wei, K.; Wang, Z. Effect of Heat Treatment on Microstructure and Mechanical Properties of Fe-Cr-Ni-Co-Mo Maraging Stainless Steel Produced by Selective Laser Melting. Mater. Sci. Eng. A 2021, 814, 141149. [Google Scholar] [CrossRef]
- dos Santos, L.P.M.; Béreš, M.; de Castro, M.O.; Sarvezuk, P.W.C.; Wu, L.; Herculano, L.F.G.; Paesano, A.; Silva, C.C.; Masoumi, M.; de Abreu, H.F.G. Kinetics of Reverted Austenite in 18 wt.% Ni Grade 300 Maraging Steel: An in-Situ Synchrotron X-Ray Diffraction and Texture Study. Miner. Met. Mater. Soc. 2020, 72, 3502–3512. [Google Scholar] [CrossRef]
- Fabian, R.J. Phase Transformation Kinetics in Laser-Powder Bed Fused Fe-Cr.Ni-Al-Maraging Stainless Steel. Master’s Thesis, University of Memphis, Memphis, TN, USA, 2022. Available online: https://digitalcommons.memphis.edu/etd/3205 (accessed on 8 March 2022).
- Bai, Y.; Wang, D.; Yang, Y.; Wang, H. Effect of Heat Treatment on the Microstructure and Mechanical Properties of Maraging Steel by Selective Laser Melting. Mater. Sci. Eng. A 2019, 760, 105–117. [Google Scholar] [CrossRef]
- Lupi, G.; Bettini, E.; Deirmina, F.; Casati, R. Microstructural and Mechanical Properties of a Novel Cobalt and Titanium Free Maraging Steel for Laser Powder Bed Fusion. J. Mater. Res. Technol. 2024, 30, 1269–1278. [Google Scholar] [CrossRef]
- Murthy, C.; Krishna, A.G.; Reddy, G. Microstructural and Mechanical Properties and Dissimilar Metal Gas tungsten Constricted Arc Welds: Maraging Steel to 13-8 Mo Stainless Steel. Def. Technol. 2019, 15, 111–121. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. E415-21 Standard Test Method for Analysis of Carbon and Low Alloy Steel by Spark Atomic Emission Spectrometry; ASTM: West Conshohocken, PA, USA, 2021. [Google Scholar]
- Teixeira, F.R.; Scotti, F.M.; Jorge, V.L.; Scotti, A. Combined Effect of the Interlayer with Travel Speed on Features of Thin Wall WAAM Under Two Cooling Approaches. Int. J. Adv. Manuf. Technol. 2023, 126, 273–289. [Google Scholar] [CrossRef]
- Sastry, K.Y.; Narayanan, R.; Shamantha, C.R.; Sundaresan, S.; Seshadri, S.K.; Radhakrishnan, V.M.; Iyer, K.J.L.; Sundararajan, S. Stress Corrosion Cracking of Maraging Steel Weldments. Mater. Sci. Technol. 2003, 19, 375. [Google Scholar] [CrossRef]
- Rohit, B.; Muktinutalapati, N.R. Austenite Reversion in 18% Ni Maraging Steel and its Weldments. Mater. Sci. Technol. 2017, 34, 253–260. [Google Scholar] [CrossRef]
- de Carvalho, L.G.; Andrade, M.S.; Plaut, R.L.; Souza, F.M.; Padilha, A.F. A Dilatometric Study of the Phase Transformations in 300 and 350 Maraging Steels During Continuous Heating Rates. Mater. Res. 2013, 16, 740–744. [Google Scholar] [CrossRef]
- da Fonseca, D.P.M.; Feitosa, A.L.M.; de Carvalho, L.G.; Plaut, R.L.; Padilha, A.F. A Short Review on Ultra-High-Strength Maraging Steels and Future Perspectives. Mater. Res. 2020, 24, e20200470. [Google Scholar] [CrossRef]
- Peters, D.T.; Cupp, C.R. The Kinetics of Aging Reaction in 18 Pct Ni Maraging Steels. Transit. Metall. Soc. AIME 1965, 236, 1420. [Google Scholar]
- Jacob, K.; Yadav, D.; Dixit, S.; Hohenwarter, A.; Jaya, B.N. High Pressure Torsion Processing of Maraging Steel 250: Microstructure and Mechanical Behaviour Evolution. Mater. Sci. Eng. A 2021, 802, 140665. [Google Scholar] [CrossRef]
- Freitas, G.H.d.O.; de Oliveira, C.A.S. Effect of Hot Deformation on Microstructure, Hardness and Precipitation Kinetics in a 350 Maraging Steel Modified by Titanium Alloy. Mater. Res. 2018, 21, e20180120. [Google Scholar] [CrossRef]
- Floreen, S.; Decker, R.F. Source Book on Maraging Steel; Decker, R.F., Ed.; ASM: Metal Park, OH, USA, 1979; pp. 20–32. [Google Scholar]
- Gupta, R.N.; Raja, V.S.; Mukherjee, M.K.; Murty, S.V.S.N. On Improving the Quality of Gas Tungsten Arc Welded 18Ni 250 Maraging Steel Rocket Motor Casing. Miner. Met. Mater. Soc. ASM Int. 2017, 48, 4655–4666. [Google Scholar] [CrossRef]
- Evans, G.M.; Bailey, N. Metallurgy of Basic Weld Metal, 1st ed.; Greening, G., Ed.; Abington Publishing: Northampton, UK, 1997; p. 240. [Google Scholar]
- Viswanathan, U.; Banerjee, S.; Krishnan, R. Effects of Aging on the Microstructure of 17-4 PH Stainless Steel. Mater. Sci. Eng. A 1988, 104, 181–189. [Google Scholar] [CrossRef]
- Vasudevan, V.K.; Kim, S.J.; Wayman, C.M. Precipitation Reactions and Strengthening Behavior in 18 Wt Pct nickel maraging steels. Met. Trans. A 1990, 21, 2655–2668. [Google Scholar] [CrossRef]
- Bui, N.; Dabosi, F. Contribution to the Study of the Effect of Molybdenum on the Ageing Kinetics of Maraging Steels. Cobalt 1972, 57, 192–201. [Google Scholar]
- Habiby, F.; ul Haq, A.; Hashmi, F.H.; Khan, A.Q. Lattice changes in the martensitic phase due to ageing in 18 wt% nickel maraging steel grade 350. In Proceedings of the International Conference on Martensitic Transformations (ICOMAT-86), Nara, Japan, 26–30 August 1986; The Japan Institute of Metals: Sendai, Japan, 1986; pp. 560–565. [Google Scholar]
- Goldberg, A.; O‘Connor, D.G. Influence of Heating Rate on Transformations in an 18 per cent Nickel Maraging Steel. Nature 1967, 213, 170–171. [Google Scholar] [CrossRef]
- Goldberg, A. Maraging Steels; Decker, R.F., Ed.; ASM: Metals Park, OH, USA, 1979; pp. 41–51. [Google Scholar]
- Saul, G.; Roberson, J.A.; Adair, A.M. Maraging Steels; Decker, R.F., Ed.; ASM: Metals Park, OH, USA, 1979; pp. 52–56. [Google Scholar]
- Brandes, E.A.; Brook, G.B. Smithells Metals Reference Book, 7th ed.; Heinemann, B., Brook, G.B., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 13–20. [Google Scholar]
- Christian, J.W. Chapter 12: Formal Theory of Transformation Kinetics. In Part 1: The Theory of Transformation in Metals and Alloys; Elsevier Science: Amsterdam, The Netherlands, 2002; p. 546. [Google Scholar]
- Krishtal, M.K. Diffusion Processes in Iron Alloys; Israel Programme for Scientific Translation Ltd.: Jerusalem, Israel, 1970; pp. 175–203. [Google Scholar]
- Mahadevan, S.; Jayakumar, T.; Rao, B.; Kumar, A.; Rajkumar, K.; Raj, B. X-Ray Diffraction Profile Analysis for Characterizing Isothermal Aging Behavior of M250 Grade Maraging Steel. Miner. Met. Mater. Soc. ASM Int. 2008, 39, 1978–1984. [Google Scholar] [CrossRef]
- Zhu, F.; Yin, Y.F.; Faulkner, R.G. Microstructural Control of Maraging Steel C300. Mater. Sci. Technol. 2011, 27, 395–405. [Google Scholar] [CrossRef]
- Mittra, J.; Kulkarni, U.; Dey, G. Hardness Based Model for Determining the Kinetics of Precipitation. Mater. Sci. Eng. A 2009, 500, 244–247. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W. Quantification of Precipitation Hardening and Evolution of Precipitates. Mater. Trans. 2002, 43, 1273–1282. [Google Scholar] [CrossRef]
- Sinha, I.; Mandal, R. Avrami Exponent Under Transient and Heterogeneous Nucleation Transformation Conditions. J. Non-Cryst. Solids 2011, 357, 919–925. [Google Scholar] [CrossRef]
- Reddy, G.M.; Rao, V.V.; Raju, A.V.S. The Effect of Post-weld Heat Treatments on the Microstructure and Mechanical Properties of Maraging Steel Laser Weldments. J. Mater. Des. Appl. 2009, 223, 149–159. [Google Scholar] [CrossRef]
- Abreu, H.F.; Silva, J.J.; Silva, M.R.; da Silva, M.J.G. Influence of Reverted Austenite on the Texture and Magnetic Properties of 350 Maraging Steel. J. Magn. Magn. Mater. 2015, 393, 99–104. [Google Scholar] [CrossRef]



| (wt%) | C | Mn | Si | Ni | Mo | Co | Ti | Al | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MA-C250 | 0.01 | 0.04 | 0.09 | 18.2 | 4.9 | 7.9 | 0.4 | 0.15 | 0.009 | 0.003 | Bal |
| ASTM-A538 | 0.03 * | 0.10 * | 0.10 * | 17–19 | 4.6–5.2 | 7–8.5 | 0.3–0.5 | 0.05–0.15 | 0.01 * | 0.01 * | Bal |
| Ip [A] | Ib [A] | IRMS [A] | VRMS [V] | de-p [mm] | F [Hz] | Ws [mm/s] | HI [J/mm] |
|---|---|---|---|---|---|---|---|
| 120 | 72 | 99 | 10 | 1 | 15 | 4 | 246 |
| Vc [°C/min] | Zone * | Peak Temp. [°C] | Temp. Range [°C] | Possible Transformation |
|---|---|---|---|---|
| 20 | I Exo | 429 | 212–513 | Recovery of martensite and formation of carbides (minor hardening) [33] Formation of coherent precipitation zones [40,41,42] |
| 40 | 437 | 255–517 | ||
| 60 | 446 | 293–524 | ||
| 80 | 451 | 315–531 | ||
| 20 | II Exo | 571 | 516–614 | Formation of the main strengthening precipitates [42,43,44,45,46,47] |
| 40 | 577 | 521–628 | ||
| 60 | 586 | 532–631 | ||
| 80 | 595 | 539–637 | ||
| 20 | III Endo | 686 | 624–730 | Reverted Austenite formation [34,40,42,43,44,45] |
| 40 | 693 | 633–737 | ||
| 60 | 702 | 647–749 | ||
| 80 | 711 | 659–756 | ||
| 20 | IV Endo | 771 | 732–797 | Martensite to austenite by shear [42,46] |
| 40 | 769 | 741–823 | ||
| 60 | 772 | 751–825 | ||
| 80 | 768 | 751–821 |
| Zone | n | k | R2 |
|---|---|---|---|
| WM | 0.57 | 2.4 | 0.99 |
| R-HAZ | 0.75 | 2.2 | 0.99 |
| IC-HAZ | 0.72 | 3.2 | 0.98 |
| SC-HAZ | 0.78 | 1.7 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran, M.A.; Peitsch, P.; Svoboda, H.G. Phase Transformation Kinetics During Post-Weld Heat Treatment in Weldments of C-250 Maraging Steel. Materials 2025, 18, 2820. https://doi.org/10.3390/ma18122820
Duran MA, Peitsch P, Svoboda HG. Phase Transformation Kinetics During Post-Weld Heat Treatment in Weldments of C-250 Maraging Steel. Materials. 2025; 18(12):2820. https://doi.org/10.3390/ma18122820
Chicago/Turabian StyleDuran, Mercedes Andrea, Pablo Peitsch, and Hernán Gabriel Svoboda. 2025. "Phase Transformation Kinetics During Post-Weld Heat Treatment in Weldments of C-250 Maraging Steel" Materials 18, no. 12: 2820. https://doi.org/10.3390/ma18122820
APA StyleDuran, M. A., Peitsch, P., & Svoboda, H. G. (2025). Phase Transformation Kinetics During Post-Weld Heat Treatment in Weldments of C-250 Maraging Steel. Materials, 18(12), 2820. https://doi.org/10.3390/ma18122820





