Effect of Hot Isostatic Pressure on the Microstructure Evolution of Ti-22Al-25Nb Alloy Formed by Selective Laser Melting
Abstract
1. Introduction
2. Materials and Methods
3. Results and Analysis
3.1. Effect of HIP Treatment on Microstructure of SLMed State
3.1.1. Microstructure Analysis of the HIPed State
3.1.2. Comparative Analysis of Microstructure and Tensile Properties Between HIPed and Direct Solution Aging Specimens
3.2. Effect of Solution Temperature on Microstructure of HIPed State
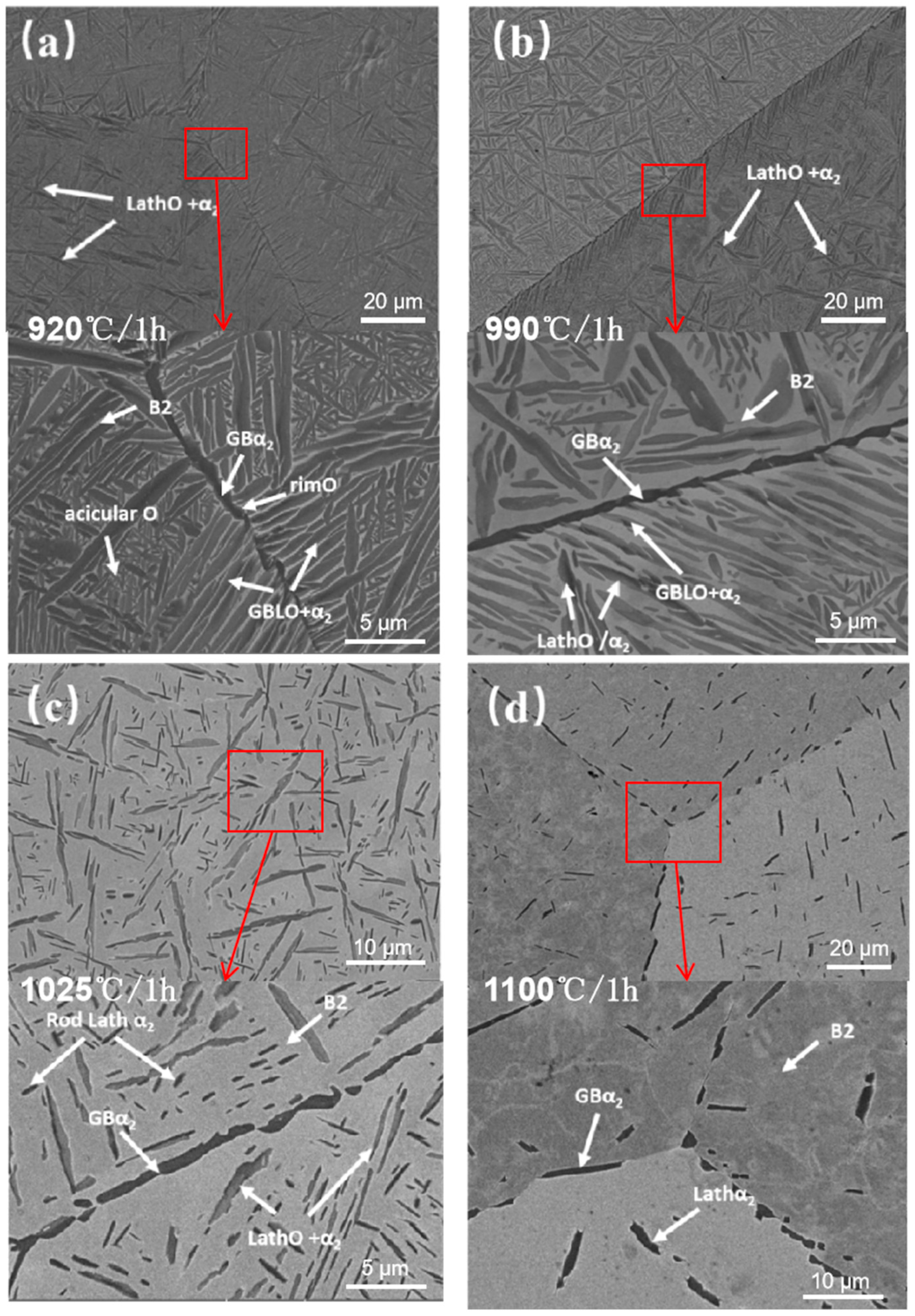
3.2.1. Microstructure Analysis of the HIPed Solid Solution Treatment
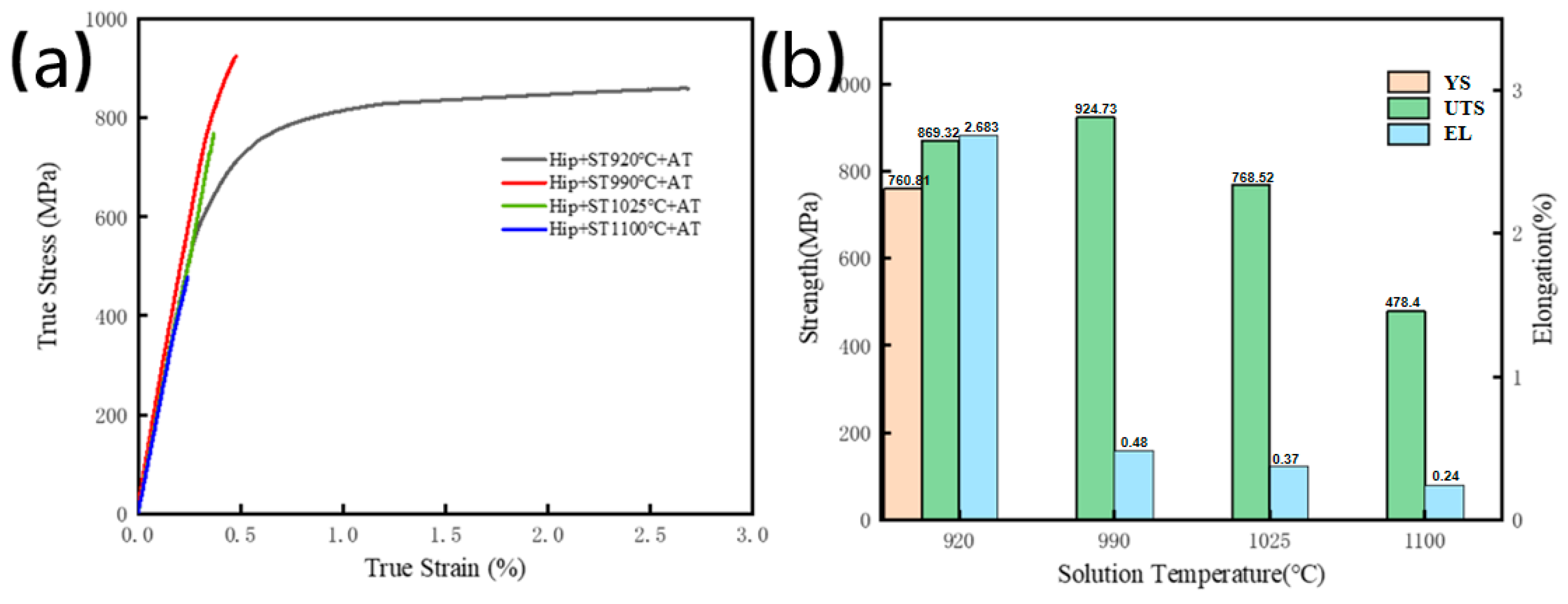
3.2.2. Effect of Solution Temperature on Tensile Properties
3.3. Effect of Aging at 800 °C on the Microstructure of the HIPed State
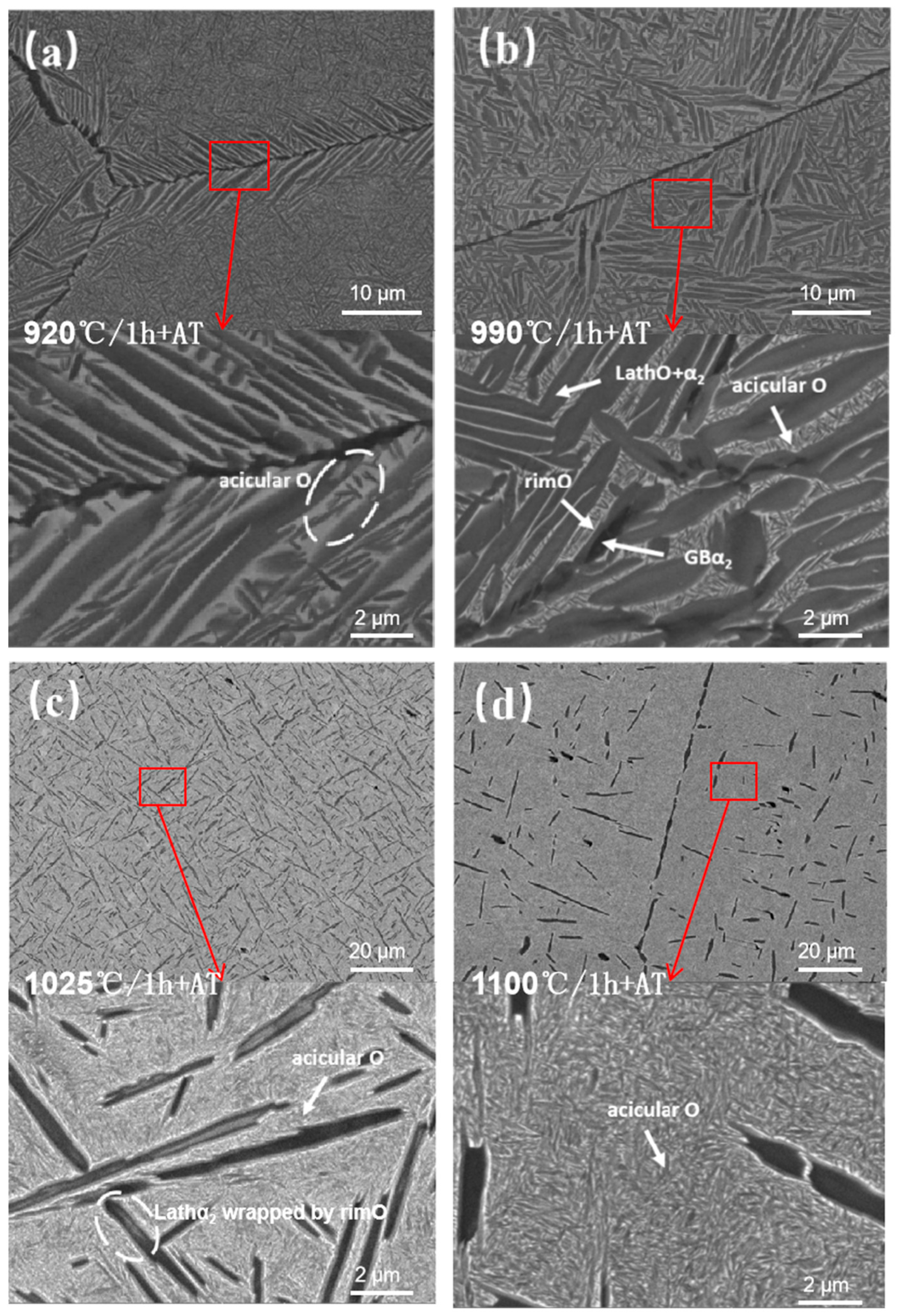
3.3.1. Microstructure Analysis of the HIPed Solution Aging Treatment
3.3.2. Effect of HIPed 800 °C Aging on the Tensile Properties
4. Summary and Conclusions
- Following (1100 °C + 300 MPa)/3 h HIP treatment, the unfused flaws inside the specimen in the SLM state were eliminated, and the pores were sealed. The HIPed state segregated the GBα2 at the grain borders, with the GBLO + α2 laths developing parallel from the grain boundaries toward the intragranular region, alongside the cross or snowflake O + α2 lath clusters and acicular O phase within the B2 phase. The GBLO + α2 was precipitated through interfacially unstable nucleation and sympathetic nucleation, according to a particular orientation relationship.
- The volume fraction of the B2 phase in the HIPed state increases with rising solid solution temperature, sequentially dissolving the acicular O phase, GBLO, lath O phase, lath α2, and GBα2 in order. The dimensions of the acicular O phase exhibit greater uniformity following solid solution at 920 °C, α2/O symbiotic laths emerge after solid solution at 990 °C, the reprecipitation of bar α2 transpires during solution treatment at 1025 °C, and the GBα2 dissolves in an intermittent chain subsequent to solid solution at 1100 °C.
- Following HIP, the aging microstructure is primarily characterized by the proliferation of the acicular O phase precipitated from the B2 phase and the retained lath O phase inside a solid solution. The precipitation of GBLO in the original solid solution is suppressed, and the GBLα2 in the original solid solution partially decomposes into rimO, resulting in coarse grain size and significant internal decomposition of α2. The phenomenon of discontinuous dissolution, attributed to a continuous distribution of the GBα2, disappears. However, a minor degree of discontinuous dissolution persists due to the significant temperature differential during the solution aging at 110 °C.
- Compared to direct solution aging specimens, the HIPed condition demonstrates increased densification but a coarser-grained structure, resulting in diminished strength and a notable boost in ductility. Following solution treatment and aging at 920 °C, the proliferation of the acicular O phase enhances ductility, resulting in optimum overall characteristics with YS of 760.81 MPa, UTS of 869.32 MPa, and EL of 2.683%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLM | Selective Laser Melting |
| HIP | Hot isostatic pressing |
| GB | Grain Boundary |
| GBL | Grain Boundary Lath |
| DSC | Differential scanning calorimetry |
| O | Orthorhombic |
| B2 | Body-centered cubic |
| VFRP | volume fraction of retained precipitates |
| YS | Yield Strength |
| UTS | Ultimate Tensile Strength |
| EL | Elongation |
References
- Fu, Y.; Su, Z. Elemental Composition, Phase Diagram, Microstructure, Fabrication Processes, and Mechanical Properties of Ti2AlNb Alloy: A Review. J. Mater. Eng. Perform. 2024, 704, 1–28. [Google Scholar] [CrossRef]
- Wei, W. Study on the Mechanical Properties of Ti-22Al-25Nb Alloy Based on Three Typical Microstructures; Northwestern Polytechnical University: Xi’an, China, 2015. [Google Scholar]
- Du, G.; Cui, L.; Lei, Q.; Zhang, H.; Li, Y.; Wang, X.; Chen, J.; Zhao, M.; Liu, Z.; Feng, S.; et al. Research Progress on O-phase Alloy Ti2AlNb. Prog. Mater. Sci. China 2018, 37, 68–73. [Google Scholar]
- Zhang, H.Y.; Zhang, Y.R.; Liang, H.Y.; Liu, Y.; Wang, J.; Chen, X.; Zhao, T.; Li, M.; Huang, S.; Yang, L.; et al. Effect of the primary O phase on thermal deformation behavior of a Ti2AlNb-based alloy. J. Alloys Compd. 2020, 846, 156458. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yan, N.; Liang, H.Y.; Liu, Y.C. Phase transformation and microstructure control of Ti2AlNb-based alloys: A review. J. Mater. Sci. Technol. 2021, 80, 203–216. [Google Scholar] [CrossRef]
- Gao, G.F.; Fu, Z.X.; Wang, Y.; Xiang, D.H.; Zhhao, B. Research progress on precision machining of Ti-Al intermetallic compounds. Rare Met. Mater. Eng. 2021, 50, 1867–1882. [Google Scholar]
- Wang, W.; Zeng, W.; Xue, C.; Liu, Y.; Zhang, H.; Li, S.; Chen, J.; Zhao, M.; Feng, S.; Yang, L.; et al. Microstructural evolution, creep, and tensile behavior of a Ti-22Al-25Nb (at%) orthorhombic alloy. Mater. Sci. Eng. A 2014, 603, 176–184. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhou, J.P.; Chen, X.K.; Liu, Y.; Zhang, H.; Li, S.; Zhao, M.; Feng, S.; Yang, L.; Wang, J.; et al. Experimental investigation on Ti2AlNb in high-efficiency short electric arc milling. Int. J. Adv. Manuf. Technol. 2024, 131, 4361–4375. [Google Scholar] [CrossRef]
- Xia, Z.W.; Gao, G.F.; Wang, Y.; Liu, Y.; Zhang, H.; Li, S.; Chen, X.; Zhao, M.; Feng, S.; Yang, L.; et al. Surface micro-texture and tribological properties in longitudinal-torsional ultrasonic-assisted milling Ti2AlNb. Int. J. Adv. Manuf. Technol. 2023, 126, 1919–1935. [Google Scholar] [CrossRef]
- Ahmed Reda Mohamed, A.; Shahin, P.M. Review of Material Selection for Corrosion-Resistant Alloy Pipelines. Eng. Sci. 2025, 33, 1373. [Google Scholar]
- Illrionov, A.G.; Stepanov, S.I.; Naschetnikov, I.A.; Popov, A.A.; Soundappan, P.; Thulasi, R.K.H.; Suwas, S. A Review-Additive Manufacturing of Intermetallic Alloys Based on Orthorhombic Titanium Aluminide Ti2AlNb. Materials 2023, 16, 991. [Google Scholar] [CrossRef]
- Shi, K.; Shan, D.B.; Xu, W.C.; Li, Y.; Zhang, H.; Wang, J.; Chen, X.; Zhao, M.; Liu, Z.; Yang, L.; et al. Near net shape forming process of a titanium alloy impeller. J. Mater. Process. Technol. 2007, 187, 582–585. [Google Scholar] [CrossRef]
- Huang, X.; He, Y.; Wang, J.; Liu, Z.; Zhang, H.; Li, S.; Chen, X.; Zhao, M.; Feng, S.; Yang, L.; et al. Research Progress on Near-net Shaping Technology of Titanium and Titanium Alloy Powder. Powder Metall. Ind. 2022, 32, 34–43. [Google Scholar] [CrossRef]
- Chen, G.; Lu, X.; Zhang, L.; Li, Y.; Wang, J.; Zhao, M.; Liu, Z.; Chen, X.; Feng, S.; Yang, L.; et al. Research Progress on Powder Preparation and Near-net Shaping of Titanium and Titanium Alloys. Mater. Sci. Technol. 2020, 28, 98–108. [Google Scholar]
- Debroy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components–process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Dong, Y.P.; Li, Y.L.; Zhou, S.Y.; Zhang, J.; Liu, X.B.; Wang, H.; Chen, M.X.; Huang, Y.; Zhang, Z.; Sun, D.L.; et al. Cost-affordable Ti-6Al-4V for additive manufacturing: Powder modification, compositional modulation and laser in-situ alloying. Addit. Manuf. 2021, 37, 101699. [Google Scholar] [CrossRef]
- Hou, Y.H.; Liu, B.; Liu, Y.; Zhang, H.; Wang, J.; Li, S.; Chen, X.; Zhao, M.; Feng, S.; Yang, L.; et al. Ultra-low cost Ti powder for selective laser melting additive manufacturing and superior mechanical properties associated. Opto-Electron. Adv. 2019, 2, 10–17. [Google Scholar] [CrossRef]
- Singh, N.P.; Hameed, R.; Ummethal, R.; Kumar, S.; Sharma, P.; Gupta, A.; Verma, N.; Singh, A.; Mehta, D.; Joshi, R.; et al. Selective laser manufacturing of Ti-based alloys and composites: Impact of process parameters, application trends, and future prospects. Mater. Today Adv. 2020, 8, 10009. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, W.P.; Wang, D.W.; Zhang, H.; Liu, Y.; Chen, X.; Zhao, M.; Feng, S.; Yang, L.; Wang, J.; et al. Selective laser melting enabled additive manufacturing of Ti-22Al-25Nb intermetallic: Excellent combination of strength and ductility, and unique microstructural features associated. Acta Mater. 2019, 173, 117–129. [Google Scholar] [CrossRef]
- Polozov, I.; Gracheva, A.; Popovich, A. Interface Characterization of Bimetallic Ti-6Al-4V/Ti2AlNb Structures Prepared by Selective Laser Melting. Materials 2022, 15, 8528. [Google Scholar] [CrossRef]
- Grigoriev, A.; Polozov, I.; Sufiiarov, V.; Shaysultanov, D.; Masaylo, D.; Popovich, A.; Salishchev, G. In-situ synthesis of Ti2AlNb-based intermetallic alloy by selective laser melting. J. Alloys Compd. 2017, 704, 434–442. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Zhao, L.; Wang, J.; Li, S.; Zhang, H.; Liu, Y.; Chen, X.; Zhao, M.; Feng, S.; et al. Effect of scanning speed on microstructure and mechanical properties of as-printed Ti-22Al-25Nb intermetallic by laser powder bed fusion. Mater. Sci. Eng. A 2023, 885, 145652. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Bai, Q.; Xie, G. Correlation of microstructure and mechanical properties of Ti2AlNb manufactured by SLM and heat treatment. Intermetallics 2021, 139, 107367. [Google Scholar] [CrossRef]
- Man, J.; Huang, L.; He, J.; Wang, J.; Li, S.; Zhang, H.; Liu, Y.; Chen, X.; Zhao, M.; Feng, S.; et al. Effect of heat treatment on microstructure and properties of Ti2AlNb alloy formed by selective laser melting. J. Mater. Res. Technol. 2024, 33, 2549–2559. [Google Scholar] [CrossRef]
- Shao, L.; Chen, Y.; Datye, A.; Wang, J.; Li, S.; Zhang, H.; Liu, Y.; Chen, X.; Zhao, M.; Feng, S.; et al. Effect of Heat Treatment on Two-Phase Ti-22Al-25Nb Alloy Phase Composition and Microhardness. Met. Sci. Heat Treat. 2023, 65, 304–308. [Google Scholar] [CrossRef]
- Sun, W.; Niu, H.; Xia, Y.; Wang, J.; Li, S.; Zhang, H.; Liu, Y.; Chen, X.; Zhao, M.; Feng, S.; et al. Combined effects of local residual stresses, internal pores, and microstructures on the mechanical properties of laser-welded Ti-6Al-4V sheets. J. Mater. Sci. Technol. 2025, 216, 178–191. [Google Scholar] [CrossRef]
- Benjamin, G.; Amine, H.; Azziz, H.; Zhang, Y.; Liu, W.; Chen, X.; Wang, J.; Li, M.; Zhao, T.; Yang, L.; et al. Micro-mechanism and mechanical properties of solid-powder hot isostatic pressing diffusion-bonded Ti2AlNb alloy. Adv. Powder Technol. 2021, 32, 3610–3623. [Google Scholar]
- Liu, Y.; Yang, X.; Jiao, H. Enhancing high-temperature creep resistance of additively manufactured Ti2AlNb alloys via vacuum hot isostatic pressing-induced tailoring of coherent interfaces. Vacuum 2025, 239, 114392. [Google Scholar] [CrossRef]
- Polozov, I.; Sufiiarov, V.; Kantyukov, A.; Shaysultanov, D.; Popovich, A.; Salishchev, G. Selective Laser Melting of Ti2AlNb-based intermetallic alloy using elemental powders: Effect of process parameters and post-treatment on microstructure, composition, and properties. Intermetallics 2019, 112, 106554. [Google Scholar] [CrossRef]
- Polozov, I.; Popovich, A.; Sufiiarov, V. Effects of Heat Treatment and Hot Isostatic Pressing on Microstructure and Mechanical Properties of Ti2AlNb-Based Alloy Fabricated by SLM. In Proceedings of the METAL 2021, Brno, Czech Republic, 26–28 May 2021. [Google Scholar]
- Polozov, I.; Starikov, K.; Popovich, A.; Sufiiarov, V.; Masaylo, D.; Salishchev, G. Mitigating inhomogeneity and tailoring the microstructure of selective laser melted titanium orthorhombic alloy by heat treatment, hot isostatic pressing, and multiple laser exposures. Materials 2021, 14, 4946. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Majumdar, B.S.; Seetharaman, V.; Miracle, D.B. Part I. The microstructural evolution in Ti-Al-Nb O+Bcc orthorhombic alloys. Met. Mater. Trans. A 1999, 30, 2305–2323. [Google Scholar] [CrossRef]
- Muraleedharan, K.; Banerjee, D.; Banerjee, S.; Nag, S.; Banerjee, R.; Chaturvedi, M.C. The α2-to-O transformation in Ti-Al-Nb alloys. Philos. Mag. A 1995, 1011–1036. [Google Scholar] [CrossRef]
- Huang, Y. Heat Treatment Organization Evolution Mechanism of Ti-22Al-25Nb Alloy; Tianjin University: Tianjin, China, 2019. [Google Scholar]
- Tang, Y. Research on the Basic Problems of Laser Additive Manufacturing Ti-22Al-25Nb Alloy; Beijing General Research Institute of Nonferrous Metals: Beijing, China, 2018. [Google Scholar]
- Liu, B.; Zhang, G.; Wu, F. Phase-Field Modeling of Crack Propagation Behavior at RimO/α2 Interfaces in Titanium Aluminides. Int. J. Plast. 2023, 165, 103612. [Google Scholar]
- Wang, L.; Xu, C.; Zhou, Y. Oxygen Diffusion Inhibition and Embrittlement Resistance of RimO-Modified Ti3Al-Based Alloys at Elevated Temperatures. Corros. Sci. 2021, 191, 109735. [Google Scholar]
- Xu, J.; Huang, K.; Sun, M. Synergistic Strengthening-Toughening Mechanisms in Ti2AlNb Alloys with RimO-Encapsulated α2 Microstructures. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar]
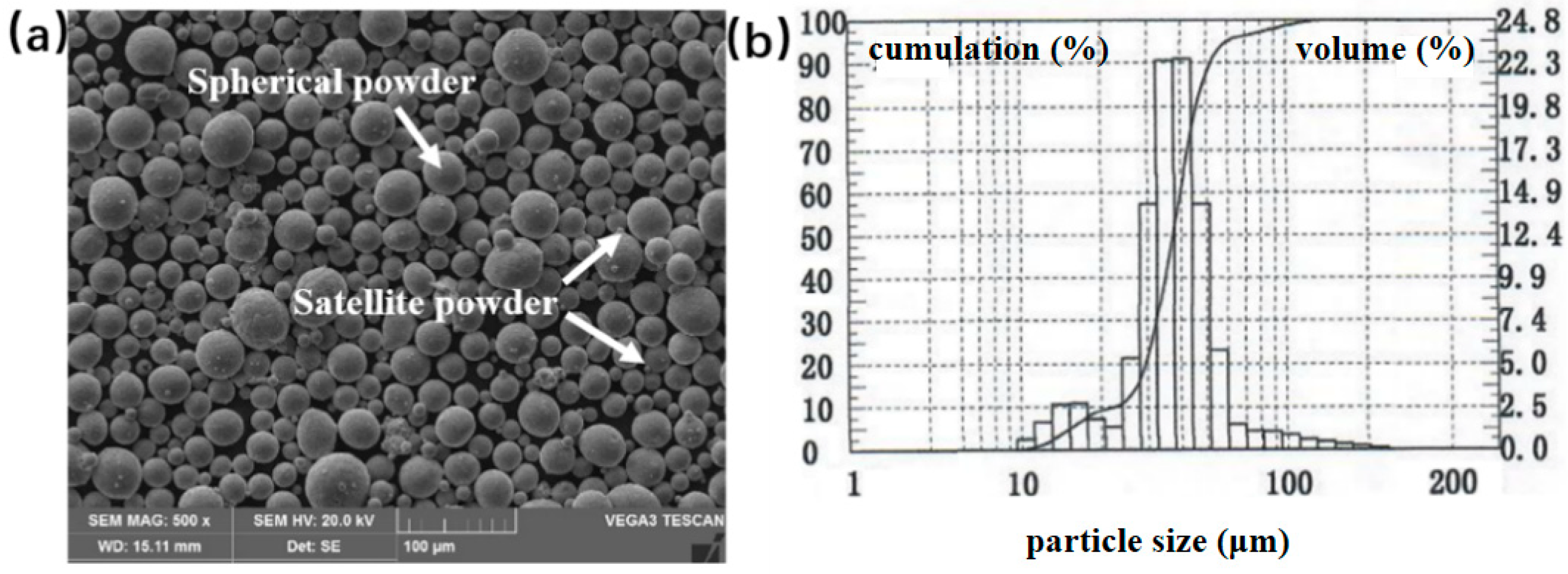
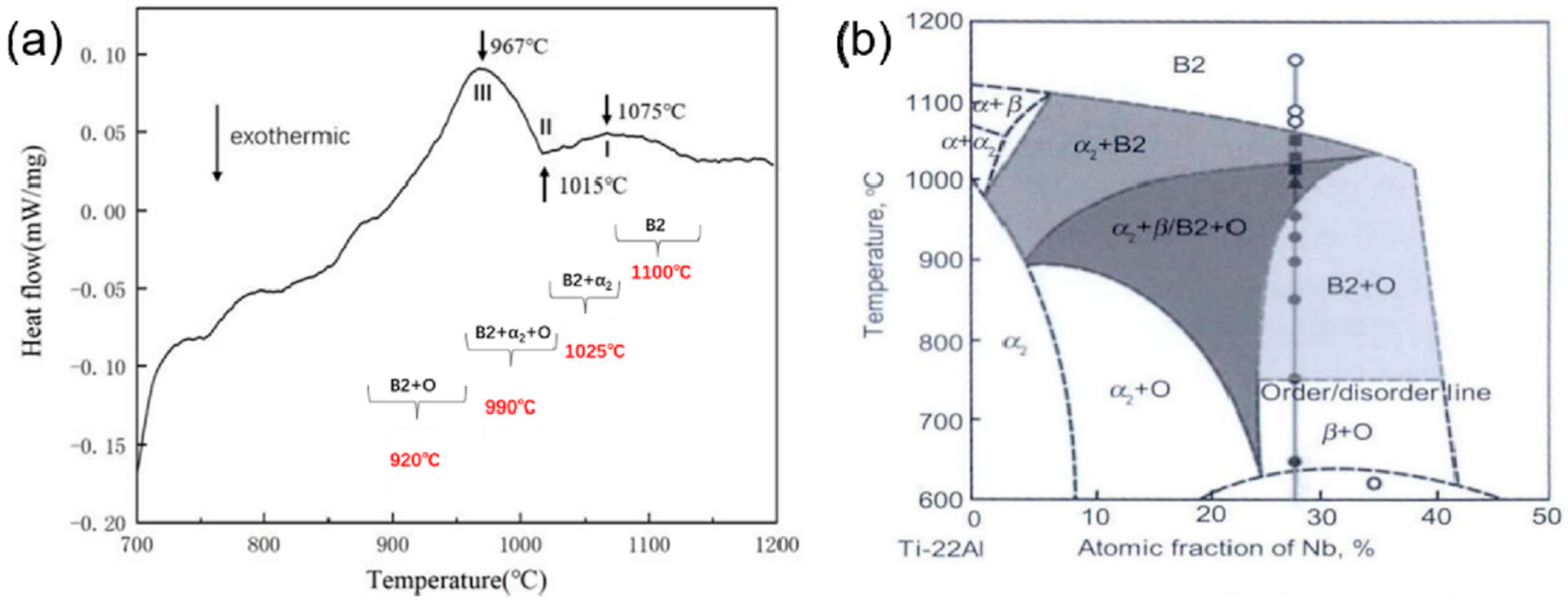
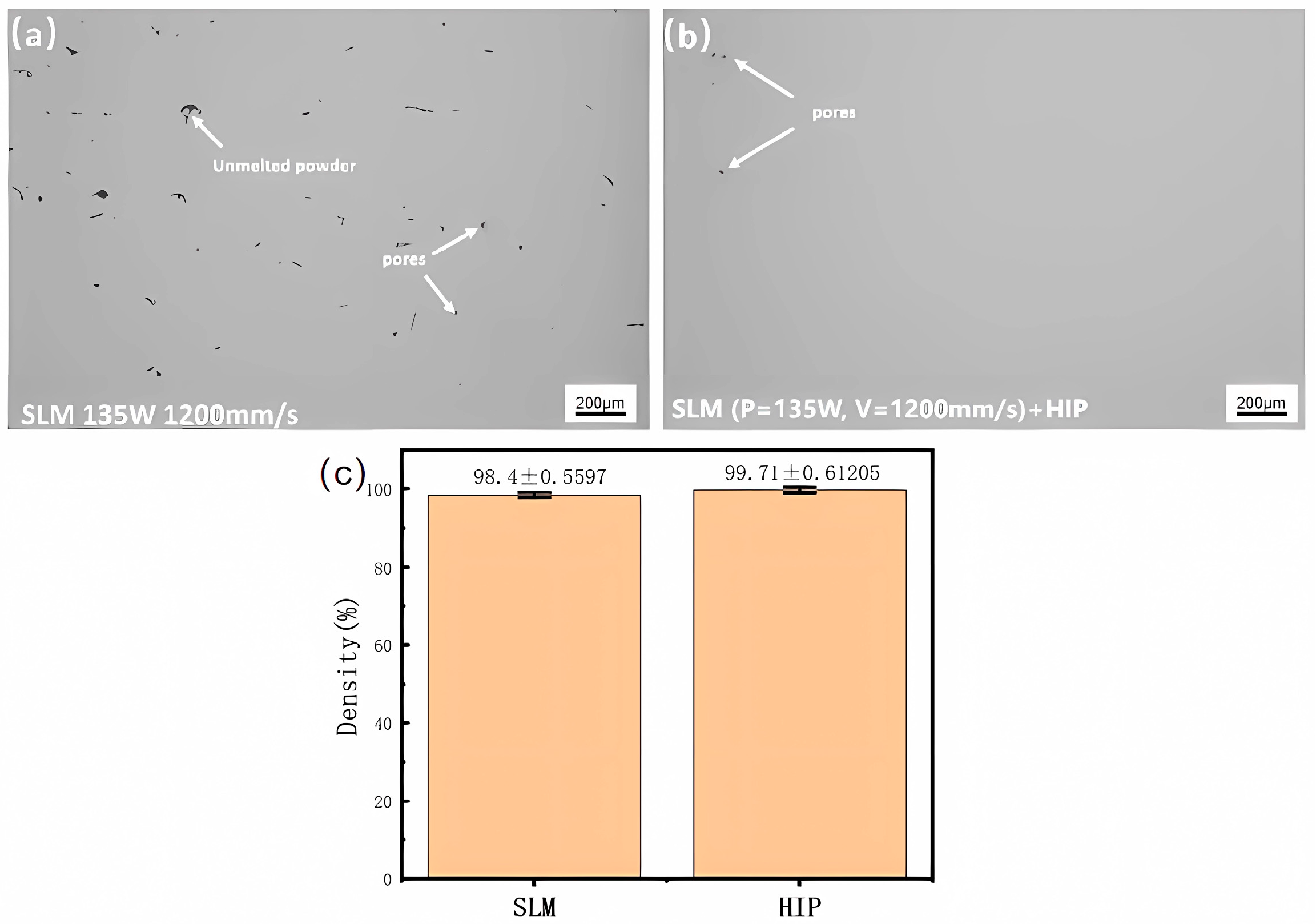


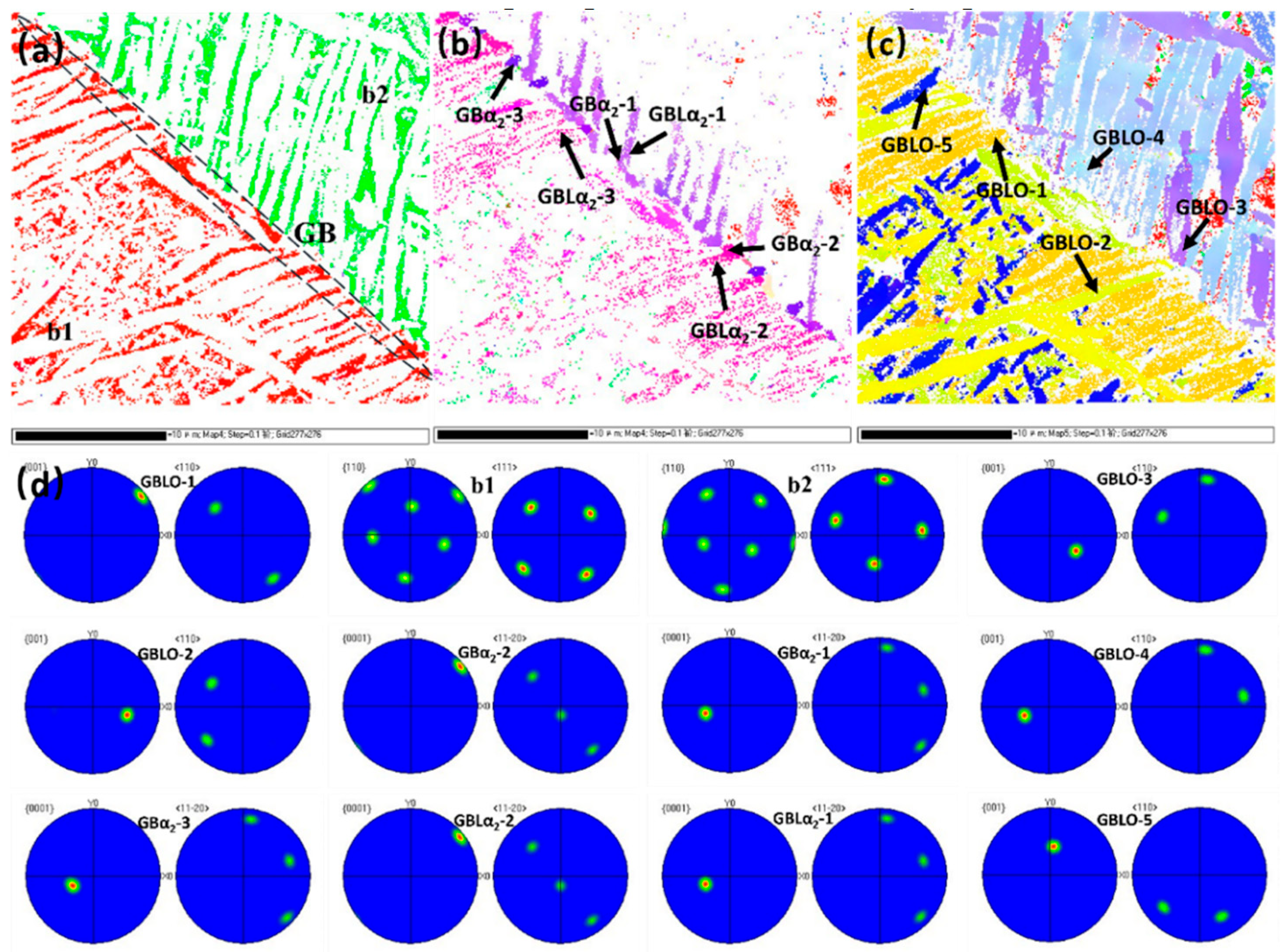



| Element | Al | Nb | Ti |
|---|---|---|---|
| at.% | 22.27 | 27.34 | 50.39 |
| Specimens | Solution | Cooling Mode | Aging | Cooling Mode |
|---|---|---|---|---|
| ST920 | 920 °C/1 h | water-cooling | — | — |
| ST990 | 990 °C/1 h | water-cooling | — | — |
| ST1025 | 1025 °C/1 h | water-cooling | — | — |
| ST1100 | 1100 °C/1 h | water-cooling | — | — |
| ST920 + AT | 920 °C/1 h | water-cooling | 800 °C/5 h | air-cooling |
| ST990 + AT | 990 °C/1 h | water-cooling | 800 °C/5 h | air-cooling |
| ST1025 + AT | 1025 °C1 h | water-cooling | 800 °C/5 h | air-cooling |
| ST1100 + AT | 1100 °C/1 h | water-cooling | 800 °C/5 h | air-cooling |
| Specimens | Solution | VFRP (%) | Main Characteristics of Precipitates |
|---|---|---|---|
| ST920 | 920 °C/1 h | 70.48 | α2 + O + B2, acicular O dissolved |
| ST990 | 990 °C/1 h | 49.22 | α2/O phase mixed laths, B2 increased |
| ST1025 | 1025 °C/1 h | 20.83 | GBLO, acicular O completely dissolved |
| ST1100 | 1100 °C/1 h | 5.47 | Only Lathα2, GBα2 dissolved |
| Specimens | Solution | VFRP (%) | Main Characteristics of Precipitates |
|---|---|---|---|
| ST920 + AT | 920 °C/1 h | 72.63 | smaller acicular O, O growth and coarsening |
| ST990 + AT | 990 °C/1 h | 74.63 | abundant acicular O, Lath O coarsening |
| ST1025 + AT | 1025 °C/1 h | 73.78 | Lath α2 wrapped rimO, α2 decomposed into O |
| ST1100 + AT | 1100 °C/1 h | 75.46 | abundant dispersed acicular O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Yang, H.; Huang, L.; Man, J.; Wu, Y.; Lin, X. Effect of Hot Isostatic Pressure on the Microstructure Evolution of Ti-22Al-25Nb Alloy Formed by Selective Laser Melting. Materials 2025, 18, 2806. https://doi.org/10.3390/ma18122806
He J, Yang H, Huang L, Man J, Wu Y, Lin X. Effect of Hot Isostatic Pressure on the Microstructure Evolution of Ti-22Al-25Nb Alloy Formed by Selective Laser Melting. Materials. 2025; 18(12):2806. https://doi.org/10.3390/ma18122806
Chicago/Turabian StyleHe, Jingjun, Haiou Yang, Linhao Huang, Jingyu Man, Yuhan Wu, and Xin Lin. 2025. "Effect of Hot Isostatic Pressure on the Microstructure Evolution of Ti-22Al-25Nb Alloy Formed by Selective Laser Melting" Materials 18, no. 12: 2806. https://doi.org/10.3390/ma18122806
APA StyleHe, J., Yang, H., Huang, L., Man, J., Wu, Y., & Lin, X. (2025). Effect of Hot Isostatic Pressure on the Microstructure Evolution of Ti-22Al-25Nb Alloy Formed by Selective Laser Melting. Materials, 18(12), 2806. https://doi.org/10.3390/ma18122806








